Abstract
A major complication with enzyme replacement therapy of Factor VIII (FVIII) in Hemophilia A (HA) is the development of anti-drug antibodies. Recently, we have shown that FVIII administration in the presence of heterogeneous phosphatidylserine (PS) nanoparticles derived from a natural source induces tolerance to FVIII, suggesting that PS converts an immunogen to a tolerogen. However, the specific structural features responsible for the immune-regulatory properties of PS is unclear. Identifying a specific PS species that is responsible is critical in order to further develop and optimize this nanoparticle. Further, clinical development of this lipid-based strategy requires optimization of the lipid particle that is homogeneous and synthetic. Here, we investigate the ability of mono-acylated Lyso-PS to induce hypo-responsiveness towards FVIII in HA mice. Administration of both PS and Lyso-PS FVIII significantly reduced anti-FVIII antibody responses despite rechallenge with FVIII. Additionally, the Lyso-PS-mediated effect was shown to be antigen-specific as mice responded normally against a rechallenge with an unrelated antigen, ovalbumin. Furthermore, the hypo-responsiveness observed with Lyso-PS may involve interactions with a specific PS receptor, TIM-4, along with increasing regulatory T-cells. These data indicate that using Lyso-PS allows for a more homogenous formulation in order to induce tolerance towards therapeutic proteins.
Keywords: Phosphatidylserine, Nanoparticles, Immunogenicity, Tolerance Induction, Biotechnology
1. INTRODUCTION
Factor VIII (FVIII) is a large, multi-domain glycoprotein that is an important clotting protein in the blood coagulation cascade. The genetic deficiency or dysfunction of FVIII manifests in the bleeding disorder, Hemophilia A (HA). The use of recombinant FVIII is the first line of therapy for patients with this disease. However, one of the main challenges with HA therapy is the development of anti-FVIII antibodies. These antibodies can be binding antibodies, which could enhance FVIII clearance, or neutralizing, also known as inhibitory antibodies, which are observed in 15–30% of patients (Lollar et al., 2001). The development of inhibitors presents a clinical challenge, as these inhibitors abrogate the activity of FVIII, potentially compromising the safety and efficacy of therapy (Klinge et al., 2002; Lollar et al., 2001). Any strategy to mitigate immunogenicity and induce tolerance towards FVIII would address an urgent clinical need.
Our previous studies have shown that pre-exposure of FVIII associated with phosphatidylserine (PS) liposomes, derived from a natural source that is heterogeneous in its structure in terms of acyl chain length and degrees of unsaturation, was able to induce tolerance and significantly reduce antibody responses towards FVIII after subsequent rechallenges with free FVIII in HA mice (Gaitonde et al., 2013). Further studies aimed to elucidate the mechanism of PS-mediated hypo-responsiveness showed that PS is able to induce the expansion of FoxP3+ regulatory T cells (Tregs) while inhibiting memory B cell production in vivo (Gaitonde et al., 2013; Ramakrishnan et al., 2015). These results indicate that PS is able to induce tolerance by converting an immunogen to a tolerogen.
Although we established that PS is able to induce tolerance, it is not yet clear whether a structurally specific PS is responsible for the PS-mediated effects that were previously observed. The conventional formulation used consists of several structurally different PS species. The identification of a specific PS species responsible for converting an immunogen to a tolerogen is essential as the design of a tolerogenic and homogeneous nanoparticle is desired for clinical development to induce more effective tolerance.
Lyso-phosphatidylserine (Lyso-PS) is a mono-acylated species with one fatty acid acyl chain and one degree of unsaturation. It has been suggested in the literature that Lyso-PS may have a critical role in immune regulation (Bellini and Bruni, 1993; Frasch and Bratton, 2012; van der Kleij et al., 2002). It has been shown that Lyso-PS can inhibit T-cell activation and induce apoptosis and clearance of neutrophils to resolve inflammation (Bellini and Bruni, 1993; Frasch and Bratton, 2012; Frasch et al., 2013). In the present work, we investigated the ability of Lyso-PS nanoparticles to induce immunological tolerance compared to our conventional PS liposomes and investigate the role of a PS receptor and Tregs in the Lyso-PS-mediated mechanism.
2. MATERIALS AND METHODS
2.1. Materials
Dimyrisotylphosphatidylcholine (DMPC), brain PS, and Lyso-PS were purchased from Avanti Polar Lipids (Alabaster, AL). Brain PS includes structural heterogeneity relating to acyl chain length and number of unsaturation, with 18-carbon chain length with no or one unsaturation being the pre-dominant species. In addition, the Lyso-PS species is an 18-carbon acyl chain length and one unsaturation (18:1). Full length recombinant human FVIII was provided as a generous gift from the Hemophilia Center of Western New York (Buffalo, NY). All solvents and buffer salts were obtained from Fisher Scientific (Fairlawn, NJ). Endograde ovalbumin (OVA) was purchased from BioVender LLC (Asheville, NC). Murine anti-TIM-4 antibody was purchased from BioLegend (San Diego, CA). Anti-FVIII monoclonal antibody ESH8 was obtained from Sekisui Diagnostics (Lexington, MA). Alkaline phosphatase-conjugated goat anti-mouse Ig antibody was purchased from Southern Biotech (Birmingham, AL). p-nitrophenylphosphate (pNPP) substrate system was purchased from SeraCare Life Sciences (Milford, MA). Horseradish peroxidase-conjugated goat anti-mouse IgG antibody and 3,3’,5,5’-tetramethylbenzidine substrate (TMB) were purchased from Sigma Aldrich (St. Louis, Missouri). Endosafe Endochrome-K® Kit was purchased from Charles River Laboratories (Charleston, SC). NUNC MaxiSorp 96 well plates were purchased from Thermo Scientific (Waltham, MA).
2.2. Animals
A colony of hemophilia mice with a targeted deletion in exon 16 of the FVIII gene, termed HA mice, was maintained onsite. An additional colony of hemophilic mice with green fluorescent protein (GFP) knocked-in to the FoxP3 gene (termed GFP-FoxP3 HA mice) was also maintained onsite and used for the antigen specificity study. This animal model allows for the ease of tracking the expression of FoxP3+ Tregs. All animal experiments were conducted under approval and following the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo, The State University of New York.
2.2. Preparation of Protein-Lipid Complexes
PS and Lyso-PS liposomes were prepared at a 30:70 molar ratio of PS or Lyso-PS to DMPC as previously described (Ramani et al., 2008). Lipid content was confirmed using a phosphate assay (Bartlett, 1959). The protein to lipid molar ratio used was 1:10,000 for all experiments. FVIII was associated with liposome formulations by a trigger-loading mechanism of incubation at 37°C for 30 minutes to allow a gentle and controlled thermal unfolding procedure to promote interaction of FVIII with the PS and Lyso-PS liposomes. This interaction results in approximately 45.2 ± 16.8% of FVIII to be associated with the PS liposomes (Purohit et al., 2003). Association efficiency of FVIII with Lyso-PS liposomes was determined with discontinuous dextran gradient centrifugation, modified from a protocol previously described (Heath et al., 1981; Shetty et al., 2015). Briefly, Lyso-PS FVIII was loaded into the bottom layer of a 0%/20%/30% dextran gradient. After ultracentrifugation at 190,000g for 1 hour at 4°C, Lyso-PS liposomes and their associated protein floated to the interface of the buffer/20% dextran bands. The amount of associated protein was determined with the aPTT assay and compared with a standard curve of known FVIII activity. All formulations were tested for endotoxin level by using Endosafe Endochrome-K endotoxin assay kit (Charleston, SC) and only endotoxin negative samples were used for in vivo studies.
2.3. Biophysical Characterization of Lipid Particles
The lipid particles alone as well as the lipid-protein complexes were characterized for the size and zeta potential. The size of the unloaded and FVIII-associated liposomes were confirmed using a NICOMP Model CW380 particle size analyzer from Particle Sizing Systems (Port Richey, FL). Zeta potential for the unloaded and FVIII-associated liposomes were analyzed for surface charge as previously described using Nanobrook Omni zeta potential analyzer from Brookhaven Instruments Corporation (Holtsville, NY) (Schneider et al., 2018). Briefly, samples were allowed to equilibrate for 60 seconds and zeta potential measured at 25°C and calculated using the Smoluchowski equation. The reported results (mean ± SD) are the average of three independent experiments.
2.4. Lyso-PS FVIII Immunogenicity Study
The relative immunogenicity of Lyso-PS FVIII and its ability to induce tolerance was evaluated in HA mice. HA mice (n=7/group) were immunized with 0.4 μg of FVIII subcutaneously (SC) in the presence and absence of PS or Lyso-PS once a week for nine weeks. Beginning on the sixth week, mice were rechallenged with 0.4 μg of free FVIII intravenously (IV) via the tail vein 24 hours after SC immunization once a week for four weeks. Two weeks after the last rechallenge, all mice were sacrificed and plasma collected via cardiac puncture in 10% v/v acid citrate dextrose solution. Plasma samples were stored at −80°C until further analysis.
2.5. Antigen Specificity Study
The antigen specificity of Lyso-PS liposomes was evaluated in GFP-FoxP3 HA mice. Mice (n=18/group) were immunized with 0.4 μg of FVIII SC in the presence and absence of Lyso-PS once a week for four weeks. Two weeks after the SC immunization, six mice from each treatment group were sacrificed and plasma was collected to assess baseline antibody development. Half of the remaining mice were then rechallenged with two weekly SC injections of 0.4 μg of free FVIII while the remaining mice were rechallenged with two weekly SC injections of 0.4 μg of free OVA. Two weeks after the last rechallenge, all remaining mice were sacrificed and plasma collected and stored at −80°C until further analysis.
2.6. Role of TIM-4 Immunogenicity Study
The involvement of T cell immunoglobulin and mucin domain containing receptor-4 (TIM-4) in the ability of Lyso-PS to induce tolerance was evaluated in GFP-FoxP3 HA mice. To investigate the role of TIM-4 receptor in Lyso-PS-mediated tolerance, we used a function blocking anti-TIM-4 antibody due to the exclusive expression of TIM-4 on antigen presenting cells (APCs) and the expected PS-mediated effects through dendritic cells (DCs) by skewing the DCs towards a tolerogenic phenotype (Gaitonde et al., 2013; Kobayashi et al., 2007; Miyanishi et al., 2007). Mice (n=7/group) were immunized with four weekly SC injections of 0.4 μg FVIII in the presence and absence of Lyso-PS. A third group of mice received a SC injection of 15 μg of anti-TIM-4 antibody thirty minutes prior to administration of the Lyso-PS FVIII formulation in close proximity. Following a two week washout period, animals were rechallenged with two weekly SC injections of 0.4 μg of free FVIII alone. Two weeks after the last rechallenge injection, all mice were sacrificed and plasma collected and stored at −80°C until further analysis.
2.7. Role of Regulatory T Cells in Lyso-PS-Mediated Effects
Mice used in the above study (described in Section 2.6 “Role of TIM-4 Immunogenicity Study”) were analyzed for Treg expression. Since the mice had GFP knocked in to the FoxP3 gene, GFP expression was used as a marker for FoxP3-Treg expression. Inguinal lymph nodes were isolated from the mice and prepared for flow cytometry using BD LSRFortessa (Pittsburgh, PA). The dot plots were analyzed using FlowJo (Ashland, OR). Further, total lymphocytes were selected based on side scatter versus forward scatter criteria. A histogram analysis was used to analyze GFP expression and the data expressed as the percentage of GFP-FoxP3+ cells in the total lymphocytes region.
2.8. Determination of Inhibitory Titers and Total Anti-FVIII Titers
Inhibitory anti-FVIII titers were quantified using an activated partial thromboplastin time (aPTT) assay following Nijmegen’s modified Bethesda assay and expressed in Bethesda Units (BU)/mL (Verbruggen et al., 1995). Total anti-FVIII titers were determined by ELISA as previously described (Ramani et al., 2008).
2.9. Determination of Anti-OVA IgG Antibodies
Anti-OVA IgG antibodies were measured using an ELISA assay modified from a procedure that was previously described (Schneider and Balu-Iyer, 2016). Ninety six-well plates were coated with 2 μg/mL of OVA in phosphate buffered saline (PBS) overnight at 4°C. The following day, the plates were washed and blocked with 5% bovine serum albumin in PBS for 1 hour at 37°C. Following incubation, the plates were then washed and serial dilutions of sample plasma were added to the plate and incubated for 1 hour at room temperature. The plates were washed again, followed by the addition of goat anti-mouse IgG-HRP detection antibody and incubated for 1 hour at room temperature. The plates were washed again, and TMB substrate was added and the plate was allowed to develop in the dark. After 15 minutes, the reaction was stopped with 1N hydrochloric acid and the absorbance was read at 450 nm. Titers were determined using a statistically significant cutoff determined from sham-treated animals as previously described (Frey et al., 1998).
2.10. Statistical Analyses
All values are represented as mean ± standard error of the mean (SEM). All statistical analyses were performed using GraphPad Prism (La Jolla, CA). One-way ANOVA followed by Tukey’s post-hoc analysis or unpaired two-tailed Student’s t-test were performed as indicated. P values < 0.05 were considered statistically significant.
3. RESULTS
3.1. Biophysical Characterization of Lipid Particles
The size and zeta potential of unloaded PS and Lyso-PS liposomes as well as the lipid-FVIII complexes are summarized in Table 1. The average size and zeta potential of PS liposomes were 193.9 ± 69.2 nm and −27.64 ± 4.00 mV, respectively. When FVIII was associated with PS, PS-FVIII complexes displayed an average size of 199.6 ± 62.3 nm and zeta potential −18.77 ± 3.51 mV. For Lyso-PS liposomes, the average size of these liposomes were found to be smaller (105.3 ± 25.8 nm), despite being prepared in identical conditions as PS liposomes. The acyl chain length and one unsaturation present in Lyso-PS could potentially increase the curvature of the vesicle that contributes to the size reduction observed. Zeta potential of Lyso-PS liposomes were measured as −24.98 ± 3.67 mV. Lyso-PS-FVIII complexes displayed an average size of 169.7 ± 62.5 nm and zeta potential of −14.96 ± 1.85 mV. Association efficiency of PS FVIII was reported to be approximately 45.2 ± 16.8% and the protein to lipid topology was determined to be surface association of FVIII through the C2 domain (Purohit et al., 2003; Ramani et al., 2008). Association efficiency of Lyso-PS FVIII was determined to be 43.8 ± 7.4%.
Table 1.
Size and zeta potential of unloaded and FVIII-associated PS and Lyso-PS liposomes. Values are represented as mean ± SD of three independent experiments.
| Unloaded Liposomes | FVIII-Associated Liposomes | |||
| PS Liposomes |
Lyso-PS Liposomes |
PS FVIII | Lyso-PS FVIII | |
| Size ± SD (nm) | 193.9 ± 69.2 | 105.3 ± 25.8 | 199.6 ± 62.3 | 169.7 ± 62.5 |
| Zeta Potential ± SD (mV) |
−27.64 ± 4.00 | −24.98 ± 3.67 | −18.77 ± 3.51 | −14.96 ± 1.85 |
3.2. Lyso-PS FVIII Immunogenicity Study
Animals were immunized with the different formulations according to the protocol described in Figure 1A. If PS and Lyso-PS induces tolerance, then pre-exposure with FVIII in the presence of these PS nanoparticles would desensitize the mice towards FVIII and would expect to be hypo-responsiveness following rechallenge with free FVIII. In order to investigate whether Lyso-PS induces tolerance towards FVIII, plasma was collected after repeated rechallenges with free FVIII. As seen in Figure 1B, mice that were treated with Lyso-PS FVIII displayed a statistically significant lower total antibody responses (26141 ± 2329 arbitrary units) compared to those treated with free FVIII (55605 ± 9186 arbitrary units). A similar trend of reduced antibody development was observed with mice treated with PS FVIII, with titer values of 34756 ± 5773 arbitrary titer units. It is important to evaluate the impact of a tolerance induction strategy using Lyso-PS on anti-FVIII inhibitor development as this is more clinically meaningful. As seen in Figure 1C, mice that were treated with either PS FVIII or Lyso-PS FVIII displayed significant reduction in inhibitor development (608 ± 93 BU/mL and 670 ± 75 BU/mL, respectively) as compared to mice treated with free FVIII (1225 ± 87 BU/mL). Taken together, the data suggests that either liposome formulation can induce immunological tolerance towards FVIII.
FIGURE 1.
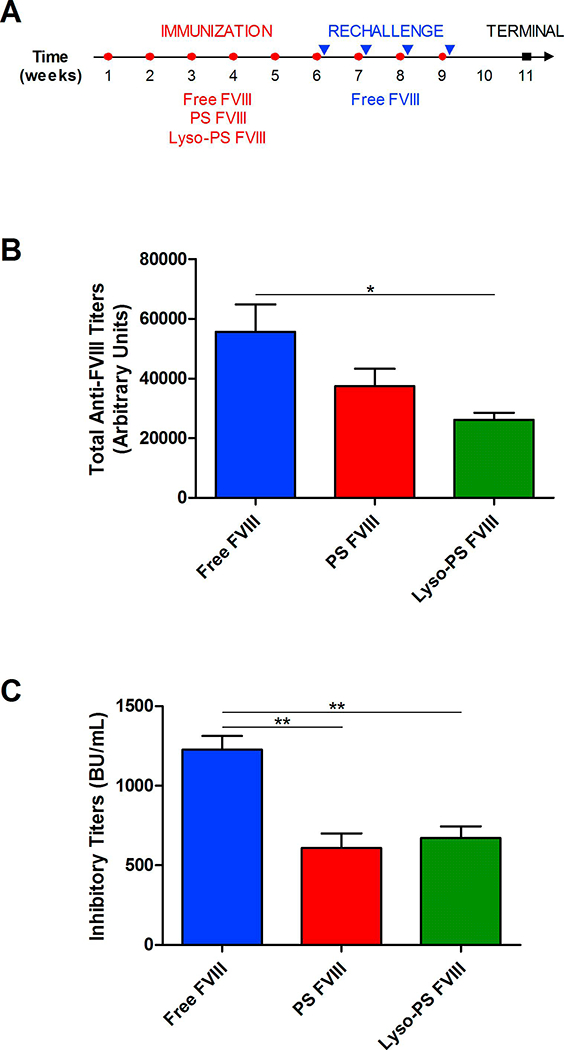
Lyso-PS induces tolerance towards FVIII in HA mice. (A) Immunization schedule utilized for Lyso-PS FVIII immunogenicity study. Open circles (○) represent immunizations and the closed triangles (▾) represent rechallenge injections of free FVIII 24 hours after immunization. The closed squares (∎) represent the terminal samples. (B) Total anti-FVII antibody titers (mean ± SEM) after rechallenge with free FVIII at week 11. (C) Inhibitory titers (mean ± SEM) after rechallenge with free FVIII at week 11. * denotes statistical significance with p < 0.05 by one-way ANOVA with Tukey’s post-hoc analysis.
3.3. Antigen Specificity Study
Previously, our lab has shown that PS is able to induce tolerance towards FVIII and this PS-mediated tolerance is antigen specific (Gaitonde et al., 2013; Ramakrishnan et al., 2015). Since we have shown that Lyso-PS is also able to induce tolerance, it is important to investigate whether the Lyso-PS-mediated effects were antigen specific as well. Lyso-PS FVIII-immunized animals were rechallenged with either free FVIII or OVA, according to the protocol in Figure 2A. If Lyso-PS exposure induces tolerance specific towards FVIII, then the FVIII rechallenge should result in hypo-responsiveness against FVIII but not for OVA. Baseline plasma samples were taken at week 6 before rechallenge in order to assess relative immunogenicity of FVIII and Lyso-PS FVIII. As seen in Figure 2B, mice that were pre-treated with Lyso-PS FVIII displayed a significant reduction in total antibody responses (3940 ± 1207 arbitrary units) compared to mice pre-treated with free FVIII (12786 ± 2646 arbitrary units). In mice that were then rechallenged with FVIII (Figure 2C), the total antibody responses were lower for the Lyso-PS FVIII pretreated group (11136 ± 3623 arbitrary units) as compared to free FVIII pre-treatment (22347 ± 4459 arbitrary units). In addition, 7/8 animals in the Lyso-PS FVIII pre-treated animals and then rechallenged with FVIII displayed total titers that were less than the mean of the free treatment group. Despite a clear trend towards decreased antibody responses of Lyso-PS, statistical significance could not be established as observed in Figure 1. This is partly due to the different HA mice strains and immunization protocol used for the experiments for Figures 1 and 2. Both pre-treatment groups showed comparable antibody responses towards OVA (Figure 2D). For FVIII pre-treatment, anti-OVA IgG (4237 ± 755 arbitrary units) were observed that were comparable to the Lyso-PS FVIII pre-treatment (3884 ± 746 arbitrary units).
FIGURE 2.
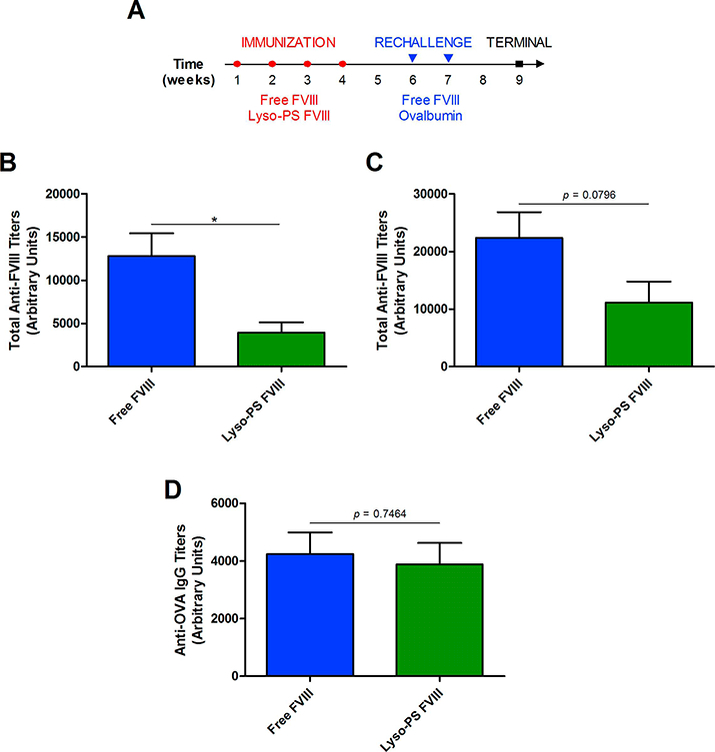
Antigen specificity of Lyso-PS-mediated tolerance induction. (A) Immunization schedule used for Lyso-PS antigen specificity study. Open circles (○) represent SC immunizations during the preexposure period and the closed triangles (▾) represent rechallenge injections of either free FVIII or OVA. The closed squares (∎) represent the terminal samples. (B) Total anti-FVII antibody titers (mean ± SEM) at baseline on week 6. (C) Total anti-FVIII antibody titers (mean ± SEM) after rechallenge with free FVIII at week 9. (D) Anti-OVA IgG titers (mean ± SEM) after rechallenge with OVA at week 9. * denotes statistical significance with p < 0.05 by unpaired two-tailed Student’s t-test.
This data suggests that Lyso-PS-mediated effects are antigen specific.
3.4. Role of TIM-4 Immunogenicity Study
In order to elucidate the mechanism of Lyso-PS-mediated tolerance induction, we sought to investigate whether the TIM-4 receptor was involved in this process. TIM-4 is exclusively expressed on APCs and we hypothesize that PS/Lyso-PS can skew DCs towards a tolerogenic phenotype (Gaitonde et al., 2013; Kobayashi et al., 2007; Miyanishi et al., 2007). If TIM-4 is involved, then the administration of a function blocking TIM-4 antibody will prevent binding of Lyso-PS to the TIM-4 receptor that would result in a reversal of Lyso-PS-mediated effects on antibody production. Mice were immunized according to the protocol in Figure 3A followed by rechallenge administration of free FVIII. From Figure 3B, animals that were pre-treated with free FVIII demonstrated the greatest total antibody responses (18673 ± 4761 arbitrary units). Mice that were pre-treated with Lyso-PS FVIII displayed significantly lower total anti-FVIII antibody responses (5578 ± 1524 arbitrary units) even after FVIII rechallenge. However, this reduction in antibody development as observed with Lyso-PS was reversed upon administration of anti-TIM-4 antibody (14416 ± 1399 arbitrary units). This data suggests that the TIM-4 receptor may be involved in the mechanism of Lyso-PS tolerance induction.
FIGURE 3.
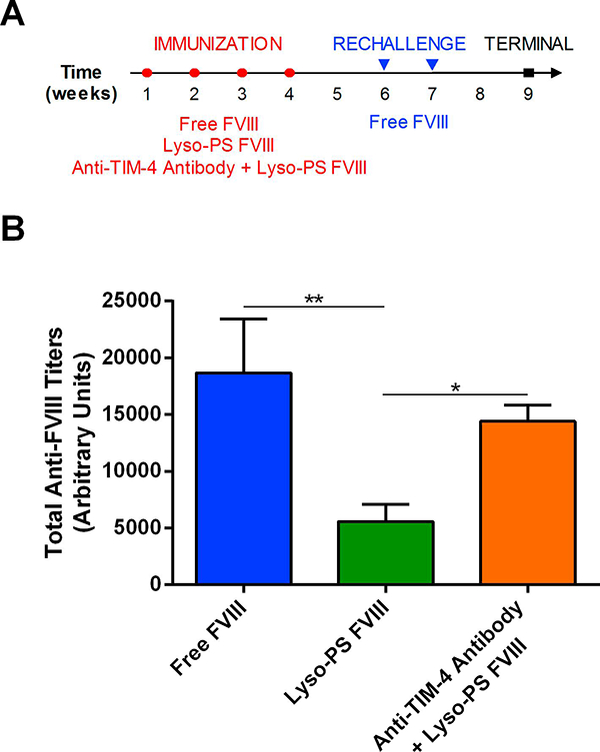
Lyso-PS-mediated hypo-responsiveness involves TIM-4 receptor. (A) Immunization schedule used for TIM-4 immunogenicity study. Open circles (○) represent SC immunizations during the pre-exposure period and the closed triangles (▾) represent rechallenge injections of free FVIII. The closed squares (∎) represent the terminal samples. (B) Total anti-FVIII antibody titers (mean ± SEM) after rechallenge with free FVIII at week 9. * denotes statistical significance with p < 0.05 by one-way ANOVA with Tukey’s post-hoc analysis.
3.5. Role of Regulatory T Cells in Lyso-PS-Mediated Effects
In order to further elucidate the mechanism of Lyso-PS-mediated effects, we investigated the role of Tregs in this mechanism. FoxP3-expressing cells were analyzed via flow cytometry as FoxP3 is a characteristic biomarker for Tregs (Hori et al., 2003). In this study, the animal model used is a HA model with GFP knocked into FoxP3, allowing for GFP expression to be a surrogate marker for Treg expression. As seen in Figure 4, the % GFP expression from Lyso-PS pre-treated animals were higher (4.45 ± 0.45%) compared to FVIII treated animals (4.02 ± 0.06%) and mice administered with anti-TIM-4 antibody prior to Lyso-PS FVIII (4.13 ± 0.20%). Although the differences were not statistically significant, these differences may still be immunologically significant since Tregs have the ability to suppress effector functions. However, given the small increase in Treg generation, other tolerance mechanisms and the involvement of other immune cells cannot be ruled out.
FIGURE 4.
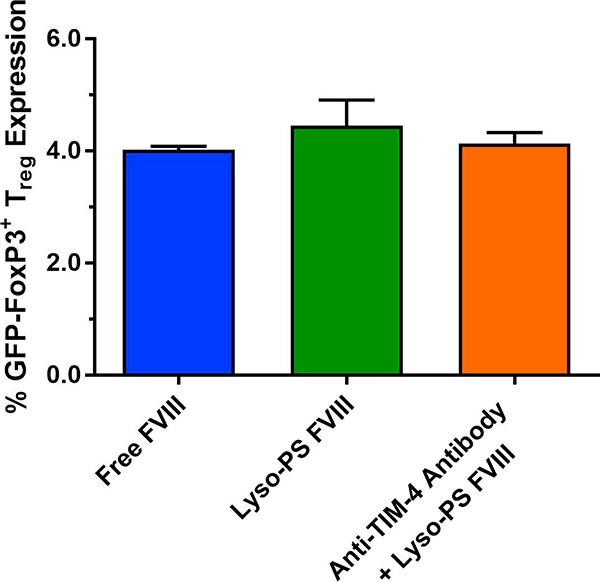
Lyso-PS-mediated hypo-responsiveness involves regulatory T cells. GFP-FoxP3+ Treg expression determined from inguinal lymph nodes isolated from mice immunized with free FVIII, Lyso-PS FVIII, and anti-TIM-4 antibody + Lyso-PS FVIII.
4. DISCUSSION
The development of antibody responses towards FVIII is one of the clinical complications that could compromise the safety and efficacy of therapy in HA patients (Lacroix-Desmazes et al., 2008; Lollar et al., 2001). Alternative treatments are available to these patients that include use of bypass agents and immune tolerance induction (ITI) therapy, where repeated FVIII administration is given to lower inhibitor development (Astermark, 2011; Kempton and White, 2009). However, the lack of uniform efficacy and cost-limitations of these therapies provide a challenge for patients. Thus, there is still an unmet clinical need to develop a safe and effective strategy to mitigate immunogenicity.
We have previously shown successful tolerance induction and hypo-responsiveness towards FVIII using PS liposomes. Mice that were pre-exposed to PS liposomes complexed with FVIII displayed a significant reduction in overall antibody development and inhibitor development, even after an aggressive rechallenge with the free protein (Gaitonde et al., 2013). Further studies to elucidate the mechanism of PS have shown that PS is able to act on multiple immune cells. We have previously demonstrated that PS is able to skew the DCs towards a tolerogenic phenotype, inhibit T cell activation, induce Tregs, and inhibit memory B cell production (Gaitonde et al., 2011; Gaitonde et al., 2013; Ramakrishnan et al., 2015). The PS tolerance induction strategy has also been extended to other disease areas such as Pompe disease and multiple sclerosis, indicating the broad applicability of this strategy (Schneider and Balu-Iyer, 2016, Glassman et al., 2018).
While we have shown that PS is able to reduce immunogenicity and induce tolerance towards the protein of interest, it is not yet clear whether there is a specific PS species that is responsible for this effect. In fact, our previous PS formulation consists of several structurally different species of PS. As a result, the identification of a specific PS species would allow the design of an optimal nanoparticle and allow for a homogeneous formulation in order to potentially induce more effective tolerance in this strategy for clinical development. Knowing that the mono-acylated Lyso-PS may be involved in immunomodulation and may offer unique structural and biophysical features (Bellini and Bruni, 1993; Frasch and Bratton, 2012; Frasch et al., 2013), we sought to investigate the impact of this particular species in its ability to induce tolerance towards FVIII.
The immunogenicity studies indicated that exposure with Lyso-PS FVIII was able to significantly reduce the overall antibody responses compared to the free FVIII control, even after an aggressive rechallenge that would mimic the initiation of clinical therapy (Figure 1B). Surprisingly, both liposome formulations displayed a significant reduction in the formulation of inhibitors and there was no apparent distinction between the two formulations (Figure 1C). Furthermore, it was observed that mice pre-treated with free FVIII or Lyso-PS FVIII developed a robust immune response towards ovalbumin, an irrelevant antigen, while observing a trend towards reduced FVIII-specific antibodies upon FVIII re-challenge, which suggests that Lyso-PS treatment may be antigen-specific (Figure 2). This data suggests that, although both formulations were able to induce tolerance subcutaneously to a similar extent, there may be several advantages to further move forward with Lyso-PS liposomes. The first advantage is that the previously used PS is from a brain source and contains severally structurally different species, with varying acyl chain lengths and degrees of saturation. Further developing and optimizing a nanoparticle using Lyso-PS would allow for a more homogeneous and synthetic formulation that would only contain one PS species in the composition. Another finding is that this data suggests that the structural characteristics of Lyso-PS with an 18-carbon acyl chain length and one unsaturation is also tolerogenic following SC administration.
PS is a naturally occurring phospholipid present in the inner leaflet of a healthy cell. However, during apoptosis, PS flips to the outer leaflet and serves as an “eat me” signal for the immune cells to clear the apoptotic cells in a non-inflammatory manner (Bar, 1996; Fadok et al., 1998; Fadok et al., 1992; Munoz et al., 2010). Our previous work with PS to reduce immunogenicity of enzyme replacement therapies has taught us that apoptosis may not be a silent event and that the exposure of PS may result in specific signaling effects that contribute to the active teaching process that PS has on the immune system to reduce immune responses towards a protein of interest.
Since we have shown that Lyso-PS can also convert an immunogen to a tolerogen, it is critical to elucidate the mechanism. It is believed that interactions between PS and its receptor are critical in mediating the immunosuppressive functions (Hoffmann et al., 2005). PS receptors that have been identified include stabilin-2 and members of the TIM family (Freeman et al., 2010; Park et al., 2008). Of these receptors, we investigated the role of TIM-4 in tolerance induction. TIM-4 is expressed exclusively on antigen presenting cells such as DCs and macrophages and has been shown to specifically recognize PS (Kobayashi et al., 2007; Miyanishi et al., 2007). Structural analysis indicate that TIM-4 contains a binding pocket specifically for the serine head group of PS. We have previously shown that TIM-4 is involved in the PS-mediated mechanism with our conventional PS formulation (Glassman et al., 2018) and here, we investigated whether that holds true for Lyso-PS as well. To our knowledge, the interaction between Lyso-PS and the TIM-4 receptor has not been identified previously. It is reasonable to expect that Lyso-PS can also interact with TIM-4 as the structural changes are within the acyl chain and not within the head group. In order to assess the involvement of TIM-4, a function blocking anti-TIM-4 antibody was administered, where it would prevent binding of Lyso-PS to the receptor and we would anticipate no reduction in antibody responses with administration of Lyso-PS FVIII. We have shown that Lyso-PS FVIII pre-treatment resulted in the hypo-responsiveness compared to free FVIII treatment. However, this reduction in antibody responses was reversed in mice that were first administered with the function blocking anti-TIM-4 antibody. This data then suggests that Lyso-PS also is able to interact with TIM-4.
Although we have looked specifically at TIM-4, recent literature suggests that Lyso-PS itself may have specific receptors it interacts with such as GPR34, G2A, and P2Y10 (Frasch and Bratton, 2012; Frasch et al., 2013; Kitamura et al., 2012). Not much is known about these receptors except that G2A is present on macrophages and that the exposure of Lyso-PS on neutrophils may interact with G2A receptors that allows for enhanced neutrophil clearance and contribute to the resolution of inflammation (Frasch and Bratton, 2012; Frasch et al., 2013). Here, we have also identified that TIM-4 may interact with Lyso-PS as well and this Lyso-PS/TIM-4 interaction may promote the signaling effects that allow for tolerance induction and hypo-responsiveness.
In summary, the biological properties of PS in its involvement of apoptosis have been harnessed and developed into a tolerogenic nanoparticle for tolerance induction towards therapeutic proteins. Identifying a specific PS species that is responsible is critical in order to further develop and optimize this nanoparticle. Based on the results described here, we hypothesize that Lyso-PS induces tolerance towards an antigen by converting an immunogen to a tolerogen and that this Lyso-PS-mediated process is antigen specific. We have also shown that the Lyso-PS mediated mechanism involves the interaction with the TIM-4 receptor and likely involves the induction of Tregs. Further investigation is necessary to fully elucidate its mechanism and optimize other factors such as the route of immunization in order to realize the full potential of this lipid-based strategy in order to translate it into the clinic.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institutes of Health R01 HL-70227 to SVB. The authors thank the Pharmaceutical Sciences Instrument Facility, University at Buffalo, The State University of New York, for the use of the shared microplate reader. We would like to thank Dr. Wojciech Krzyzanski for the use of the Auto Hematology analyzer. We are grateful to the Hemophilia Center of Western New York for providing the recombinant Factor VIII. We would like to thank Dr. Raymond Kelleher and the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York.
ABBREVIATIONS
- FVIII
Factor VIII
- PS
phosphatidylserine
- HA
Hemophilia A
- Tregs
regulatory T cells
- Lyso-PS
Lyso-phosphatidylserine
- DMPC
dimyrisotylphosphatidylcholine
- OVA
ovalbumin
- GFP
green fluorescent protein
- SC
subcutaneous
- IV
intravenous
- APC
antigen presenting cell
- aPTT
activated partial thromboplastin time
- BU
Bethesda Unit
- ITI
immune tolerance induction
- DC
dendritic cell
Footnotes
DECLARATIONS OF INTEREST
This work was supported by a grant from the National Institutes of Health R01 HL-70227 to SVB. Authors declare no other conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Astermark J, 2011. Immune tolerance induction in patients with hemophilia A. Thrombosis research 127 Suppl 1, S6–9. [DOI] [PubMed] [Google Scholar]
- Bar PR, 1996. Apoptosis--the cell’s silent exit. Life Sciences 59, 369–378. [DOI] [PubMed] [Google Scholar]
- Bartlett GR, 1959. Phosphorus assay in column chromatography. J Biol Chem 234, 466–468. [PubMed] [Google Scholar]
- Bellini F, Bruni A, 1993. Role of a serum phospholipase A1 in the phosphatidylserine-induced T cell inhibition. FEBS letters 316, 1–4. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM, 1998. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell death and differentiation 5, 551–562. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM, 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. Journal of immunology 148, 2207–2216. [PubMed] [Google Scholar]
- Frasch SC, Bratton DL, 2012. Emerging roles for lysophosphatidylserine in resolution of inflammation. Progress in lipid research 51, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch SC, Fernandez-Boyanapalli RF, Berry KA, Murphy RC, Leslie CC, Nick JA, Henson PM, Bratton DL, 2013. Neutrophils regulate tissue Neutrophilia in inflammation via the oxidant-modified lipid lysophosphatidylserine. J Biol Chem 288, 4583–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH, 2010. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunological reviews 235, 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Di Canzio J, Zurakowski D, 1998. A statistically defined endpoint titer determination method for immunoassays. Journal of immunological methods 221, 35–41. [DOI] [PubMed] [Google Scholar]
- Gaitonde P, Peng A, Straubinger RM, Bankert RB, Balu-Iyer SV, 2011. Phosphatidylserine reduces immune response against human recombinant Factor VIII in Hemophilia A mice by regulation of dendritic cell function. Clinical immunology 138, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde P, Ramakrishnan R, Chin J, Kelleher RJ, Bankert RB, Balu-Iyer SV, 2013. Exposure to Factor VIII Protein in the Presence of Phosphatidylserine Induces Hypo-responsiveness toward Factor VIII Challenge in Hemophilia A Mice. J Biol Chem 288, 17051–17056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman FY, Schneider JL, Ramakrishnan R, Dingman RK, Ramanathan M, Bankert RB, Balu-Iyer SV, 2018. Phosphatidylserine Is Not Just a Cleanup Crew but Also a Well-Meaning Teacher. Journal of pharmaceutical sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath TD, Macher BA, Papahadjopoulos D, 1981. Covalent attachment of immunoglobulins to liposomes via glycosphingolipids. Biochimica et biophysica acta 640, 66–81. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA, 2005. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. Journal of immunology 174, 1393–1404. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S, 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061. [DOI] [PubMed] [Google Scholar]
- Kempton CL, White GC 2nd, 2009. How we treat a hemophilia A patient with a factor VIII inhibitor. Blood 113, 11–17. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Makide K, Shuto A, Ikubo M, Inoue A, Suzuki K, Sato Y, Nakamura S, Otani Y, Ohwada T, Aoki J, 2012. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J Biochem 151, 511–518. [DOI] [PubMed] [Google Scholar]
- Klinge J, Ananyeva NM, Hauser CA, Saenko EL, 2002. Hemophilia A--from basic science to clinical practice. Seminars in thrombosis and hemostasis 28, 309–322. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ, 2007. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix-Desmazes S, Navarrete AM, Andre S, Bayry J, Kaveri SV, Dasgupta S, 2008. Dynamics of factor VIII interactions determine its immunologic fate in hemophilia A. Blood 112, 240–249. [DOI] [PubMed] [Google Scholar]
- Lollar P, Healey JF, Barrow RT, Parker ET, 2001. Factor VIII inhibitors. Adv Exp Med Biol 489, 65–73. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S, 2007. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439. [DOI] [PubMed] [Google Scholar]
- Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M, 2010. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nature reviews. Rheumatology 6, 280–289. [DOI] [PubMed] [Google Scholar]
- Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS, 2008. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell death and differentiation 15, 192–201. [DOI] [PubMed] [Google Scholar]
- Purohit VS, Ramani K, Kashi RS, Durrani MJ, Kreiger TJ, Balasubramanian SV, 2003. Topology of factor VIII bound to phosphatidylserine-containing model membranes. Biochimica et biophysica acta 1617, 31–38. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan R, Davidowitz A, Balu-Iyer SV, 2015. Exposure of FVIII in the Presence of Phosphatidyl Serine Reduces Generation of Memory B-Cells and Induces Regulatory T-Cell-Mediated Hyporesponsiveness in Hemophilia A Mice. Journal of pharmaceutical sciences 104, 2451–2456. [DOI] [PubMed] [Google Scholar]
- Ramani K, Miclea RD, Purohit VS, Mager DE, Straubinger RM, Balu-Iyer SV, 2008. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. Journal of pharmaceutical sciences 97, 1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Balu-Iyer SV, 2016. Phosphatidylserine Converts Immunogenic Recombinant Human Acid Alpha-Glucosidase to a Tolerogenic Form in a Mouse Model of Pompe Disease. Journal of pharmaceutical sciences 105, 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JL, Dingman RK, Balu-Iyer SV, 2018. Lipidic Nanoparticles Comprising Phosphatidylinositol Mitigate Immunogenicity and Improve Efficacy of Recombinant Human Acid Alpha-Glucosidase in a Murine Model of Pompe Disease. Journal of pharmaceutical sciences 107, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty KA, Kosloski MP, Mager DE, Balu-Iyer SV, 2015. Soy phosphatidylinositol containing nanoparticle prolongs hemostatic activity of B-domain deleted factor VIII in hemophilia A mice. Journal of pharmaceutical sciences 104, 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, Espevik T, de Jong EC, Kapsenberg ML, Golenbock DT, Tielens AG, Yazdanbakhsh M, 2002. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem 277, 48122–48129. [DOI] [PubMed] [Google Scholar]
- Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E, 1995. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thrombosis and haemostasis 73, 247–251. [PubMed] [Google Scholar]


