Abstract
Cryptococcus neoformans is a human fungal pathogen that often causes infections in immunocompromised individuals. Upon inhalation into the lungs C. neoformans differentiates into cells with altered size and morphology, including production of large titan cells. Titan cells possess thickened cell wall and dense, cross-linked capsule when compared to in vitro grown cells. In addition, titan cells have increased cell wall chitin that is associated with a detrimental anti-inflammatory immune response. Here we examined the cell wall and capsule composition of in vitro, in vivo typical-sized and in vivo titan cells using High Performance Liquid Chromatography (HPLC). The monomer composition of cell wall polysaccharides showed that in vivo C. neoformans cells contained more glucosamine and less glucose than in vitro cells, suggesting alteration in abundance of both chitin and glucans, respectively. Low levels of galactosamine were also detected in carbohydrates from both in vivo and vitro cells. Within the in vivo cell population, differences in the proportions of cell wall and capsule monomers between typical and titan cells were also observed. Taken together, these results demonstrate that C. neoformans reshapes its cell wall and capsule composition during infection. These cell wall and capsule alterations likely help C. neoformans escape recognition by, and allow modulation of, the host immune system.
Keywords: C. neoformans, Cell wall, Capsule, Titan cells, Cryptococcus
Introduction
Cryptococcosis is a life-threatening infection caused by Cryptococcus neoformans and often observed in immunocompromised individuals including patients with HIV/AIDS, cancer chemotherapy patients and organ transplant recipients (Mitchell and Perfect, 1995, Park et al., 2009, Jarvis et al., 2010). Even though the absolute number of Cryptococcus-related deaths has declined since previous estimates, cryptococcal meningitis still causes 15% of all HIV/AIDS-related deaths globally (Rajasingham et al., 2017). In Sub-Saharan Africa alone, the region with the greatest burden, cryptococcal meningitis kills around 140,000 people per year and global mortality is estimated at around 181,000 people (Rajasingham et al., 2017).
A critical step during cryptococcal infection involves the formation of enlarged cells called “titan cells” (Okagaki et al., 2010, Zaragoza et al., 2010). While typical C. neoformans cells are 5–7 µm in diameter, the titan cells can be up to 100 µm in diameter (Okagaki et al., 2010, Zaragoza et al., 2010). Titan cells are also characterized by their increased DNA content, thick cell wall, and a significantly denser crosslinked capsule (Okagaki et al., 2010, Zaragoza et al., 2010). Changes in titan cell walls are important for survival of C. neoformans within the host, and increased chitin content in titan cell wall signals the host immune system to generate a detrimental immune response that promotes disease progression in mice (Wiesner et al., 2015). However, it is not known whether there are other in vivo cell wall changes in titan or typical cells, and how these changes might affect C. neoformans pathogenesis.
Mammalian cells lack a cell wall; therefore, the fungal cell wall is an ideal target for development of antifungal drugs. In fungi, the cell wall plays important roles including protecting the cell from osmotic pressure, various environmental stresses, and is also involved in interaction between the fungal cell and its environment (Bowman and Free, 2006, Erwig and Gow, 2016). C. neoformans also possesses a polysaccharide capsule that is attached to the cell wall and covers its cell body (Zaragoza et al., 2009). The presence or absence of capsule, as well as its size and structure depends on different environmental stimuli such as temperature, growth medium, pH, the presence of CO2, and infected organ type (Rivera et al., 1998, Zaragoza and Casadevall, 2004, O’Meara and Alspaugh, 2012). Capsule polysaccharides can be divided into two groups; those attached to the cell wall and exopolysaccharides that are secreted into the surrounding environment or culture media (Zaragoza et al., 2009). Exopolysaccharides are composed of 90–95% glucuronoxylomannan (GXM), 5–8% glucuronoxylomannogalactan (GXMGal), and a few mannoproteins (Juneann, 1988, Cherniak and Sundstrom, 1994, Vaishnav et al., 1998, Bose et al., 2003, McFadden et al., 2006, Heiss et al., 2009, Zaragoza et al., 2009). Most of the known information on cryptococcal capsule comes from studies that examined the polysaccharides recovered from culture media (Turner et al., 1992, Cherniak and Sundstrom, 1994, Cleare and Casadevall, 1999, McFadden et al., 2006, Heiss et al., 2009, Zaragoza et al., 2009), however one study showed that the attached capsule is different from polysaccharides secreted to the environment (Frases et al., 2008). In addition, most, if not all, previous studies used Cryptococcus cells generated in in vitro conditions. The exact structure and composition of Cryptococcus capsule during infection is not well understood.
The composition and organization of individual cell wall components can vary depending on the fungal species, growth conditions and the stage of growth (Reiss et al., 1986, Abad et al., 2010, Gow et al., 2011). The cell wall of C. neoformans is mainly composed of α- and β-glucan, chitin and its deacetylated form chitosan (Reiss et al., 1986, Banks et al., 2005, Baker et al., 2007, Reese et al., 2007, Baker et al., 2011). In addition to these main components, the cell wall of C. neoformans also contains melanin, lipids and mannoproteins (Vartivarian et al., 1989, Wang et al., 1995, Siafakas et al., 2007). However, this information comes mainly from in vitro studies that used acapsular mutant strains or strains with mutations impacting the cell wall (Reiss et al., 1986, James et al., 1990, Reese et al., 2007). Acapsular mutants were used to avoid contamination from the capsule polysaccharides that are normally attached to the outer layer of the C. neoformans cell wall. This is not ideal as acapsular mutant strains are avirulent in mice (Fromtling et al., 1982, Chang and Kwon-Chung, 1994), and could possess additional defects in their cell wall structure. Thus, the complete cell wall composition of a wild-type C. neoformans strain is currently not known, and the potential for cell wall remodeling under different growth conditions has not been explored.
Modification of the cell wall composition/structure likely constitutes a crucial step in the initial establishment and maintenance of C. neoformans infection (Okagaki et al., 2010, Zaragoza et al., 2010, Okagaki and Nielsen, 2012, Zaragoza and Nielsen, 2013, Wiesner et al., 2015). Except for preliminary studies examining chitin content in titan cells, it is not known how other cell wall changes, if any, in in vivo C. neoformans cells (both titan and typical cells) affect C. neoformans pathogenesis and the host immune response. The aim of this study was to identify the cell wall composition of both in vitro grown and in vivo cells, both titan and typical cells, of a wild type strain of C. neoformans.
Materials and methods
Ethics statement
Mice were handled in accordance with guidelines defined by the University of Minnesota Animal Care and Use Committee (IACUC). All animal experiments were done in concordance with the Animal Welfare Act, United States federal law, and NIH guidelines.
Strains and media
C. neoformans KN99α (WT) (Nielsen et al., 2003), and acapsular C. neoformans cap67 and cap59 mutant strains (Jacobson et al., 1982, Chang and Kwon-Chung, 1994) were used in this study. Strains from stored glycerol stocks (kept at −80 °C) were grown at 30 °C for 24–48 h on yeast-peptone-dextrose (YPD) (BD, Hercules, CA). C. neoformans cells from the YPD agar plate were transferred to YPD broth and grown overnight at 30 °C.
Production and isolation of titan and typical cells
C. neoformans titan and typical cells were produced as previously described (Okagaki et al., 2010, Wiesner et al., 2015). Briefly, overnight YPD-grown C. neoformans cells were collected by centrifugation, washed twice with sterile phosphate-buffered saline (PBS) and resuspended in sterile PBS at a concentration of 1 × 106 cells/ml based on hemocytometer count. Groups of 6–8-week-old female A/J mice (Jackson Laboratory, Bar Harbor, MA) were anesthetized by intraperitoneal pentobarbital injection and infected intranasally with 5 × 104 cells in 50-µl PBS. At 14 days post-infection, mice were sacrificed by CO2 inhalation. The lungs were harvested and then homogenized in 10 ml PBS supplemented with collagenase (1 mg/ml) (Zaragoza et al., 2010). Cell homogenates were incubated for 1 h at 37 °C with agitation, and washed several times with double distilled water. The C. neoformans cells were resuspended in sterile double distilled water.
To separate titan and normal-sized (which we refer to as “typical”) cell populations, we used a previously described method (Gerstein et al., 2015). As previously, C. neoformans cells (mixture of titan and typical cells) from lung homogenates were filtered using CellMicroSieves (BioDesign Inc. of New York, Carmel, NY) with a 20-µm pore size that allows typical cells to pass through the membrane. The CellMicroSieves were then rinsed with sterile PBS to remove any remaining typical cells from the filter. To recover the titan cell population, the CellMicroSieves were inverted and the membrane was washed with PBS. The resulting cell populations was concentrated by centrifugation at 12,000×g for 1 min.
γ-Radiation treatment
In vivo C. neoformans cells with enlarged capsule were exposed to γ-radiation to remove layers of the capsule polysaccharide as described previously with the following modifications (Zaragoza et al., 2010). Cells isolated from the lungs of infected mice were washed in PBS and resuspended in sterile double distilled water. The cells were then transferred to a 24-well flat-bottom plate and irradiated for 45 min for a total irradiation dose of 560 Gy (56,000 rad). After irradiation, cell suspensions were transferred into Eppendorf tubes, centrifuged, and the supernatant was separated from the irradiated cell pellet by centrifugation. The supernatant containing capsule polysaccharide was freeze-dried and stored at −20 °C until further analysis. The irradiated cell pellet was used in cell wall extraction experiments.
Cell wall and capsule extraction
Cell wall/capsules were extracted using a modified version of previously described methods (Munro et al., 2003, Mora-Montes et al., 2007, Mora-Montes et al., 2012). Briefly, C. neoformans cells were either grown in YPD medium or isolated from lungs of infected mice as described above. Cells were washed with sterile double distilled water and broken with glass beads using a BIO101/Savant FastPrep FP120 machine (20 cycles of 45 s each cycle, at a speed of 4 m/s). The homogenates were centrifuged at 4000 rpm for 5 min, and the pellet (broken cells) was washed 5 times with 1 M NaCl, resuspended in buffer (500 mM Tris–HCl buffer [pH 7.5], 2% [wt/vol] SDS, 0.3 M β-mercaptoethanol, 1 mM EDTA) and boiled twice at 100 °C for 10 min. Extracted cell wall pellet was collected, washed with sterile double distilled water, freeze dried and the dry weight of recovered cell walls was recorded.
Hydrolysis of cell wall/capsule polysaccharides
Purified cell wall/capsule was hydrolyzed using a modification of several previously described methods (Morrison, 1988, Mora-Montes et al., 2007, Mora-Montes et al., 2012). Six different approaches were used to hydrolyze cell wall/capsule polysaccharide (Table 1).
Table 1.
Cell wall hydrolysis approaches.
| Hydrolysis method |
Carbohydrates detected (μg/mg of dry cell wall) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Step-1 at 37 °C | Step-2 at 100 °C | Glucosamine | Glucose | Mannose | Xylose | Galactose | Total (μg/ml) | |
| Approach -1 (N = 6) | – | 3 h | 7.3 ± 1.1 | 130.4 ± 34.1 | 9.7 ± 3.9 | ND | ND | 147.3 ± 38.2 |
| Approach -2 (N = 4) | 20 h | – | 0.6 ± 0.5 | 76.7 ± 7.9 | 4.2 ± 1.5 | ND | ND | 81.5 ± 9.0 |
| Approach -3 (N = 5) | 20 h | 30 min | 6.5 ± 1.2 | 415.0 ± 78.6 | 39.1 ± 5.6 | 7.0 ± 2.7 | 1.1 ± 0.7 | 468.7 ± 83.6 |
| Approach -4 (N = 5) | 20 h | 3 h | 11.3 ± 0.5 | 625.9 ± 19.8 | 55.5 ± 8.6 | ND | 3.6 ± 1.5 | 696.3 ± 20.3 |
| Approach -5 (N = 5) | 20 h | 4 h | 6.5 ± 1.6 | 266.8 ± 39.7 | 24.8 ± 12.4 | 0.4 ± 0.2 | ND | 348.4 ± 62.6 |
| Approach -6 (N = 2) | 72 h | 30 min | 1.3 ± 0.5 | 77.0 ± 1.2 | ND | 0.9 ± 0.1 | ND | 79.2 ± 0.6 |
| Approach -7 (N = 3) | 72 h | 3 h | 5.1 ± 1.2 | 154.7 ± 35.7 | 2.2 ± 1.4 | ND | ND | 162.0 ± 33.2 |
ND: not detected.
Approaches 1 and 2
Trifluoroacetic acid (TFA) (0.75 ml) was added to dried cell wall/capsule polysaccharides in 1.5 ml screw-cap tubes (SARSTEDT) and incubated at 37 °C for 20 h (approach 1) or at 100 °C for 3 h (approach 2), then evaporated to dryness at 70–80 °C in a thermal block. After all acid was evaporated, double distilled water (1.0 ml) was added to each sample as a washing step, and mixed by pipetting. Samples were then evaporated to dryness by incubating open tubes at 70–80 °C. Dried hydrolyzed samples were stored at -20 °C until analyzed by High Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD).
Approaches 3–7
TFA (0.75 ml) was added to dried cell wall/capsule polysaccharide and incubated at 37 °C for 20 h. Aliquots (0.375 ml) were transferred to two new tubes and myo-inositol (1.125 ml, 0.75 mg/ml) was added to each tube as a positive control to confirm hydrolysis. Preliminary studies comparing the addition of water versus myo-inositol showed no effect of the myo-inositol addition on other components within the reaction (data not shown). One aliquot was heated at 100 °C for 30 min (approach 3), and the other heated at 100 °C for 3 h (approach 4) or for 4 h (approach 5). Both samples were then evaporated to dryness. After all acid was evaporated, double distilled water (1.0 ml) was added to each sample as a washing step, and mixed by pipetting. Samples were then evaporated to dryness by incubating tubes at 70–80 °C. Dried hydrolyzed samples were stored at −20 °C until analyzed by HPAEC-PAD. Approaches 6 and 7 were similar to approaches 3 and 4 except that the dried cell wall/capsule polysaccharides were resuspended in TFA and incubated at 37 °C for 72 h, instead of 20 h, before being incubated at 100 °C for 30 min and 3 h respectively.
Carbohydrate quantification by HPAEC-PAD
The hydrolyzed cell wall/capsule samples were analyzed by HPAEC-PAD as previously described with minor modifications (Munro et al., 2003, Mora-Montes et al., 2007, Mora-Montes et al., 2012). For monosaccharide analysis of the Cryptococcus cell wall component, 10 µl aliquots of acid-hydrolyzed samples were injected in a Dionex HPAEC system equipped with a CarboPac SA-10 analytical column (4 × 250 mm) and a CarboPac SA-10 guard column (4 × 50 mm). The CarboPac SA-10 analytical column was used because the most commonly used CarboPac PA-10 column was unable to separate xylose and mannose (Supplemental Table 1 and Supplemental Fig. 1). Samples were eluted with 12 mM NaOH at a flow rate of 1.2 ml/min for 10 min. The column was washed with 100 mM NaOH for 5–10 min and equilibrated with 12 mM NaOH for 10 min prior to the next run. Monosaccharides were detected by their oxidation at a gold electrode surface of the PAD. Seven monomers were used as standards: d-fucose, d-glucose, d-glucosamine, d-mannose, d-galactose, d-galactosamine, and d-xylose. The monomer standards were dissolved in double distilled water and run under the same conditions. Myo-inositol was used as a hydrolysis control for Approaches 3–7.
Results
Detection of C. neoformans carbohydrates depends on the method used
To analyze the total C. neoformans cell surface carbohydrate content, we used a wild type strain, KN99α, that is virulent in the mouse model of infection (Nielsen et al., 2003). Using previously described methods (Munro et al., 2003, Mora-Montes et al., 2007, Mora-Montes et al., 2012), we measured the total carbohydrate content of C. neoformans cells from in vitro cultures and from lungs of infected mice (in vivo cells). The major characteristic differentiating in vivo and in vitro-grown cells is the presence of a large capsule surrounding the C. neoformans cell body in vivo (Fig. 1A). C. neoformans capsule is predominantly composed of GXM and GXMGal polysaccharides (Frases et al., 2008, Zaragoza et al., 2009). Preliminary analysis of carbohydrates of both in vitro and in vivo cells identified different proportions of glucosamine, glucose and mannose in the two cell populations (Fig. 1B). However, monosaccharides such as galactose and xylose that would be expected to come from in vivo capsule were not detected (Fig. 1B). Different hydrolysis methods that varied the temperature and time of hydrolysis were tested to optimize detection of all expected monosaccharides (Table 1). These experiments showed that hydrolysis for 20 h at 37 °C followed by a 30-min incubation at 100 °C (Approach 3) recovered all carbohydrates expected to be present in the cell wall and capsule of C. neoformans (Table 1). In addition, a CarboPac SA 10 column was needed to differentiate galactose, galactosamine, glucose, glucosamine, mannose, and xylose simultaneously (Supplemental Table 1 and Supplemental Figs. 1 and 2).
Fig. 1.
Total carbohydrate content of in vitro and in vivo C. neoformans cells. A, In vitro-grown and in vivo C. neoformans cells stained with India ink. B, Carbohydrate content of in vitro and in vivo C. neoformans cells. C. neoformans KN99α strain was grown in vitro overnight at 30 °C in YPD broth, or isolated from the lungs of infected mice at 14 days post-infection. Cell walls were extracted, freeze-dried and hydrolyzed with TFA for 3 h at 100 °C. Glucosamine, glucose, mannose, galactose and xylose were quantified using high-performance anion-exchange chromatography with pulse amperometric detection (HPAEC-PAD). Data represent means of results from 2 independent biological replicates (N = 6 for in vitro samples, and N = 8 for in vivo samples). ND: not detected, YPD: yeast-peptone-dextrose, CW: cell wall. Scale bar = 10 μm.
We next measured the carbohydrate content of the wild type strain (KN99α) and the previously used acapsular mutants cap67 and cap59 grown in the same in vitro conditions. The results show that, when grown in vitro, C. neoformans KN99α, cap67 and cap59 strains had similar glucosamine (monomer component of cell wall chitin and chitosan), and glucose (monomer component of α- or β-glucans) (Fig. 2A and B). The mannose content was significantly higher in the wild type strain compared to the acapsular mutant strains (Fig. 2C). Low levels of galactose and xylose (components of GXM and GXMGal) were detected in the wild type strain and, as expected, xylose was absent in the acapsular mutant strains (Fig. 2D and E). Surprisingly, low levels of galactose, a component of the capsular GXMGal were detected in the acapsular mutants (Fig. 2E). Very low levels (less than 0.1% of total carbohydrate content) of galactosamine were also detected in both the wild type and acapsular mutants, with acapsular mutants having more galactosamine than the wild type strain (data not shown). These results show that KN99α, cap67 and cap59 strains have overall similar cell wall carbohydrate composition when grown in vitro. As the wild type strain is known to form a small capsule in vitro (Fig. 1A), the presence of xylose in the wild type, but not in the acapsular mutant strains indicates that xylose is mainly a capsular component. The presence of galactose in the acapsular cell wall extracts could be because the GXMGal was secreted into the environment or was still loosely attached to the cell wall in these mutants, as previously suggested (Turner et al., 1984, Cherniak, 1988).
Fig. 2.
Cell wall composition of in vitro grown C. neoformans KN99α, cap67 and cap59 strains. C. neoformans KN99α, cap67 and cap59 strains were grown in vitro overnight at 30 °C in YPD broth. Cell walls were extracted, freeze-dried and hydrolyzed with TFA. Absolute monosaccharide amounts are expressed as micrograms per 1 mg of dry weight. Relative carbohydrate content expressed as percentages of the recovered total carbohydrate materials. Glucosamine (A), glucose (B), mannose (C), xylose (D), and galactose (E) were quantified using HPAEC-PAD. Data represent mean of results from at least 3 replicates (N = 5 for KN99α, N = 3 for cap67 and cap59). T-test was used to compare mean of the wild type (WT) strain to those of acapsular mutants. *P < 0.05, **P < 0.01. YPD: yeast-peptone-dextrose, ND: not detected.
Differences in C. neoformans cell wall composition in vitro and in vivo
C. neoformans titan cells from in vivo mouse models possess a thicker cell wall compared to in vitro grown cells (Zaragoza et al., 2010). Furthermore, we previously showed that titan cells have increased cell wall chitin compared to in vivo typical cells (Wiesner et al., 2015). However, it was not known whether other cell wall components also change in in vivo C. neoformans cells. We used hydrolysis approach-3 to measure the cell wall composition of the wild type strain KN99α grown in vitro, and isolated from the lungs of infected mice. In vivo cells contained significantly higher glucosamine and less glucose than in vitro grown cells (Fig. 3A and B). In vivo cryptococcal cells also contained increased mannose and xylose compared to in vitro grown cells (Fig. 3C and D). Galactose content was slightly higher in in vitro cells than in in vivo cells (Fig. 3E). To determine whether these differences were due to the capsule on the in vivo cells, we also analyzed the cell wall composition of irradiated in vivo cells in which most of the capsule was removed (Fig. 4). Irradiated in vivo cells contained similar mannose and xylose as the in vitro cells (Fig. 3C and D), showing the increase in these components in the non-irradiated in vivo cells was due to additional capsule formation. Galactose was not detected in irradiated cells suggesting that galactose is a capsular component that was removed by the irradiation (Fig. 3E).
Fig. 3.
Differences between cell wall and capsule carbohydrate composition of in vivo and in vitro C. neoformans cells. C. neoformans KN99α strain was either grown in vitro overnight at 30 °C in YPD broth, or isolated from lungs of infected mice. A portion of the in vivo C. neoformans cell sample was treated with γ-radiation for 45 min to remove the capsule polysaccharides. Cell walls were extracted from irradiated and non-irradiated C. neoformans cells, freeze-dried and hydrolyzed with TFA. Absolute monosaccharide amounts are expressed as micrograms per 1 mg of dry weight. Relative carbohydrate content is expressed as percentage of the recovered total cell wall material. Glucosamine (A), glucose (B), mannose (C), xylose (D), and galactose (E) were quantified using HPAEC-PAD. Data represent mean of at least 4 replicates per group. T-test or Mann-Whitney test were used to compare mean carbohydrate composition of in vitro-grown, in vivo and in vivo irradiated C. neoformans cells. *P < 0.05, **P < 0.01, ***P = <0.001. YPD: yeast-peptone-dextrose, TFA: trifluoroacetic acid.
Fig. 4.
Irradiation removes C. neoformans capsule. C. neoformans KN99α cells were isolated from the lungs of infected mice 14 days after infection. A portion of the cell sample was treated with γ-radiation (560 Gy) for 45 min to remove capsule polysaccharides. Both irradiated and non-irradiated cells were stained with India ink, and imaged using differential interference contrast (DIC). Scale bar = 10 μm.
These data show that in vivo and in vitro cells have different cell wall and capsule composition (Fig. 3A–E). In vivo cells have increased glucosamine and decreased glucose indicating higher chitin and lower glucan content of the in vivo cell wall compared to the in vitro cell wall. In addition, the in vivo cells have a thickened capsule with increased levels of mannose, xylose and galactose when compared to in vitro cells.
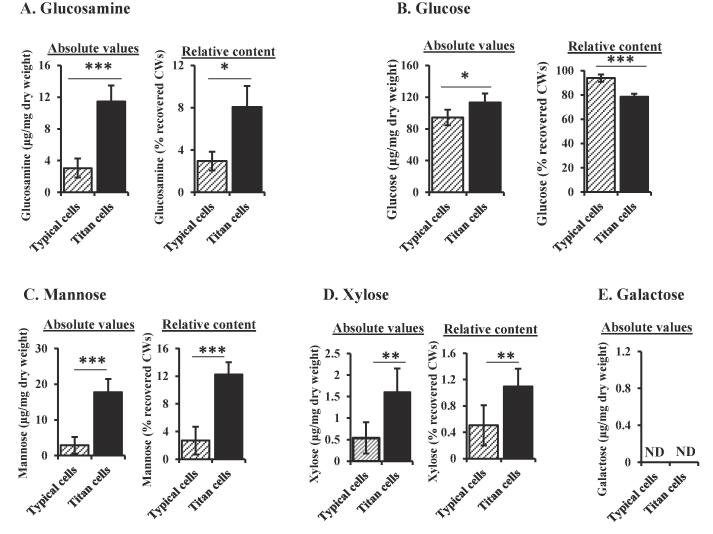
Titan and typical C. neoformans cells have different cell wall composition
In vivo C. neoformans cells vary in size, from cells less than 3 μm in cell body diameter to cells more than 100 μm in diameter (Feldmesser et al., 2001, Okagaki et al., 2010, Zaragoza et al., 2010, Alanio et al., 2015). The in vivo C. neoformans cells were divided into two groups; typical cells (less than 10 μm of cell body diameter), and titan cells greater than 10 μm in cell body diameter. The titan cell population has increased DNA content, a dense cross-linked capsule and thick cell wall (Okagaki et al., 2010, Zaragoza et al., 2010). We examined the cell wall composition of both typical and titan cells after γ-irradiation to remove most of the capsule. Glucosamine was significantly increased in titan cell walls compared to typical cells (Fig. 5A), confirming previous studies showing increased levels of chitin in titan cells (Wiesner et al., 2015). While the absolute glucose content was higher in titan cells (Fig. 5B), the relative cell wall glucose content as percentage of the total recovered cell wall material decreased in titan cells (78.5%) compared to typical cell walls (93.8%) (Fig. 5B). The cell wall of titan cells also contained significantly higher mannose and xylose (Fig. 5C and D). Galactose was not detected in these γ-irradiated cell wall samples (Fig. 5E), suggesting no GXMGal capsule component remained associated with the cell wall after γ-irradiation. The detected mannose and xylose could be structural components of the actual C. neoformans cell wall, cell wall mannoproteins, or the small portion of the capsule still attached to the irradiated C. neoformans cells (Fig. 4), also described previously (Zaragoza et al., 2010).
Fig. 5.
Cell wall composition of C. neoformans typical and titan cells. C. neoformans KN99α cells were isolated from lungs of infected mice. Titan cells were separated from typical cells by filtration and treated with γ-radiation for 45 min to remove capsule polysaccharides. Cell walls were extracted from irradiated C. neoformans cells, freeze-dried and hydrolyzed with TFA. Absolute monosaccharide amounts are expressed as micrograms per 1 mg of dry weight. Relative carbohydrate content is expressed as percentage of the total recovered cell wall material. Glucosamine (A), glucose (B), mannose (C), xylose (D), and galactose (E) were quantified using HPAEC-PAD. Data represents the mean of 4 replicates per group. T-test was used to compare mean carbohydrate composition of typical and titan cell walls. *P < 0.05, **P < 0.01, ***P = <0.001. TFA: trifluoroacetic acid, ND: not detected.
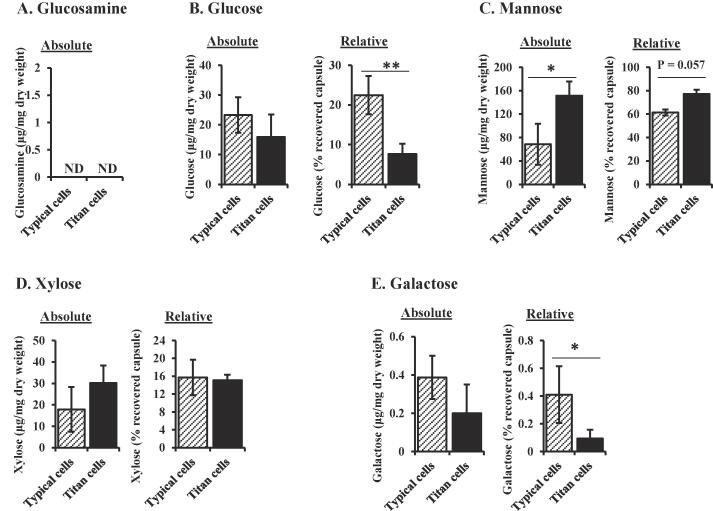
Capsule composition differences between titan and typical cells
Treatment of C. neoformans cells with γ radiation removes most of the capsule (Fig. 4). We analyzed the composition of this dissociated capsule from in vivo typical and titan cells. Glucosamine was not detected in the dissociated capsule of either titan or typical cells (Fig. 6A), suggesting the capsule preparation was not contaminated with cell wall debris. Analysis of the dissociated capsule revealed that titan cell capsule contained significantly higher relative mannose content than that of typical cells (Fig. 6C). Conversely, the relative amount of glucose and galactose decreased in the capsule of titan cells compared to typical cells, although this difference was not significant when absolute values were compared (Fig. 6B and E). Absolute xylose content was increased in the titan cell capsule (Fig. 6D), however the relative xylose content (percentage) was not significantly different from typical cells (15.1% vs 15.7% respectively) (Fig. 6D). These results show that, in addition to the cell wall composition, capsule composition differs between titan and typical cells, with titan cells containing higher mannose and xylose than typical cells.
Fig. 6.
Typical and titan capsule composition. C. neoformans KN99α cells were isolated from lungs of infected mice. Titan cells were separated from typical cells by filtration and treated with γ-radiation for 45 min to remove the capsule polysaccharides. The dissociated capsule was collected in the supernatant, freeze-dried and hydrolyzed with TFA. Absolute monosaccharide amounts were expressed as micrograms per 1 mg of dry weight. Relative carbohydrate content is expressed as percentage of the total recovered capsule material. Glucosamine (A), glucose (B), mannose (C), xylose (D), and galactose (E) were quantified using HPAEC-PAD. Data represent mean of at least 3 replicates in each group. (N = 4 for typical cells and N = 3 for titan cells). T-test or Mann-Whitney test were used to compare mean carbohydrate composition of the capsule of typical and titan cells. *P < 0.05, **P < 0.01. TFA: trifluoroacetic acid, ND: note detected.
Discussion
Morphology changes play an important role in the initiation and maintenance of C. neoformans infections. During infection, C. neoformans cells of varying cell body size and capsule size are observed (Feldmesser et al., 2001, Okagaki et al., 2010, Zaragoza et al., 2010, Alanio et al., 2015). These structural and morphological characteristics allow C. neoformans to evade and modulate the host immune system (Almeida et al., 2001, Okagaki and Nielsen, 2012, Wiesner et al., 2015). We previously showed that increases in chitin, a C. neoformans cell wall component, during titan cell formation induces a detrimental Th2 immune response in mice (Wiesner et al., 2015). However, it was not known if there are other cell wall changes in in vivo C. neoformans. Here we examined the cell wall composition of both in vitro-grown and in vivo C. neoformans cells to identify changes that occur when C. neoformans transitions from in vitro to in vivo growth conditions. Cell walls of C. neoformans cells from infected mice contained significantly higher glucosamine, and lower glucose than the cell wall of in vitro-grown cells. In addition to differences between in vitro and in vivo cell walls, we found that in vivo titan cells have a different cell wall composition than that of in vivo typical cells. Titan cell walls contained higher glucosamine, mannose and xylose than typical cells.
The HPLC analysis used here identified changes in the total carbohydrate content between different C. neoformans cell types. However, this technique cannot determine the origin of the observed changes for specific polysaccharides with similar monomer carbohydrate components. For example, we observed that the cell wall glucosamine content increased in in vivo C. neoformans cells, but HPLC cannot determine whether these changes in glucosamine come from changes in chitin, chitosan, chito-oligomers, or glucosamine-derived polysaccharides. We also observed changes in total cell surface glucose and mannose content. Similarly, our method cannot specify if these carbohydrate changes reflect changes in α- or β-glucans, or changes in cell wall mannans, mannoproteins, or capsule components (GXM, GXMGal) trapped in the cell wall, respectively. In spite of these limitations, this study identified substantial cell surface modifications that occur during C. neoformans infection and likely dramatically impact the way C. neoformans is perceived by the host immune system.
The increase in total glucosamine in in vivo cell wall, especially in titan cells, supports our previous observation using different methods (Wiesner et al., 2015) that C. neoformans has increased chitin (polymer of N-acetylglucosamine) in vivo. This increase in chitin correlates with a detrimental Th2 immune response in mice (Wiesner et al., 2015). In addition, the glucosamine-derived molecules chitin and chitosan are known to play key roles in cell wall integrity, capsule structure/attachment to the cell wall, and in the virulence of C. neoformans (Baker et al., 2007, Rodrigues et al., 2008, Fonseca et al., 2009, Baker et al., 2011, Wiesner et al., 2015). Thus, modification of in vivo C. neoformans not only changes cell wall composition but also interactions with the host immune system and the resulting immune response.
Increase in fungal cell wall chitin can also affect how the fungus reacts to antifungal drugs. In another fungal pathogen, Candida albicans, increased chitin is associated with increased resistance to echinocandins, a class of antifungal drugs targeting cell wall β,1–3 glucans (Walker et al., 2010). The observed increase in in vivo cell wall glucosamine, and decreased glucose mirror cell wall changes observed when C. albicans is treated with Caspofungin, an antifungal that inhibits cell wall β,1–3 glucan synthesis (Walker et al., 2008), and likely explains why echinocandin drugs show promising anti-cryptococcal activity in vitro (Bartizal et al., 1997, Franzot and Casadevall, 1997), but are ineffective in vivo (Abruzzo et al., 1997).
Glucans are polysaccharides of glucose monomers, and the cell wall of C. neoformans is composed of both α- and β-glucans (Bacon et al., 1968, James et al., 1990, Reese et al., 2007). In our experiments, we showed that in vivo cell wall contains less glucose than in vitro cell wall, and titan cell wall contained less glucose than typical cell wall. Cell wall α-glucans are important for capsule attachment to the C. neoformans cell body (Reese and Doering, 2003). Disrupting α-1,3-glucan production in C. neoformans produces cells that shed capsule material into the environment but lack the surface capsule normally attached to the cell body (Reese and Doering, 2003, Reese et al., 2007). Titan cells have a thick and highly cross-linked capsule attached to their cell wall (Fig. 7 and (Zaragoza et al., 2010)). Thus, the reduction we see in total glucose content of the titan cell wall is unlikely due to a lack of α-glucans as capsule attachment appears to be unaffected. C. neoformans cell wall also contains two types of β-glucans; β-1,3-glucans and β-1,6-glucans (James et al., 1990, Feldmesser et al., 2000). The proportion of each β-glucan type can change depending on the cryptococcal strain, growth condition, and exposure to caspofungin, an antifungal drug that targets β-1,3-glucans (Feldmesser et al., 2000). The specific function of β-1,6-glucan in the C. neoformans cell wall is not well understood, but disruption of β-1,6-glucan production results in capsules that are enlarged, more diffuse, and more permeable than wild type cells (Gilbert et al., 2010). In vivo titan cells have large cross-linked capsules with reduced permeability (Zaragoza et al., 2010), suggesting that β-1,6-glucans are either unaffected or increased in our in vivo samples. Fungal β-glucans are recognized by the Dectin-1 receptor of host immune cells (Brown et al., 2003, Gow et al., 2007). Fungal pathogens such as C. albicans are known to evade Dectin-1 recognition by switching to hyphal forms that do not expose β-glucans at the cell surface (Gantner et al., 2005). Similarly, our data show C. neoformans cell wall contains lower glucan levels, which likely explains why Dectin-1 receptor has no function in the immune response to C. neoformans (Nakamura et al., 2007). The combination of low glucan in in vivo cell walls and a thick capsule likely reduce the surface exposure of β-glucans on in vivo C. neoformans cells and prevent direct contact between β-glucans and the Dectin-1 receptor. Direct contact between Dectin-1 receptor and the microbial surface, not soluble ligands, is required for the activation of the receptor and subsequent signaling (Goodridge et al., 2011, Lin et al., 2016).
Fig. 7.
Schematic representation of C. neoformans typical and titan cell surface. Electron microscope (EM) images and schematic diagram of titan and typical cell wall and capsule. The cell wall of C. neoformans is surrounded by a thick polysaccharide capsule composed of GXM and GXMGal. The cell wall is composed of polysaccharides arranged into inner and outer layers. The inner wall contains chitin, chitosan, β-glucans, melanin and cell wall proteins. The outer layer is composed of α-glucans that mediate the attachment of the capsule to the cell wall. Based on our findings, titan cells contain more chitin in their inner wall, and the outer wall of titan cell walls also have a layer of mannan fibrils in addition to α-glucans. Attached to α-glucans is the capsule that is more dense and highly cross-linked in titan cells than in typical cells. Scale bar = 1 μm.
Large increases in mannose were detected in the titan cell wall, and could be components of cell wall mannans, mannoproteins, or capsule components trapped in the cell wall during extraction. Mannoproteins are located in the inner layer of the C. neoformans cell wall (Vartivarian et al., 1989). These mannoproteins were suggested to be non-structural cell wall components that eventually migrate to the cell surface, and are secreted in the environment (Vartivarian et al., 1989). Mannoproteins are also found in the supernatant and cell wall extracts from in vitro C. neoformans cultures, and small amounts of mannose were found among monosaccharides extracted from C. neoformans cells (Murphy et al., 1988, James et al., 1990, Levitz et al., 2001, Park et al., 2012). Mannose detected in titan cell walls accounted for approximately 12% of the total cell wall. This amount seems high for residual capsule GXM and GXMGal transiting the cell wall for subsequent secretion. An alternate hypothesis is that titan cell walls contain a significant amount of mannoproteins or structural mannans. The presence of significant mannoprotein fractions in the cell walls could also explain the presence of xylose as previous studies showed that C. neoformans mannoproteins might contain xylose and xylose-phosphate structures (Reilly et al., 2011, Park et al., 2012). Some mannoproteins are recognized as primary cryptococcal antigens (Murphy et al., 1988, Levitz et al., 2001, Mansour et al., 2002), while other mannoproteins such as MP4 and App1 dampen the host immune response against C. neoformans (Coenjaerts et al., 2001, Luberto et al., 2003). During infection, titan cells confer protection from phagocytosis to the entire cryptococcal population (Crabtree et al., 2012, Okagaki and Nielsen, 2012). Whether this protection is associated with increased production of specific mannoproteins and their impact on immune responses poses an intriguing idea. Finally, and perhaps most intriguing is the possibility that titan cells contain increased structural mannans. Studies in both Pneumocystis and C. albicans have revealed structural mannan layers that generate distinct vertical fibral structures when imaged by electron microscopy (EM) (Gow et al., 2011, Erwig and Gow, 2016, Ma et al., 2016). EM images of titan and typical cell walls (Fig. 7) show similar vertical structures on the exterior surface of the titan cell wall (adjacent to the capsule), and these structures are absent in typical cells.
Capsule dissociated from titan cells contained more mannose, but less galactose and glucose, than capsule from typical cells. All these carbohydrate monomers were previously found to be components of the Cryptococcus capsule (Cherniak and Sundstrom, 1994, Vaishnav et al., 1998, Frases et al., 2008). Glucose represented up to 20% of capsule dissociated from typical cells. This glucose may represent glucans released from the cell wall by γ-radiation treatment (Frases et al., 2008). Alternatively, the glucose may be a structural component of the capsule essential for the attachment of the capsule to the cell wall. Our observation that glucose levels were higher than xylose, a known capsule component of GXM and GXMGal, and the absence of glucosamine in the detached capsule argue for the latter hypothesis. Differences in capsule polysaccharides between in vivo typical and titan cells may represent different epitopes between these two types of cells, and likely impact how these two types of cells are perceived by and/or interact with the host immune system.
In addition to the carbohydrate content of the known cell wall and capsule polysaccharides, we observed smaller amounts of galactosamine in both in vitro and in vivo cell extracts. To our knowledge galactosamine has not been previously detected in C. neoformans cell wall or capsule, although one study identified a UGT1 (UDP-galactose transporter) gene responsible for the transport of both UDP-galactose and UDP-galactosamine in vitro (Li et al., 2017). This finding together with our observation suggest the presence of both galactose and galactosamine in C. neoformans polysaccharides. The significance of this observation in relation to possible differences in host immune response remain to be explored. Studies in Aspergillus fumigatus found that galactosaminogalactan (GAG), a polysaccharide of galactose and N-acetylgalactosamine, impacts the immune response (Fontaine et al., 2011, Gravelat et al., 2013, Lee et al., 2015). This observation supports the need to study galactosamine in C. neoformans and its potential role in pathogenesis.
Our data, both EM images and HPLC analysis, and previous studies support a model in which C. neoformans typical and titan cells possess a different cell surface (Fig. 7). Upon initial pulmonary infection, a subset of C. neoformans cells differentiate into enlarged titan cells with a thick cell wall and a highly crosslinked capsule (Feldmesser et al., 2001, Okagaki et al., 2010, Zaragoza et al., 2010). The cell wall of C. neoformans is composed of (a) an inner cell wall layer that contains chitin/chitosan, melanin and β-glucan, and (b) an outer layer that contains α-glucans that mediate the attachment the capsule to the cell wall (James et al., 1990, Nosanchuk et al., 2000, Rosas et al., 2000, Reese and Doering, 2003, Banks et al., 2005, Baker et al., 2007, Reese et al., 2007, Erwig and Gow, 2016). Titan cell walls contain increased chitin and melanin and less β-glucan than typical cells (Fig. 5 and (Zaragoza et al., 2010, Wiesner et al., 2015)). EM images suggest that titan cells, but not typical cells, also possess structural mannan in their outer cell wall in addition to α-glucans (Fig. 5, Fig. 7). Lastly, our results suggest that titan cells have increased capsule GXM, but similar GXMGal than typical cell capsule (Fig. 6, Fig. 7). In addition to polysaccharides the C. neoformans cell surface also contains proteins and lipids (Vartivarian et al., 1989, Levitz et al., 2001, Djordjevic et al., 2005), however these were not analyzed in the present study. The proposed model of in vivo C. neoformans cell wall and capsule presented in Fig. 7 was deduced from our observations, together with observations from earlier studies that measured total carbohydrate content. The exact organization and localization of individual cell wall and capsule components needs to be verified by further studies.
The in vivo cell surface analysis performed here was from C. neoformans cells isolated from the lungs of infected mice. Previous studies specifically examining capsule structure have shown differences based on anatomical location with differences in capsule structure of cells grown in vitro and those isolated from the lungs, spleen, kidney, liver, heart and brain of infected mice (Rivera et al., 1998, Charlier et al., 2005). Further research is necessary to investigate whether similar cell wall changes occur across different organs. Importantly, we have shown dramatic differences in the composition of the cell wall in vivo versus in vitro, and between different in vivo cell types that likely impact interaction with the host. Better understanding of how these differences alter the host-pathogen interaction and the immune response will highlight key changes necessary for C. neoformans pathogenesis.
Funding information
This work was supported by the National Institutes of Health (R01AI080275 and R21AI22352), the NIH Fogarty International Center (R25TW009345), the University of Minnesota Center for Translational Science Institute (UL1TR000114), Wellcome Trust (086827, 075470, 097377, 101873 & 200208) and MRC Centre for Medical Mycology (N006364/1). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflicts of interest
None.
Acknowledgements
We thank Kyle Smith, Marina Yoder and Laura Okagaki-Vraspir for technical assistance, and the University of Minnesota Center for Immunology core facility for instrumentation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tcsw.2017.12.001.
Appendix A. Supplementary data
References
- Abad A., Fernandez-Molina J.V., Bikandi J., Ramirez A., Margareto J., Sendino J., Hernando F.L., Ponton J., Garaizar J., Rementeria A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010;27(4):155–182. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Abruzzo G.K., Flattery A.M., Gill C.J., Kong L., Smith J.G., Pikounis V.B., Balkovec J.M., Bouffard A.F., Dropinski J.F., Rosen H., Kropp H., Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 1997;41(11):2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanio A., Vernel-Pauillac F., Sturny-Leclere A., Dromer F. Cryptococcus neoformans host adaptation: toward biological evidence of dormancy. MBio. 2015;6(2) doi: 10.1128/mBio.02580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida G.M., Andrade R.M., Bento C.A. The capsular polysaccharides of Cryptococcus neoformans activate normal CD4(+) T cells in a dominant Th2 pattern. J. Immunol. 2001;167(10):5845–5851. doi: 10.4049/jimmunol.167.10.5845. [DOI] [PubMed] [Google Scholar]
- Bacon J.S.D., Jones D., Farmer V.C., Webley D.M. The occurrence of α(1–3)glucan in Cryptococcus, Schizosaccharomyces and Polyporus species, and its hydrolysis by a Streptomyces culture filtrate lysing cell walls of Cryptococcus. Biochim. Biophys. Acta, Gen. Subj. 1968;158(2):313–315. doi: 10.1016/0304-4165(68)90153-0. [DOI] [PubMed] [Google Scholar]
- Baker L.G., Specht C.A., Donlin M.J., Lodge J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell. 2007;6(5):855–867. doi: 10.1128/EC.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L.G., Specht C.A., Lodge J.K. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryot. Cell. 2011;10(9):1264–1268. doi: 10.1128/EC.05138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks I.R., Specht C.A., Donlin M.J., Gerik K.J., Levitz S.M., Lodge J.K. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell. 2005;4(11):1902–1912. doi: 10.1128/EC.4.11.1902-1912.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartizal K., Gill C.J., Abruzzo G.K., Flattery A.M., Kong L., Scott P.M., Smith J.G., Leighton C.E., Bouffard A., Dropinski J.F., Balkovec J. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872) Antimicrob. Agents Chemother. 1997;41(11):2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose I., Reese A.J., Ory J.J., Janbon G., Doering T.L. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot. Cell. 2003;2(4):655–663. doi: 10.1128/EC.2.4.655-663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman S.M., Free S.J. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28(8):799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Herre J., Williams D.L., Willment J.A., Marshall A.S., Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003;197(9):1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.C., Kwon-Chung K.J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 1994;14(7):4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C., Chretien F., Baudrimont M., Mordelet E., Lortholary O., Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am. J. Pathol. 2005;166(2):421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R. Soluble Polysaccharides of Cryptococcus neoformans. In: McGinnis M.R., editor. Current Topics in Medical Mycology. Springer; New York, NY: 1988. pp. 40–54. [DOI] [PubMed] [Google Scholar]
- Cherniak R., Sundstrom J.B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect. Immun. 1994;62(5):1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare W., Casadevall A. Scanning electron microscopy of encapsulated and non-encapsulated Cryptococcus neoformans and the effect of glucose on capsular polysaccharide release. Med. Mycol. 1999;37(4):235–243. [PubMed] [Google Scholar]
- Coenjaerts F.E., Walenkamp A.M., Mwinzi P.N., Scharringa J., Dekker H.A., van Strijp J.A., Cherniak R., Hoepelman A.I. Potent inhibition of neutrophil migration by cryptococcal mannoprotein-4-induced desensitization. J. Immunol. 2001;167(7):3988–3995. doi: 10.4049/jimmunol.167.7.3988. [DOI] [PubMed] [Google Scholar]
- Crabtree J.N., Okagaki L.H., Wiesner D.L., Strain A.K., Nielsen J.N., Nielsen K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012;80(11):3776–3785. doi: 10.1128/IAI.00507-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic Julianne T., Del Poeta M., Sorrell Tania C., Turner Kylie M., Wright Lesley C. Secretion of cryptococcal phospholipase B1 (PLB1) is regulated by a glycosylphosphatidylinositol (GPI) anchor. Biochem. J. 2005;389(Pt 3):803–812. doi: 10.1042/BJ20050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig L.P., Gow N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016;14(3):163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- Feldmesser M., Kress Y., Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147(Pt 8):2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- Feldmesser M., Kress Y., Mednick A., Casadevall A. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J. Infect. Dis. 2000;182(6):1791–1795. doi: 10.1086/317614. [DOI] [PubMed] [Google Scholar]
- Fonseca F.L., Nimrichter L., Cordero R.J., Frases S., Rodrigues J., Goldman D.L., Andruszkiewicz R., Milewski S., Travassos L.R., Casadevall A., Rodrigues M.L. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot. Cell. 2009;8(10):1543–1553. doi: 10.1128/EC.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine T., Delangle A., Simenel C., Coddeville B., van Vliet S.J., van Kooyk Y., Bozza S., Moretti S., Schwarz F., Trichot C., Aebi M., Delepierre M., Elbim C., Romani L., Latgé J.-P. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7(11):e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzot S.P., Casadevall A. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob. Agents Chemother. 1997;41(2):331–336. doi: 10.1128/aac.41.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frases S., Nimrichter L., Viana N.B., Nakouzi A., Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot. Cell. 2008;7(2):319–327. doi: 10.1128/EC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R.A., Shadomy H.J., Jacobson E.S. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia. 1982;79(1):23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- Gantner B.N., Simmons R.M., Underhill D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24(6):1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein A.C., Fu M.S., Mukaremera L., Li Z., Ormerod K.L., Fraser J.A., Berman J., Nielsen K. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. MBio. 2015;6(5):e01340–01315. doi: 10.1128/mBio.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N.M., Donlin M.J., Gerik K.J., Specht C.A., Djordjevic J.T., Wilson C.F., Sorrell T.C., Lodge J.K. KRE genes are required for β-1,6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in Cryptococcus neoformans. Mol. Microbiol. 2010;76(2):517–534. doi: 10.1111/j.1365-2958.2010.07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge H.S., Reyes C.N., Becker C.A., Katsumoto T.R., Ma J., Wolf A.J., Bose N., Chan A.S., Magee A.S., Danielson M.E., Weiss A., Vasilakos J.P., Underhill D.M. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N.A., van de Veerdonk F.L., Brown A.J., Netea M.G. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 2011;10(2):112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N.A.R., Netea M.G., Munro C.A., Ferwerda G., Bates S., Mora-Montes H.M., Walker L., Jansen T., Jacobs L., Tsoni V., Brown G.D., Odds F.C., Van der Meer J.W.M., Brown A.J.P., Kullberg B.J. Immune recognition of Candida albicans β-glucan by dectin-1. J. Infect. Dis. 2007;196(10):1565–1571. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelat F.N., Beauvais A., Liu H., Lee M.J., Snarr B.D., Chen D., Xu W., Kravtsov I., Hoareau C.M.Q., Vanier G., Urb M., Campoli P., Al Abdallah Q., Lehoux M., Chabot J.C., Ouimet M.-C., Baptista S.D., Fritz J.H., Nierman W.C., Latgé J.P., Mitchell A.P., Filler S.G., Fontaine T., Sheppard D.C. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 2013;9(8):e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C., Klutts J.S., Wang Z., Doering T.L., Azadi P. The structure of Cryptococcus neoformans galactoxylomannan contains beta-d-glucuronic acid. Carbohydr. Res. 2009;344(7):915–920. doi: 10.1016/j.carres.2009.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E.S., Ayers D.J., Harrell A.C., Nicholas C.C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J. Bacteriol. 1982;150(3):1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P.G., Cherniak R., Jones R.G., Stortz C.A., Reiss E. Cell-wall glucans of Cryptococcus neoformans CAP 67. Carbohydr. Res. 1990;198(1):23–38. doi: 10.1016/0008-6215(90)84273-w. [DOI] [PubMed] [Google Scholar]
- Jarvis J.N., Meintjes G., Williams A., Brown Y., Crede T., Harrison T.S. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect. Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneann W.M. Influence of cryptococcal antigens on cell-mediated immunity. Rev. Infect. Dis. 1988;10:S432–S435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- Lee M.J., Liu H., Barker B.M., Snarr B.D., Gravelat F.N., Al Abdallah Q., Gavino C., Baistrocchi S.R., Ostapska H., Xiao T., Ralph B., Solis N.V., Lehoux M., Baptista S.D., Thammahong A., Cerone R.P., Kaminskyj S.G.W., Guiot M.-C., Latgé J.-P., Fontaine T., Vinh D.C., Filler S.G., Sheppard D.C. The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog. 2015;11(10):e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S.M., Nong S., Mansour M.K., Huang C., Specht C.A. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc. Natl. Acad. Sci. U.S.A. 2001;98(18):10422–10427. doi: 10.1073/pnas.181331398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.X., Ashikov A., Liu H., Griffith C.L., Bakker H., Doering T.L. Cryptococcus neoformans UGT1 encodes a UDP-Galactose/UDP-GalNAc transporter. Glycobiology. 2017;27(1):87–98. doi: 10.1093/glycob/cww078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Wester M.J., Graus M.S., Lidke K.A., Neumann A.K. Nanoscopic cell-wall architecture of an immunogenic ligand in Candida albicans during antifungal drug treatment. Mol. Biol. Cell. 2016;27(6):1002–1014. doi: 10.1091/mbc.E15-06-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberto C., Martinez-Marino B., Taraskiewicz D., Bolanos B., Chitano P., Toffaletti D.L., Cox G.M., Perfect J.R., Hannun Y.A., Balish E., Del Poeta M. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Invest. 2003;112(7):1080–1094. doi: 10.1172/JCI18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Chen Z., Huang D.W., Kutty G., Ishihara M., Wang H., Abouelleil A., Bishop L., Davey E., Deng R., Deng X., Fan L., Fantoni G., Fitzgerald M., Gogineni E., Goldberg J.M., Handley G., Hu X., Huber C., Jiao X., Jones K., Levin J.Z., Liu Y., Macdonald P., Melnikov A., Raley C., Sassi M., Sherman B.T., Song X., Sykes S., Tran B., Walsh L., Xia Y., Yang J., Young S., Zeng Q., Zheng X., Stephens R., Nusbaum C., Birren B.W., Azadi P., Lempicki R.A., Cuomo C.A., Kovacs J.A. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat. Commun. 2016;7:10740. doi: 10.1038/ncomms10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M.K., Schlesinger L.S., Levitz S.M. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J. Immunol. 2002;168(6):2872–2879. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- McFadden D.C., De Jesus M., Casadevall A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J. Biol. Chem. 2006;281(4):1868–1875. doi: 10.1074/jbc.M509465200. [DOI] [PubMed] [Google Scholar]
- Mitchell T.G., Perfect J.R. Cryptococcosis in the era of AIDS–100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 1995;8(4):515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Montes H.M., Bates S., Netea M.G., Díaz-Jiménez D.F., López-Romero E., Zinker S., Ponce-Noyola P., Kullberg B.J., Brown A.J.P., Odds F.C., Flores-Carreón A., Gow N.A.R. Endoplasmic reticulum α-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host–fungus interaction. Eukaryot. Cell. 2007;6(12):2184–2193. doi: 10.1128/EC.00350-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Montes H.M., McKenzie C., Bain J.M., Lewis L.E., Erwig L.P., Gow N.A. Interactions between macrophages and cell wall oligosaccharides of Candida albicans. Methods Mol. Biol. 2012;845:247–260. doi: 10.1007/978-1-61779-539-8_16. [DOI] [PubMed] [Google Scholar]
- Morrison I.M. Hydrolysis of plant cell walls with trifluoroacetic acid. Phytochemistry. 1988;27(4):1097–1100. [Google Scholar]
- Munro C.A., Whitton R.K., Hughes H.B., Rella M., Selvaggini S., Gow N.A. CHS8-a fourth chitin synthase gene of Candida albicans contributes to in vitro chitin synthase activity, but is dispensable for growth. Fungal Genet. Biol. 2003;40(2):146–158. doi: 10.1016/s1087-1845(03)00083-5. [DOI] [PubMed] [Google Scholar]
- Murphy J.W., Mosley R.L., Cherniak R., Reyes G.H., Kozel T.R., Reiss E. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect. Immun. 1988;56(2):424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Kinjo T., Saijo S., Miyazato A., Adachi Y., Ohno N., Fujita J., Kaku M., Iwakura Y., Kawakami K. Dectin-1 is not required for the host defense to Cryptococcus neoformans. Microbiol. Immunol. 2007;51(11):1115–1119. doi: 10.1111/j.1348-0421.2007.tb04007.x. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Cox G.M., Wang P., Toffaletti D.L., Perfect J.R., Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect. Immun. 2003;71(9):4831–4841. doi: 10.1128/IAI.71.9.4831-4841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk J.D., Rosas A.L., Lee S.C., Casadevall A. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet. 2000;355(9220):2049–2050. doi: 10.1016/S0140-6736(00)02356-4. [DOI] [PubMed] [Google Scholar]
- O’Meara T.R., Alspaugh J.A. The Cryptococcus neoformans capsule: a sword and a shield. Clin. Microbiol. Rev. 2012;25(3):387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki L.H., Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell. 2012;11(6):820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki L.H., Strain A.K., Nielsen J.N., Charlier C., Baltes N.J., Chretien F., Heitman J., Dromer F., Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6(6):e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Park J.-N., Lee D.-J., Kwon O., Oh D.-B., Bahn Y.-S., Kang H.A. Unraveling unique structure and biosynthesis pathway of N-linked glycans in human fungal pathogen Cryptococcus neoformans by glycomics analysis. J. Biol. Chem. 2012;287(23):19501–19515. doi: 10.1074/jbc.M112.354209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingham R., Smith R.M., Park B.J., Jarvis J.N., Govender N.P., Chiller T.M., Denning D.W., Loyse A., Boulware D.R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017 doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A.J., Doering T.L. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 2003;50(4):1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- Reese A.J., Yoneda A., Breger J.A., Beauvais A., Liu H., Griffith C.L., Bose I., Kim M.J., Skau C., Yang S., Sefko J.A., Osumi M., Latge J.P., Mylonakis E., Doering T.L. Loss of cell wall alpha(1–3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 2007;63(5):1385–1398. doi: 10.1111/j.1365-2958.2006.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly M.C., Aoki K., Wang Z.A., Skowyra M.L., Williams M., Tiemeyer M., Doering T.L. A xylosylphosphotransferase of Cryptococcus neoformans acts in protein O-glycan synthesis. J. Biol. Chem. 2011;286(30):26888–26899. doi: 10.1074/jbc.M111.262162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss E., White E.H., Cherniak R., Dix J.E. Ultrastructure of acapsular mutant Cryptococcus neoformans cap 67 and monosaccharide composition of cell extracts. Mycopathologia. 1986;93(1):45–54. doi: 10.1007/BF00437014. [DOI] [PubMed] [Google Scholar]
- Rivera J., Feldmesser M., Cammer M., Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 1998;66(10):5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M.L., Alvarez M., Fonseca F.L., Casadevall A. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot. Cell. 2008;7(4):602–609. doi: 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas A.L., Nosanchuk J.D., Feldmesser M., Cox G.M., McDade H.C., Casadevall A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 2000;68(5):2845–2853. doi: 10.1128/iai.68.5.2845-2853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafakas A.R., Sorrell T.C., Wright L.C., Wilson C., Larsen M., Boadle R., Williamson P.R., Djordjevic J.T. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J. Biol. Chem. 2007;282(52):37508–37514. doi: 10.1074/jbc.M707913200. [DOI] [PubMed] [Google Scholar]
- Turner S.H., Cherniak R., Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr. Res. 1984;125(2):343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- Turner S.H., Cherniak R., Reiss E., Kwon-Chung K.J. Structural variability in the glucuronoxylomannan of Cryptococcus neoformans serotype A isolates determined by 13C NMR spectroscopy. Carbohydr. Res. 1992;233:205–218. doi: 10.1016/s0008-6215(00)90932-7. [DOI] [PubMed] [Google Scholar]
- Vaishnav V.V., Bacon B.E., O'Neill M., Cherniak R. Structural characterization of the galactoxylomannan of Cryptococcus neoformans Cap67. Carbohydr. Res. 1998;306(1–2):315–330. doi: 10.1016/s0008-6215(97)10058-1. [DOI] [PubMed] [Google Scholar]
- Vartivarian S.E., Reyes G.H., Jacobson E.S., James P.G., Cherniak R., Mumaw V.R., Tingler M.J. Localization of mannoprotein in Cryptococcus neoformans. J. Bacteriol. 1989;171(12):6850–6852. doi: 10.1128/jb.171.12.6850-6852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.A., Gow N.A.R., Munro C.A. Fungal echinocandin resistance. Fungal Genet. Biol. 2010;47(2):117–126. doi: 10.1016/j.fgb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.A., Munro C.A., de Bruijn I., Lenardon M.D., McKinnon A., Gow N.A. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008;4(4):e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Aisen P., Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 1995;63(8):3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner D.L., Specht C.A., Lee C.K., Smith K.D., Mukaremera L., Lee S.T., Lee C.G., Elias J.A., Nielsen J.N., Boulware D.R., Bohjanen P.R., Jenkins M.K., Levitz S.M., Nielsen K. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015;11(3):e1004701. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O., Casadevall A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol. Proced. Online. 2004;6:10–15. doi: 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O., Garcia-Rodas R., Nosanchuk J.D., Cuenca-Estrella M., Rodriguez-Tudela J.L., Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6(6):e1000945. doi: 10.1371/journal.ppat.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O., Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr. Opin. Microbiol. 2013;16(4):409–413. doi: 10.1016/j.mib.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O., Rodrigues M.L., De Jesus M., Frases S., Dadachova E., Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.