Abstract
Human Epidermal Growth Factor Receptor type 2 (HER2) gene amplification and/or protein overexpression is observed in patients suffering from HER2+ breast cancer. This subtype of breast cancer has improved prognosis due to availability of anti-HER2 therapy. However, drug resistance and tumor recurrence still remains a major concern. Cancer Stem Cells (CSCs) are believed to constitute the subset of cell population that is resistant to drug treatment and possesses characteristics of stem cells. CSCs enable the tumors to thrive despite major insults. This review provides a comprehensive idea about the concept of CSCs in context of HER2+ breast cancer by providing the description of the markers that are used for the identification of CSCs and by elucidating the signaling pathways that are associated with HER2+ breast CSCs. Furthermore, the review also describes the interaction of HER2 with those signaling pathways and the future of targeting CSCs in HER2+ breast cancer.
Keywords: Breast cancer, Cancer stem cells (CSCs), Drug resistance, Human epidermal growth factor receptor type 2 (HER2), Signaling pathways
Introduction
Breast cancer is the most common cancer among women worldwide. It remains the second leading cause of cancer-related death among women in the United States, and about one in eight U.S. women would be diagnosed with breast cancer in her lifetime. In 2016, about 40,450 U.S. women are expected to die due to breast cancer.1, 2 Breast cancer is a heterogeneous disease and could be categorized into four major subtypes – Luminal A, Luminal B, HER2 and triple negative (basal-like). These breast cancer subtypes are summarized in the table below (Table 1).3, 4
Table 1.
Subtypes of breast cancer.
| Molecular subtype | Prevalence | Characteristics | Treatment response |
|---|---|---|---|
| Luminal A | 40% | ER+ and/or PR+, HER2+, low Ki67 | Best Prognosis. Respond to endocrine therapy (tamoxifen, fulvestrant and aromatase inhibitors). Less response to chemotherapy as compared to Luminal B. |
| Luminal B | 20% | ER+ and/or PR+, HER2+/−, high Ki67 | Better respond to chemotherapy as compared to endocrine therapy due to high Ki67. Poor prognosis as compared to Luminal A |
| HER2 | 15–20% | ER-, PR- and HER2+ | Improved prognosis due to drugs like humanized therapeutic monoclonal antibodies trastuzumab & pertuzumab, taxane based chemotherapy, antibody drug conjugate T-DM1 and dual EGFR/HER2 tyrosine kinase inhibitor lapatinib. |
| Triple Negative/Basal like | 10–15% | ER-, PR-, HER2- | Worst prognosis due to lack of targeted therapy. Combination of surgery, radiation therapy and chemotherapy. |
Listed are the major subtypes of breast cancer. Also included are their characteristics, prevalence rate and treatment response.
ER+: estrogen receptor positive; ER-: estrogen receptor negative.
PR+: progesterone receptor positive; PR-: progesterone receptor negative.
HER2+: human epidermal growth factor receptor 2 positive.
HER2-: human epidermal growth factor receptor 2 negative.
EGFR: epidermal growth factor receptor; T-DM1: trastuzumab emtansine.
The current review would primarily focus on HER2+ subtype of breast cancer.
HER2 signaling pathway
HER2 subtype of breast cancer is associated with gene amplification and/or protein overexpression of Human epidermal growth factor receptor 2 (HER2/neu), also known as ErbB2 which leads to aggressive tumor growth and poor clinical outcome. HER2 belongs to HER family of receptor tyrosine kinase. Other members of the family include HER1 (also known as Epidermal Growth Factor Receptor or EGFR and ErbB1), HER3 (also known as ErbB3) and HER4 (also known as ErbB4). HER receptors contain an extracellular ligand binding domain containing two cysteine-rich regions, lipophilic alpha helical transmembrane domain and an intracellular cytoplasmic domain.
Specific ligand binds to the HER receptor at the ligand binding domain, which then leads to homodimerization or heterodimerization of the HER receptors. The receptor dimerization is followed by autophosphorylation and trans-phosphorylation of specific tyrosine residues within the intracellular tyrosine kinase domain. Depending upon the ligand involved and the resulting HER dimer formed, specific tyrosine residues would be autophosphorylated and would affect the recruitment of adaptor proteins and downstream activation of signaling pathways. The most common effectors of HER signaling include mitogen-activated protein kinase (MAPK) pathway, Phosphoinositide-3-kinase (PI3K)/Akt signaling pathway and protein kinase C (PKC) activation. The signaling pathways are involved in cell survival, cell proliferation, adhesion, migration, differentiation and apoptosis. Biological response depends on the ligand involved, the dimer formed as well as on cellular context. The known ligands for HER receptors are listed in the table below (Table 2). There are no known ligands for HER2 and HER3 lacks intrinsic tyrosine kinase activity and for the same reason HER2 and HER3 are preferred dimerization partners.5, 6 Signaling through HER receptors is attenuated by ligand mediated endocytosis of receptors.
Table 2.
Ligands for HER family receptors.
| HER receptor | Ligands |
|---|---|
| HER1 | Amphiregulin, Epidermal Growth Factor (EGF), Epigen, Epiregulin, β-Cellulin, Heparin-Binding EGF-like Growth Factor (HBEGF) and Transforming Growth Factor – α |
| HER3 | Neuregulin1 and Neuregulin2 |
| HER4 | Neuregulin1, Neureulin2, Neuregulin3, Neuregulin4, β-Cellulin, Heparin-Binding EGF-like Growth Factor (HBEGF) and Epiregulin |
HER2 therapy
First line treatment for advanced, HER2+ breast cancer patients includes combination of therapeutic humanized monoclonal antibodies pertuzumab and trastuzumab and taxane-based chemotherapy, unless the patient has severe heart problems. Along with being HER2+, if the cancer is hormone receptor positive, the treatment includes combination of hormonal therapy and anti-HER2 therapy.
In case of de novo resistance to trastuzumab or if the tumor recurrence is observed within 12 months, trastuzumab-emtansine (T-DM1) is used as a second line of treatment. T-DM1 is an antibody drug conjugate consisting of therapeutic humanized monoclonal antibody trastuzumab linked to cytotoxic agent DM1.
Third line treatment includes dual HER2-EGFR tyrosine kinase inhibitor lapatinib along with capecitabine or other combinations of chemotherapy and anti-HER2 therapy.3
Other potential therapeutics
Cyclin dependent kinase inhibitors
Cell cycle dysregulation is one of the most important hallmarks of cancer. Cyclin dependent kinase 4/6 (CDK 4/6) inhibitors are currently being investigated extensively. The CDK 4/6 acts by forming a complex with Cyclin D1 and phosphorylating retinoblastoma protein (Rb). The phosphorylation results in inactivation of Rb and allows cells to transition from the G1 phase into S phase of the cell cycle. The inhibitors act by preventing the interaction between Cyclin D1 and CDK 4/6, and thus blocking the entry from the G1 phase into S phase. Recently, the FDA approved the use of CDK 4/6 inhibitor palbociclib in combination with letrozole for the treatment of postmenopausal women suffering from ER+/HER2-advanced metastatic breast cancer. Other CDK 4/6 inhibitors that have been developed are abemaciclib and LEE01.
In the HER2 setting, palbociclib, either alone, or in combination with trastuzumab showed growth inhibitory effects on HER2+ cell lines. Currently, a phase II clinical trial has been undertaken to assess the safety and efficacy of palbociclib and trastuzumab, with or without letrozole, in post-menopausal patients with HER2-positive locally advanced or metastatic breast cancer (MBC) who have received prior chemotherapy and trastuzumab for their metastatic disease.7
Immune checkpoint inhibitors
Tumor antigens are recognized by T lymphocytes, which mediate an immune response against cancer cells. During this attack by T and other immune cells, the immune system enhances the activation of certain molecules known as checkpoints. Checkpoints prevent the immune reaction from damaging normal tissues in the body.
Cancer cells are known to upregulate the expression of immune checkpoint molecules in order to escape the attack of the immune system. Such checkpoint molecules include cytotoxic T lymphocyte–associated protein-4 (CTLA-4) and a co-inhibitory receptor called Programmed death 1 (PD-1), which is overexpressed on tumor-infiltrating lymphocytes (TILs). PD-L1 is the ligand for PD-1 and is overexpressed in various forms of cancer, including breast cancer. PD-1 expressing TILs are associated with poor overall survival in HER2+ breast cancer. Moreover, a positive correlation is observed between the expression of PD-L1 and anti PD-1 therapy.
PD-1 inhibitors have shown promising results during the initial evaluation in triple negative breast cancer. Furthermore, Anti-PD1 antibodies showed encouraging results in HER2+ breast cancer during the preclinical studies, as synergistic effects were observed with the combination of trastuzumab and anti-PD1 antibodies. Currently, a phase II clinical trial is being undertaken to assess the efficacy of using anti-PD-1 monoclonal antibody and trastuzumab in patients with trastuzumab-resistant, HER2-positive metastatic breast cancers.8
Recent work by Müller et al demonstrates that T-DM1 promotes the immune system. Despite primary resistance to immunotherapy, the combination of T-DM1 with anti CTLA-4 and anti PD-1 inhibitors renders HER2+ breast cancer susceptible to immune attack.9
Combining chemotherapy with immunotherapy would be an exciting strategy that needs to be thoroughly investigated.
Drug resistance and cancer stem cell hypothesis
Despite improved prognosis of the HER2 subtype of breast cancer due to the availability of targeted agents, drug resistance and tumor recurrence still remains a major concern. A fraction of patients are intrinsically resistant to drug treatment (denovo drug resistance) whereas others acquire drug resistance over the course of treatment (acquired drug resistance). Reports indicate that breast cancer recurrence might occur up to 25 years after the treatment of primary tumor.10
Possible mechanisms for trastuzumab resistance include – overexpression of other HER family receptors, Insulin-like growth factor 1 receptor (IGF1R) upregulation, increased expression and activity of c-Met (mesenchymal-epithelial transition factor) receptor, overexpression of EphA2 receptor, overexpression of PDK1, increased activation of PI3K signaling pathway, increased Src activity, loss of tumor suppressor phosphatase and tensin homolog (PTEN), loss of p27kip1, increased expression of membrane associated glycoprotein MUC4, HER2 mutation(s) that alters the binding of trastuzumab to HER2, expression of amino-terminus truncated form of HER2-p95HER2 which sterically hinders the binding of HER2 and trastuzumab, expression of a splice variant that removes exon 16 in the extracellular domain of HER2 and rare incidence of antibody development against trastuzumab. Moreover, the inability of the host immune system to exhibit an antibody-dependent cell-mediated cytotoxicity (ADCC) response could contribute to de novo resistance.11, 12, 13
Lapatinib resistance may be mediated by the following
-
I
Activation of compensatory pathways: These include signaling through other HER family receptor tyrosine kinases as well as through c-Met and AXL, altered signaling through PI3 Kinase/Akt/mTOR and Src family of non-receptor tyrosine kinase, overexpression of Protein Tyrosine Kinase 6 (PTK6), activation of multiple tyrosine kinases, increased levels of receptor tyrosine kinase ligands and activation of estrogen receptor (ER) pathway.
-
II
Mutations in HER2 tyrosine kinase domain – Various HER2 amino acid substitutions have been identified to be involved in mediating lapatinib resistance. The HER2 L755S and T798I and EGFR T790M were associated with high level of lapatinib resistance.
-
III
Gene Amplification – Various genes that are amplified and overexpressed in HER2+ breast cancer can lead to lapatinib resistance. One such gene implicated in mediating resistance is NIBP (TRAPPC9, trafficking protein particle complex 9).14
The cancer stem cell hypothesis states that tumors contain a subpopulation of cells that possess the properties of stem cells and are called cancer stem cells (CSCs) or tumor initiating cells (TICs). These cells drive the growth of the tumor and are responsible for resistance to radiation and chemotherapy and tumor recurrence. CSCs are at the apex of the hierarchy and can lead to formation of other tumorigenic CSCs through self-renewal and can form the non-tumorigenic bulk by differentiation.15
There is controversy in the literature regarding the term “Cancer Stem Cells”. CSCs may or may not arise from normal stem cells. However, they do fulfill the criteria of a true stem cell through their ability of self-renewal and capability to recapitulate all the cell types in a given tissue. Hence, for the purpose of this review, the term CSCs would be used hereafter.16
Epithelial to Mesenchymal Transition (EMT) is a phenomenon by which the cells lose their epithelial characteristics and acquire mesenchymal traits. It is characterized by the loss of epithelial markers like E-cadherin and cytokeratin and enhancement of mesenchymal markers like N-cadherin, fibronectin and vimentin. EMT as well as its reverse process Mesenchymal to Epithelial Transition (MET) are critical processes during embryonic development. The pioneering work done by Robert Weinberg's group (Mani et al) demonstrates that induction of EMT in non-tumorigenic immortalized human mammary epithelial cells using ectopic overexpression of EMT transcription factors such as Snail or Twist results in fibroblast like mesenchymal looking cells displaying traits of CSCs as assessed by mammosphere formation and through levels of CD44 high and CD24 low. The markers used for the assessment of CSCs are discussed in the later part of the review. Moreover, the authors demonstrated that breast CSCs express elevated levels of EMT markers and that EMT promotes the generation of CSCs.17
In contrast to the above mentioned idea, the work done by Tsuji et al reveals that EMT and CSCs are mutually exclusive.18 Another recent report also indicated that tumor initiating capacity is EMT independent in breast cancer cell lines. The authors concluded that even though EMT and MET had an effect on CD44 high/CD24 low levels, cell proliferation, invasion, drug resistance and radio resistance, it had no impact on tumor-initiating capacity which is an important measure for the assessment of a bonafide CSC. EMT mediated CSC formation might be context dependent and further research is required to confirm the role of EMT in formation of breast CSCs.
Various markers have been proposed to identify breast CSCs. These include – in vitro markers like mammosphere formation, surface expression of CD44 high/CD24 low and elevated expression of aldehyde dehydrogenase (ALDH). In vivo measurement of CSCs is based on the limiting dilution assay whereby the tumor take is assessed by injecting a range of cells i.e. 100k cells/site, 1k cells/site, 100 cells/site and 10 cells/site. The CSC frequency is calculated based on the tumor take. Secondary and tertiary mammosphere formation in vitro and serial transplantation in-vivo is used to assess the self-renewal capacity of the CSC.
Recently, cell lineage markers are used in tumor models to identify the hierarchy of CSCs and various studies have demonstrated that heterogeneity exists between CSC populations and that the cells have the capacity to interconvert between stem and non-stem states.19
Whether different markers identify the same subpopulation or different was unclear and recent work by Liu et al suggests that ALDH is a better marker for epithelial like breast CSCs and CD44 high/CD24 low is a better indicator of mesenchymal like CSCs. Epithelial like CSCs proliferate rapidly and are more localized, whereas mesenchymal like CSCs are more invasive and are quiescent.20
The review deals primarily with breast CSCs in the HER2+ subtype of breast cancer. However, it is important to note that HER2 is selectively expressed in HER2-, ER+ luminal breast CSCs and the HER2+ CSCs of HER2-breast cancer could be targeted by employing anti-HER2 agents as listed before.21 The HER2+ CD44 High/CD24 Low breast CSCs isolated from the HER2 negative breast cancer cells showed enhanced ALDH activity, invasiveness and in vivo tumorigenesis, as compared to HER2-breast CSCs.22
HER2+ CSCs display a distinct genotype as compared to non-HER2+ CSCs through altered epigenetic regulation. HER2 strongly regulates genes related to stem cell and progenitor cell control.23 Moreover, HER2+ CSCs display elevated levels of genes involved in S/G2/M transition and reduced expression of genes involved in differentiation and immune response.24 The following table summarizes the features of HER2+ CSCs and non-HER2+ CSCs (Table 3).
Table 3.
HER2+ CSCs verus Non-HER2+ CSCs.
| Parameter | HER2+ CSC | Non-HER2+ CSC |
|---|---|---|
| HER2 overexpression | Observed | Not observed |
| Subtypes | Lumial A, Luminal B and HER2+ | All subtypes |
| Anti-HER2 agents | Effective for treatment | Not effective for treatment |
| Phenotype | High ALDH activity, mammosphere formation, invasiveness and tumorigenesis, as compared to Non-HER2+ CSC. Higher self-renewal and replicative potential. |
Low ALDH activity, mammosphere formation, invasiveness and tumorigenesis, as compared to HER2+ CSC. Lower self-renewal and replicative potential, as compared to HER2+ CSC. |
| Genotype | Increased regulation of genes related to stem cells and progenitor cell control. Increased expression of genes related to S/G2/M transition. Reduced expression of genes involved in differentiation and immune response. | Decreased regulation of genes related to stem cells and progenitor cell control, decreased expression of genes related to S/G2/M transition. Increased expression of genes involved in differentiation and immune response, as compared to HER2+ CSC. |
According to work by Magnifico et al, HER2+ CSCs are enriched with higher HER2 mRNA levels and HER2 surface expression as compared to the HER2+ bulk cells.25 Contrary to this, recent work by Diessner et al demonstrated that CD44 high/CD24 low HER2+ breast CSCs have lower surface expression of HER2, and possess higher endocytic activity which renders them susceptible to targeting by T-DM1. Moreover, the higher clinical efficacy of T-DM1 as compared to trastuzumab could be attributed to its ability to preferentially and efficiently target CSCs, rather than the bulk cells.26 Sorting of HER2 high and HER2 low populations from the mammospheres of Luminal A subtype MCF7 cells showed an increase in stem cell properties and markers such as OCT4, NANOG and SOX2 in HER2 low population as compared to HER2 high population. OCT4, SOX2 and NANOG are known to induce pluripotency and thereby affect the stem cell potential. Also, xenografts of HER2 low sorted cells from MCF7 mammospheres showed enhanced levels of CSC markers and CSC properties, as compared to xenografts of HER2 high fraction.27
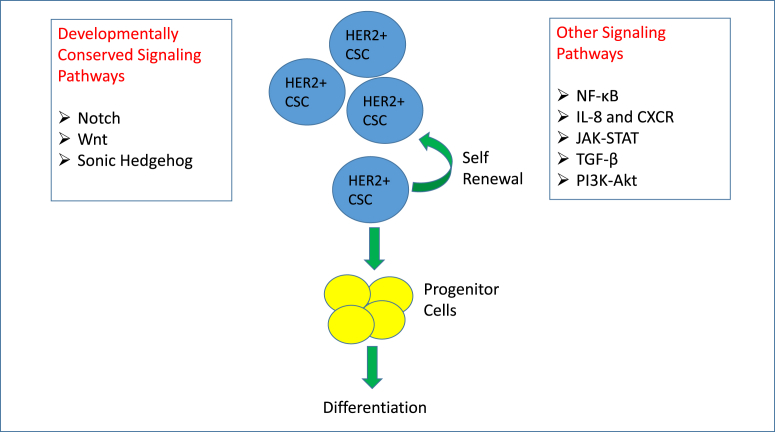
Developmentally conserved signaling pathways such as Notch, Wnt, and Sonic Hedgehog are deemed to be critical for the formation of CSCs. Besides these, various other signaling pathways and signaling crosstalk could contribute to formation of breast CSCs (Fig. 1).
Fig. 1.
Signaling Pathways that have been implicated in the formation of HER2+ breast cancer stem cells. Breast cancer stem cells that originated from HER2+ breast cancer have both the capacity to self-renew and differentiate into progenitors and bulk tumor cells. Numerous pathways have been shown to promote self-renewal and differentiation. Notch, Wnt, and Sonic Hedgehog pathways are developmentally conserved and promote both normal and cancer stem functions. Other signaling pathways such as NF-κB, IL-8-CXCR, JAK-STAT, TGF-β, and PI3K-AKT have also been implicated in promoting self-renewal and differentiation of cancer stem cells.
Developmentally conserved stemness related signaling pathways and CSCs in HER2+ breast cancer
Notch signaling
Canonical Notch signaling functions in a paracrine fashion with the ligand being expressed on the signal sending cell and the receptor being expressed on the signal receiving cell. In humans, there are four known Notch receptors (Notch 1–4) and five known Notch ligands (Jagged1, Jagged2, DLL1, DLL3 and DLL4). Upon interaction with the receptor, the ligand gets ubiquitinylated by an E3 ubiquitin ligase (Neuralized or Mindbomb). This ubiquitinylation generates the pulling force that enables the subsequent S2 (mediated by ADAM17/TACE – A Disintegrin And Metalloproteinase 17/Tumor necrosis factor Alpha Converting Enzyme) and S3 (mediated by γ-Secretase) cleavages of the receptor. The S3 cleavage generates Notch Intracellular Domain (NICD) which translocates to the nucleus, binds directly to CSL, a transcriptional repressor. Once bound, NICD recruits the coactivator, mastermind like 1 (MAML1) as well as other coactivators while simultaneously releasing corepressors to initiate the transcription of Notch target genes.28
Crosstalk between HER2 and Notch signaling was demonstrated by Osipo et al whereby they found that HER2 inhibition results in Notch1 activation and that Notch activation leads to trastuzumab resistance. Dual inhibition of HER2 and Notch signaling using trastuzumab and Notch1 siRNA or γ-Secretase Inhibitor (GSI) could prevent or reverse trastuzumab resistance in vitro.29 In-vivo studies reveal that combined blockade of HER2 and Notch signaling is required in order to prevent tumor recurrence and to reverse drug resistance.30 Recent work by Abravanel et al indicates that Notch signaling remains activated in a subset of dormant tumor cells after HER2 inhibition and promotes tumor recurrence. The study was based on the bioinformatics analyses of breast cancer patients and mouse model of recurrent mammary tumorigenesis.10 In contrast to these studies, Magnifico et al showed that HER2 levels are controlled by Notch signaling and that Notch inhibition through GSI or Notch1 siRNA as well as HER2 inhibition using trastuzumab was able to reduce the levels of CSCs.25 Notch1 and Notch4 receptors are known to play important roles in the formation of breast CSCs. Findings indicate that the Notch4 receptor is involved in the transition of breast cancer stem cell to progenitor cell and that Notch1 is critical for progenitor proliferation and luminal differentiation. Moreover, based on this finding, targeting Notch4 would be more effective than Notch1 to prevent tumor recurrence.31
Wnt signaling
Canonical Wnt signaling comprises the binding of Wnt ligand with Frizzled receptor and other coreceptors like lipoprotein receptor-related protein (LRP)-5/6, Ryk, or Ror2. Nineteen Wnt ligands and 10 Frizzled receptors have been identified in humans. The interaction results in destabilization of the destruction complex which consists of Glycogen synthase kinase 3 beta (GSK3β), axin, protein phosphatase 2A (PP2A), casein kinase 1α (CK1α) and adenomatous polyposis coli (APC). The process requires the Dishevelled phosphoprotein. Due to the destabilization of the destruction complex, β-catenin is stabilized, which then translocates to the nucleus and acts as a transcriptional coactivator to mediate T-cell factor (TCF)/lymphocyte enhancer factor (LEF)-dependent transcription. In the absence of Wnt ligand, the destruction complex phosphorylates β-catenin which results in ubiquitinylation-mediated proteasomal degradation of β-catenin.32
HER2 interacts with the Wnt signaling pathway through its downstream mediators AKT and Extracellular signal-regulated kinases (ERK). AKT and ERK kinases are known to phosphorylate and inhibit GSK3β that leads to translocation of β-catenin to the nucleus and transcription of Wnt-target genes through TCF/LEF family of transcription factors.33 Trastuzumab resistant cell lines showed an upregulation of Wnt signaling pathway ligand Wnt3. Increased Wnt3 levels resulted in activation of Wnt/β-catenin signaling pathway. Moreover, Wnt3 inhibition restored trastuzumab sensitivity and reduced invasiveness.34 Tumor spheres cultured in-vitro from the tumors derived from ErbB2 positive transgenic mice showed an upregulation of Wnt/β-catenin target genes as compared to mouse mammospheres and mammospheres induced to differentiate in-vitro. This suggests that Wnt signaling is enriched in HER2 breast CSCs relative to mammary epithelial stem/progenitor cells or differentiated mammary epithelial cells. While pharmacological inhibition of Wnt signaling using PKF118-310 inhibited the sphere formation and colony formation by primary tumor cells and primary mammary epithelial cells, as well as by tumorsphere- and mammosphere-derived cells, it affected the self-renewal of only primary tumor derived and tumorsphere-derived cells. Primary tumor cells treated with PKF118-310 or cells derived from PKF118-310 treated mice failed to generate tumors in mice after transplantation suggesting the importance of inhibiting Wnt signaling for eradicating HER2 breast CSCs in-vitro and in-vivo.35
Hedgehog signaling
Canonical Hedgehog signaling depends on the interaction of the Hedgehog ligand released from the secretory cell with the Patched (Ptch) receptor expressed on a different cell. The interaction relieves the Smoothened (Smo) complex that is inhibited by Ptch in the absence of the ligand. The signal transduction results in the liberation of the activated Gli-1 protein which translocates to the nucleus and initiates the transcription of Hedgehog target genes. Gli-1 and Ptch-1 mediate negative feedback loop.36
Hedgehog signaling has been primarily studied in hormone receptor positive and triple negative subtypes of breast cancer. There is very little published information describing crosstalk between HER2 and the Sonic Hedgehog pathway.37 However, HER2 activates the Hedgehog pathway via PI3K-Akt signaling in human esophageal adenocarcinoma.38 Recent efforts to understand the Hedgehog related protein expression in breast cancer focused on tissue microarray data from 334 human cases and revealed that Sonic hedgehog (Shh) expression was higher in the HER2 subtype and Gli-1 expression was higher in the luminal B HER2 positive subtype. Furthermore, the Smo expression was higher in HER2 and triple negative subtypes. The Gli-2 expression was higher in all the subtypes except triple negative and predicted poor overall survival.39
Other signaling pathways
Other than stemness related developmentally conserved signaling pathways, various other signaling pathways are involved in crosstalk with HER2 and are implicated in formation of HER2 breast CSCs.
NF-κB signaling pathway
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) consists of five transcription factors – NF-κB1/p105, NF-κB2/p100, RelA/p65, RelB, and c-Rel, which can homodimerize or heterodimerize. NF-κB is activated by numerous stimuli. These include growth factors, cytokines, viral and bacterial products, reactive oxygen species (ROS), ultraviolet and ionizing radiation, DNA damage and oncogenic stress. The stimuli activate inhibitor of IκB kinase (IKK) complex. IKK1/IKKα, IKK2/IKKβ, and NEMO/IKKγ are the principal components of the complex. In the absence of stimuli, NF-κB forms a complex with IκB and is sequestered in the cytosol. Upon stimulation, activated IKK phosphorylates IκB which results in ubiquitinylation and proteasomal degradation of IκB. This releases NF-κB dimers from the complex and the dimers then translocate from cytoplasm into the nucleus and initiate the transcription of target genes.40
HER2 activates NF-κB signaling pathway in a canonical fashion. However, IKKα plays a major role rather than IKKβ and is involved in the phosphorylation of p65 subunit at serine 536. IKKα is primarily associated with invasive phenotype in HER2+ breast cancer.41 IKKα is also important for self-renewal as the cells derived from tumors of IKKα defective knock-in mice, failed to generate secondary mammospheres. However, no difference in NF-κB activity was observed between the wild type and IKKα defective knock-in mice.42
NF-κB inhibition resulted in decrease in CD44 + cells in HER2 dependent tumors and reduced mammosphere formation in cell lines derived from HER2 dependent tumors. The inhibition of CSCs could be possibly due to reduced expression of Nanog and Sox2 which are critical embryonic stem cell regulators. In this study, the phenotype was attributed to IKKβ dependent NF-κB activation.
Lentiviral shRNA kinome screen performed on non-adherent HER2/Neu mouse tumorsphere revealed non-canonical IκB kinase TBK1 as a vital target. Inhibition of TBK1 lead to cellular senescence and growth inhibition of HER2+ breast cancer cells. Senescence was attributed to inhibition of active p65-NfκB and induction of the cell cycle inhibitor p16(ink4a).43
These studies reveal the distinct role for IKKα and IKKβ and the possibility of differential regulation of NF-κB pathway through canonical/non-canonical signaling.
As mentioned before, PTEN loss is commonly observed in patients that are resistant to trastuzumab treatment. Korkaya and colleagues have shown in a preclinical model, that PTEN knockdown in HER2+ breast cancer cells results in trastuzumab-resistant breast CSCs through inflammatory loop mediated by IL-6 and NF-κB.44
IL-8 and CXCR signaling pathway
IL-8 binds and activates CXCR-1/2 which belongs to the seven transmembrane domain class A, rhodopsin-like guanine-protein-coupled receptors (GPCRs). IL-8 and granulocyte chemotactic protein-2 (GCP-2) activate CXCR1. CXCR2 is known to be activated by IL-8 and various other ligands. Like other GPCRs, CXCR-1/2 associate with heterotrimeric G proteins, α, β and γ subunits. Upon ligand binding, guanosine diphosphate is converted to guanosine triphosphate which results in the separation of Gα subunit from Gβγ subunit. The Gα subunit and the Gβγ subunit can activate numerous other signaling pathways. The major ones include – PI3K/Akt signaling pathway, phospholipase C/protein kinase C (PLC/PKC) pathway and MAP Kinase signaling pathway. Other signaling pathways include Rho, Rac, Focal adhesion kinase and Janus Kinase/Signal Transducers and Activators of Transcription (JAK/STAT) pathway.45
HER2 overexpression is associated with an increase in levels of IL-8 and other CXCR 1/2 agonists. IL-8 acts through CXCR 1/2 and leads to downstream activation of SRC kinases which then transactivate HER2 and leads to mammosphere formation. Combination of HER2 inhibition and CXCR 1/2 inhibition was found to be more effective in targeting HER2 breast CSCs.46
JAK-STAT signaling pathway
Janus kinase- JAK1, JAK2, JAK3 and TYK2 are the members of JAK family of kinases. Upon binding of a cytokine to a cytokine receptor, JAK kinases are recruited and activated. The activation of JAK kinases leads to recruitment of the STAT3/STAT5 transcription complex on specific phosphotyrosines of the cytokine receptor. The transcription complex is then phosphorylated by JAK kinases which results in dissociation of STATs from the cytokine receptor and formation of stable homodimers and heterodimers that translocate to the nucleus. Upon translocation into the nucleus, the STATs bind to specific DNA sequences at the promoter region of the genes involved in apoptosis, cell proliferation and differentiation.47
HER2 overexpression leads to phosphorylation of STAT-3 resulting in enhancement of stem cell markers (Oct-4, Sox-2 and CD44), tumorsphere formation and trastuzumab resistance. STAT3 inhibition abolished the CSC phenotype and resulted in reduced tumorsphere formation and a decrease in stem cell markers. Combined inhibition of HER2 and STAT3 showed synergistic effect on suppression of cell growth in-vitro.48
TGF-β signaling pathway
There are three known isoforms of Transforming growth factor – β (TGF-β) – TGF-β1, -β2 and β3. Other ligands of TGF-β superfamily include activins, bone morphogenetic proteins, inhibins, and nodals. The canonical signaling pathway is initiated when the TGF-β superfamily ligand binds to TGF-β type II receptor (TβRII) and initiates dimerization with the TGF-β type I receptor (TβRI) and activation of the TβRI kinase. Receptor associated Smad proteins (R-Smads) – Smad2 and Smad3 are phosphorylated by the activated TβRI. The phosphorylated Smad binds Smad4 which enables them to translocate to the nucleus and initiate the transcription of TGF-β target genes. TβRI and TβRII belong to transmembrane serine/threonine kinases. Seven isoforms of TβRI and five isoforms of TβRII are identified in humans and different combinations of TβRI and TβRII have been identified for different ligands. TGF-β is also known to signal in a Smad independent manner.49
TGF-β is known to be important for embryonic development. However, during mammary gland development, it acts as a growth inhibitor initially and is later on known to promote EMT, drug resistance and metastasis. TGF-β acts as a dual edge sword and its effects are context dependent.50 HER2 interacts with TGF-β at various levels. This involves i. Transcriptional regulation of Smad target genes and pathways, ii Smad independent activation of PI3K/Akt pathway and iii alteration of tumor microenvironment through secretion of HER ligands, TGF-β and angiogenic mediators.51 CSCs isolated from MMTV-Her2/neu tumors show enrichment of TGF-β responsive gene signature and enhanced TGF-β signaling. Integrin-β3 signaling is imperative for TGF-β signaling and integrin-β3-TGFβ axis is important for self-renewal and maintenance of CSCs. TGF-β receptor inhibition led to reduction of CSCs without affecting the overall cell survival.52
PI3-kinase-Akt signaling pathway
The phosphoinositide-3-kinase (PI3-kinase) is a heterodimer comprised of a regulatory subunit (p85) and a catalytic subunit (p110). The pathway is activated when the p85 subunit binds to phosphorylated tyrosine residues of receptor tyrosine kinases, i.e. insulin-like growth factor 1 receptor (IGF1R), epidermal growth factor receptor (EGFR), or HER2. PI3-kinase mediates the conversion of membrane bound phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). AKT and phosphoinositide-dependent-kinase-1 (PDK1) contain pleckstrin homology domains that bind to PIP2 and PIP3. This results in the translocation of AKT and PDK1 to the plasma membrane. PDK1 partially activates AKT by phosphorylating threonine308. Complete activation of AKT is achieved by phosphorylation of serine473, mediated by mammalian target of rapamycin (mTOR) containing TORC2 complex. AKT then phosphorylates various substrates leading to multiple effects on protein synthesis, apoptosis, cell proliferation and metabolism. AKT is known to activate mTOR which exists in two different complexes – mTORC1 and mTORC2. AKT activates with mTORC1 by phosphorylating Tsc2 and relieving the inhibition of mTORC1which then plays a crucial role in downstream signaling, affecting protein synthesis and cellular metabolism. Phosphatase and tensin homolog (PTEN) is the phosphatase that mediates the conversion of PIP3 to PIP2 and attenuates the PI3-kinase-Akt signaling. It is known to act as a tumor suppressor and its loss or mutation is implicated in breast cancer.53
HER2 signaling leads to downstream activation of PI3K-Akt signaling pathway as discussed before. Reports cite the role of HER2-PI3K-FoxO-Survivin loop that is activated in trastuzumab resistant cells and that pharmacologic inhibition of PI3K along with trastuzumab is effective in targeting the CSCs in the fraction trastuzumab resistant cells in-vitro and in-vivo.54 Another study reveals that when activating mutant PIK3CA is present in HER2+ tumor, it results in anti-HER2 therapy drug resistance, EMT and increased levels of CSCs. PI3K inhibitor was successfully shown to reverse the drug resistance and CSC formation.55
CSCs and tumor microenvironment
The tumor microenvironment is the environment within which the tumor exists and its constituents are cancer associated fibroblasts, leukocytes, mesenchymal stem cells, lymphatic and blood endothelial cells, extracellular matrix and the signaling molecules. The tumor microenvironment is believed to provide a niche to CSCs which helps them to thrive and maintain an immature phenotype. The microenvironment helps the CSCs to evade the immune system and to undergo EMT and thus metastasize. The microenvironment is responsible for the genetic changes and epigenetic variation. CSCs activate or secrete various factors in order to maintain their survival. The tumor microenvironment also plays a pivotal role in the regulation of bidirectional CSC and non-CSC switch.56 This could be possibly attributed to EMT/MET plasticity that is exhibited by the tumor cells, in order to establish themselves in the changing microenvironment at the primary and metastatic sites.57
Tumor niche consists of a combination of cells that carry wild type HER2 and the ones that harbor activating HER2 mutations in the tyrosine kinase domain. Recent work by Wang et al demonstrates that the mutations impact the tumor microenvironment and mediates the upregulation of growth factors like Vascular Endothelial Growth Factor (VEGF), TGF-β and EGFR ligands like amphiregulin and TGF-α. The secreted growth factors promote autocrine and paracrine signaling which favors tumor growth.58
Cancer associated fibroblasts are known to mediate trastuzumab resistance in HER2+ breast cancer through secretion of IL-6, expansion of CSCs and by activation of multiple signaling pathways like NF-kB, JAK/STAT3 and PI3K/AKT.59
HER2 is selectively expressed in the CSCs of HER2-subtype of breast cancer and this involvement of HER2 is regulated by the tumor microenvironment. HER2 expression is induced by Receptor activator of nuclear factor kappa-B (RANK) ligand produced by bone osteoblasts. Moreover, higher levels of HER2 are reported under bone metastatic setting as compared to primary tumor and the circulating tumor cells of HER2-breast cancer are known to express HER2. Currently, clinical trials are under way to evaluate the efficacy of using RANK ligand inhibitor denosumab along with adjuvant therapy for the prevention of recurrence in the bone or any other part in women with early stage breast cancer and who are prone for recurrence.60
Future directions and unmet needs of basic and clinical sciences
This review describes list of signaling pathways and their important crosstalk with HER2. However, it is important to note that each of the above mentioned signaling pathways can crosstalk with each other and that there might be multiple nodes that impinge on other signaling pathways leading to a complex web of cancer signaling crosstalk.
It is critical to identify the roles of each distinct signaling pathways – i.e. to identify the pathway/(s) that are important in formation of CSCs, survival of CSCs and proliferation of CSCs in HER2+ breast cancer. Furthermore, it would be vital to identify and target the central node that necessary for the activation of multiple signaling pathways that are important for survival and proliferation of breast CSCs in HER2+ breast cancer. Also, targeting certain signaling pathways might result in activation of compensatory pathways posing an important challenge for designing drug molecules in order to target CSCs in HER2+ breast cancer. Furthermore, there exists a plasticity between the CSCs and the non-CSCs. To eradicate the tumor completely, it would be necessary to develop a combinatorial approach to target the non-CSCs along with CSCs, as non-CSCs are capable of transitioning to CSC state and vice-versa.
Another important aspect involves identification and targeting of dormant tumor cells. Dormancy can result from cells completely withdrawing from the cell cycle, or by cells proliferating at a slower rate. Dormancy is the persistent state of disease without any signs or symptoms, until tumor relapse is observed. Dormant tumor cells are known to possess characteristics of CSCs. Possible approaches to target dormant tumor cells include targeting angiogenesis, microenvironment, signaling pathways and activating the immune system. An important challenge exists in terms of identifying, isolating and characterizing dormant tumor cells, as no marker exists for dormant tumor cells and they exist in a lower frequency. Better understanding of the dormant cells can help in the development of appropriate therapies to prevent the transition from dormancy to metastasis, or tumor relapse.61
Despite our extensive efforts to identify and target CSCs in HER2+ breast cancer, no strategy has yet been developed to isolate and target the HER2+ breast CSCs in a clinical setting. We have not yet found an Achilles heel and several questions remain unanswered – Will it be possible to attain rational targeting of few signaling pathways to kill CSCs in HER2+ breast cancer? Did evolution create plastic CSCs in order to survive various assaults?
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Institutes of Health grant (R01 CA160378-04) awarded to Dr. Clodia Osipo.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Breast Cancer Facts – National Breast Cancer Foundation. Website http://www.nationalbreastcancer.org/breast-cancer-facts. (accessed 20.03.15).
- 2.Breast Cancer Statistics – Susan G Komen®. Website http://ww5.komen.org/BreastCancer/Statistics.html. Updated February 3, 2016. (accessed 04.02.16).
- 3.Goldberg K. ASCO Issues Two New Guidelines on Treating Patients with Advanced, HER2-positive Breast Cancer. American Society of Clinical Oncology Website http://www.asco.org/press-center/asco-issues-two-new-guidelines-treating-patients-advanced-her2-positive-breast-cancer. Updated May 5, 2014. (accessed 20.03.15).
- 4.Schnitt S.J. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23(Suppl 2):S60–S64. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 5.Yarden Y., Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 6.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 7.Mayer E.L. Targeting breast cancer with CDK inhibitors. Curr Oncol Rep. 2015;17:443. doi: 10.1007/s11912-015-0443-3. [DOI] [PubMed] [Google Scholar]
- 8.Lavaud P., Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med. 2014;12:132. doi: 10.1186/s12916-014-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller P., Kreuzaler M., Khan T. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7:315ra188. doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- 10.Abravanel D.L., Belka G.K., Pan T.C. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J Clin Invest. 2015;125:2484–2496. doi: 10.1172/JCI74883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rexer B.N., Arteaga C.L. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17:1–16. doi: 10.1615/critrevoncog.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta K., Osipo C. Trastuzumab resistance: role for Notch signaling. ScientificWorldJournal. 2009;9:1438–1448. doi: 10.1100/tsw.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 14.D'Amato V., Raimondo L., Formisano L. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat Rev. December 2015;41:877–883. doi: 10.1016/j.ctrv.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Frank N.Y., Schatton T., Frank M.H. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan C.T. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4:203–205. doi: 10.1016/j.stem.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mani S.A., Guo W., Liao M.J. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuji T., Ibaragi S., Shima K. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens T.W., Naylor M.J. Breast cancer stem cells. Front Physiol. 2013;4:225. doi: 10.3389/fphys.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S., Cong Y., Wang D. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ithimakin S., Day K.C., Malik F. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013;73:1635–1646. doi: 10.1158/0008-5472.CAN-12-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duru N., Fan M., Candas D. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18:6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahmatpanah F., Jia Z., Chen X., Jones F.E., McClelland M., Mercola D. Expression of HER2 in breast cancer promotes a massive reorganization of gene activity and suggests a role for epigenetic regulation. J Data Min Genomics Proteomics. 2012;3 doi: 10.4172/2153-0602.1000e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J.C., Voisin V., Bader G.D. Seventeen-gene signature from enriched Her2/Neu mammary tumor-initiating cells predicts clinical outcome for human HER2+:ERalpha- breast cancer. Proc Natl Acad Sci U S A. 2012;109:5832–5837. doi: 10.1073/pnas.1201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnifico A., Albano L., Campaner S. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–2021. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 26.Diessner J., Bruttel V., Stein R.G. Targeting of preexisting and induced breast cancer stem cells with trastuzumab and trastuzumab emtansine (T-DM1) Cell Death Dis. 2014;5:e1149. doi: 10.1038/cddis.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oak P.S., Kopp F., Thakur C. Combinatorial treatment of mammospheres with trastuzumab and salinomycin efficiently targets HER2-positive cancer cells and cancer stem cells. Int J Cancer. 2012;131:2808–2819. doi: 10.1002/ijc.27595. [DOI] [PubMed] [Google Scholar]
- 28.Hori K., Sen A., Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126(Pt 10):2135–2140. doi: 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osipo C., Patel P., Rizzo P. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene. 2008;27:5019–5032. doi: 10.1038/onc.2008.149. [DOI] [PubMed] [Google Scholar]
- 30.Pandya K., Meeke K., Clementz A.G. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer. 2011;105:796–806. doi: 10.1038/bjc.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison H., Farnie G., Howell S.J. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao T.P., Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi H., Chang S.S., Hsu J.L., Hung M.C. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene. 2014;33:1073–1081. doi: 10.1038/onc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y., Ginther C., Kim J. Expression of Wnt3 activates Wnt/beta-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol Cancer Res. 2012;10:1597–1606. doi: 10.1158/1541-7786.MCR-12-0155-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallett R.M., Kondratyev M.K., Giacomelli A.O. Small molecule antagonists of the Wnt/beta-catenin signaling pathway target breast tumor-initiating cells in a Her2/Neu mouse model of breast cancer. PLoS One. 2012;7:e33976. doi: 10.1371/journal.pone.0033976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flemban A., Qualtrough D. The potential role of hedgehog signaling in the Luminal/Basal phenotype of breast Epithelia and in breast Cancer invasion and metastasis. Cancers. 2015;7:1863–1884. doi: 10.3390/cancers7030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasper M., Jaks V., Fiaschi M., Toftgard R. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30:903–911. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kebenko M., Drenckhan A., Gros S.J. ErbB2 signaling activates the Hedgehog pathway via PI3K-Akt in human esophageal adenocarcinoma: identification of novel targets for concerted therapy concepts. Cell Signal. 2015;27:373–381. doi: 10.1016/j.cellsig.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Im S., Choi H.J., Yoo C. Hedgehog related protein expression in breast cancer: gli-2 is associated with poor overall survival. Korean J Pathol. 2013;47:116–123. doi: 10.4132/KoreanJPathol.2013.47.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y., Shen S., Verma I.M. NF-kappaB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merkhofer E.C., Cogswell P., Baldwin A.S. Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene. 2010;29:1238–1248. doi: 10.1038/onc.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Y., Luo J.L., Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci U S A. 2007;104:15852–15857. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Z., Liu J.C., Chung P.E., Egan S.E., Zacksenhaus E. Targeting HER2(+) breast cancer: the TBK1/IKKepsilon axis. Oncoscience. 2014;1:180–182. doi: 10.18632/oncoscience.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korkaya H., Kim G.I., Davis A. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh J.K., Simoes B.M., Howell S.J., Farnie G., Clarke R.B. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15:210. doi: 10.1186/bcr3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh J.K., Farnie G., Bundred N.J. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19:643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintas-Cardama A., Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res. 2013;19:1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung S.S., Giehl N., Wu Y., Vadgama J.V. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J Oncol. 2014;44:403–411. doi: 10.3892/ijo.2013.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheen Y.Y., Kim M.J., Park S.A., Park S.Y., Nam J.S. Targeting the transforming growth factor-beta signaling in Cancer therapy. Biomol Ther. 2013;21:323–331. doi: 10.4062/biomolther.2013.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moses H., Barcellos-Hoff M.H. TGF-beta biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S.E. The functional crosstalk between HER2 tyrosine kinase and TGF-beta signaling in breast cancer malignancy. J Signal Transduct. 2011;2011:804236. doi: 10.1155/2011/804236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo P.K., Kanojia D., Liu X. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene. 2012;31:2614–2626. doi: 10.1038/onc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauring J., Park B.H., Wolff A.C. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw. 2013;11:670–678. doi: 10.6004/jnccn.2013.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakrabarty A., Bhola N.E., Sutton C. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013;73:1190–1200. doi: 10.1158/0008-5472.CAN-12-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanker A.B., Pfefferle A.D., Balko J.M. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc Natl Acad Sci U. S. A. 2013;110:14372–14377. doi: 10.1073/pnas.1303204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao D., Vahdat L.T., Wong S., Chang J.C., Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S.E., Yu Y., Criswell T.L. Oncogenic mutations regulate tumor microenvironment through induction of growth factors and angiogenic mediators. Oncogene. 2010;29:3335–3348. doi: 10.1038/onc.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao Y., Zhang Y., Qu Q. Cancer-associated fibroblasts induce trastuzumab resistance in HER2 positive breast cancer cells. Mol Biosyst. 2015;11:1029–1040. doi: 10.1039/c4mb00710g. [DOI] [PubMed] [Google Scholar]
- 60.Korkaya H., Wicha M.S. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013;73:3489–3493. doi: 10.1158/0008-5472.CAN-13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banys M., Hartkopf A.D., Krawczyk N. Dormancy in breast cancer. Breast Cancer (Dove Med Press) 2012;4:183–191. doi: 10.2147/BCTT.S26431. [DOI] [PMC free article] [PubMed] [Google Scholar]