Abstract
The proposal that humans can develop cognitive maps of their environment has a long and controversial history. We suggest an individual-differences approach to this question instead of a normative one. Specifically, there is evidence that some people derive flexible map-like representations from information acquired during navigation whereas others store much less accurate information. Our research uses a virtual-reality paradigm in which two routes are learned and must be related to each other. It defines 3 groups: Integrators, Non-integrators, and Imprecise Navigators. These groups show distinctive patterns of spatial skills and working memory, as well as personality. We contrast our approach with research challenging the cognitive map hypothesis, and offer directions for rapprochement between the two views.
Keywords: Cognitive maps, spatial navigation
All mobile species must find their way around the world to survive and reproduce. Wide-ranging comparative research shows that evolved navigation systems exhibit significant commonalities across species, but also reveals interesting differences due not only to variations in sensory capacities but also due to varied evolutionary pressures (e.g., Rosati & Hare, 2012; Wiener, Shettleworth, Bingman, Cheng, Healy, Jacobs, Jeffery, Mallot, Menzel & Newcombe, 2011). Tolman (1948) highlighted cross-species commonality in writing about “cognitive maps in mice and men”, showing regrettable word choice in using the noun “men”, but also launching a controversy about “cognitive maps” that continues to this day. The cognitive map view of navigation is that it involves representing space in an allocentric format that allows recovery of distances and directions between locations and flexible planning of routes (Gallistel, 1989; O’Keefe & Nadel, 1978). This view is widely endorsed by rodent neurophysiological researchers who study neurons that code spatial properties like location (place cells), orientation (head direction cells), boundaries (border cells), and distance (grid cells). Recent neuroimaging work in humans has supported the existence of many of these same properties in the human brain (for review, see Epstein, Patai, Julian, & Spiers, 2017). An alternative view is that “mice and men” simply rely on snapshot memories of locations, and route-following response strategies for navigation (e.g., Shettleworth, 2010; Warren, Rothman, Schnapp & Ericson, 2017). We have argued recently that this debate may admit of a simple solution. People differ considerably in their ability to learn large-scale environments and navigate within them (Hegarty & Waller, 2006; Wolbers & Hegarty, 2010). Thus, some participants in experiments may encode accurate internal maps, and others may encode spatial information imperfectly or in fragments, or rely entirely on route-based strategies.
Route Integration in the Real World
The individual-differences approach to navigation began with research by Ishikawa and Montello (2006), who devised a testing method called the route integration paradigm. Participants experience two separated routes and try to learn the names and locations of distinctive places (like buildings). Later, they experience a connecting route. People in Ishikawa and Montello’s experiment, who were driven by the experimenter around the hills above Santa Barbara on successive days, differed dramatically in what they learned. Some people related the two routes effectively, immediately, and seemingly easily. Some people learned to relate the routes over time. Both groups arguably formed cognitive maps, at least eventually. However, some people never integrated the routes, i.e., did not ever form a cognitive map.
Because active rather than passive movement might facilitate spatial learning, Schinazi, Nardi, Newcombe, Shipley and Epstein (2013) did a very similar experiment, but led their participants on a walking tour along two routes and later, two connecting routes, in a campus environment. Like Ishikawa and Montello, we found substantial individual differences, although variation in the walking environment was most marked before participants experienced the connecting route. We also found that perspective-taking skills assessed on a paper-and-pencil test were correlated with individual differences in real-world spatial learning, a relation also found in other studies (Wolbers & Hegarty, 2010).
Route Integration in Virtual Reality
Variation in perspective-taking skills is interesting, but it would be nice to know more about the correlates of individual differences in cognitive map-making. A practical challenge to individual differences research in navigation is that experimentation in a real-world environment poses logistical difficulties, such as cancellations due to bad weather and the necessity to transport participants to unfamiliar areas. Furthermore, we can’t test diverse geographic and cultural populations in a standard real-world environment; transporting all our participants to Santa Barbara or Philadelphia is unrealistic! Especially problematic for individual-differences research, logistical factors limit sample size, making it difficult to gather large enough samples to have the power to probe variation. To address these problems, Weisberg et al. (2014) devised a virtual learning environment modeled after the real-world route-integration paradigm (Figure 1).
Figure 1.
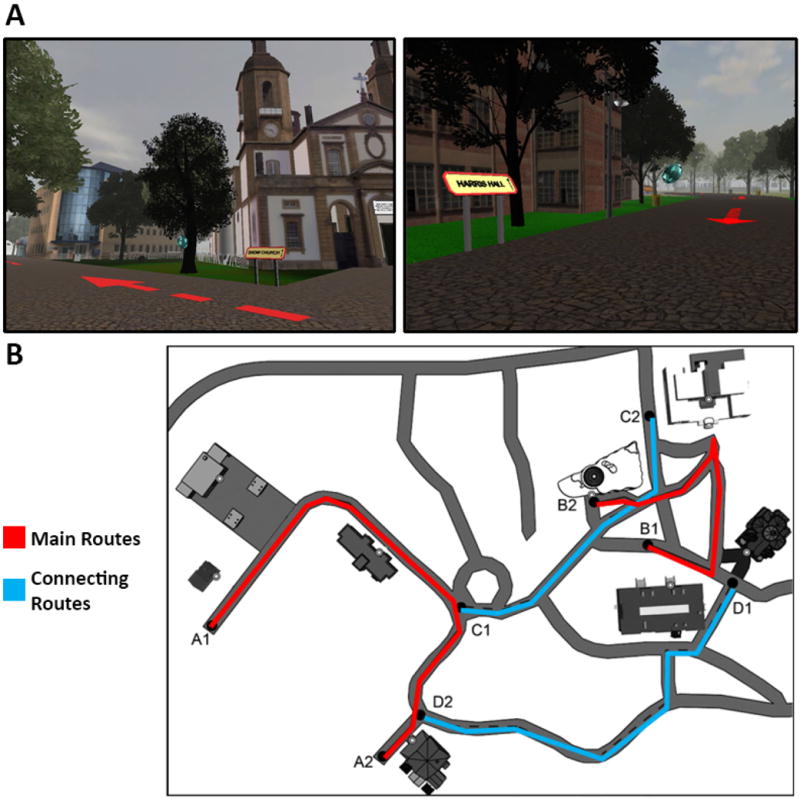
Screen shots from the Virtual Silcton desktop virtual environment (A), and an overhead map (B), which depicts the main routes (highlighted in red) and connecting routes (highlighted in blue), which participants learned. Buildings are shown in the screen shots, along with signs and blue gems, which were used to indicate the presence of a building nearby. Buildings are shown in the map as schematic depictions. White circles represent the front door of each building. The overhead map was never shown to participants.
The virtual environment had the same spatial configuration as the real-world study (Schinazi et al., 2013), but replaced the buildings with virtual models of similar saliency, size, and style. In the virtual route-integration paradigm (which we call Virtual Silcton), participants virtually travelled along two routes, which were indicated by arrows along each path, and learned four buildings per route. Then, they travelled along two connecting routes, which connected the first two routes. Finally, they completed two navigation tests: an onsite pointing task in which they were dropped at each building location and had to point to all other buildings; and a model-building task, in which they had to drag and drop building images around a map to recreate the configuration. Crucially, the route integration paradigm allows us to distinguish between buildings that were traveled between directly because they were learned along the same initial route (within-route) and buildings that were never directly traveled between (between-route). The connecting routes provide a path between segments of the two routes, but inferences must still be made about how the buildings along the two routes relate to each other overall. As a result, between-route judgments require much more difficult spatial inference.
Individual differences emerged for both within- and between-route judgments, and a cluster analysis based on between- and within-route pointing scores suggested the existence of three groups1 (Figure 2). One group, Integrators, performed well on both within- and between-route judgments. A second group, Non-integrators, performed well on within-route judgments, but relatively poorly on between-route judgments. A third group, Imprecise Navigators, performed relatively poorly (although above chance) on both types of pointing judgments. These results, which we have now replicated across four separate samples in two published studies (Weisberg et al., 2014; Weisberg & Newcombe, 2016) suggest a two-step process in which routes are learned first, and then related to each other.
Figure 2.
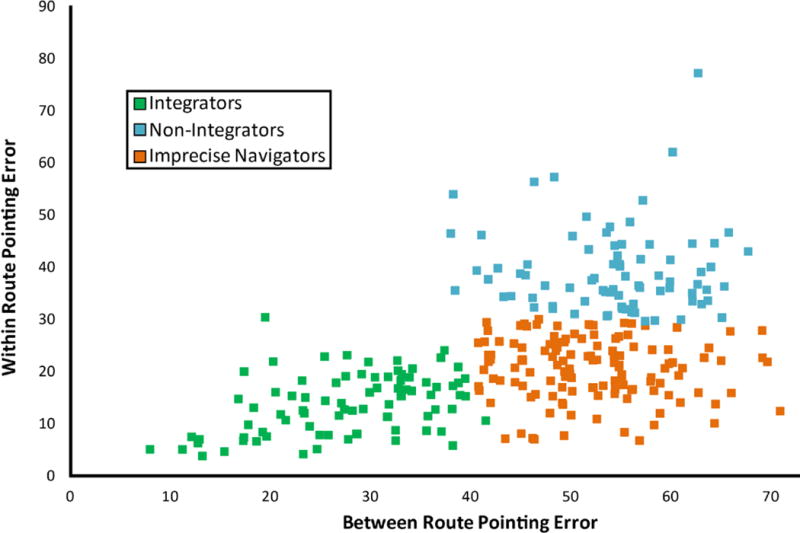
A scatter plot of performance on the two types of pointing trials from Virtual Silcton. Each dot represents one participant’s averaged data on the two types of trials. Colors refer to results from cluster analyses, dividing participants into three groups. Between route pointing trials refer to trials which required participants to point across the two main routes (i.e., from a building along one main route to a building along the other main route). Within route pointing refers to trials which required participants to point within the same main route. Pointing was measured in degrees of error, with 90° as chance performance. Data are from 294 participants from the four studies reported in Weisberg & Newcombe (2016), including the data which were also presented in Weisberg, Schinazi, Newcombe, Shipley, & Epstein (2014).
Cognitive Correlates
A key question is whether individual differences on these aspects of learning an environment are mirrored by individual differences in related aspects of cognition. We have now investigated whether the three groups of navigators show distinct patterns of performance on related tasks, both in the spatial domain and in terms of domain-general cognitive abilities.
In terms of spatial cognition, it is important to remember that it is a variegated domain, not a monolithic one (Newcombe, in press). Some common tests of spatial ability are conceptually and empirically linked to large-scale navigation, while other tests seem to tap small-scale spatial tasks and depend on somewhat different neural systems. The widely-used mental rotation test (MRT; Vandenberg & Kuse, 1978), wherein participants must match an object made up of cubes with its rotated equivalents, is likely a measure of small-scale spatial ability. However, despite its conceptual distinctness, it shows varied performance across the three Silcton groups: Integrators outperform Non-Integrators who outperform Imprecise Navigators (Weisberg & Newcombe, 2016). Perspective taking is more closely related to navigation (Lambrey, Doeller, Berthoz & Burgess, 2011) and data from Schinazi et al.’s real-world study of navigation revealed a strong relationship between perspective taking ability and navigation performance. Recently, in a study of children and adolescents, Nazareth et al. (under review) found that perspective taking accounted for a wider range of navigational behaviors in Virtual Silcton than mental rotation, and more variance when included in regression equations along with MRT. Thus, the correlation with MRT may reflect shared variance between MRT and PT, rather than mental rotation per se.
In terms of domain-general cognitive capacities, Integrators and Non-Integrators both outperform Imprecise Navigators on verbal and spatial working memory measures. This fact suggests that a general working memory capacity might underlie the ability to store navigationally-relevant data. Learning within-route relations has both verbal and spatial components, including remembering the associations between the names of buildings, the appearance of the buildings, and buildings’ location. However, different aspects of spatial working memory may relate to integrating two routes in a large-scale environment as well as to within-route learning (Blacker, Weisberg, Newcombe, & Courtney, in press). Importantly, these results obtained with a statistical control for general intelligence, and the three groups do not differ substantially in g, although Imprecise Navigators are significantly worse than the other two groups when the data from several studies are aggregated (Weisberg & Newcombe, 2016).
Ability Versus Preference
There is another approach to individual differences in navigation that emphasizes flexibility in the application of navigation strategies (Shelton, Marchette, & Furman, 2013). Their task, called the dual solution paradigm (DSP), taps navigation strategy and preferences in a virtual maze, i.e., whether individuals prefer a place-based or response-based approach to navigation (Marchette, Bakker, & Shelton, 2011). Navigators must learn to locate a set of objects in a maze, but can find them using a learned familiar route, or using a novel shortcut. We have administered the DSP as well as Silcton to assess whether the conceptualizations are the same or different. Our data suggest that Integrators can be successful with either navigation strategy, whereas Imprecise Navigators are only successful if they use the route-based strategy.
In addition, Integrators and Non-Integrators seem to store knowledge about Virtual Silcton in a way that would support multiple strategies. Specifically, when we asked Silcton participants whether buildings were first encountered on the same route or on different routes, we found that Integrators performed as well as Non-Integrators. If Integrators formed a global cognitive map and disregarded the route knowledge (i.e., which buildings were along which routes) as irrelevant to learning the whole environment, they would have performed worse on this task. Instead, this suggests that Integrators scaffolded the integration of the two routes on strong knowledge of both routes.
Motivational and Emotional Correlates
One possibility that could explain the individual differences we see in Silcton is that some navigators have different motivational or emotional dispositions, or different personalities. Let’s take motivation first. Some people might worry that Imprecise Navigators just don’t try too hard to succeed at the task. However, we don’t think that’s the case, because we ran a version of the Silcton study (Study 3, Weisberg & Newcombe, 2016) in which we told participants they would be entered in a bonus raffle if they finished in the top half of all participants. Performance was not measurably improved by this incentive, and the same pattern of three groups still emerged.
What about emotion, especially anxiety? It is natural to wonder whether Imprecise Navigators may simply be fearful and apprehensive about navigating. Anxiety about doing mathematics drains working memory and lowers math performance (Beilock, 2008), so navigation could be a similar case. Indeed, across several studies, Imprecise Navigators scored higher on a spatial anxiety self-report measure, which assesses how fearful and apprehensive people feel in various navigational situations. But we need to remember that a correlation can run two ways. Spatial anxiety is also negatively correlated with self-ratings of navigation proficiency on the Santa Barbara Sense of Direction Scale. Similarly, the SBSOD is correlated with high Emotional Stability on the Big 5 personality test, that is, with low anxiety, withdrawal and self-consciousness (Condon, Wilt, Cohen, Revelle, Hegarty & Uttal, 2015). Thus, anxiety about navigation may impede success, but the alternative is that people may simply be anxious about performing a task that they are aware they do poorly.
What about personality? Perhaps Integrators are good navigators because they are adventurous, and relish the challenge of learning a new environment. Interestingly, ratings on the SBSOD are related to Openness on the Big 5 personality test, which is a measure of curiosity, ingenuity and adventurousness, and to Extraversion, which measures energy, enthusiasm and approach behavior (Condon et al., 2015). In addition, Condon et al. found that SBSOD is related to Conscientiousness, which measures attention to detail, organization and diligence. These data paint a picture of Integrators as both eager to learn and willing to work hard at cognitive tasks, underscoring the idea that forming cognitive maps is possible, but not automatic or effortless.
Status of the Cognitive Map Controversy
We see two possible issues in assigning spatial representations to the brain in the form of a cognitive map. First, what is meant by “map?” Here, we define a map as a recording of metric associations between properties of the world. This recording can be on paper (traditional maps), digitally instantiated (global positioning system displays), or in the brain (a cognitive map). Note that maps need not be veridical – even in physical maps, all transformations of a sphere onto a plane will introduce distortion. Maps can also be distorted for other reasons, through errors in recording, due to emphasizing certain features (e.g., enlarging a landmark by making it larger). Introducing systematic distortions of the metric content changes the representation of space from a map to what we would call a schematic. A schematic retains some metric associations (e.g., directions), but systematically changes others, as in a subway map.
The second possible issue is how the brain instantiates a map. The term “cognitive map” has been criticized for implying a completely unified representation in which all possible spatial relations are represented equivalently (Downs, 1981). Indeed, Warren et al. (2017) have recently argued that there can be no cognitive map, because human cognition exhibits distortions, which mean the underlying representation must be non-Euclidean. In their study, participants have no trouble with wormholes in virtual environments. The wormholes distort Euclidean space by automatically transporting navigators to bypass a section of the maze. Navigators do not become lost or disoriented (and in fact are perfectly happy to take the wormhole “shortcut”). Warren et al argue that because the gaps between wormholes are not represented, the cognitive map must not be Euclidean, and therefore is not a map at all. Instead, they claim, human navigation behavior is better characterized by a labeled graph – a mathematical structure by which objects are related through pairwise connections of varying strength. The labels on the graph refer to rough distances and directions between pairs of locations. However, there is recent neuroimaging work showing that the hippocampus has similar representations of locations that are either close together in space or close together as experienced in time (i.e., even through teleporting). This finding suggests that the reason for the distorted metric information may be because the hippocampus normally builds time-dependent representations of space (Deuker, Bellmund, Schroder, & Doeller, 2016). The lack of a way to take account of wormholes may be an idiosyncrasy of tricking an evolved system built for the natural world by forcing it to try to cope with an environment possible only in virtual reality.
Despite the seeming dichotomy of the “cognitive maps—yes or no” debate, we see areas of commonality. The two descriptions might be reconciled if we postulate that spatial relations in large-scale space exhibit a hierarchical representation, in which local areas, or routes, are represented in detail, but the relations among them are represented more coarsely (Chrastil & Warren, 2014; Jacobs & Menzel, 2014; Jacobs & Schenk, 2003; Kuipers, 2000; Wolbers & Hegarty, 2010). In some cases, it makes sense to think of navigable space as a network – a set of connections between locations, with rough information about direction and distance. After learning, this may be how we can navigate in a car in cities with one-way streets. In other cases, it makes sense to think of navigable space as a map with distortions. In an area like a forest or dessert, with few distinct landmarks, we can nevertheless maintain orientation. We think our data on Virtual Silcton provide evidence in support of the idea that some navigators do form cognitive maps. Minimally, they can do something a map affords easily, which a graph does not: they can calculate distances and directions between places between which they have never directly travelled.
Recommended Reading.
Waller, D. E., & Nadel, L. E. (2013). Handbook of spatial cognition. American Psychological Association. A comprehensive collection of chapters on various topics related to spatial cognition in general and navigation behavior specifically. The collection includes chapters on individual differences as well as a variety of approaches across navigation topics: computational modeling, animal and human behavior and neuroscience, applications, and the roles of language, perception, and memory.
Warren, W. H., Rothman, D. B., Schnapp, B. H., & Ericson, J. D. (2017). (See References). An empirical article that outlines the cognitive graph theory alternative to cognitive map theory.
Weisberg, S. M., & Newcombe, N. S. (2016). (See References). The largest individual differences studies we have published using Virtual Silcton, with full methodological details and findings.
Wolbers, T., & Hegarty, M. (2010). (See References). A theoretical approach to individual differences in navigation that breaks down aspects of navigation behavior with cognitive and neural constituents.
Acknowledgments
Work on this project was funded by a grant to the Spatial Intelligence and Learning Center from the National Science Foundation, SBE-1041707, and a grant from the National Institute of Health to S.M.W., F32-DC-015203.
Footnotes
It is important to note that 10–15° is approximately ceiling performance for pointing judgments, and that most Integrators and Non-Integrators are in or around this range. This is because small deviations in pointing to the buildings themselves, instead of precisely pointing to the front door, yield degrees of error in that range, despite participants pointing accurately at some part of the building.
References
- Beilock S. Math performance in stressful situations. Current Directions in Psychological Science. 2008;17(5):339–343. doi: 10.1111/j.1467-8721.2008.00602.x. [DOI] [Google Scholar]
- Blacker KJ, Weisberg SM, Newcombe NS, Courtney SM. Keeping track of where we are: Spatial working memory in navigation. Visual Cognition. 2017 doi: 10.1080/13506285.2017.1322652. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrastil ER, Warren WH. From cognitive maps to cognitive graphs. PLOS ONE. 2014;9(11):e112544. doi: 10.1371/journal.pone.0112544.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon DM, Wilt J, Cohen CA, Revelle W, Hegarty M, Uttal DH. Sense of direction: General factor saturation and associations with the Big-Five traits. Personality and Individual Differences. 2015;86:38–43. [Google Scholar]
- Downs RM. Maps and metaphors. The Professional Geographer. 1981;33(3):287–293. [Google Scholar]
- Deuker L, Bellmund JLS, Navarro Schröder T, Doeller CF. An event map of memory space in the hippocampus. eLife. 2016 doi: 10.7554/eLife.16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nature Neuroscience. 2017;20:1504–1513. doi: 10.1038/nn.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. Animal Cognition - the Representation of Space, Time and Number. Annual Review of Psychology. 1989;40:155–189. doi: 10.1146/annurev.ps.40.020189.001103. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Waller D. In: Handbook of Visuospatial Thinking. Shah P, Miyake A, editors. Cambridge University Press; Cambridge: 2006. [Google Scholar]
- Ishikawa T, Montello RD. Spatial knowledge acquisition from direct experience in the environment: Individual differences in the development of metric knowledge and the integration of separately learned places. Cognitive Psychology. 2006;52:93–129. doi: 10.1016/j.cogpsych.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Menzel R. Navigation outside of the box: what the lab can learn from the field and what the field can learn from the lab. Movement Ecology. 2014;2(1):3. doi: 10.1186/2051-3933-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs LF, Schenk F. Unpacking the cognitive map: The parallel map theory of hippocampal function. Psychological Review. 2003;110(2):285–315. doi: 10.1037/0033-295x.110.2.285. [DOI] [PubMed] [Google Scholar]
- Kuipers B. The spatial semantic hierarchy. Artificial intelligence. 2000;119(1–2):191–233. [Google Scholar]
- Lambrey S, Doeller C, Berthoz A, Burgess N. Imagining being somewhere else: neural basis of changing perspective in space. Cerebral cortex. 2011;22(1):166–174. doi: 10.1093/cercor/bhr101. [DOI] [PubMed] [Google Scholar]
- Marchette SA, Bakker A, Shelton AL. Cognitive mappers to creatures of habit: differential engagement of place and response learning mechanisms predicts human navigational behavior. Journal of Neuroscience. 2011;31(43):15264–15268. doi: 10.1523/JNEUROSCI.3634-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth A, Weisberg SM, Margules K, Newcombe NS. Cognitive maps reach adult levels in early adolescence: The advent of individual differences (under review) [Google Scholar]
- Newcombe NS. Three kinds of spatial cognition. In: Wixted J, editor. Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience. 4th (in press) [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Rosati AG, Hare B. Chimpanzees and bonobos exhibit divergent spatial memory development. Developmental Science. 2012;15(6):840–853. doi: 10.1111/j.1467-7687.2012.01182.x. [DOI] [PubMed] [Google Scholar]
- Schinazi VR, Nardi D, Newcombe NS, Shipley TF, Epstein RA. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 2013;23:515–528. doi: 10.1002/hipo.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton AL, Marchette SA, Furman AJ. A mechanistic approach to individual differences in spatial learning, memory, and navigation. The Psychology of Learning and Motivation. 2013;59:223–259. [Google Scholar]
- Shettleworth SJ. Cognition, Evolution, and Behavior. Oxford University Press; US: 2010. [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychological Review. 1948;55:189–209. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Vandenberg SG, Kuse AR. Mental rotations, a group test of three-dimensional spatial visualization. Perceptual and Motor Skills. 1978;47(2):599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Wang RF, Spelke ES. Human spatial representation: Insights from animals. Trends in Cognitive Sciences. 2002;6:376–382. doi: 10.1016/s1364-6613(02)01961-7. [DOI] [PubMed] [Google Scholar]
- Warren WH, Rothman DB, Schnapp BH, Ericson JD. Wormholes in virtual space: From cognitive maps to cognitive graphs. Cognition. 2017;166:152–163. doi: 10.1016/j.cognition.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Weisberg SM, Newcombe NS. How do (some) people make a cognitive map? Routes, places, and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2016;42(5):768. doi: 10.1037/xlm0000200. [DOI] [PubMed] [Google Scholar]
- Weisberg SM, Schinazi VR, Newcombe NS, Shipley TF, Epstein RA. Variations in cognitive maps: Understanding individual differences in navigation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2014;40(3):669. doi: 10.1037/a0035261. [DOI] [PubMed] [Google Scholar]
- Wiener J, Shettleworth S, Bingman VP, Cheng K, Healy S, Jacobs LF, Jeffret KJ, Mallot HA, Menzel R, Newcombe NS. Animal navigation: A synthesis. In: Menzel R, Fische J, editors. Animal thinking: Contemporary issues in comparative cognition. Cambridge, MA: MIT Press; 2011. pp. 51–76. (Strugmann Forum Report, Vol 8, J. Lupp, Series ed). [Google Scholar]
- Wolbers T, Hegarty M. What determines our navigational abilities? Trends in cognitive sciences. 2010;14(3):138–146. doi: 10.1016/j.tics.2010.01.001. [DOI] [PubMed] [Google Scholar]


