Abstract
The control of the location and activity of stem cells depends on spatial regulation of gene activities in the stem cell niche. Using computational and experimental approaches, we have tested, and found support for, a hypothesis for gene interactions that specify the Arabidopsis apical stem cell population. The hypothesis explains how the WUSCHEL gene product, synthesized basally in the meristem, induces CLAVATA3-expressing stem cells in the meristem apex, but, paradoxically, not in the basal domain where WUSCHEL itself is expressed. The answer involves the activity of the small family of HAIRY MERISTEM genes, that prevent the activation of CLAVATA3, and which are expressed basally in the shoot meristem.
One Sentence Summary: The domain of stem cells in the Arabidopsis shoot apical meristem is founded on WUS expression but bounded by HAM expression.
Distinct cell types in multicellular organisms form specific patterns during development, and how the patterns are regulated is a critical question. In Arabidopsis shoot apical meristems (SAMs), stem cells reside at and near the apex while the cells specifying the stem cells are located more basally (1). Along the apical-basal axis, the homeodomain transcription factor WUSCHEL (WUS) and the secreted peptide CLAVATA3 (CLV3) form a negative feedback loop mediating communication between the stem cells and the beneath rib meristem cells (2–9). CLV3 is highly expressed in the apical stem cells (2–4), while WUS transcript is confined to the center of rib meristem (5–6). WUS protein moves apically via plasmodesmata, cell-cell connections, into the stem cells (10–12) to activate CLV3 expression, reportedly through direct binding to the CLV3 promoter (10,12). However, how the apical-basal patterns of CLV3 and WUS mRNAs are initiated and maintained are unknown. One central question is, why is CLV3 activated only by WUS that has transited into the apical stem cells but is not activated in the interior cells where WUS protein is at highest concentration, and where WUS is actively expressed? It has been proposed that either WUS requires an as-yet unidentified signal from the epidermis (L1) of the meristem for CLV3 activation (13–14), or that WUS can convert itself from a repressor to an activator when its concentration is low (12). Here, we propose a different mechanism. We previously found that members of the HAIRY MERISTEM (HAM) family, GRAS-domain transcription factors, function as interacting partners of WUS to control the production of shoot stem cells (15). The HAM proteins are involved in meristem regulation and the CLV3-WUS pathway (15–18) and CLV3 is ectopically expressed in the rib meristem of a ham multiple mutant (16). Using computational and experimental approaches, we show here that in the SAMs, WUS activates CLV3 only in the absence of HAM, and in the initiating meristems, an apical-basal gradient of HAM defines the patterning of the CLV3 expression domain.
Imaging of fluorescent reporters for both HAM and CLV3 in the same living SAMs shows that the expression patterns of HAM and CLV3 mRNAs are nearly complementary, with opposite concentration gradients along the apical-basal axis (Fig. 1; Fig. S1; Movie S1-S3). HAM1 and HAM2 are highly expressed in the rib meristem and peripheral zone in the corpus (Fig.1, Fig. S1), where CLV3 expression is reduced. In contrast, HAM1 and HAM2 expression is not detected in the L1 and L2 layers of the central zone, where CLV3 is highly expressed (Fig.1, Fig. S1). In addition, we previously showed that HAM1, HAM2 and WUS are co-expressed in the same cells at the center of corpus (15) and HAM protein was not detected in the central zone (15). These images (Fig.1, Fig. S1) and previous reports (4, 7,10–12,15) have revealed the distinct and overlapping expression patterns of WUS, CLV3, and HAM in the SAM (Fig. S2), which leads to the hypothesis that in the apical stem cells where HAM is absent, CLV3 mRNA production is activated by WUS; at the basal part of the SAM where HAM proteins are present, the ability of WUS to activate CLV3 mRNA production is suppressed.
Fig. 1.
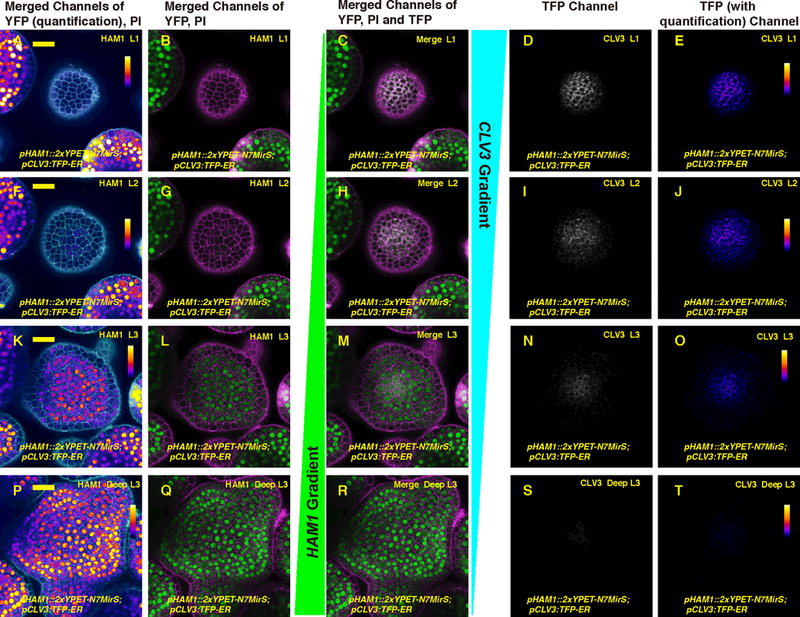
Expression patterns of HAM1 and CLV3 in the SAM are largely complementary. (A-T) Expression of pHAM1::2xYPET-N7MirS and pCLV3::TFP-ER in transverse optical sections from top to bottom through the same Arabidopsis SAM, including L1 (A-E), L2 (F-J), cells just below the L2 (K-O), and deeper layers in the center, and all layers on the meristematic periphery (P-T). Panels (from left to right): YFP (quantification indicated by color) and propidium iodide (PI) counterstain (white); YFP (green) and PI counterstain (purple); merge of three channels: YFP (green), PI (purple) and TFP (gray); TFP (gray); and TFP (quantification indicated by color). Scale bar (A-T): 20 μm; color bar: fire quantification of signal intensity.
To test this hypothesis, we first established a new 3D+t computational model to simulate the patterns of CLV3 and WUS transcripts and the movement of CLV3 and WUS proteins during meristem development (See Methods). The model incorporated the current knowledge of the CLV-WUS feedback (2–8,19), and included an activator of WUS transcription, the Organizing Center (OC) signal at the center of the meristem corpus, and it took the concentration gradient of HAM as an input. We modeled the movement of WUS protein as a passive diffusion-like transport, as previously reported (13–14). Most importantly, we defined WUS as the activator of CLV3 transcription when HAM is absent, but not when HAM protein is present. We represented CLV3 peptide as a repressor of WUS mRNA level and modeled its rapid apoplastic movement between cells (8). This model was able to reproduce the specific patterns of WUS transcript, WUS protein, and CLV3 transcript in a wild type SAM, and these patterns were resistant to perturbations introduced by cell growth and divisions (Movie S4-S6, S8-S9). In addition, a simplified 1D+t cell layer model was able to predict the patterns of WUS and CLV3 along the apical-basal axis (Movie S7), suggesting the apical-basal polarity of gene expression can be uncoupled from lateral cell proliferation. By contrast, if HAM was converted into an activator (either together with WUS or in addition to WUS) of CLV3 transcription, we were not able to reproduce the wild-type CLV3 mRNA pattern (Fig. S3A-B).
We further tested the hypothesis and validated the computational model by introducing a spatial perturbation of gene expression both in silico and in vivo. The fact that HAM protein is absent at the center of the meristem L1 layer (Fig. 1A-E, Fig. S1A-E) promoted us to test the impact on CLV3 patterning of specifically expressing HAM in the L1 layer. Our model predicted that CLV3 mRNA in the L1 layer would be dramatically reduced due to the absence of activation in the L1, and the peak of CLV3 expression would shift into deeper cell layers (Fig. 2A-B). We reproduced this perturbation experimentally by generating pATML1::HAM1m-GFP (L1-HAM) transgenic plants that express a HAM1-GFP fusion protein in L1 (Fig. S4A-F, Movie S10-S11) from an epidermis-specific promoter (20). We found that the activity of the CLV3 reporter in L1 of the L1-HAM SAM was reduced compared either to the level in cells below L2 from the same L1-HAM SAM or the level in L1 in a wild type SAM (Fig. 2C-L). In addition, the SAMs of L1-HAM plants were substantially enlarged compared to the wild type (Fig. 2C-D; Fig. S5A-B), demonstrating the functional significance of keeping HAM levels low and CLV3 high in the epidermis.
Fig. 2.
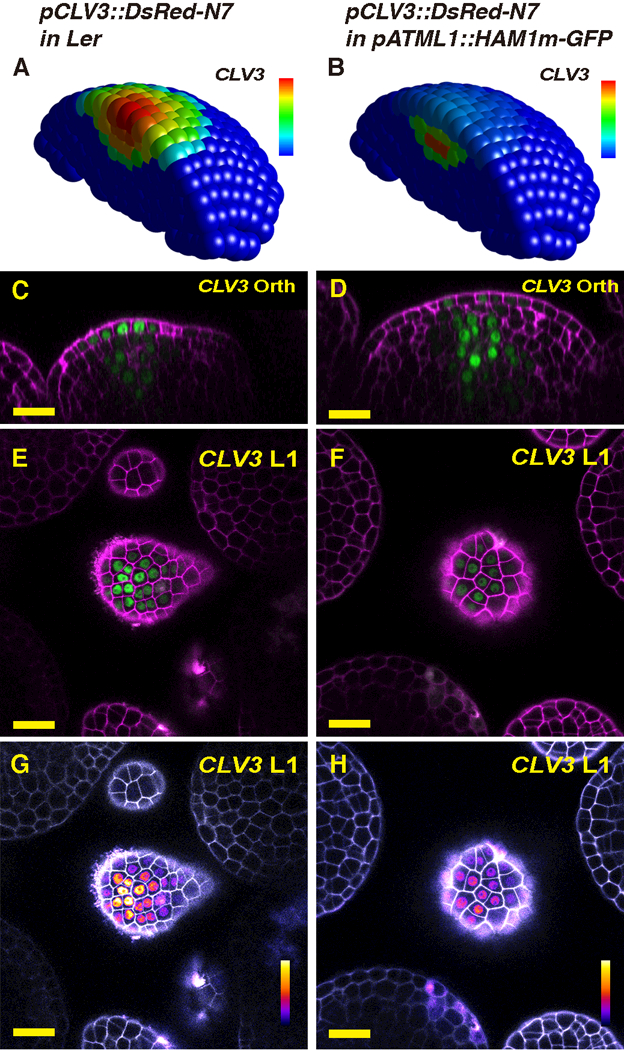
Results of HAM expression changes in the L1. (A-B) The simulated CLV3 expression domain in 3D in both wild type (A) and the pATML1::HAM1m-GFP transgenic plant (B) in which HAM1 is over-expressed in the epidermis. The CLV3 mRNA levels are indicated by color, with a gradient from red (maximum, 0.86 arbitrary units, a.u.) to blue (0). (C-H) Validation of the computational simulation through confocal live-imaging of a pCLV3::DsRed-N7 (green) reporter in a pATML1::HAM1m-GFP transgenic plant. Orthogonal (C-D) and transverse section (E-H) views of the same wild type (Ler) plant (C, E, G) or the same L1-HAM transgenic plant (D, F, H) are shown. PI counterstain is represented in purple (C-F) or gray (G-H), and the relative pCLV3::DsRed-N7 signal intensity is indicated by color (G-H). Scale bar: 20 μm; color bar (G-H): fire quantification of signal intensity. Here and elsewhere in this report, the whole SAM template was used for all of the simulations, but only a half of the SAM is represented, for visualization of cells in inner layers.
The model also predicted that a partial repression of HAM (18, 21) is sufficient to alter the CLV3 pattern (Fig. S6A-B), which is consistent with the experimental results and quantitative analyses (Fig. S6A-F). This partial repression of HAM led to a substantial increase in CLV3 mRNA levels (Fig. S6C-D), more cells expressing CLV3 in the SAMs (Fig. S6E), and a basal shift of the CLV3 expression peak (Fig. S6F).
A series of genetic perturbations was introduced further to dissect the roles of HAM and WUS in CLV3 patterning along the apical-basal axis in the SAM. The model made the following predictions in parallel (Fig. 3A-D): When HAM is absent in a SAM, CLV3 mRNA would be locally activated by WUS, with the concentration peak in the basal part of the SAM (Fig. 3A-B). When HAM is present in a SAM but the transcriptional activity of WUS is reduced, the CLV3 mRNA would still be expressed in the apical part of the SAM, with the level significantly reduced (Fig. 3C). Furthermore, when HAM is absent and the transcriptional activity of WUS is reduced, CLV3 would then be expressed in the basal part of the SAM at a reduced level (Fig. 3D). To test these predictions, we examined the CLV3 mRNA in the SAMs of wild type, ham1;2;3 triple loss-of-function mutants, wus-7 partial loss-of-function mutants (15, 22), and wus-7;ham1;2;3 quadruple mutants (Fig. 3E-H). As assessed using in situ hybridization experiments, the CLV3 expression domain in ham1;2;3 shifted to the center of the rib meristem (Fig. 3F), as observed previously (16), while CLV3 mRNA level was reduced locally in the central zone in a wus-7 SAM (Fig. 3G). In the wus-7; ham1;2;3 SAM, CLV3 mRNA expression shifted to the rib meristem, and its level was significantly reduced compared to that in ham1;2;3 (Fig. 3H). In addition, the wus-7; ham1;2;3 mutant displayed a much reduced SAM size (Fig. 3H), consistent with the previous finding that HAM and WUS work together in control of stem cell homeostasis (15). In addition to the partial loss of function (Fig. 3C), the computational model predicted that when WUS activity is completely lost (Fig. 3I-L), CLV3 mRNA would be absent since this major activator of CLV3 is absent, regardless whether HAM is present (Fig. 3K) or not (Fig. 3L). These predictions were validated through the CLV3 mRNA in situ hybridization in the SAMs of wild type, ham1;2;3, wus-1 null mutants (5), and wus-1;ham1;2;3 quadruple mutants (Fig. 3M-P; Fig. S7). We found that CLV3 mRNA was undetectable in both wus-1 and wus-1;ham1;2;3 plants, and wus-1;ham1;2;3 showed a terminated meristem similar to that in wus-1 (Fig. 3O-P). These results (Fig. 3) reveal distinct roles of HAM and WUS in determining CLV3 mRNA pattern in an established SAM: WUS maintains overall CLV3 level while HAM defines the apical-basal positioning of the CLV3 expression domain.
Fig. 3.
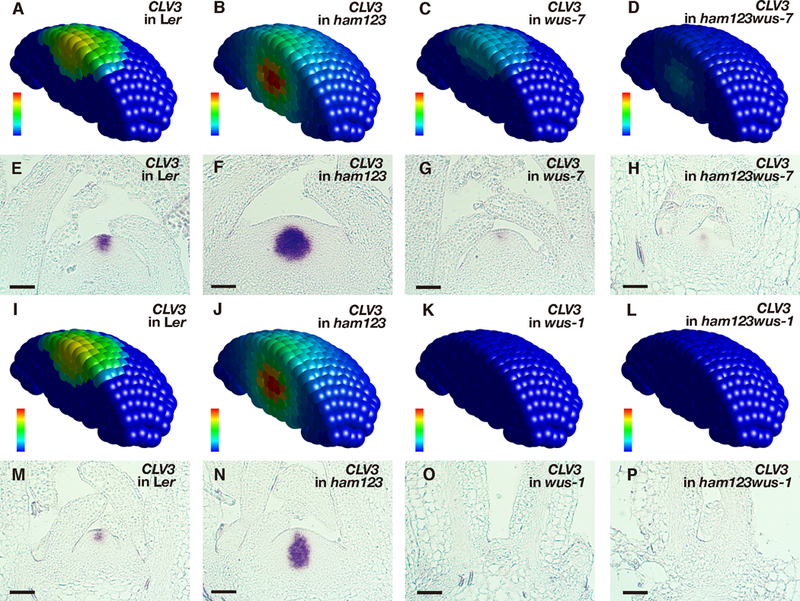
Model prediction and experimental validation in SAMs of different genotypes. (A-D) Simulated CLV3 mRNA levels in 3D in different genotypes including wild type (A), ham1;2;3 (B), wus-7 (C), and wus-7;ham1;2;3 (D). (E-H) Validation of the computational simulation through in situ hybridization to CLV3 RNA in the SAMs of wild type (Ler) (E), ham1;2;3 (F), wus-7 (G), and wus-7;ham1;2;3 (H) at the same developmental stage (30 days after germination, DAG) in the same experimental conditions. (I-L) Simulated CLV3 mRNA levels in 3D in different genotypes including wild type (I), ham1;2;3 (J), wus-1 (K), and wus-1;ham1;2;3 (L). (M-P) Validation of the computational simulation through RNA in situ hybridization of CLV3 in the SAMs of wild type (Ler) (M), ham1;2;3 (N), wus-1 (O), and wus-1;ham1;2;3 (P) at the same developmental stage (22 DAG) in the same experimental conditions. Simulated CLV3 mRNA level (A-D, I-L) in each individual cell is indicated by color, with the gradient from red (maximum, 1.15 a.u) to blue (none), and the simulations in (I) and (J) are the same as in (A) and (B), respectively. Scale bar (E-H, M-P): 50 μm.
We further examined whether the HAM-WUS-CLV3 regulatory loop defines the initiation of polarity during de novo meristem formation from leaf axils (Fig. 4A-P, Fig. S8-S9). Differently from the already established apical meristem, in the initiating axillary meristem (AM) (Stages S2 and S3) (23–24), expression of CLV3 is first seen in the corpus (24). To seek the underlying mechanism, we first examined the expression pattern of HAM1 at early stages of AM initiation. At S2, HAM1 is evenly expressed in the initiating meristem with no gradient from the epidermis to the interior cells (Fig. 4A). At S3, HAM1 is also expressed throughout the initiating meristem, lacking a clear gradient (Fig. 4E). Both patterns are distinct from the HAM1 pattern in the established SAMs (Fig. 1) (15). When these HAM1 patterns (Fig. 4A, E) were used as inputs for AM simulation (Fig. 4B, F), the model predicted that the CLV3 domains in wild type would be confined to the basal part of the initiating meristems (Fig. 4C, G), suggesting that when a HAM concentration gradient does not exist or is very shallow, the CLV3 mRNA pattern is predominantly determined by WUS concentration. These predictions are consistent with our experimental results (Fig. 4D, H) and previous observations (24). At late stages of AM initiation (Fig. 4I, M), the concentration gradient of HAM1 from epidermis to interior cells has been established, comparable to its pattern in the SAMs (Fig. 1). Using this information as input (Fig. 4J, N), the model predicted that at these stages (Fig. 4K, O), CLV3 would be expressed predominantly in the apical domain of the initiating AMs; this was experimentally validated (Fig. 4L, P; 24). Thus the dynamic gradient of HAM drives the CLV3 pattern dynamics during de novo formation of a new stem cell niche (Movie S12).
Fig. 4.
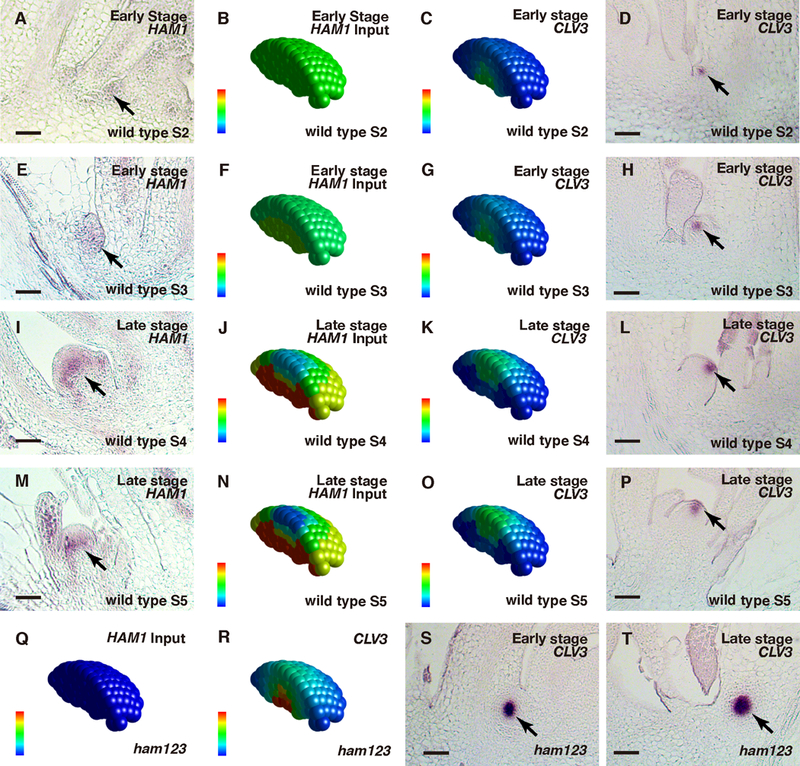
Patterning during the de novo formation of axillary stem cell niches in wild type (A-P) and in ham1;2;3 (Q-T). (A, E, I, M) In situ hybridization to HAM1 RNA in wild type, at early (A, E) and late (I, M) stages of axillary meristem (AM) initiation. (B, F, J, N) Levels of HAM concentration in wild-type at early (B, F) and late stages (J, N) as input. (C, G, K, O) Simulated CLV3 mRNA levels at early (C, G) and late stages (K, O) in wild type. (D, H, L, P) Validation of the simulation through the in situ hybridization to CLV3 mRNA in a wild type AM at early (D, H) and late stages (L, P). (Q) HAM concentration was set at zero in ham1;2;3 at all stages as the input. (R) Simulated CLV3 mRNA levels at different developmental stages in ham1;2;3. (S-T) Validation of the simulation through RNA in situ hybridization of ham1;2;3 AMs. The initiation of AMs in ham1;2;3 was disturbed, and did not follow the well-characterized developmental stages (23–24). The early (S) and late (T) stages of AM initiation in ham1;2;3 were defined based on the distance of leaf axils from the same main SAM in longitudinal sections. In each individual cell, the relative HAM protein level (input) is indicated by color (blue: 0, red: maximal level 1), and the relative CLV3 mRNA level (output) is also indicated by color (blue: 0, red: 1.23 a.u). Arrows: HAM1-expressing cells (A, E, I, M) and CLV3-expressing cells (D, H, L, P, S-T) during new meristem initiation. Scale bar: 50 μm.
We then simulated the patterns of CLV3 mRNA expression at different stages during AM initiation when HAM activity is absent (Fig. 4Q). The model predicted that CLV3 expression domains would be confined to deep cell layers at all times (Fig. 4R, Movie S13-S15), which is consistent with the experimental result (Fig. 4S, T). The computational and experimental results together demonstrated that the lack of either a gradient or expression of HAM leads to similar CLV3 RNA patterns, suggesting that the HAM concentration gradient in a meristem acts in specifying the apical-basal polarity of the CLV3 mRNA pattern. Additionally, the defects in CLV3 patterning during meristem initiation (Fig. 4S, T) could be the molecular basis of the shoot branching phenotypes of the ham1;2;3 mutants (16–18).
Differently from previous models (13–14, 25), this work, complemented by a recent theoretical study (26), reveals a regulatory circuit involving three components—WUS, HAM and CLV3—that sustains both the initiation and the maintenance of the apical-basal polarity of distinct cell types in the plant apical stem cell niche.
Supplementary Material
Acknowledgments:
The authors are grateful to H. Yang for sharing the HAM1m DNA construct, and to A. Garda from Caltech and C. Layug from Purdue for technical support.
Funding:The work at Caltech was funded by NIH grant R01 GM104244, and by the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation (through Grant GBMF3406) to E.M.M. The work at Purdue was funded by a start-up package and support from the Purdue Center for Plant Biology to Y.Z.
Footnotes
Data and materials availability: all data are available in the manuscript or the supplementary material.
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Competing interests: The authors declare that they have no competing interests.
References and Notes:
- 1.Meyerowitz EM, Cell 88, 299–308 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM, Science 283, 1911–1914 (1999) [DOI] [PubMed] [Google Scholar]
- 3.Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R, Science 289, 617–619 (2000) [DOI] [PubMed] [Google Scholar]
- 4.Brand U, Grunewald M, Hobe M, Simon R. Plant physiology 129, 565–575 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laux T, Mayer KF, Berger J, Jurgens G, Development 122, 87–96 (1996) [DOI] [PubMed] [Google Scholar]
- 6.Mayer KF et al. , Cell 95, 805–815 (1998) [DOI] [PubMed] [Google Scholar]
- 7.Schoof H et al. , Cell 100, 635–644 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Nimchuk ZL, Tarr PT, Ohno C, Qu X, Meyerowitz EM, Current biology 21, 345–352 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA, Meyerowitz EM, Development 142, 1043–1049 ( 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav RK et al. , Genes & development 25, 2025–2030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daum G, Medzihradszky A, Suzaki T, Lohmann JU, PNAS 111, 14619–14624 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perales M et al. , PNAS 113, E6298–E6306 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsson H et al. , Bioinformatics 21, 232–240 (2005) [Google Scholar]
- 14.Gruel J et al. , Science advances 2, e1500989 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y et al. Nature 517(7534):377–380 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze S, Schafer BN, Parizotto EA, Voinnet O, Theres K, The Plant journal 64, 668–678 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Engstrom EM et al. Plant physiology 155, 735–750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Mai YX, Zhang YC, Luo Q, Yang HQ, Molecular plant 3, 794–806 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Muller R, Borghi L, Kwiatkowska D, Laufs P, Simon R, The Plant cell 18, 1188–1198 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu P, Porat R, Nadeau JA, O’Neill SD, The Plant cell 8, 2155– 2168 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llave C, Xie Z, Kasschau KD, Carrington JC, Science 297, 2053–2056 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Graf P et al. , The Plant cell 22, 716–728 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long J, Barton MK, Developmental biology 218, 341–354 (2000) [DOI] [PubMed] [Google Scholar]
- 24.Xin W, Wang Z, Liang Y, Wang Y, Hu Y, Journal of plant physiology 214,1–6 (2017) [DOI] [PubMed] [Google Scholar]
- 25.Chickarmane VS et al. , PNAS 109, 4002– 4007 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruel J, Deichmann J, Landrein B, Hitchcock T, Jönsson H, bioRxiv doi: 10.1101/237933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy GV et al. , Development 131, 4225–4237 (2004) [DOI] [PubMed] [Google Scholar]
- 28.Cunha A et al. , Methods in cell biology 110, 285–323 (2012) [DOI] [PubMed] [Google Scholar]
- 29.Clough SJ, Bent AF, The Plant journal 16, 735–743 (1998) [DOI] [PubMed] [Google Scholar]
- 30.Roeder AH, Cunha A, Ohno CK, Meyerowitz EM, Development 139, 4416–4427 (2012) [DOI] [PubMed] [Google Scholar]
- 31.Li W et al. , Science signaling 6, ra23cr (2013)23572147 [Google Scholar]
- 32.Zhang X et al. , The Plant cell 25, 83–101 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krizek BA, Developmental genetics 25, 224–236 (1999) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


