Abstract
Fish are an important source of nutrients which may reduce risk of adverse health outcomes such as cardiovascular disease; however, fish may also contain significant amounts of environmental pollutants such as mercury, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and perfluorinated compounds (PFCs, also called perfluoroalkyl compounds), which confer increased risk for adverse health effects. The Wisconsin Departments of Health Services and Natural Resources developed a survey instrument, along with a strategy to collect human biological samples to assess the risks and benefits associated with long-term fish consumption among older male anglers in Wisconsin. The target population was men aged 50 years and older, who fish Wisconsin waters and live in the state of Wisconsin. Participants provided blood and hair samples and completed a detailed (paper) questionnaire, which included questions on basic demographics, health status, location of catch and species of fish caught/eaten, consumption of locally caught and commercially purchased fish, and awareness and source of information for local and statewide consumption guidelines. Biological samples were used to assess levels of PCBs, PBDEs, PFCs (blood), and mercury (hair and blood). Quantile regression analysis was used to investigate the associations between biomarker levels and self-reported consumption of fish from the Great Lakes and other areas of concern, other locally caught fish, and commercially purchased fish (meals per year). Respondents had a median age of 60.5 (interquartile range: 56, 67) years. The median fish consumption was 54.5 meals per year, with most fish meals coming from locally-caught fish. Participants had somewhat higher mercury levels compared with the US general population, while levels of other contaminants were similar or lower. Multivariate regression models showed that consumption of fish from the Great Lakes and areas of concern was associated with higher levels of each of the contaminants with the exception of PBDEs, as was consumption of locally caught fish from other water bodies. All commercial fish consumption was also associated with both hair and blood mercury. When looking at specific PCB, PBDE and PFC analytes, consumption of fish from the Great Lakes and areas of concern was associated with higher levels of each of the individual PCB congeners examined, as well as higher levels of all of the PFCs examined, with the exception of PFHxS. Among the PFCs, locally caught fish from other water bodies was also associated with higher levels of each of the congeners examined except PFHxS. Finally, all commercial fish was associated with higher levels of PFHxS.
Keywords: Fish consumption, Great lakes, Anglers, Persistent, Pollutant
Introduction
Fish represent a dietary source of lean protein and important nutrients such as omega-3 fatty acids. However, fish may also contain high levels of contaminants, including persistent compounds such as mercury, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and perfluorinated compounds (PFCs, also called perfluoroalkyl compounds). Given the increasing importance of fish in the US diet (Loke et al., 2012) and the health effects which have been associated with these compounds, monitoring contaminant levels in fish and fish consumers is important for preventing adverse health effects through appropriate fish consumption guidelines, monitoring exposures, and tracking health impacts in the population.
Mercury has long been recognized as a neurotoxicant, and has also been associated with increased risk for cardiovascular disease (ATSDR, 1999, 2013). Polychlorinated biphenyls are a group of 209 different compounds formerly used in electronic equipment manufacturing for their insulative and conductive properties. Although production in the US was banned in 1979 due to health concerns, PCBs persist in the environment, and have been linked to a wide range of adverse health effects including certain cancers, endocrine and reproductive disorders, and neurodevelopmental delays (ATSDR, 2000, 2011). Polybrominated diphenyl ethers are used as flame retardants in a variety of consumer products; like PCBs, they are persistent and lipophilic (ATSDR, 2004). Although there are fewer studies of human health effects compared with PCBs, there is some evidence that thyroid and neurodevelopmental effects could be of concern. Perfluorinated compounds are also found in a variety of consumer products, used for non-stick/non-stain applications (ATSDR, 2009b; Steenland et al., 2010). Similarly to PBDEs there are fewer studies of human health effects available, but some evidence for health effects including changes in liver enzymes, and lower birth weight.
Fish from many waters, including the Great Lakes, may be contaminated with each of these pollutants, although levels vary by specific location, age and type of fish, and other factors (EPA, 2009a).
The Wisconsin Department of Natural Resources (DNR) state waters monitoring program has noted declines in mercury concentrations in fish on the whole, but also that the rate of decline is different depending the latitude of the specific water body (Rasmussen et al., 2007). Wisconsin DNR data have also shown that PCB concentrations have decreased dramatically in Lake Michigan salmon species from 1975-2010, but the rate of decline is much less in recent years compared with the earliest years of data collected (Rasmussen et al., 2014). Similarly, longitudinal biomonitoring among a group of Great Lakes fish consumers has shown a decreased in serum PCBs over time, although the rate of decline was relatively slow at 3.5% per year (Knobeloch et al., 2009). PBDE concentrations in Great Lakes walleye and trout have been decreasing from 1980 to 2009 (Crimmins et al., 2012); however, the congener composition of PBDEs is also changing, with larger proportions of the highly brominated PBDEs in more recent years, which may reflect changing industrial use patterns. Biomonitoring among Great Lakes fish consumers has shown a similar phenomenon—total PBDE body burden increased from 1994–1995 to 2004–2005, and the composition also changed with an increasing proportion of the more highly brominated PBDE 153, relative to PBDE 47 (Turyk et al., 2010). Regarding PFCs in the Great Lakes region, there are fewer data available, partly because these chemicals have been in use for a shorter time compared with PCBs or PBDEs, but limited data suggest that the concentrations of many PFC congeners are increasing over time (Furdui et al., 2008). Recent data collected by the Environmental Protection Agency provide a baseline for future monitoring of PFC levels in the Great Lakes (Stahl et al., 2014); these data showed high detection frequency for certain PFCs including PFOS (100%), PFDA (92%), and PFUnA (90%). Thus, contaminants in Great Lakes fish remain a concern for consumers.
Anglers and their families are a particularly vulnerable population because they tend to consume more locally caught Great Lakes Basin fish. Given the increased risk of exposure to persistent contaminants and potential for adverse health, current fish consumption guidelines in Great Lakes states are designed to encourage consumption of fish that is high in nutrients yet low in contaminants. In Wisconsin, such guidelines were first issued in 1976, by the Departments of Health Services (DHS) and Natural Resources (DNR) and targeted licensed, mostly male anglers. As fetal exposure adverse impacts became known there was a shift in focus to emphasize advice to women of childbearing age. Older male fish consumers may have increased vulnerability to adverse health effects due to higher body burden of contaminants (Knobeloch et al., 2006, 2009; Turyk et al., 2012), and increased risk of stroke and heart disease (de Goede et al., 2012; He et al., 2002; Salonen et al., 1995). Accordingly, the Wisconsin Fish Consumption Advisory Program (WFCAP) has renewed efforts to reach aging male anglers, developed a survey instrument, along with a strategy to collect biological samples, which will be used to assess the risks and benefits associated with long-term fish consumption among older male anglers in Wisconsin. Our goal in this study is to examine the association between fish consumption and biomarkers of exposure to persistent contaminants in this angler cohort.
Materials and methods
Participants were recruited from those who previously participated in an online survey administered by the DHS, and had indicated they would be interested in future studies (n = 111; see (Christensen et al., 2015; Imm et al., 2013) for details). An additional 43 persons (who had not participated in the online survey) were recruited via flyers and other methods (Fig. 1). The study was conducted in 2012–2013.
Fig. 1.
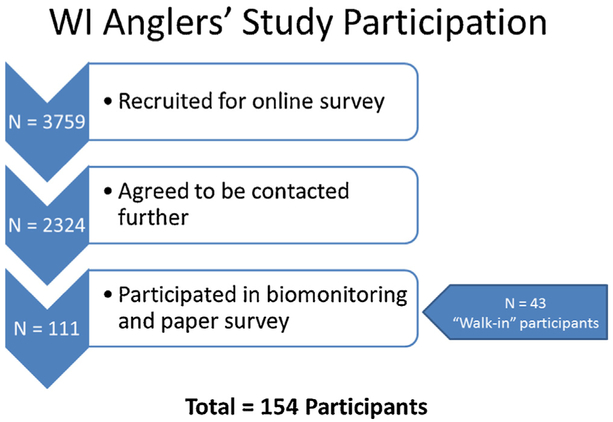
Participation for the Wisconsin Anglers’ Study.
The target population was men aged 50 years and older, who fish Wisconsin waters and live in the state of Wisconsin. Participants provided blood and hair samples and completed a detailed (paper) questionnaire, which included questions on basic demographics, current health status, location of catch and species of fish caught/eaten, consumption of locally caught and commercially purchased fish, awareness of local and statewide consumption guidelines, and source of information on consumption guidelines. The Survey of the Health of Wisconsin program conducted follow-up phone calls and coordinated bio-sample collection in the homes of study participants (Nieto et al., 2010). The study was reviewed by the University of Wisconsin Human Subjects Review Board and determined to be exempt, as it was conducted for the purpose of public health research.
Biological samples
Biological samples were used to assess serum levels of contaminants PCBs, PBDEs, PFCs, and mercury in blood, and mercury in hair. Each participant provided 47 mLs of whole blood and a small hair sample. Fatty acids (triglycerides) and lipids (cholesterol) were also measured in blood. All blood collection vials were frozen immediately at −20 degrees, with the exception of the vial for fatty acids analysis (frozen at −80 degrees in vertical position) and the vial for lipids analysis (refrigerated in vertical position for no longer than one week). The Wisconsin State Laboratory of Hygiene (WSLH) analyzed the serum collected from whole blood samples. Mercury levels in whole blood were measured following EHD CLIN TOX Method 12 CT/Clinical Blood Mercury by Inductively Coupled Plasma–Mass Spectrometry, with a reporting limit of 0.3 μg/L. PCB and PBDE congeners were measured in serum following the WSLH Method ESS ORG 1810, Blood Serum for PCB Congeners, Pesticides and PBDE Congeners, 2012. In brief, serum sample extracts were analyzed for PCBs congeners and PBDEs using high-resolution capillary column gas chromatography with an electron capture detector (HP6890 N equipped with a PC-based Chemstation) after multiple cleanup steps. Quality control procedures included matrix spikes, surrogates, certified reference materials, and method blanks for each batch of 10 samples. Limits of detection (LODs) for the various PCB congeners ranged from 0.07 to 0.2 ng/mL, with the exception of congeners 3 and 4/10 (co-eluting) which had higher LODs of 0.7 and 6.0 μg/L, respectively. LODs for the PBDE congeners ranged from 0.025 to 0.50 ng/mL. PFCs present in serum were extracted by an ion-pairing liquid/liquid extraction with MTBE, evaporated to dryness under nitrogen gas, reconstituted in 50:50 2 mM ammonium acetate:MeOH and then analyzed on an Applied Biosystems API 4000 triple quadrupole mass spectrometer. Quality control procedures included matrix spikes, surrogates, certified reference materials, and method blanks for each batch of 10 samples. The LOD for all PFC congeners was 0.12 ng/mL. Mercury levels in hair samples were measured following EPA Method 1631, Revision E using cold vapor atomic absorption fluorescence. This method (ESS INO METHOD 541.1, Rev 5, Total Mercury by Oxidation, Purge & Trap, and Cold Vapor Atomic Fluorescence Spectrometry (CVAFS)) has a detection limit of 0.011 ug/g for hair.
Marshfield Laboratory analyzed serum samples for triglycerides and cholesterol. For both tests, an enzymatic/timed endpoint method was performed on Beckman DXC analyzer. Serum lipids were used as an adjustment factor in multivariate models where the outcome was a lipophilic compound.
Data analysis
All analyses were performed using SAS/STAT software version 9.31. The biomarker data were analyzed to determine distribution among study participants, and associations between and among contaminant levels, blood lipids, and reported fish consumption. Consumption was based on questions regarding the number of times participants reported having eaten fish or shellfish from any source in the past 30 days, or in the past 12 months (for fish from specific sources, including the Great Lakes, other areas of concern [Menominee River, Fox River/Lower Green Bay, Sheboygan River, St. Louis River and Bay, or the Milwaukee Estuary, including the lower Milwaukee and inner harbor, Kinnickinnic, and Menomonee Rivers and harbor], other locally caught fish, and fish purchased at a restaurant, or store/market). The frequency was assumed to translate to number of meals eaten during the time periods specified, and the consumption levels for all fish and all shellfish were converted to a yearly basis (i.e., number of meals consumed per year). A priori, the decision was made to focus the analysis on fish consumption (rather than shellfish) as the main predictor, and on summed PCBs and summed PBDEs (rather than individual congeners), blood and hair mercury, and PFOA and PFOS (among the PFCs measured) as the main outcomes of interest.
As with many biomarkers, most of the contaminants examined were not normally distributed (both untransformed and after natural logarithm transformation). We also wanted to explore the hypothesis that fish consumption may have a different effect at different levels of each of the biomarkers examined—for example, eating more fish could have a greater or lesser effect on PFOA levels at the lower end of the distribution of PFOA compared to the higher end of the distribution. This possibility is particularly important in environmental public health when examining risk factors for high levels of contaminants. For example, there are multiple sources of exposure to PBDEs (e.g., (Imm et al., 2009)) and PFCs which create a ‘background’ level of exposure not associated with fish consumption; these other sources may predominate at low levels of fish consumption, such that fish-associated contribution to body burden only become discernable as fish consumption increases. Accordingly, we used quantile regression in order to examine the possibility that predictor effects were different at different response levels. With quantile regression, the response being modeled is the conditional quantile of the outcome, rather than the mean (as in ordinary linear regression) (Cade and Noon, 2003; Koenker and Bassett, 1978). Instead of estimating a single slope, multiple slopes are estimated for different (conditional) levels of the outcome, providing a more complete picture of the effect of exposure. In order to provide a wide representation of the exposure-response relationships, quantile regression modeling results (beta coefficients) are presented for the 10th, 50th, and 90th percentile of each outcome. Quantile regression modeling was performed using the PROC QUANTREG procedure in SAS. Unadjusted (univariate) models were examined first, with a single predictor (representing a specific category of fish consumption) for each biomarker. After examining potential collinearity between predictor variables, multivariate models were constructed to investigate the effect of multiple fish consumption variables, on each biomarker. Heteroscedasticity of estimated beta coefficients across the specified quantiles was evaluated using Wald tests, and coefficients across quantiles were plotted to evaluate the relationship between fish consumption and biomarker levels, across quantiles.
Results
Demographic characteristics of study participants are shown in Table 1. Respondents were largely non-Hispanic white men in their 60’s, with nearly full life residence in the state of Wisconsin. Most had at least some college education, and about half were working, and half retired. Nearly all respondents were classified as either overweight (42.2%) or obese (46.8%) according to their height and weight.
Table 1.
Demographic characteristics of study participants.
| Total n | 154 |
|
|---|---|---|
| Mean (SD) | Median (IQR) | |
| Agea | 61.7 (7.7) | 60.5 (56, 67) |
| Years living in Wisconsin | 54.8 (15.2) | 58 (50, 64) |
| Total cholesterol (mg/dL) | 190.7 (36.3) | 192 (172, 215) |
| Triglycerides (mg/dL) | 235.2 (143.9) Percent (n)* |
197 (138, 278) |
| Race/ethnicity | ||
| Identification as Hispanic/Latino | 0.7 (1) | |
| Identification as white (alone or in combination) | 98.7 (152) | |
| Educational attainment | ||
| High school or less | 31.2 (39) | |
| Some college or two-year degree | 14.4 (18) | |
| College degree (four-year) or more | 54.4 (68) | |
| Missing | (29) | |
| Employment status | ||
| Working (full or part-time, or self-employed) | 50.0 (77) | |
| Retired | 46.1 (71) | |
| Other | 3.9 (6) | |
| Marital status | ||
| Married (or marriage-like relationship) | 85.7 (132) | |
| Other | 14.3 (22) | |
| Household income | ||
| <$14,999 | 2.0 (3) | |
| $00,0–$34,999 | 16.2 (24) | |
| $35,000–$49,999 | 16.9 (25) | |
| $50,000–$74,999 | 25.7 (38) | |
| ≥$75,000 | 38.5 (57) | |
| Not answered | 0.7 (1) | |
| Smoked at least 100 cigarettes in lifetime | 58.8 (90) | |
| Smoke cigarettes daily | 12.6 (12) | |
| BMI categoryb | ||
| Underweight (<18.5) | 0 (0) | |
| Normal (18.5–25) | 11.0 (17) | |
| Overweight (25–30) | 42.2 (65) | |
| Obese (≥30) | 46.8 (72) | |
| Total cholesterol (mg/dL) category | ||
| Desirable (<200) | 59.1 (91) | |
| Borderline (200–239) | 33.8 (52) | |
| High (≥240) | 7.1 (11) | |
| Triglycerides (mg/dL) category | ||
| Desirable (<150) | 29.2 (45) | |
| Borderline (150–199) | 21.4 (33) | |
| High (200–499) | 44.2 (68) | |
| Very high (≥500) | 5.2 (8) | |
For percentages, calculations exclude missing values.
Two individuals were missing date of birth; these were set to the median value in the study population.
One individual was missing weight; this was set to the median value in the study population. One individual gave height in feet as ‘2;’ height was set to the median value in the study population.
When comparing the individuals who participated in the biomonitoring component (n = 111) and the larger study group from which they were recruited (i.e., all participants in the online survey described in Imm et al. (2013), n = 3740); there were no differences between the two groups with respect to race/ethnicity, marital status, employment status, income, or time living and fishing in Wisconsin. Those who participated in the biomonitoring component did tend to be slightly, more highly educated, and all lived in a county bordering Lakes Superior or Michigan.
Reported levels of fish and shellfish consumption, and biomarker levels of contaminants, are displayed in Table 2 (note that since median values are displayed, the number of fish meals for each category of contribution will not sum to the median total number of fish meals consumed). Participants consumed a median of 66.5 fish and shellfish meals per year, with the majority of this coming from fish, and specifically from locally caught (rather than commercially purchased) fish. For comparison, information on fish consumption was also gathered from two additional populations. First, data from the 2011 to 2012 cycle of the National Health and Nutrition Examination Survey (CDC and NCHS, 2014) was used to provide information on fish consumption for a nationally representative sample of the general US population. For non-Hispanic white men aged 50 and older, the median consumption of fish was 33 meals per year, compared with a median of 54.5 meals per year among the angler study population. Similarly, the median consumption of combined fish and shellfish was approximately 50 meals per year among US men, compared to a median of 66.5 among the anglers. Second, data from a random sample of fishing license holders in Great Lakes states showed that even that population had lower consumption compared with this angler population–for the random sample (which included both men and women of all ages), the average number of fish meals eaten in the past year was 20.5, comprising 5.9 sport-caught fish meals and 15.1 purchased fish meals (Connelly et al., 2012).
Table 2.
Fish consumption and biomarker levels of contaminants among study participants, and among NHANES participants.
| Median (IQR) |
||
|---|---|---|
| Anglers (This study) | NHANES, non-Hispanic white men aged ≥50 yearsb | |
| Fish and shellfish consumption (meals per year) | ||
| Total fish and shellfish | 66.5 (47, 114) | 50.4 (23.9, 96.8) |
| Total fish | 54.5 (36, 93) | 33.3 (16.9, 64.6) |
| Fish from Great Lakes | 7 (2, 24) | – |
| Fish from areas of concerna | 0 (0, 3) | – |
| Other locally caught fish | 12 (6, 36) | – |
| Fish from restaurant | 10 (4, 20) | – |
| Fish from store | 4.5 (0, 12) | – |
| Total shellfish | 9 (4, 18) | 23.1 (12.2, 47.9) |
| Shellfish from restaurant | 4 (1, 6) | – |
| Shellfish from store | 4 (1, 10) | – |
| Contaminants | ||
| Perfluoro-n-octanoic acid, (ng/mL) (PFOA) | 2.5 (1.8, 3.3); 10th, 90th percentiles: 1.1, 4.3 | 3.78 (2.63, 5.27) |
| Perfluorooctane sulfonate, (ng/mL) (PFOS) | 19.0 (9.8, 28.0); 10th, 90th percentiles: 5.5, 42.0 | 13.67 (9.84, 19.78) |
| Hair mercury, (μg/g) | 0.5 (0.3, 1.0); 10th, 90th percentiles: 0.17, 2.0 | – |
| Blood mercury, (μg/L) | 2.5 (1.3, 4.0); 10th, 90th percentiles: 0.7, 7.2 | 0.97 (0.48, 2.18) |
| ΣPolybrominated diphenyl ethers, (ng/mL) (PBDEs) | 0.2 (0.1, 0.4); 10th, 90th percentiles: 0.1, 0.6 | 0.25 (0.10, 0.51) |
| ΣPolychlorinated biphenyls, (ng/mL) (PCBs) | 1.3 (0.6, 2.5); 10th, 90th percentiles: 0.3, 5.1 | 1.59 (1.30, 2.39) |
Areas of concern include: Menominee River, Fox River/Lower Green Bay, St. Louis River and Bay, Sheboygan River, Milwaukee Estuary (including the lower Milwaukee and inner harbor, Kinnickinnic, and Menomonee Rivers and harbor)
For NHANES participants, all results are adjusted for survey sampling and design. Fish and shellfish consumption values are taken from the 2011–2012 cycle; consumption was ascertained for the previous 30 days, thus yearly consumption was calculated by multiplying by (365.25/30). For contaminant levels, values are taken from the 2011–2012 (blood mercury and PFOA) and 2003-2004 (PBDEs and PCBs) cycles of the NHANES. Total PBDEs are calculated as the sum of the following congeners for NHANES: 17, 28, 47, 66, 85, 99, 100, 153, 154, 183; and for the survey participants: 17, 28, 47, 49, 66, 71, 77, 85, 85, 99, 100, 119, 126, 138, 153, 154, 156, 183, 184, 191, 196, 197, 206, 207, 209. Total PCBs are calculated as the sum of the following congeners for NHANES: 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 118, 128, 138, 146, 149, 151, 153, 167, 170, 172, 177, 178, 180, 183, 187, 194, 195, 196, 199, 206; and for the survey participants: 4, 6, 7, 8, 9, 10, 11, 15, 16, 17, 18, 19, 22, 25, 26, 27, 28, 31, 32, 33, 37, 40, 41, 42, 44, 45, 46, 47, 48, 49, 52, 53, 56, 60, 63, 64, 66, 70, 71, 74, 77, 82, 83, 84, 85, 87, 89, 91, 92, 95, 97, 99, 101, 105, 110, 118, 128, 130, 132, 135, 138, 141, 144, 146, 149, 151, 153, 158, 163, 167, 170, 171, 172, 174, 177, 178, 180, 183, 185, 187, 190, 193, 194, 195, 196, 199, 201, 202, 203, 206, 208.
Although the exact number of fish meals was not ascertained for specific species or individual water bodies, participants were asked about consumption of certain species over the past year (at least 6 meals caught by self or someone they know), and about consumption of fish caught in specific water bodies over the past year (at least one meal caught by self or someone they knew). The most commonly consumed species included walleye (66.7%), bluegill (64.3%) and yellow perch (63.0%). At least 30% of participants also reported consuming crappie (48.1%), northern pike (34.4%), coho salmon (32.7%) and chinook salmon (30.1%). The least commonly consumed species were catfish and chubs (7.1% for each), muskellunge (<1%) and carp (0%). Regarding specific water bodies, few participants reported eating fish from any of the Great Lakes aside from Lakes Michigan (64.9%) or Superior (26.6%). Among water bodies of concern, participants most commonly reported eating fish from the Fox River and Lower Green Bay River/Lower Green Bay (27.3%) and the Menominee River (16.9%); fewer than 10% reported eating fish from the other water bodies listed (Sheboygan River, St. Louis River and Bay, or the Milwaukee River and Estuary).
Contaminant levels in the angler study population were compared to those for the US general population of non-Hispanic white men aged 50 years and older, using estimates from the National Health and Nutrition Examination Survey. Blood mercury was notably higher among anglers compared with the general US population (median of 2.5 compared with 1 μg/L), and the median level of PFOS was also somewhat higher among anglers (median of 19 compared with 13.7 ng/mL) but levels of other contaminants were similar. Interestingly, while PFOS was increased in the angler population, the levels of PFOA were slightly lower compared to the general population sample (2.5 compared with 3.8 ng/mL).
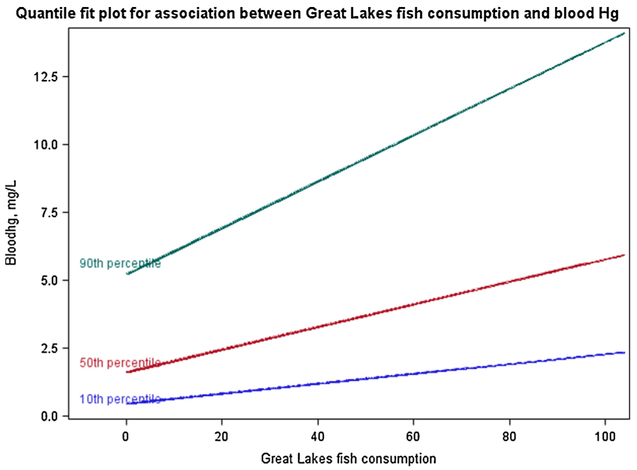
Table 3 shows the unadjusted quantile regression modeling results for the association between fish consumption and each contaminant. Results are interpreted as follows, using the example of ‘Fish purchased in restaurant’ and blood mercury: increasing consumption of Fish purchased in restaurant by one unit (one meal per year) results in a 0.014 μg/L increase in blood mercury, at the conditional 10th percentile of blood mercury. In general, the effect of each predictor on a given response is greater with increasing quantile (i.e., the effect at the 90th percentile > the effect at the median > the effect at the 10th percentile). For example, the beta coefficient for consumption of Great Lakes fish ranged from 0.018 at the 10th percentile of blood mercury, to 0.042 at the median, to 0.086 at the 90th percentile (Fig. 2). In many cases, the beta coefficients at the different quantiles were not significantly different from each other—for example, as shown by overlapping confidence intervals of the beta coefficients for hair mercury and locally-caught fish from the Great Lakes at the 50th and 90th percentiles. In these cases, a straightforward linear regression may have led to similar conclusions, but would mask potential non-linearity that, although not statistically significant, may be important to understand the exposure-response relationship.
Table 3.
Univariate associations between fish consumption (meals per year) and levels of contaminants, modeled using a quantile regression model for the 10th, 50th, and 90th percentiles.
| Beta (95% CI)* |
||||
|---|---|---|---|---|
| Predictor | Response | 10th percentile | 50th percentile | 90th percentile |
| Locally-caught fish–Great Lakes | Blood | 0.018 (0.001, 0.029) | 0.042 (0.009, 0.072) | 0.086 (−0.010, 0.161) |
| Locally-caught fish–AOCS | Hg, (μg/L) | 0.018 (0.000, 0.022) | 0.020 (0.000, 0.072) | −0.052 (−0.053, 0.000) |
| Locally-caught fish–other | 0.013 (0.000, 0.019) | 0.016 (0.005, 0.033) | 0.009 (−0.021, 0.151) | |
| Fish purchased in restaurant | 0.014 (−0.015, 0.026) | 0.017 (−0.028, 0.049) | 0.052 (0.013, 0.315) | |
| Fish purchased in store | 0.014 (−0.038, 0.024) | 0.016 (−0.012, 0.048) | 0.031 (−0.018, 0.411) | |
| Locally-caught fish–Great Lakes | Hair | 0.003 (0.000, 0.008) | 0.013 (0.006, 0.017) | 0.019 (0.004, 0.053) |
| Locally-caught fish–AOCS | Hg, (μg/g) | 0.003 (0.000, 0.006) | 0.003 (−0.005, 0.023) | −0.012 (−0.022, 0.000) |
| Locally-caught fish–other | 0.002 (−0.001, 0.004) | 0.005 (0.000, 0.012) | 0.006 (−0.005, 0.037) | |
| Fish purchased in restaurant | 0.001 (−0.002, 0.007) | 0.010 (0.002, 0.021) | 0.008 (0.006, 0.085) | |
| Fish purchased in store | 0.000 (−0.008, 0.003) | 0.004 (−0.005, 0.022) | 0.014 (−0.002, 0.097) | |
| Locally-caught fish–Great Lakes | ΣPBDE, (ng/mL) | 0.000 (−0.028, 0.003) | 0.002 (0.000, 0.004) | 0.006 (0.000, 0.034) |
| Locally-caught fish–AOCS | 0.000 (0.000, 0.001) | −0.002 (−0.007, 0.001) | 0.000 (−0.003, 0.000) | |
| Locally-caught fish–other | 0.000 (−0.002, 0.000) | −0.001 (−0.002, 0.001) | −0.001 (−0.002, 0.018) | |
| Fish purchased in restaurant | 0.001 (−0.005, 0.002) | 0.000 (−0.001, 0.004) | −0.002 (−0.004, 0.016) | |
| Fish purchased in store | 0.002 (−0.005, 0.002) | 0.001 (0.000, 0.002) | −0.003 (−0.003, 0.049) | |
| Locally-caught fish–Great Lakes | ΣPCB, (ng/mL) | 0.008 (−0.020, 0.017) | 0.012 (0.008, 0.044) | 0.097 (0.032, 0.241) |
| Locally-caught Fish–AOCS | −0.003 (0.000, 0.014) | 0.018 (−0.014, 0.000) | 0.033 (−0.056, 0.000) | |
| Locally-caught fish–other | −0.004 (−0.011, 0.001) | 0.013 (−0.011, 0.023) | 0.003 (−0.025, 0.147) | |
| Fish purchased in restaurant | 0.001 (−0.029, 0.023) | 0.019 (−0.026, 0.028) | −0.023 (−0.037, 0.136) | |
| Fish purchased in store | −0.003 (−0.048, 0.004) | 0.006 (−0.021, 0.019) | −0.026 (−0.034, 0.194) | |
| Locally-caught fish–Great Lakes | PFOA, (ng/mL) | 0.018 (−0.129, 0.025) | 0.007 (0.000, 0.026) | 0.007 (−0.014, 0.066) |
| Locally-caught fish–AOCS | 0.000 (0.000, 0.018) | 0.009 (−0.068, 0.030) | ||
| Locally-caught fish–other | 0.004 (−0.030, 0.013) | 0.012 (0.004, 0.021) | 0.015 (−0.005, 0.050) | |
| Fish purchased in restaurant | −0.006 (−0.050, 0.026) | 0.007 (−0.016, 0.029) | 0.010 (−0.015, 0.130) | |
| Fish purchased in store | −0.006 (−0.059, 0.010) | 0.000 (−0.012, 0.036) | 0.012 (−0.020, 0.121) | |
| Locally-caught fish–Great Lakes | PFOS, (ng/mL) | 0.110 (−0.011, 0.191) | 0.175 (0.022, 0.370) | 1.250 (0.014, 1.749) |
| Locally-caught fish–AOCS | 0.141 (−, 0.165) | 0.200 (−1.172, 0.475) | 0.083 (−0.125, −) | |
| Locally-caught fish–other | 0.083 (−0.164, 0.145) | 0.116 (0.027, 0.306) | 0.198 (−0.011, 0.961) | |
| Fish purchased in restaurant | 0.042 (−0.083, 0.208) | 0.125 (−0.043, 0.460) | 0.144 (0.094, 2.283) | |
| Fish purchased in store | −0.046 (−0.374, 0.154) | −0.022 (−0.238, 0.235) | 0.217 (−0.173, 0.459) | |
Bold font indicates an association that is statistically significant at the p < 0.05 level.
Fig. 2.
Association between Great Lakes fish consumption and blood mercury, for the (conditional) 10th, 50th, and 90th percentiles of blood mercury; beta coefficients are calculated from the univariate quantile regression model.
Significant associations were found between: fish purchased in restaurants and both hair and blood mercury, and PFOS; Great Lakes fish with both hair and blood mercury, PCBs and PFOS; other locally-caught fish (not from Great Lakes or AOCs) with blood mercury, PFOA and PFOS.
While many associations were identified in the unadjusted modeling, for multivariate modeling the correlation among the various consumption parameters is important to consider. Spearman correlation coefficients are displayed in Table 4—the correlations between the different categories of locally caught fish (Great Lakes, Areas of Concern, other locally caught fish) were low, indicating these could likely be included as independent predictors for multivariate modeling. However, in multivariate modeling, locally caught fish from areas of concern was not included as a separate predictor-due to the small range of this variable, inclusion caused notable model instability. Instead, a combined variable was created as the sum of locally-caught fish from areas of concern and from the Great Lakes, since these groupings of water bodies share common attributes with respect to type and distribution of both fish species, and contaminants. There was also a statistically significant correlation between fish purchased from a store and fish purchased from a restaurant, which we addressed for modeling by creating a combined variable (sum) of’all commercial fish’.
Table 4.
Spearman correlations (p-value) between fish consumption parameters.
| Locally-caught fish–Great Lakes |
Locally-caught fish–AOCS |
Locally-caught fish–other |
Fish purchased in restaurant |
Fish purchased in store |
|
|---|---|---|---|---|---|
| Locally-caught fish—Great Lakes | 1.00 (−) | 0.13 (0.10) | 0.01 (0.91) | −0.002 (0.98) | 0.05 (0.55) |
| Locally-caught fish—AOCS | 1.00 (−) | 0.08 (0.30) | 0.05 (0.58) | 0.04 (0.59) | |
| Locally-caught fish—other | 1.00 (−) | 0.16 (0.05) | 0.10 (0.20) | ||
| Fish purchased in restaurant | 1.00 (−) | 0.39 (<0.001) | |||
| Fish purchased in store | 1.00 (−) |
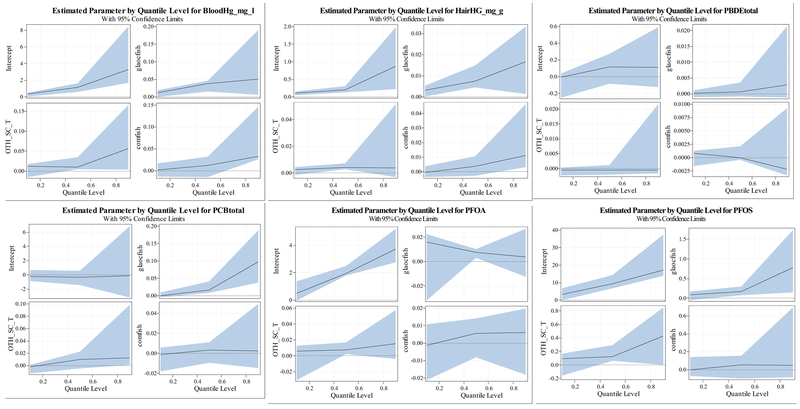
Results of quantile regression models including as predictors all commercially purchased fish, locally-caught fish from the Great Lakes fish, and non-Great Lakes locally-caught fish, are shown in Table 5. Quantile process plots showing the estimated model coefficients across quantiles are shown in Fig. 3. Additionally, total serum lipids were included as a predictor for the lipophilic contaminants (PCBs, PBDEs), but not for mercury and PFOA. As before, there are several significant associations between consumption of locally caught fish and each of the contaminants-consumption of fish from the Great Lakes and areas of concern was associated with higher levels of each of the contaminants with the exception of PBDEs, as was consumption of locally caught fish from other water bodies. All commercial fish consumption was also associated with both hair and blood mercury at the highest (90th) percentile examined. In the multivariate models, significant heteroscedasticity (Wald test p-value <0.05) was seen for the effect of locally-caught fish (both Great Lakes and AOC, as well as other locally-caught) on blood mercury, effect of other locally-caught fish on PFOS, and of Great Lakes and AOC fish on PCB levels.
Table 5.
Multivariate associations between fish consumption (meals per year) and levels of contaminants, modeled using a quantile regression model for the 10th, 50th, and 90th percentiles.
| Beta (95% CI)* |
||||
|---|---|---|---|---|
| Predictor | Response | 10th percentile | 50th percentile | 90th percentile |
| All commercially purchased fish (store, restaurant) | Blood | 0.002 (−0.014, 0.016) | 0.012 (−0.015, 0.032) | 0.033 (0.026, 0.146) |
| Locally-caught fish–Great Lakes and AOCs | Hg, (μg/L) | 0.012 (0.000, 0.019) | 0.037 (0.016, 0.046) | 0.050 (0.006, 0.190) |
| Locally-caught fish–other water bodies | 0.012 (−0.015, 0.017) | 0.010 (0.005, 0.035) | 0.056 (0.004, 0.166) | |
| All commercially purchased fish (store, restaurant) | Hair | −0.001 (−0.004, 0.004) | 0.004 (−0.003, 0.010) | 0.011 (0.003, 0.046) |
| Locally-caught fish–Great Lakes and AOCs | Hg, (μg/g) | 0.003 (0.000, 0.005) | 0.007 (0.005, 0.015) | 0.017 (0.001, 0.033) |
| Locally-caught fish–other water bodies | 0.002 (−0.001, 0.004) | 0.004 (0.003, 0.007) | 0.004 (−0.003, 0.051) | |
| All commercially purchased fish (store, restaurant) | ΣPBDE, (ng/mL) | 0.001 (−0.002, 0.001) | 0.000 (0.000, 0.002) | −0.002 (−0.003, 0.009) |
| Locally-caught fish–Great Lakes and AOCs | 0.000 (−0.001, 0.001) | 0.001 (−0.001, 0.004) | 0.003 (−0.001, 0.021) | |
| Locally-caught fish–other water bodies | 0.000 (−0.002, 0.000) | 0.000 (−0.002, 0.001) | 0.000 (−0.002, 0.022) | |
| Total lipids | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | 0.001 (0.000, 0.001) | |
| All commercially purchased fish (store, restaurant) | ΣPCB, ng/mL | −0.001 (−0.018, 0.006) | 0.003 (−0.009, 0.011) | 0.002 (−0.015, 0.049) |
| Locally-caught fish–Great Lakes and AOCs | 0.000 (−0.005, 0.009) | 0.017 (0.008, 0.042) | 0.098 (0.038, 0.188) | |
| Locally-caught fish–other water bodies | −0.002 (−0.013, 0.001) | 0.010 (−0.005, 0.023) | 0.012 (0.002, 0.099) | |
| Total lipids | 0.001 (−0.001, 0.001) | 0.002 (0.000, 0.003) | 0.004 (−0.001, 0.008) | |
| All commercially purchased fish (store, restaurant) | PFOA, ng/mL | −0.001 (−0.021, 0.011) | 0.006 (−0.008, 0.014) | 0.006 (−0.018, 0.020) |
| Locally-caught fish–Great Lakes and AOCs | 0.016 (−0.032, 0.022) | 0.007 (0.003, 0.010) | 0.004 (−0.013, 0.027) | |
| Locally-caught fish–other water bodies | 0.006 (−0.031, 0.012) | 0.007 (0.002, 0.017) | 0.015 (−0.004, 0.058) | |
| All commercially purchased fish (store, restaurant) | PFOS, ng/mL | 0.000 (−0.071, 0.143) | 0.055 (−0.099, 0.156) | 0.050 (−0.085, 0.693) |
| Locally-caught fish–Great Lakes and AOCs | 0.090 (−0.048, 0.170) | 0.175 (0.081, 0.293) | 0.782 (0.151, 1.732) | |
| Locally-caught fish–other water bodies | 0.093 (−0.159, 0.165) | 0.123 (0.063, 0.297) | 0.427 (0.002, 0.850) | |
Bold font indicates an association that is statistically significant at the p < 0.05 level.
Fig. 3.
Quantile process plots for the association between fish consumption parameters and each contaminant. Legend: “glaocfish” indicates locally-caught fish from the Great Lakes and Areas of Concern, “oth_sc_t” indicates locally-caught fish from all other areas, and “comfish” indicates all commercially purchased fish.
In the main analysis, the sum of all PCB and PBDE congeners were analyzed, as was PFOA. However, information was also collected on specific congeners and additional PFCs. In extended analyses, these were examined as outcomes if at least 50% of values were above the limit of detection (values < LOD were substituted with zero; results using instead the LOD/sqrt(2) or using multiply imputed values provided very similar or identical results). As with the main analysis, total lipids were included as a predictor when modeling PCBs and PBDEs as outcomes, but not for PFCs. Results are shown in Table 6. Consumption of fish from the Great Lakes and areas of concern was associated with higher levels of each of the individual PCB congeners examined, as well as higher levels of all of the PFCs examined, with the exception of PFHxS. Among the PFCs, locally caught fish from other water bodies was also associated with higher levels of each of the congeners examined except PFHxS. Finally, all commercial fish was associated with higher levels of PFHxS. The associations for PFOS were the strongest among the PFC congeners examined, possibly due to the higher blood levels of this analyte compared with the other PFCs (median of 19 ng/mL, compared with a median of 2.5 for PFOA and <2 for the other PFCs examined).
Table 6.
Multivariate associations between fish consumption (meals per year) and specific PBDE, PCB, and PFC analytes, modeled using a quantile regression model for the 10th, 50th, and 90th percentiles.
| Beta (95% CI)* |
||||
|---|---|---|---|---|
| Predictor | Response | 10th percentile | 50th percentile | 90th percentile |
| Polybrominated diphenyl ethers (PBDEs), (ng/mL) | ||||
| All commercially purchased fish (store, restaurant) | PBDE47 | 0.000 (−0.001, 0.000) | 0.000 (0.000, 0.001) | −0.001 (−0.002, 0.002) |
| Locally-caught fish–Great Lakes and AOCs | −0.001 (−0.001, 0.000) | 0.000 (−0.001, 0.000) | 0.002 (−0.001, 0.012) | |
| Locally-caught fish–other water bodies | −0.001 (−0.001, 0.000) | 0.000 (−0.001, 0.000) | 0.000 (−0.001, 0.004) | |
| Total lipids | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | |
| All commercially purchased fish (store, restaurant) | PBDE99 | – | – | – |
| Locally-caught fish–Great Lakes and AOCs | – | – | – | |
| Locally-caught fish–other water bodies | – | – | – | |
| Total lipids | – | – | – | |
| Polychlorinated biphenyls (PCBs), (ng/mL) | ||||
| All commercially purchased fish (store, restaurant) | PCB132/153/105 (co-eluting) | – | 0.001 (−0.002, 0.003) | −0.002 (−0.003, 0.010) |
| Locally-caught fish–Great Lakes and AOCs | – | 0.003 (0.001, 0.008) | 0.023 (−0.002, 0.027) | |
| Locally-caught fish–other water bodies | – | 0.000 (−0.002, 0.003) | 0.003 (−0.003, 0.012) | |
| Total lipids | – | 0.000 (0.000, 0.001) | 0.001 (0.000, 0.001) | |
| All commercially purchased fish (store, restaurant) | PCB163/138 (co-eluting) | – | 0.000 (−0.001, 0.002) | 0.000 (−0.002, 0.008) |
| Locally-caught fish–Great Lakes and AOCs | – | 0.002 (0.001, 0.005) | 0.014 (0.001, 0.023) | |
| Locally-caught fish–other water bodies | – | 0.001 (−0.001, 0.002) | 0.004 (−0.001, 0.009) | |
| Total lipids | – | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.001) | |
| All commercially purchased fish (store, restaurant) | PCB170/190 (co-eluting) | – | 0.000 (−0.001, 0.001) | 0.000 (−0.001, 0.001) |
| Locally-caught fish–Great Lakes and AOCs | – | 0.002 (0.001, 0.003) | 0.004 (0.002, 0.007) | |
| Locally-caught fish–other water bodies | – | 0.000 (0.000, 0.001) | 0.001 (−0.001, 0.005) | |
| Total lipids | – | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | |
| All commercially purchased fish (store, restaurant) | PCB180 | 0.001 (−0.005, 0.003) | 0.001 (−0.001, 0.002) | −0.001 (−0.002, 0.005) |
| Locally-caught fish–Great Lakes and AOCs | −0.001 (−0.004, 0.003) | 0.002 (0.000, 0.005) | 0.010 (0.004, 0.016) | |
| Locally-caught fish–other water bodies | 0.000 (−0.003, 0.000) | 0.000 (−0.001, 0.002) | 0.001 (−0.002, 0.013) | |
| Total lipids | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.001) | |
| All commercially purchased fish (store, restaurant) | PCB187 | – | 0.000 (−0.001, 0.001) | −0.001 (−0.001, 0.003) |
| Locally-caught fish–Great Lakes and AOCs | – | 0.001 (0.000, 0.003) | 0.007 (0.002, 0.009) | |
| Locally-caught fish–other water bodies | – | 0.000 (−0.001, 0.001) | 0.000 (−0.001, 0.006) | |
| Total lipids | – | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | |
| Perfluorinated compounds (PFCs), (ng/mL) | ||||
| All commercially purchased fish (store, restaurant) | PFDA | 0.000 (−0.009, 0.004) | 0.002 (−0.003, 0.005) | 0.003 (0.000, 0.015) |
| Locally-caught fish–Great Lakes and AOCs | 0.004 (−0.001, 0.007) | 0.006 (0.004, 0.015) | 0.017 (0.009, 0.042) | |
| Locally-caught fish–other water bodies | 0.003 (−0.008, 0.004) | 0.004 (0.002, 0.007) | 0.016 (0.002, 0.030) | |
| All commercially purchased fish (store, restaurant) | PFHpS | 0.001 (−0.004, 0.003) | 0.002 (−0.001, 0.005) | 0.001 (−0.001, 0.019) |
| Locally-caught fish–Great Lakes and AOCs | 0.005 (−0.005, 0.005) | 0.003 (0.001, 0.004) | 0.007 (−0.001, 0.020) | |
| Locally-caught fish–other water bodies | 0.002 (−0.006, 0.004) | 0.002 (0.001, 0.007) | 0.004 (−0.001, 0.020) | |
| All commercially purchased fish (store, restaurant) | PFHxS | −0.001 (−0.007, 0.011) | 0.010 (−0.004, 0.033) | 0.135 (0.015, 0.167) |
| Locally-caught fish–Great Lakes and AOCs | 0.007 (−0.008, 0.016) | 0.002 (−0.002, 0.011) | −0.001 (−0.017, 0.122) | |
| Locally-caught fish–other water bodies | 0.009 (−0.014, 0.011) | 0.009 (−0.001, 0.018) | 0.010 (−0.001, 0.117) | |
| All commercially purchased fish (store, restaurant) | PFNA | −0.002 (−0.007, 0.007) | 0.005 (−0.008, 0.009) | 0.023 (−0.002, 0.054) |
| Locally-caught fish–Great Lakes and AOCs | 0.007 (−0.004, 0.008) | 0.021 (0.004, 0.033) | 0.057 (0.026, 0.115) | |
| Locally-caught fish–other water bodies | 0.008 (−0.015, 0.011) | 0.013 (0.004, 0.021) | 0.018 (0.005, 0.043) | |
| All commercially purchased fish (store, restaurant) | PFOA | −0.001 (−0.021, 0.011) | 0.006 (−0.008, 0.014) | 0.006 (−0.018, 0.020) |
| Locally-caught fish–Great Lakes and AOCs | 0.016 (−0.032, 0.022) | 0.007 (0.003, 0.010) | 0.004 (−0.013, 0.027) | |
| Locally-caught fish–other water bodies | 0.006 (−0.031, 0.012) | 0.007 (0.002, 0.017) | 0.015 (−0.004, 0.058) | |
| All commercially purchased fish (store, restaurant) | PFOS | 0.000 (−0.071, 0.143) | 0.055 (−0.099, 0.156) | 0.050 (−0.085, 0.693) |
| Locally-caught fish–Great Lakes and AOCs | 0.090 (−0.048, 0.170) | 0.175 (0.081, 0.293) | 0.782 (0.151, 1.732) | |
| Locally-caught fish–other water bodies | 0.093 (−0.159, 0.165) | 0.123 (0.063, 0.297) | 0.427 (0.002, 0.850) | |
| All commercially purchased fish (store, restaurant) | PFuDA | 0.000 (−0.002, 0.002) | 0.002 (−0.002, 0.003) | 0.001 (0.000, 0.018) |
| Locally-caught fish–Great Lakes and AOCs | 0.001 (0.000, 0.003) | 0.005 (0.003, 0.011) | 0.016 (0.009, 0.032) | |
| Locally-caught fish–other water bodies | 0.002 (−0.004, 0.002) | 0.002 (0.001, 0.003) | 0.004 (0.000, 0.013) | |
Bold font indicates an association that is statistically significant at the p < 0.05 level. Empty cells (−) indicate no estimated effect due to model instability. Models for PBDE and PCB summed congeners are additionally adjusted for total lipids.
Discussion
Nationally and globally, fish are an increasingly important part of the human diet. Over the past few decades, fish consumption has increased by about 30% in the United States (Loke et al., 2012), and fish locally-caught from Great Lakes water bodies represent an important and commonly consumed food source for area residents. Contamination of Great Lakes basin waters is more widespread than previously appreciated with new chemicals being identified (such as PFCs) as levels of older legacy chemicals decline. In this study, we evaluated fish consumption and levels of contaminants in blood, among Wisconsin male anglers aged 50 years and older. The anglers in this study had relatively high fish consumption, with a large proportion comprised of locally caught fish. Consumption was higher in this population compared with both the US general population (overall and for older non-Hispanic white men (CDC and NCHS, 2014)), and with a random sample of anglers in fishing license holders in Great Lakes states (Connelly et al., 2012). Contaminant levels among the random sample of fishing license holders were not available, but in comparison with the US general population, the anglers in this study had notably higher blood mercury, perhaps linked to their higher fish consumption. However, levels of other contaminants (PFOA, PBDEs, PCBs) were lower than or comparable to those in the US general population. It should be noted that the general population (NHANES) data were collected in 2011–2012 (blood mercury and PFOA), a similar time frame as the angler study population data collection, but that the time frame for PBDEs and PCBs was earlier (2003–2004). Thus, the difference in contaminant levels between the angler study population and NHANES data may be due to differences in exposure routes and sources, including other dietary sources aside from fish (e.g., PCBs in ambient air, or PFCs from consumer products with non-stick properties), but also may be due to temporal trends for PBDEs and PCBs. In the case of PBDEs and PCBs there were also differences in the specific congeners measured, which affects the ability to make direct comparisons between the two groups.
In this population, higher fish consumption was associated with higher levels of the contaminants evaluated in this study with the exception of PBDEs. For associations with locally-caught fish there were generally greater effects with increasing quantile of contaminant level (i.e., as consumption increased, so did the biomarker level). In addition to Congener-specific analysis showed that consumption of fish from the Great Lakes and areas of concern was associated with higher levels of each of the PCB congeners examined, but no associations were found with individual PBDE congeners. Both categories of locally-caught fish (Great Lakes and areas of concern, other water bodies) were associated with all of the individual PFCs examined except PfHxS. Commercial fish consumption was associated with higher PFHxS.
While fish consumption has been previously studied with respect to legacy contaminants such as PCBs and mercury, far less data are available for PFCs. Our findings regarding associations with PFCs are thus particularly interesting; PFCs are a relatively newer class of contaminants (compared with mercury and PCBs) where the major source is dietary, and specifically, seafood consumption (e.g., (D’Hollander et al., 2010; Denys et al., 2014; Falandysz et al., 2006; Haug et al., 2010; Holzer et al., 2011)). Some of these studies were conducted specifically among anglers, providing support to the observation of increased biomarker concentration with increased fish consumption among anglers in this study (Falandysz et al., 2006; Holzer et al., 2011). Further, a growing number of studies are identifying potential health effects associated with exposure to PFCs (e.g.,(Domingo, 2012)). Further, many epidemiology studies have looked only at the most widely used PFCs – PFOA and PFOS – although usage of other PFCs may be increasing due to changes in industrial usage patterns (e.g., Glynn et al., 2012; Kato et al., 2011).
There were some limitations to this study. First, the cross-sectional design means that only ‘point-in-time’ associations can be evaluated, rather than cause-effect relationships. The study population was essentially a convenience sample of those who agreed to participate from a larger group of online survey respondents, with the addition of ‘walk-in’ participants; thus, inference to a larger population of older male anglers may not be possible. However, we did find that the individuals who participated in the biomonitoring study described here were largely similar to the larger survey respondent population from which they were recruited, although there were some differences with respect to age, educational attainment, and geography (residence in a county bordering a Great Lake). There may be error in self-reported characteristics, including self-reported fish and shellfish consumption. This type of error should not be different based on contaminant levels however—such non-differential error would generally have the effect of biasing effect estimates toward the null. Similarly, there may be measurement errors in the biomarker levels (e.g., due to laboratory variation); however, these should not be different according to fish consumption. Regarding contaminant levels, one factor which is both a strength as well as a limitation, is that measured biomarker levels are aggregated over all exposure sources and routes. A strength of this approach is that no sources or routes are ‘missed’ but a limitation is that exposure cannot be traced back to any one factor (such as fish). While for some contaminants the dominant source of exposure is indeed fish (e.g., methylmercury (Mahaffey et al., 2004, 2009; Mergler et al., 2007)), for others, such as the PFCs, important exposure routes such as contaminated water and consumer products (ATSDR, 2009a; EPA, 2009b), are not addressed by this analysis due to lack of information on non-fish sources of exposure. Finally, the analyses shown here may not account for all potential confounders; fish consumption and biomarker levels may be influenced by a number of factors aside from fish consumption. Seasonality could be one such factor, if fish consumption varied by season, and if contaminant levels varied by season for reasons other than varying consumption of fish (e.g., variations in level of ambient PCBs in air). As a sensitivity analysis, the multivariate quantile regression models were extended to include either month of data collection, or season of data collection. Neither of these time related variables was significant when looking at mercury (hair or blood) or PFOA as outcomes. For PCBs and PBDEs, certain months or seasons were significantly associated, but in most cases estimates were unstable, with confidence bounds including infinity. However, there was some evidence that data collection during the fall season was associated with lower PBDE levels compared with the winter season, while data collection during the spring and summer was associated with lower PCB levels compared with the winter season. Strengths of this study include a variety of biomarkers measured, including specific PFCs, and specific PBDE and PCB congeners. We were able to examine the effects and associations for different types of fish separately, namely those for locally-caught freshwater fish, in comparison with (largely marine) commercially purchased fish. The study population was restricted to male anglers aged 50 years and older, and there was relatively little variation in demographic characteristics (e.g., race, socio-economic status) which should limit residual confounding and confounding by unmeasured or uncontrolled factors.
In conclusion, this analysis shows that fish consumption – especially Great Lakes fish consumption - is associated with higher levels of persistent contaminants. Commercial fish consumption also contributes to contaminant body burdens and biomarker studies need to collect consumption information on all sources of fish in order to fully capture potential exposure routes. Thus, the risks and benefits of fish consumption must be balanced when evaluating guidelines for older male anglers. Public health needs to be vigilant and maintain rigorous biomonitoring programs of both the biota and humans.
Acknowledgements
The authors would like to thank the University of Wisconsin-Madison Survey Center for their work with setting up and managing the online survey, as well as all the survey participants. We would also like to thank our partners including the Survey of the Health of Wisconsin (SHOW) program, in particular Christine McWilliams who served as the ancillary study coordinator for this project along with Susan Wright who assisted in overseeing field data collection. SHOW is funded by the Wisconsin Partnership Program PERC Award (233 PRJ 25DJ), the National Institutes of Health’s Clinical and Translational Science Award (5UL 1RR025011), and the National Heart, Lung, and Blood Institute (1 RC2 HL101468).
Funding/support
The Research described in this article has been funded by the US Environmental Protection Agency Great Lakes National Program Office under Assistance no. GL-00E00452-0. It has not been subjected to the Agency’s required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
Footnotes
SAS/STAT software, Version 9.3 of the SAS System for Windows. Copyright © 2013 Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
References
- ATSDR, 1999. Toxicological Profile for Mercury U.S. Departmen of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA, Available at: ⟨http://www.atsdr.cdc.gov/toxprofiles/tp46.pdf⟩. [Google Scholar]
- ATSDR, 2000. Toxicological Profile for Polychlorinated Biphenyls (PCBs) U.S. Department of Health and Human Services. Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA, Available at: ⟨http://www.atsdr.cdc.gov/toxprofiles/tp17.pdf⟩. [PubMed] [Google Scholar]
- ATSDR, 2004. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA, Available at: ⟨http://www.atsdr.cdc.gov/ToxProfiles/tp68.pdf⟩. [Google Scholar]
- ATSDR, 2009a. Toxicological Profile for Perfluoroalkyls. (Draft for Public Comment) U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, GA. [Google Scholar]
- ATSDR, 2009b. Draft Toxicological Profile for Perfluoroalkyls U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA, Available at: ⟨http://www.atsdr.cdc.gov/toxprofiles/tp200.pdf⟩. [PubMed] [Google Scholar]
- ATSDR, 2011. Addendum for Polychlorinated Biphenyls, Supplement to the 2000 Toxicological Profile for Polychlorinated Biphenyls U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA, Available at: ⟨http://www.atsdr.cdc.gov/toxprofiles/pcbs_addendum.pdf⟩. [Google Scholar]
- ATSDR, 2013. Addendum for Organic Mercury Compounds (Alkyl and Dialkyl Mercury Compounds), Supplement to the 1999 Toxicological Profile for Mercury U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA, Available at: ⟨http://www.atsdr.cdc.gov/toxprofiles/mercury_organic_addendum.pdf⟩. [Google Scholar]
- Cade BS, Noon BR, 2003. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ 1,412–420. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), 2014. National Health and Nutrition Examination Survey Data U.S. Department of Health and Human Services Centers for Disease Control and Prevention, Hyattsville, MD, pp. 2011–2012. [Google Scholar]
- Christensen KY, Raymond MR, Thompson BA, Schrank CS, Williams MC, Anderson HA, 2015. Comprehension of fish consumption guidelines among older male anglers in Wisconsin. J. Community health. [DOI] [PubMed] [Google Scholar]
- Connelly NA, Lauber TB, Niederdeppe J, Knuth BA, 2012. Factors Affecting Fish Consumption among Licensed Anglers Living in the Great Lakes Region HDRU Publ. No. 12-3. Dept of Nat. Resour., N.Y.S. Coll. Agric. And Life Sci, Cornell Univ, Ithaca, NY, pp. 78, Available at: ⟨http://www2.dnr.cornell.edu/hdru/pubs/fishpubs.html#risk⟩. [Google Scholar]
- Crimmins BS, Pagano JJ, Xia X, Hopke PK, Milligan MS, Holsen TM, 2012. Polybrominated diphenyl ethers (PBDEs): turning the corner in Great Lakes trout 1980–2009. Environ. Sci. Technol 46, 9890–9897. [DOI] [PubMed] [Google Scholar]
- D’Hollander W, de Voogt P, De Coen W, Bervoets L, 2010. Perfluorinated substances in human food and other sources of human exposure. Rev. Environ. Contam. Toxicol 208, 179–215. [DOI] [PubMed] [Google Scholar]
- de Goede J, Verschuren WM, Boer JM, Kromhout D, Geleijnse JM, 2012. Gender-specific associations of marine n-3 fatty acids and fish consumption with 10-year incidence of stroke. PLoS ONE 7, e33866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys S, Fraize-Frontier S, Moussa O, Le Bizec B, Veyrand B, Volatier JL, 2014. Is the freshwater fish consumption a significant determinant of the internal exposure to perfluoroalkylated substances (PFAS)? Toxicol. Lett 231, 233–238. [DOI] [PubMed] [Google Scholar]
- Domingo JL, 2012. Health risks of dietary exposure to perfluorinated compounds. Environ. Int 40, 187–195. [DOI] [PubMed] [Google Scholar]
- EPA, 2009a. State of the Great Lakes 2009 Prepared by Environment Canada and United States Environmental Protection Agency, Available at: ⟨http://binational.net/wp-content/uploads/2014/11/En161-3-1-2009E.pdf⟩. [Google Scholar]
- EPA U, 2009b. Long-Chain Perfluorinate Chemicals (PFCs) Action Plan, Available at: ⟨http://www.epa.gov/opptintr/existingchemicals/pubs/pfcs_action_plan1230_09.pdf⟩.
- Falandysz J, Taniyasu S, Gulkowska A, Yamashita N, Schulte-Oehlmann U, 2006. Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic Coast? Environ. Sci. Technol 40, 748–751. [DOI] [PubMed] [Google Scholar]
- Furdui VI, Helm PA, Crozier PW, Lucaciu C, Reiner EI, Marvin CH, Whittle DM, Mabury SA, Tomy GT, 2008. Temporal trends of perfluoroalkyl compounds with isomer analysis in lake trout from Lake Ontario (1979–2004). Environ. Sci. Technol 42, 4739–4744. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO, 2012. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ. Sci. Technol 46, 9071–9079. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK, 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int 36, 772–778. [DOI] [PubMed] [Google Scholar]
- He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, Ascherio A, 2002. Fish consumption and risk of stroke in men. JAMA 288, 3130–3136. [DOI] [PubMed] [Google Scholar]
- Holzer J, Goen T, Just P, Reupert R, Rauchfuss K, Kraft M, Muller J, Wilhelm M, 2011. Perfluorinated compounds in fish and blood of anglers at Lake Mohne, Sauerland area, Germany. Environ. Sci. Technol 45, 8046–8052. [DOI] [PubMed] [Google Scholar]
- Imm P, Anderson HA, Schrank C, Knobeloch L, 2013. Fish consumption and advisory awareness amongolder Wisconsin fishermen WMJ 112,111–116 (Official publication of the State Medical Society of Wisconsin; ). [PubMed] [Google Scholar]
- Imm P, Knobeloch L, Buelow C, Anderson HA, 2009. Household exposures to polybrominated diphenyl ethers (PBDEs) in a Wisconsin Cohort. Environ. Health Perspect 117, 1890–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM, 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ. Sci. Technol 45, 8037–8045. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Steenport D, Schrank C, Anderson H, 2006. Methylmercury exposure in Wisconsin: a case study series. Environ. Res 101, 113–122. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Turyk M, Imm P, Schrank C, Anderson H, 2009. Temporal changes in PCB and DDE levels among a cohort of frequent and infrequent consumers of Great Lakes sportfish. Environ. Res 109,66–72. [DOI] [PubMed] [Google Scholar]
- Koenker R, Bassett G, 1978. Regression quantiles. Econometrica 46, 33–50. [Google Scholar]
- Loke M, Geslani C, Takenaka B, Leung PS, 2012. An overview of seafood consumption and supply sources: Hawai’i versus U.S University of Hawaii, Honolulu, HI, pp. 9, Available at: ⟨http://www.fpir.noaa.gov/SFD/pdfs/seafood/EI-22.pdf⟩ (Economic Issues; EI-22). [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC, 2004. Blood organic mercury and dietary mercury intake: national health and nutrition examination survey, 1999 and 2000. Environ. Health Perspect 112, 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA, 2009. Adult women’s blood mercury concentrations vary regionally in the United States: association with patterns of fish consumption (NHANES 1999–2004). Environ. Health Perspect 117, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D, Anderson H, Chan LH, Mahaffey KR, Murray M, Sakamoto M, Stern AH, 2007. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio 36, 3–11. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Peppard PE, Engelman CD, McElroy JA, Galvao LW, Friedman EM, Bersch AJ, Malecki KC, 2010. The survey of the health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Pub. Health; 10, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen PW, Schrank C, Williams MC, 2014. Trends of PCB concentrations in Lake Michigan coho and chinook salmon, 1975–2010. J. Great Lakes Res 40, 748–754. [Google Scholar]
- Rasmussen PW, Schrank CS, Campfield PA, 2007. Temporal trends of mercury concentrations in Wisconsin walleye (Sander vitreus), 1982–2005 Ecotoxicology (London, England: ) 16,541–550. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R, 1995. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death i eastern Finnish men. Circulation 91, 645–655. [DOI] [PubMed] [Google Scholar]
- Stahl LL, Snyder BD, Olsen AR, Kincaid TM, Wathen JB, McCarty HB, 2014. Perfluorinated compounds in fish from U.S. urban rivers and the Great Lakes. Sci. Total Environ 499,185–195. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA, 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect 118, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Steenport D, Buelow C, Imm P, Knobeloch L, 2010. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere 81, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Bhavsar SP, Bowerman W, Boysen E, Clark M, Diamond M, Mergler D, Pantazopoulos P, Schantz S, Carpenter DO, 2012. Risks and benefits of consumption of Great Lakes fish. Environ. Health Perspect 120, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]