Abstract
The pituitary homeobox1 gene (Ptx1) was initially identified as encoding a pituitary-restricted transcription factor for the proopiomelanocortin (POMC) gene. In order to elucidate the expression pattern of the Ptx1 protein, we investigated the localization of the protein in adult rat pituitary gland and in various pituitary cell lines. We produced an antibody specific for Ptx1 protein, and confirmed its specificity by Western blot analysis. Immunohistochemically, many nuclei in the anterior pituitary cells as well as in the intermediate cells were positive for Ptx1 staining with this specific antibody. Im- munohistochemical double staining revealed the presence of Ptx1 not only in all types of hormone-secreting cells but also in some folliculo-stellate (FS) cells. Furthermore, the expression of Ptx1 mRNA was confirmed in various pituitary cell lines and in the FS cell line by using the reverse transcriptase-polymerase chain reaction (RT-PCR) method. Our studies indicated that Ptx1 may not only play a role as a basic transcriptional factor for production of various hormones, but may also play some important role(s) in FS cells. Possible synergistic actions with other factors remain to be investigated. The novel finding of Ptx1 in FS cells is of particular interest, and may suggest that FS cells and hormone-secreting cells are derived from a common cellular ancestor.
Keywords: Pituitary, Ptx1, Folliculo-stellate (FS) cells, Rat (Wistar Imamichi)
Introduction
The production of hormones in the anterior pituitary gland is under the control of several pituitary-specific transcriptional factors. Pit-1 has been reported by several investigators to be a key factor in functional differentiation, resulting in production of growth hormone (GH), prolactin (PRL), and thyroid-stimulating hormone β(TSHβ) in rats and humans (Bodner et al. 1988; Bodner and Karin 1987; Mangalam et al. 1989; Simmons et al. 1990; Voss and Rosenfeld 1992; Lew et al. 1993; Andersen and Rosenfeld 1994). Our previous studies on human pituitary adenomas disclosed the possibility of Pit-1 participation in the functional differentiation of the tumor cells into cells that produce GH, PRL, and TSH (Sanno et al. 1994, 1996a, 1996b). It has also been reported that Pit- 1 functions synergistically with cell membrane receptors and members of nuclear receptor families (Sanno et al. 1996c, 1997). Some recently recognized participants in pituitary differentiation include Rpx/Hesx-1, P- Lim/mLIM-3, P-OTX/Ptx1, and Prop-1. The Rpx/Hesx-1 gene encodes a homeodomain factor that is initially expressed in the mesendoderm and anterior neural plate. It is subsequently expressed only in Rathke’s pouch, the primordium of the anterior pituitary gland, which appears as an invagination of the roof of the oral cavity (Thomas et al. 1995; Hermesz et al. 1996; Gage et al. 1996). The P-Lim/mLIM-3 gene encodes an LIM homeodomain factor which is expressed during the formation of Rathke’s pouch (Bach et al. 1995, 1997; Sheng et al. 1996). P- OTX/Ptx1 was first reported to promote the transcription of the POMC gene (Lamonerie et al. 1996). Further Prop-1 was identified as another pituitary-specific homeodomain factor (Sornson et al. 1995). It has since been clarified that these transcriptional factors may act in synergy with other transcriptional factors and function in the differentiation and development of pituitary cells.
Ptx1 has been reported to promote transcription of the POMC gene; however, Szeto et al. (1996) have reported that Ptx1 was capable of transactivating expression of the α-subunit (α-SU), GH, and PRL promoters as well as the POMC promoter, but was not able to effectively activate the Pit-1 and TSHP promoters. Subsequently, Tremblay et al. (1998) reported that Ptx1 expression was detected not only in POMC cells but also in all types of hormone-secreting cells in the murine anterior pituitary gland and in various pituitary hormone-secreting cell lines, and that Ptx1 may act in synergy with SF-1 and Pit-1. Ptx1 mRNA was expressed detectably at embryonic day 9.5 and expressed very strongly at embryonic day 17.5 in the anterior and intermediate lobes, and the expression was also observed in the adult murine pituitary (Szeto et al. 1996).
Besides these hormone-secreting cells in the anterior pituitary gland, folliculo-stellate (FS) cells comprise a significant proportion of the anterior gland, with an intimate structural relationship with the hormone-secreting cells (Baes and Denef 1987). Suggested cellular lineages of FS cells have included macrophage and glial cell lineages, but the actual lineage remains a matter of controversy (Coates and Doniach 1988; Young et al. 1967; Kagayama 1965; Couly and Le Douarin 1985; Vila- Porcile and Oliver 1984). In order to further elucidate the relationship between Ptx1 and individual hormones by localizing Ptx1 in the adult rat pituitary gland, we performed immunohistochemical analyses of Ptx1 and compared its localization with those of the anterior hormone-secreting cells and FS cells by double staining. Ptx1 mRNA expression was also analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR) in various pituitary cell lines to further characterize the relationships between Ptx1 production, and functional cell lineage and FS cells.
Materials and methods
Animals and tissues
Adult male and female Wistar Imamichi rats (250–300 g) were maintained in a specific pathogen-free environment and kept under standard housing conditions (20±2°C, light-dark cycle 8:00–20:00 h/day). They were given commercial food and water. The rats were sacrificed by decapitation after diethyl-ether treatment in the morning. The rat pituitary glands were fixed overnight in 4°C in Bouin’s solution and embedded in paraffin. Sections (4 μm) were mounted on 3-amino-propylethoxysilane-coated glass slides.
Cell lines
To investigate the expression of mRNA and protein in various pituitary hormone-secreting cells, the following cell lines were used: murine αT1–1 (α-SU-secreting cells), αT3–1 (α-SU-secreting cells), TαT-1 (α, βTSH-secreting, Pit-1-positive cells), and LβT-2 and LβT-4 cells (α, βLH-secreting cells) (Windle et al. 1990; Alarid et al. 1996), which were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Iwaki Glass, Japan) supplemented with 10% fetal bovine serum (FBS); and murine AtT-20 [adrenocorticotropic hormone (ACTH)-secreting cells] (Buonassisi et al. 1962), rat MtT/SM [growth hormone (GH)- and prolactin (PRL)- secreting cells], MtT/E (no hormone secretion) (Inoue et al. 1992), and murine TtT/GF cells (folliculo-stellate-like cells) (Inoue et al. 1990), which were cultured in medium containing (1:1) DMEM and Ham’s nutrient mixture F12 (Ham F-12, Iwaki Glass, Japan) supplemented with 10% normal horse serum (NHS) and 2.5% FBS. Murine αT1–1, αT3–1, TαT-1, LβT-2, and LβT-4 cell lines were established in Dr. Pamela L. Mellon’s laboratory, and rat MtT/SM and MtT/E and murine TtT/GF cell lines were established by Dr. Kinji Inoue.
Anti-Ptx1 antibody
To prepare immunogen, murine Ptx1 peptide (amino acid positions 31–50; FHLARAADPREPLENSASES) was conjugated with an equal amount of keyhole limpet hemocyanin (KLH) by using dimethylsuberimidate. Female Japanese White rabbits (Clea Japan, Tokyo, Japan) were injected subcutaneously with the peptide- KLH conjugate in emulsified Freund’s complete adjuvant (Calbio- chem-Behring Co., La Jolla, CA) 5 times at 2-week intervals. Ten days after the fifth immunization, the rabbits were bled, and sera were separated.
Western blot analysis
Homogenates were prepared from whole adult rat pituitary glands and aliquots of the extracts (15 μg protein) were used per lane for electrophoresis. The homogenates were subjected to 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then the separated proteins were electro-transferred to nitrocellulose membranes. The membranes were preincubated in phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) and 0.01% NaN3, and then incubated with the anti- Ptx1-specific antiserum diluted 1:250 or with the antiserum neutralized with 10 μg/ml Ptx1 peptide (31–50) overnight at 4°C in PBS containing 0.05% Tween-20 (PBST) and 2% NHS. After the incubation, the membranes were incubated with horseradish peroxidase (HRP)-labeled anti-rabbit Ig diluted 1:400 in PBST for 30 min at room temperature (RT). The staining was visualized using a solution containing 12.5 mg 3,3’-diaminobenzidine-4HCl (DAB) and 10 μl H2O2 in 50 ml PBST.
Immunohistochemistry and immunocytochemistry
For the immunohistochemical staining of Ptx1, the polyclonal antibody against murine Ptx1 described above was used at the dilution of 1:4000 for the avidin-biotin-peroxidase complex (ABC) method (Vector Laboratories, CA). The specificity for Ptx1 was also examined immunohistochemically by an absorption test on the pituitary gland. For the absorption test, the anti-serum was incubated with the Ptx1 peptide (31–50), which had been attached to CNBr-activated Sepharose-4B (Pharmacia Biotech, Sweden) for 1 h at RT, and then the complex was centrifuged and the supernatant was passed through a 0.45-μm filter. A double-staining technique was performed to examine the possible co-localization of Ptx1 and pituitary hormones or S-100 protein. Briefly, the Ptx1 protein was stained using the ABC method, yielding a brown color with DAB, and then the tissues were incubated in glycine-HCl buffer (pH 2.2) to remove the immuno-complex. Subsequently, localization of the pituitary hormones or S-l00 protein on the same sections was detected by an indirect method using an alkaline phosphatase-conjugated second antibody (DAKO Co., CA), which yielded a blue color by using fast blue salt.
For immunostaining of cultured cells, the cells were fixed with 10% formaldehyde in PBS for 30 min, preincubated in PBS containing 2% NHS and 0.1% Tween 20 for 10 min, and then stained with anti-Ptx1 antibody by using the ABC method as described above.
The anti-pituitary hormone antibodies and their sources and working dilutions were as follows: anti-human (h) GH polyclonal antibody (1:400), anti-hACTH monoclonal antibody (clone 02A3, 1:400) (DAKO Co., CA), anti-hPOMC monoclonal antibody (clone 1E4, 1:5000) (Biogenesis, UK), anti-rat (r) PRL polyclonal antibody (1:20000), anti-rTSHβ polyclonal antibody (1:40000), anti-rFSHβ polyclonal antibody (1:5000), anti-rLHβ polyclonal antibody (1:2500), and anti-rα-SU polyclonal antibody (1:4000) (from the National Institute of Diabetes and Digestive and Kidney Disease, USA). For the identification of FS cells, anti-cow S-100 protein antibody (DAKO Co., CA) was used at the working dilution of 1:200.
RT-PCR
Total RNA was extracted with the TRIzol Reagent (Gibco BRL, USA) and treated with DNase I (Promega Biotech Inc., USA) and then 5 μg total RNA was reverse transcribed with Ready-To-Go (T-primed first-strand kit) (Pharmacia Biotech, Sweden). For PCR, the following oligonucleotide primers were used to amplify Ptx1 sequences: upstream 5′-CCG TGA ACT GAA TGT AGG GAA-3′ and downstream 5′-AGA GCT GAG CCC TTC TCC TC-3′. These primers produce a 298-bp DNA product. The following oligonucleotide primers for P-actin were used for internal standardization: upstream 5’-TGG CAC CAC ACC/T TTC TAC AAT GAG-3’ and downstream 5′-GGG TCA TCT TC/TT CA/GC GGT TGG-3′. PCR was carried out with Amplitaq Gold (Perkin-Elmer, USA). After an initial denaturing step at 95°C for 10 min, amplification was carried out with 45 cycles of denaturation at 95°C for 60 s and annealing and extension at 58°C for 60 s. The size of the products was estimated by 2% agarose gel electrophoresis with ethidium bromide staining.
Results
Western blot analysis
Western blot analysis was carried out to confirm the specificity of the anti-Ptx1 antiserum in rat pituitary (Fig. 1). The rat pituitary homogenate blotted on the membrane was reacted with the anti-Ptx1 antiserum or the antiserum neutralized with 10 μg/ml Ptx1 peptide (31–50). As a result of this analysis, a single band was detected at 35 kDa (Fig. 1, lane 1) which was consistent with the molecular weight estimated from the murine Ptx1 amino acid sequence staining of this band abolished by preincubation of the antibody with Ptx1 antigen (Fig. 1, lane 2).
Fig. 1.

Western blot analysis to confirm the specificity of the anti- Ptx1 antibody (lane 1). The Ptx1 protein was detected as single band at 35 kDa (lane 2). The Ptx1 signal was decreased by use of preadsorbed antibody
Immunohistochemical staining of Ptx1 in adult rat pituitary
Immunohistochemically, almost all nuclei in the anterior and intermediate pituitary cells of the rat pituitary gland were positive for Ptx1 (Fig. 2a). The negative control and preadsorption test confirmed the specificity of the staining. In the immunohistochemical preadsorption test, the nuclear staining was abolished when cells were stained with the preadsorbed serum (data not shown). Among the fixatives we tested, Bouin’s solution gave the best reactions for nuclear staining. The other fixatives tested included 4% paraformaldehyde and 10% neutral formalin (data not shown). Immunohistochemical double staining for Ptx1 and pituitary hormones or S-100 protein showed that the Ptx1-positive nuclei were present not only in all types of hormone-secreting cells, but also in S-100-positive cells (Fig. 2b-i). Staining for Ptx1 protein was positive in the nuclei of most GH- and PRL-secreting cells, and in 71.9% and 72.2% of POMC-secreting cells in the anterior and intermediate lobes, respectively. In TSH, LH/FSH-, and α-SU-expressing cells, Ptx1-positive nuclei were detected in about 20–40% of the cells. Ptx1 expression was also found in the nuclei of 25.2% and 20.0% of S-100-positive cells (FS cells) in the anterior and intermediate lobe, respectively (Table 1).
Fig. 2a-i.
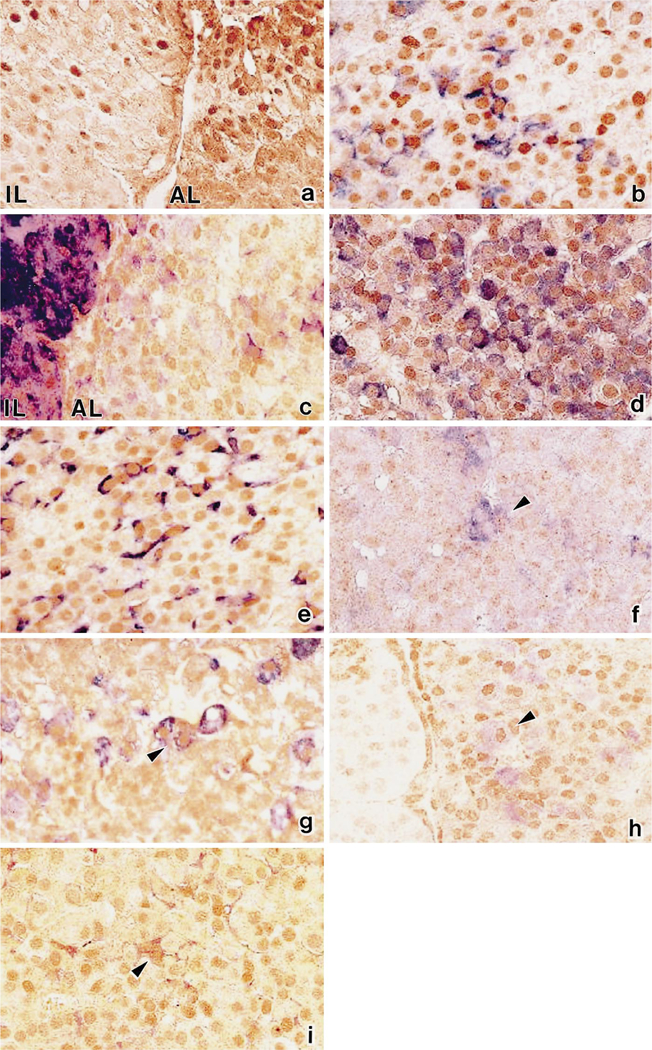
Immunohistochemical co-localization of Ptx1 (brown) and pituitary hormones or S-100 protein (blue) in rat pituitary. Ptxl was detected in the nuclei of intermediate lobe (IL) and anterior lobe (AL) (a), and it was expressed in the nuclei of cells containing POMC (b), ACTH (c), GH (d), PRL (e), TSHβ (f), α-SU (g), FSHβ (h), and S-100 protein (i). ×600(a-i)
Table 1.
Immunohistochemical co-localization of Ptx1 and pituitary hormones or S-100 protein in adult rat pituitary (a anterior lobe, i intermediate lobe)
| Expression of Ptx1 | Hormone or protein | ||||||
|---|---|---|---|---|---|---|---|
| GH | PRL | POMC | TSHβ | LHβ:FSHβ | α-SU | S-100 | |
| Ptx1-positive nuclei/counted nuclei | 300/300 | 259/259 | a: 248/345 i: 52/72 |
10/46 | 28/92:34/99 | 43/111 | a: 26/103 i: 6/30 |
| Ptx1-expressing cells (%) | 100 | 100 | a: 71.9 i: 72.2 |
21.7 | 30.4:34.3 | 38.7 | a: 25.2 i: 20.0 |
Co-localization was detected by observation of double staining
Detection of Ptx1 mRNA by RT-PCR
In the various pituitary cell lines, Ptx1 mRNA expression was detected by RT-PCR not only in the hormone-secreting cell lines, such as AtT-20, MtT/SM, αT1–1, αT3–1, LβT-2, LβT-4, and TαT-1, but also in the TtT/GF (FS) cell line (Fig. 3). From our sequence analysis of PCR products in the rat, we found that the homology between murine and rat Ptx1 genes was 87.8% (data not shown).
Fig. 3.
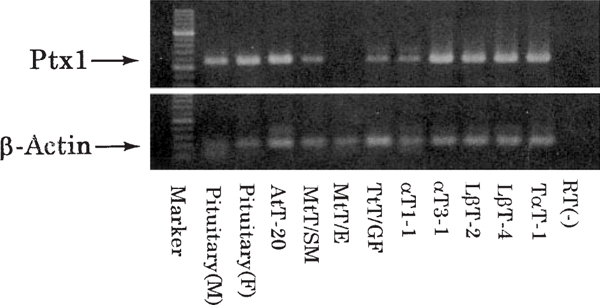
RT-PCR analysis of expression of Ptx1 mRNA and β-actin mRNA.Ptx1 mRNA was detected in male and female rat pituitary glands and all hormone-secreting cell lines examined. The Ptx1 PCR products were detected at 298 bp. The negative control without RT showed no formation of the PCR product from the rat pituitary RNA. The β-actin primers were used as internal standards, and yielded PCR products in all samples. The β-actin PCR products were detected at 105 bp
Ptx1 in cultured cell lines
Expression of Ptx1 was investigated immunocytochemically in the same cell lines in which mRNA expression was examined. Ptx1 was detected in the nuclei of all cell lines examined, including TtT/GF (FS cell line) (Fig. 4). The intensity of expression and proportion of cells expressing Ptx1 were lower in TtT/GF than in the other cell lines.
Fig. 4a, b.
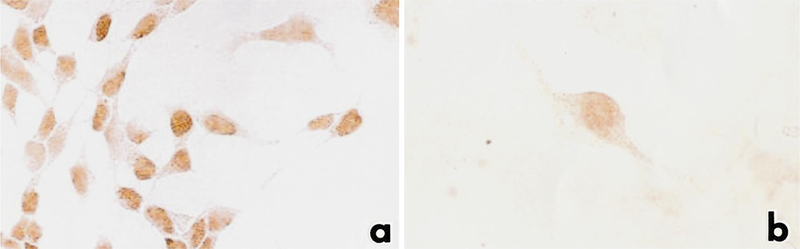
Immunocytochemical detection of Ptx1 in pituitary cell lines. Ptx1 was detected in the nuclei of the αT3–1 cell line (a) and TtT/GF cell line (b). x650 (a,b)
Discussion
We investigated Ptx1 expression by immunohistochemical double staining and RT-PCR in the rat pituitary gland and in various pituitary cell lines. Ptx1, a transcriptional factor, was initially reported to promote the transcription of the POMC gene (Lamonerie et al. 1996), but in the same year Szeto et al. (1996) reported that Ptx1 was capable of transactivating the α-SU, POMC, GH, and PRL promoters but not the Pit-1 and TSHβ promoters, and that it was able to act synergetically with Pit-1. In order to investigate the function of Ptx1 in vivo, we studied the expression of Ptx1 immunohistochemically in the rat pituitary and in various cell lines. We confirmed the specificity of the anti-Ptx1 antibody used in this study both by immunoadsorption before use in Western blotting and by an immunohistochemical preadsorption test before staining cells in the rat pituitary gland. As shown in Fig. 1, a single band was detected at 35 kDa, and the band was abolished when the preadsorbed antibody was used. The band was noted at the molecular mass which was predicted based on the murine Ptx1 amino acid sequence. Tremblay et al. (1998) obtained an antibody to murine Ptx1 peptide 24–56, and used it to detect Ptx1 in the murine pituitary gland and various kinds of pituitary cell lines by Western blotting. However, in their study, the band detected had a molecular weight larger than 35 kDa; thus, they may have detected a precursor protein. Further, our immunohistochemical study indicated that Ptx1 staining was positive in most nuclei in anterior and intermediate pituitary cells, and it was co-localized at various frequencies with all the kinds of hormone-secreting cells tested. This is consistent with the possibility that Ptx1 is a widely acting DNA-binding transcriptional factor. As Ptx1 was localized predominantly in the GH, PRL, and POMC-expressing cells, it suggested that Ptx1 has some functional role in the transcriptional control of GH, PRL, and POMC production. Tremblay et al. (1998) found that Ptx1 expression in αT3–1, an α-SU-secreting cell line, was stronger than that in AtT-20, an ACTH-secreting cell line. They also concluded that Ptx1 has a relation to the production of α-SU, based on immunohistochemical double staining. Our immunohistochemical study disclosed that Ptx1 staining was co-localized with only 38.7% of α-SU-secreting cells in the adult rat pituitary, and suggested that its involvement in transcription control of the gene for α-SU in the adult rat pituitary would be limited. Moreover, the localization of Ptx1 in luteinizing hormone/follicle-stimulating hormone (LH/FSH)-expressing cells suggested that it may also control the transcription of LH/FSH to a limited extent, similarly to α-SU. There have been many reports on the relationship of SF-l to the production of LH/FSH (Keri and Nilson l996; Ingraham et al. l994; Halvorson et al. l996); thus, it could be speculated that SF-1 acts synergistically with Ptx1 to some extent in controlling the production of LH/FSH (Tremblay et al. l998).
Furthermore, we investigated the expression of Ptx1 mRNA in various cell lines by RT-PCR, and it was particularly noteworthy that the expression was found in TtT/GF, an FS cell line, as well as in all kinds of hormone-secreting cell lines examined. From our sequence analyses of PCR products in rat, we found that the homology between the murine and rat Ptx1 genes was 87.8%. From these results, it was suggested that Ptx1 is not only related to the production of pituitary hormones but also to the functions of FS cells.
The functions and histogenesis of FS cells have been controversial with respect to not only the physiology but also the morphology. Based on this background, it should be particularly emphasized that Ptx1 may play some role(s) in the function on FS cells. FS cells have been shown to produce not only S-l00 protein and glial fibrillary acidic protein (GFAP) (Ishikawa et al. l983; Eng et al. l97l; Shirasawa et al. l983), but also various cytokines (Sawada et al. l994; Bernton et al. l987; Spangelo l989). We speculate that Ptx1 plays some role in the production of various cytokines by FS cells. The hitherto unreported finding that Ptx1 was detected in the nuclei of FS cells, which are thought to be supporting cells, suggests that Ptx1 may act on not only differentiation of hormone-secreting cells but also on development and formation of the pituitary gland. The detection of Ptx1 in FS cells suggests the possibility that they share a common cellular ancestor with the hormone-secreting cells.
Acknowledgements
We are grateful to Dr. Noboru Yanaihara for producing anti-Ptx1 antibody (Yanaihara Ins., Japan) and Dr. Yukio Kato (Biosignal Research Center, Gunma University, Japan) for primer design of β-actin.
This work was supported in part by a Grant-in Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (Project No. 09470055) and by the Takeda Research Foundation.
Contributor Information
R.Y. Osamura, Department of Pathology, Tokai University School of Medicine, Boseidai Isehara City, Kanagawa 259-1193, Japan, osamura@is.icc.u-tokai.ac.jp; Tel: +81 463 93 1121; Fax: +81 463 91 1370
A. Teramoto, Department of Neurosurgery, Nippon Medical School, Tokyo
P.L. Mellon, Department of Reproductive Medicine and Neuroscience, School of Medicine, University of California, San Diego, USA
K. Inoue, Department of Regulation Biology, Faculty of Science, Saitama University, Saitama, Japan
S. Yoshimura, Department of Molecular Life Science, Tokai University School of Medicine, Kanagawa, Japan
References
- Alarid ET, Windle JJ, Whyte DB, Mellon PL (1996) Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122: 33l9–3329 [DOI] [PubMed] [Google Scholar]
- Andersen B, Rosenfeld MG (1994) Pit-l determines cell types during development of the anterior pituitary gland. J Biol Chem 47:29335–29338 [PubMed] [Google Scholar]
- Bach I, Rhodes SJ, Pearse IIRV, Heinzel T, Gloss B, Scully KM, Sawchenko PE, Rosenfeld MG (1995) P-Lim, a LIM homeo- domain factor, is expressed during pituitary organ and cell commitment and synergized with Pit-l. Proc Natl Acad Sci U S A 92:2720–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach I, Carriere C, Ostendorff HP, Andersen B, Rosenfeld MG (1997) A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev 11:1370–l380 [DOI] [PubMed] [Google Scholar]
- Baes M, Denef C(1987) Evidence that stimulation of growth hormone release by epinephrine and vasoactive intestinal peptide is based on cell-to-cell communication in the pituitary. Endocrinology 20:280–290 [DOI] [PubMed] [Google Scholar]
- Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HG (1987) Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science 238:519–521 [DOI] [PubMed] [Google Scholar]
- Bodner M, Karin M (1987) A pituitary-specific Trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cell. Cell 50:267–275 [DOI] [PubMed] [Google Scholar]
- Bodner M, Castrillo J, Theill LE, Deerinck T, Ellisman M, Karin M (1988) The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell 55:505–5l8 [DOI] [PubMed] [Google Scholar]
- Buonassisi BV, Sato G, Cohen AI (1962) Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A 48:1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PJ, Doniach I (1988) Development of folliculo-stellate cells in the human pituitary. Acta Endocrinol (Copenh) 119: 16–20 [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM (1985) Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol 110:422–439 [DOI] [PubMed] [Google Scholar]
- Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B (1971) An acidic protein isolated from fibrous astrocytes. Brain Res 28:351–354 [DOI] [PubMed] [Google Scholar]
- Gage JP, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA (1996) The Ames dwarf gene, df is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol 10:1570–1581 [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW (l996) Stimulation of luteinizing hormone β gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem 271:6645–6650 [DOI] [PubMed] [Google Scholar]
- Hermesz E, Mackem S, Mahon KA (1996) Rpx: a novel anterior-restricted homeobox gene progressively activates in the prechodal plate, anterior neural plate and Rathke’s pouch of the mouse embryo. Development 122:41–52 [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen W, Nachtigal MW, Abbud R, Nilson JH, Parker KL (1994) The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 8:2302–2312 [DOI] [PubMed] [Google Scholar]
- Inoue K, Hattori M, Sakai T, Inukai S, Fujimoto N, Ito A (1990) Establishment of a folliculo-stellate-like cell line from a murine thyrotropic pituitary tumor. Endocrinology 126:2313–2320 [DOI] [PubMed] [Google Scholar]
- Inoue K, Matsumoto H, Koyama C, Shibata K, Nakazato Y, Ito A (1992) Establishment of a series of pituitary clonal cell lines differing in morphology, hormone secretion, and response to estrogen. Endocrinology 131:3110–3116 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Nogami H, Shirasawa N (1983) Novel clonal strains from adult rat anterior pituitary producing S-l00 protein. Nature 303:711–713 [DOI] [PubMed] [Google Scholar]
- Kagayama M (1965) The follicular cell in the pars distalis of the dog pituitary gland: an electron microscope study. Endocrinology 77:1053–1060 [DOI] [PubMed] [Google Scholar]
- Keri RA, Nilson JH (1996) A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone β subunit promoter in gonadotropes of transgenic mice. J Biol Chem 271:10782–10785 [DOI] [PubMed] [Google Scholar]
- Lamonerie T, Tremblay JJ, Lanctot C, Therrien M, Gauthier Y, Drouin J (1996) Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev 10:1284–1295 [DOI] [PubMed] [Google Scholar]
- Lew D, Brady H, Klausing K, Yaginuma K, Theill LE, Stauber C, Karin M, Mellon PL (1993) GHF-1 promoter-targeted immortalization of a somatotropic progenitor cell results in dwarfism in transgenic mice. Genes Dev 7:683–693 [DOI] [PubMed] [Google Scholar]
- Mangalam HJ, Albert VR, Ingraham HA, Kapiloff M, Wilson L, Nelson C, Elsholtz H, Rosenfeld MG (1989) A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev 3:946–958 [DOI] [PubMed] [Google Scholar]
- Moore BW(1965) A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun 19:739–744 [DOI] [PubMed] [Google Scholar]
- Sanno N, Teramoto A, Matsuno A, Inada K, Itoh J, Osamura RY (1994) Clinical and immunohistochemical studies on TSH-secreting pituitary adenoma: its multihormonality and expression of Pit-1. Mod Pathol 7:893–899 [PubMed] [Google Scholar]
- Sanno N, Teramoto A, Matsuno A, Itoh J, Takekoshi S, Osamura RY (1996a) In situ hybridization analysis of Pit-1 mRNA and hormonal production in human pituitary adenomas. Acta Neuropathol 91:263–268 [DOI] [PubMed] [Google Scholar]
- Sanno N, Teramoto A, Matsuno A, Osamura RY (1996b) Expression of human Pit-1 product in the human pituitary and pituitary adenomas. Arch Pathol Lab Med 120:73–77 [PubMed] [Google Scholar]
- Sanno N, Teramoto A, Matsuno A, Takekoshi S, Itoh J, Osamura RY (1996c) Expression of Pit-1 an estrogen receptor messenger RNA in prolactin-producing pituitary adenomas. Mod Pathol 9:526–533 [PubMed] [Google Scholar]
- Sanno N, Sugawara A, Teramoto A, Abe Y, Yen PM, Chin WW, Osamura RY (1997) Immunohistochemical expression of retinoid X receptor isoforms in human pituitaries and pituitary adenomas. Neuroendocrinology 65:299–306 [DOI] [PubMed] [Google Scholar]
- Sawada T, Koike K, Kanda Y, Sakamoto Y, Nohara A, Ohmichi M, Hirota K, Miyake A(1994) In vitro effects of CINC/gro, a member of the interleukin-8 family, on hormone secretion by rat anterior pituitary cells. Biochem Biophys Res Commun 202:155–160 [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Zhadanov AB, Mosinger B Jr., Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang S, Mahon KA, Westphal H (1996) Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272:1004–1007 [DOI] [PubMed] [Google Scholar]
- Shirasawa N, Kihara H, Yamaguchi S, Yoshimura F (1983) Pituitary folliculo-stellate cells immunostained with S-100 protein antiserum in postnatal, castrated and thyroidectomized rats. Cell Tissue Res 231:235–249 [DOI] [PubMed] [Google Scholar]
- Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW (1990) Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 4:695–711 [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP , Zuo L, Gleiberman AS, Andersen B, Beamer WG , Rosenfeld MG (1995) Pituitary lineage determination by the prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333 [DOI] [PubMed] [Google Scholar]
- Spangelo BL, Judd AM, Isakson PC, MacLeod RM (1989) Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology 125:575–577 [DOI] [PubMed] [Google Scholar]
- Szeto DP, Ryan AK, O’Connell SM, Rosenfeld MG (1996) P- OTX: a PIT-1-interacting homeodomain factor expressed during anterior pituitary gland development. Proc Natl Acad Sci U S A 93:7706–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PQ, Johnson BV, Rathjen J, Rathjen PD (1995) Sequence, genomic organization, and expression of the novel homeobox gene Hesx1. J Biol Chem 270:3869–3875 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctot C, Drouin J (1998) The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim- homeodomain gene Lim3/Lhx3. Mol Endocrinol 12:428–441 [DOI] [PubMed] [Google Scholar]
- Vila-Porcile E, Oliver L (1984) The problem of the folliculo-stellate cells in the pituitary gland In: Motta PM (ed) Ultrastructure of endocrine cells. Martimus Nijhoff, Boston, pp 64–76 [Google Scholar]
- Voss JW, Rosenfeld MG (1992) Anterior pituitary development: short tales from dwarf mice. Cell 70:527–530 [DOI] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL (1990) Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4:597–603 [DOI] [PubMed] [Google Scholar]
- Young BA, Foster CL, Cameron E (1967) Ultrastructural changes in the adenohypophysis of pregnant and lactating rabbits. J Endocrinol 39:437–443 [DOI] [PubMed] [Google Scholar]


