Abstract
Proliferating cells depend on glucose uptake more than quiescent cells for their growth. While glucose transport in keratinocytes is mediated largely by the Glut1 facilitative transporter, the keratinocyte-specific ablation of Glut1 did not compromise mouse skin development and homeostasis. Ex vivo metabolic profiling revealed altered sphingolipid, hexose, amino acid, and nucleotide metabolism in Glut1-deficient keratinocytes, suggesting metabolic adaptation. On the other hand, Glut1-deficient keratinocytes in culture displayed metabolic and oxidative stress and impaired proliferation. Similarly, Glut1 deficiency impaired in vivo keratinocyte proliferation and migration within wounded or UV-damaged mouse skin. Notably, both genetic and pharmacological Glut1 inactivation reduced hyperplasia in mouse models of psoriasis-like disease. Topical application of a Glut1 inhibitor also reduced inflammation in these models. Glut1 inhibition decreased expression of pathology-associated genes in human psoriatic skin organoids. Thus, Glut1 is selectively required for injury- and inflammation-associated keratinocyte proliferation, and its inhibition offers a novel treatment strategy for psoriasis.
Keywords: Glut-1, glucose metabolism, keratinocyte, psoriasis, wound healing, UV irradiation
INTRODUCTION
Glucose is a preferred bioenergetic and synthetic substrate for rapidly proliferating cells. However, recent studies have begun to highlight the diverse array of metabolic substrates that cells can use to promote growth. For example, although cancer cells consistently show a high capacity to utilize glucose as a metabolic substrate1, they are capable of using diverse substrates (e.g. amino acids, acetate, lactate) for energy generation and anabolic growth in vivo2–6. In some tissues, the preferential utilization of glycolysis has been linked to a cell’s phenotype. For example, increased Glut1 expression and glucose utilization promote effector T cell function while fatty acid oxidation promotes regulatory CD4+ T cell and memory CD8+ T cell fates7,8. In endothelial cells, glycolysis promotes vessel branching and migration while fatty acid oxidation is required for proliferation9,10. Thus, tissue functions are regulated in part through the controlled flow of metabolic substrates.
The facilitative glucose transporters (Glut’s) regulate the availability of glucose for most tissues. The thirteen GLUT family members are regulated in a tissue- and stimulus- specific fashion, suggesting important roles in shaping or maintaining cell function11. Glut1 is the most widely expressed facilitative glucose transporter and regulates basal glucose uptake in most tissues12, including the basal cells of the epidermis13. Consistent with a role in promoting keratinocyte proliferation, Glut1 expression is increased in wound healing, in psoriatic plaques, or after UV-induced hyperplasia14–16. However, the precise role for Glut1 and glucose transport in regulating keratinocyte function has not been definitively characterized.
Here, we specifically deleted Glut1 in keratinocytes to determine how glucose metabolism affects epidermal development and function. Consistent with its known role in energy generation and biosynthesis, Glut1 deficient keratinocytes show markedly impaired proliferation in vitro. Yet, Glut1 deficiency had no impact on epidermal development or function in vivo. Gene expression analyses, metabolic profiling, and in vitro rescue experiments revealed that alternative hexoses, amino acid catabolism, and fatty acid oxidation could all contribute to metabolic programs that allowed for epidermal function in the absence of Glut1. However, Glut1 deficiency impaired the physiological proliferation triggered by full-thickness wounds and UV irradiation, and genetic or chemical inhibition of Glut1 rendered the skin resistant to imiquimod or IL-23 induced psoriasiform hyperplasia. Thus, glucose metabolism is selectively essential for proliferating keratinocytes highlighting a potential therapeutic target for pathological hyperproliferation.
RESULTS
Glut1 Is Required for Glucose Uptake and Proliferation
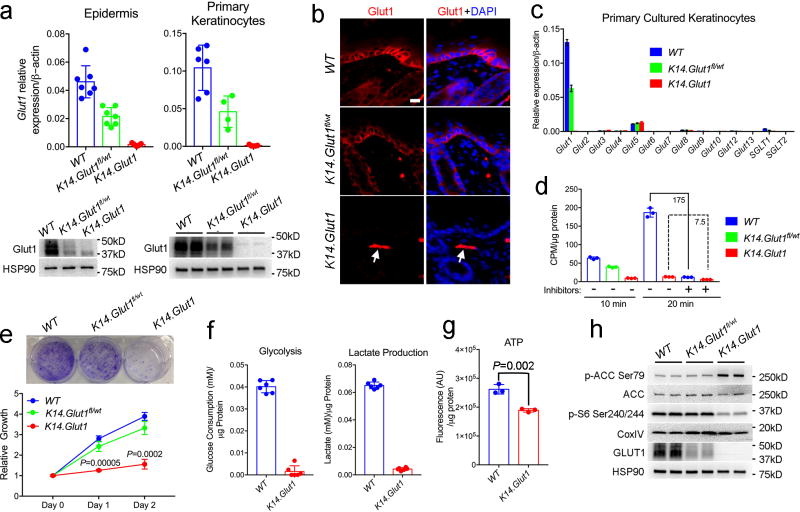
Although the expression of Glut1 in keratinocytes is well established, the regulation of Glut1 and expression of additional glucose transporters in the skin has not been explored. Immunofluorescence revealed that Glut1 was primarily expressed in the more proliferative basal layers of both human and mouse skin (Fig. S1a). Consistent with the reported role of Glut1 in cell proliferation, the expression of Glut1 was high in undifferentiated keratinocytes but decreased following either Ca2+ or confluence induced differentiation (Fig. S1b, c). The expression of other facilitative (Glut1-13) and sodium dependent glucose transporters (SGLT1-2) in mouse epidermis, mouse and human primary keratinocytes, immortalized human keratinocytes (HEK001), and squamous cell cancers were assessed17; only Glut1 was highly expressed (Fig. S1d–h). Next, Glut1 was deleted in epidermal keratinocytes by crossing Glut1fl/fl mice to K14-Cre expressing mice (hereafter K14.Glut1)18. In these mice, Glut1 mRNA expression was reduced by >95% in both the epidermis and primary cultured keratinocytes compared to their Glut1fl/fl (hereafter WT) littermates. Western Blot and immunofluorescence confirmed that Glut1 could not be detected in the epidermis of K14.Glut1 mice (Fig. 1a, b, S1i–j). Quantitative RT-PCR revealed no significant compensatory upregulation of any other glucose transporters in keratinocytes from K14.Glut1 mice (Fig. 1c). [3H] 2-deoxyglucose (2-DG) uptake assays in primary Glut1 deficient keratinocytes confirmed that Glut1 deletion blocked glucose transport. 2-DG uptake was decreased by ~96% in keratinocytes from K14.Glut1 versus WT littermates. The residual low level of glucose uptake in keratinocytes from K14.Glut1 mice was comparable to passive levels of transport, which occurred in the presence of chemical inhibitors of glucose transport (Fig. 1d).
Figure 1. Primary Keratinocytes Showed Impaired Proliferation upon Glut1 Deletion.
(a) Glut1 mRNA (n=4 mice per genotype) and protein levels in the epidermis and primary keratinocytes harvested from one week old WT, K14.Glut1fl/wt, and K14.Glut1 mice. (b) Immunostaining of Glut1 in skins from one-week old mice. Glut1 staining is absent in epidermis from K14.Glut1 mice. Arrow indicates persistence of Glut1 staining in the dermis (e.g. dermal endothelial cell). Similar results obtained in 3 independent experiments. (c) mRNA abundance of Glut/SGLT family members in primary cultured WT, Glut1fl/wt K14Cre, and keratinocytes from K14.Glut1 mice (n=4 mice per genotype). (d) 2-Deoxy-D-glucose uptake in primary cultured keratinocytes obtained from one week old WT, K14.Glut1fl/wt, and K14.Glut1 mice with or without glucose inhibitor pretreatment. Similar results obtained in uptake assays from keratinocytes from 3 independent mice. (e) Growth rate as assessed by Crystal Violet staining and relative cell number in primary WT, K14.Glut1fl/wt, and keratinocytes from K14.Glut1 mice. Identical numbers of cells were seeded in triplicate wells in 6 well plates at day 0. For Crystal Violet staining, cells were stained 3 days after seeding and similar results were obtained in 3 keratinocytes preparations. Keratinocytes from K14.Glut1 mice show significantly decreased growth compared to keratinoctyes from WT controls at day 1 and 2 after plating. (f) Glucose consumption and lactate production over 12 hours as measured from the media of primary cultured WT and K14.Glut1 (n=3 mice per genoptye) keratinocytes. (g) ATP levels (luminescence) were determined in primary keratinocytes from WT and K14.Glut1 (n=3 mice per genotype) and normalized to protein levels. (h) Western Blot analysis of the expression of p-ACC Ser79, ACC, p-S6 Ser240/244, Glut1 in primary keratinocytes obtained from WT, Glut1fl/wt K14Cre and K14.Glut1 mice. Data shown as mean±s.d. P values were calculated by two-tailed t-test. Results were confirmed in at least 2 independent experiments.
Because Glut1 plays important roles in cell proliferation19,20, we tested whether keratinocytes from K14.Glut1 mice showed defects in proliferation. The growth of primary Glut1 deficient keratinocytes was markedly impaired compared WT keratinocytes as assessed by Crystal Violet staining and cell number (Fig. 1e). GLUT1 was also essential for proliferation in human keratinocytes in vitro. The chemical inhibition of glucose transport through an established inhibitor of glucose transport, WZB11721, impaired glucose uptake and significantly slowed cell growth compared to vehicle control (Fig. S2a–b). Consistent with the 2-DG uptake studies, keratinocytes from K14.Glut1 mice consumed less glucose and produced less lactate (~5%) than their WT counterparts (Fig. 1f). ATP levels were also significantly lower in these keratinocytes (Fig. 1g). Decreased ATP levels can cause energetic stress and activate the 5’ AMP-activated protein kinase (AMPK). Indeed, downstream targets of AMPK like acetyl-CoA carboxylase 1 (ACC) showed increased phosphorylation in the knockout keratinocytes. Consistent with this energetic stress, phosphorylation of S6 ribosomal protein, a target of the mTOR and a growth-promoting pathway, was inhibited in keratinocytes from K14.Glut1 mice compared to WT controls (Fig. 1h).
Glut1 Contributes to Redox Homeostasis
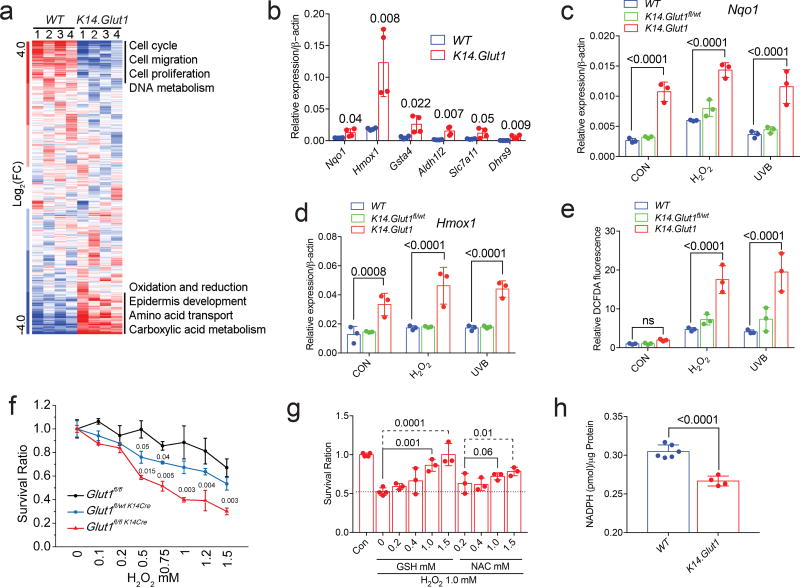
To discover additional pathways contributing to the impaired proliferation in glucose deficiency, we performed microarray analysis on keratinocytes from K14.Glut1 mice and their WT littermates (GSE102955, Fig. 2a, S3a). Consistent with the significantly decreased proliferative capacity of Glut1 deficient keratinocytes, hierarchical clustering and gene ontology analyses revealed that genes related to the cell cycle progression and cell division were markedly impaired compared to keratinocytes from their WT littermates. Normal cells respond to oxidative stress by routing glucose through the pentose phosphate pathways (PPP) to regenerate NADPH22,23. Consistent with glucose’s role in ROS clearance, transcripts related to redox homeostasis were amongst the most markedly elevated genes in keratinocytes from K14.Glut1 mice compared WT animals (Fig. 2a, S3a). Twenty-four genes related to oxidative stress were more than 2 fold higher in Glut1 deficient keratinocytes compared to WT keratinocytes (Fig. 2b, S3b). Other oxidative stress response genes including Nqo1 and Hmox1 (HO1) were significantly upregulated in Glut1 deficient keratinocytes both under basal growth conditions and when cells were treated with H2O2 or UVB compared to WT keratinocytes (Fig. 2c, d). Confirming the impact of Glut1 on cellular levels of oxidative stress, measurements of DCFDA fluorescence revealed that keratinocytes from K14.Glut1 mice showed significantly higher levels of reactive oxygen species both under basal growth conditions and after treatment with H2O2 or UVB compared to WT keratinocytes (Fig. 2e). Glut1 deficient keratinocytes were significantly more sensitive to H2O2 and UVB induced cell death than WT keratinocytes even when controlling for their slower growth (Fig. 2f). The supplementation of antioxidants (GSH and NAC) rescued H2O2-induced toxicity in keratinocytes from K14.Glut1 mice (Fig. 2g). In primary human keratinocytes, the acute inhibition of GLUT1 through WZB117 also increased the sensitivity of the cells to H2O2 (Fig. S2c) and increased the expression of redox homeostasis genes (Fig. S2d). Both genetic and chemical inhibition of glucose transport lowered levels of NADPH compared to their respective controls (Fig. 2h, S2e–f), suggesting that decreased flux through the PPP contributed to the observed defects in redox homeostasis. MitoSOX staining further revealed that the acute (but not chronic) chemical inhibition of glucose transport increased mitochondrial oxidative stress compared to vehicle control (Fig. S2g), suggesting mitochondria as an additional source of oxidative stress in keratinocytes deprived of glucose. Despite Nrf2’s established role in mediating the response to oxidative stress24, total and nuclear levels of Nrf2 were not higher in keratinocytes from K14.Glut1 mice (Fig. S4a–b). Thus, Glut1 is required for redox homeostasis in keratinocytes at least in part through its roles in regenerating NADPH.
Figure 2. Glut1 Is Required for Proliferation and Redox Homeostasis in Primary Keratinocytes.
(a) Hierarchical clustering of transcriptional profiles from keratinocytes from WT and K14.Glut1 mice. Labels identify gene clusters showing enrichment gene ontology analyses (n=8 mice per genotype). (b) RT-PCR measurement shows significant upregulation of the indicated redox genes in keratinocytes from K14.Glut1 and WT mice (n=4 mice per genotype). (c–d) Nqo1 and Hmox1 mRNA abundance in indicated with and without H2O2 (100µM) or UVB (10mj/cm2) stimulation for 6 hours (n=3 mice per genotype). (e) FACS analysis of the fluorescence after DCFDA staining of the indicated keratinocytes with and without H2O2 (100µM, 30min) and UVB (10mj/cm2, 30min) stimulation (n=3 independent mice per genotype). (f) Keratinocytes were incubated with different concentrations of H2O2 for 24 hours. Survival is plotted as a ratio of number of surviving cells compared to the number of plated cells per genotype without H2O2 treatment (n=3 mice per group). (g) Keratinocytes were pre-treated with Glutathione (GSH) or N-acetyl-cysteine (NAC), and then incubated with H2O2 for 24 hours. Survival is plotted relative to untreated controls. (h) Keratinocytes from K14.Glut1 mice show significantly lower levels of NADPH (n=4 mice per genoptype). Data are shown as mean±s.d. P values (indicated about relevant comparison) were calculated by two-tailed t-test (b, h), two-way ANOVA with Tukey’s tests (c–e), and one-way ANOVA with Dunnett tests (g). Results were confirmed in at least 2 independent experiments.
Glut1 Is Not Essential for Normal Skin Development
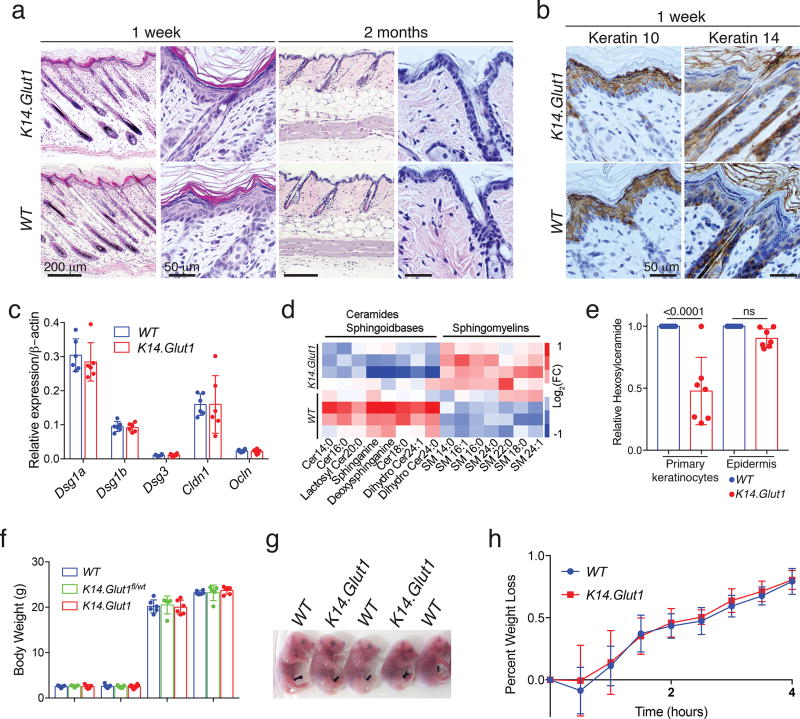
Given the striking impact of Glut1 deletion on the keratinocyte growth, we assessed the in vivo impact of Glut1 deletion. Notably, K14.Glut1 mice were overtly normal. We observed no abnormalities in the skin of young or adult K14.Glut1 mice relative to their WT littermates (Fig. S5a). Both male and female K14.Glut1 mice demonstrated normal skin histology at one week and 2 months (Fig. 3a). The expression and localization of epidermal keratins (K10 and K14) (Fig. 3b, S5b–d), loricrin (Fig. S5e), and differentiation and adhesion genes (Fig. 3c) showed no differences between WT and K14.Glut1 littermates, suggesting that epidermal differentiation was normal.
Figure 3. Glut1 is Dispensable for Normal Epidermal Development and Differentiation and its Deletion Induces Metabolic Reprogramming.
(a) Histologic section (H&E stained) of skin from one week- and 2 month- old WT, Glut1fl/wt K14Cre and K14.Glut1 mice show no histologic differences between the mice. Similar results obtained in 4 independent experiments. (b) Immunohistochemistry for Keratin 10 (K10) and K14 show no differences in the respective suprabasal and basal expression for the epidermal keratins. (c) RT-PCR measurement of keratinocyte adhesion genes—Dsg1a (Desmoglein 1a), Dsg1b, Dsg3, Cldn1 (Claudin 1), Ocln (Occludin)—in the epidermis show no differences between K14.Glut1 and WT epidermis (n=4 mice per genotype). (d) Epidermal lipids were isolated from flash frozen epidermis and analyzed by LC-MS/MS. Heat map representation of epidermal lipids demonstrates decreased free ceramides and increased sphingomyelins in the skin of K14.Glut1 mice (n=4 mice per genotype). (e) In vitro keratinocytes, but not epidermis, from K14.Glut1 mice, showed significantly decreased levels of hexosylceramides. Each point represents a distinct hexosylceramide species (n=4 mice per genotype). (f) Body weights of the 5-day-old and 2-month-old female and male mice of the indicated genotype, showed no significant differences between littermates (n=5 mice per genotype). (g) Toluidine Blue staining of E18.5 embryos indicated no differences in epidermal permeability between WT and K14.Glut1 mice. Similar results obtained in 3 independent stainings. (h) Neonatal WT (n=8 mice) and K14.Glut1 (n=5 mice) littermates showed no differences in weight loss suggesting no differences transepidermal water loss was intact. Data are shown as mean±s.d. P values were calculated by one-way ANOVA with Sidak correction (e). Results were confirmed in at least 2 independent experiments.
Given previous reports that glycosylated ceramides and UDP-glucose are essential intermediates for epidermal ceramide maturation, lamellar body formation, and stratum corneum development25,26, we performed an analysis of epidermal lipids. LC-MS/MS revealed that the epidermis of K14.Glut1 mice showed unchanged levels of phospholipids, lower levels of free ceramides and sphingoidbases, and higher levels of sphingomyelins (Fig. 3d, S5f, Lipidomics Data Set). Notably, while hexosylceramide levels were significantly decreased in keratinocytes from K14.Glut1 mice, they were not significantly decreased in the epidermis of K14.Glut1 neonatal mice compared to WT (Fig. 3e). Moreover, K14.Glut1 mice showed no significant differences in weight from WT littermates either as pups or adults (Fig. 3f). Compared to WT embryos, K14.Glut1 embryos showed both a normal toluidine blue exclusion assay (Fig. 3g) and no defects in transepidermal water loss as assessed by a weight time-course analysis of newborn mice (Fig. 3h). Finally, in contrast to the previously described defects in redox homeostasis, Glut1-deficient epidermis showed no marked alterations in the expression of redox homeostasis genes or evidence of energetic stress by Western blot analyses (Fig. S5g–h). Thus, while keratinocytes from K14.Glut1 mice showed marked alterations in lipid metabolism, redox homeostasis, and energetic stress in vitro, these defects are rescued in vivo allowing the normal skin development.
Metabolic Compensation for Glut1 Deficiency
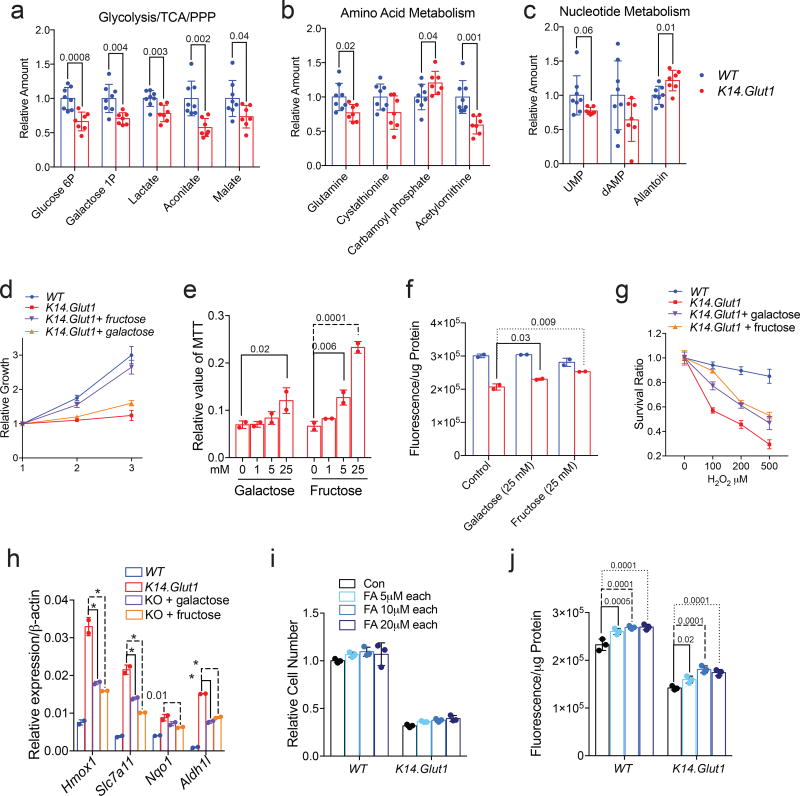
To gain insight on how K14.Glut1 mice adapted to the loss of glucose uptake, neonatal epidermis was harvested for the analysis of intracellular metabolites by LC-MS/MS (Metabolomics Data Set). Principal component analysis showed different metabolic profiles for epidermis from K14.Glut1 versus WT mice (Fig. S6a). Quantitative pathway enrichment analysis of epidermal metabolites from Glut1 deficient mice compared to littermate controls revealed the significant enrichment of several expected pathways including nucleotide sugar metabolism, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway (Fig. S6b). Significantly lower levels of numerous metabolites, including Glucose-6P, lactate, aconitate, malate, and fumarate, confirmed the expected impairments in glycolysis and the TCA cycle (Fig. 4a). Metabolomics also revealed significant changes in amino acid metabolism and the urea cycle including changes in glutamine, cystathionine, citrulline, carbamoyl phosphate and acetylornithine (Fig. 4b). Consistent with the increased catabolism of amino acids, the expression of numerous amino acid transporters were significantly higher in keratinocytes from K14.Glut1 mice compared to WT controls (Fig. S6c). Finally, Glut1 deletion also impacted nucleotide metabolism as evidenced by lower levels of uridine monophosphate (UMP) and dAMP and higher allantoin in epidermis from K14.Glut1 compared to WT controls (Fig. 4c).
Figure 4. Alternative Hexoses and Fatty Acids Can Partially Rescue Glut1 Deficiency in Keratinocytes.
(a–c) Metabolites were extracted from the epidermis of WT and K14.Glut1 mice and analyzed by LC-MS/MS (n=8 mice per genotype). The labeled metabolites represent a subset of metabolites showing significant changes in the (a) glycolysis/TCA/PPP, (b) amino acid metabolism/urea cycle, and (c) nucleotide metabolism. (d) Primary keratinocytes from K14.Glut1 mice were supplemented with galactose (25 mM) or fructose (25 mM) and cell proliferation was assessed by the MTT assay. Similar results obtained in 3 independent experiments. (e) Different concentrations of the indicated hexose were supplemented to keratinocytes from K14.Glut1 mice and cell proliferation was assessed by MTT assay after 36 hours (n=2 mice per genotype). (f) ATP concentration in keratinocytes from K14.Glut1 and WT mice was measured with and without hexose supplementation for 36 hours (n=2 mice per genotype). (g) Keratinocytes from K14.Glut1 and WT mice were incubated with different concentrations of H2O2 for 24 hours with or without hexose supplementation. Survival is calculated relative to the non-H2O2 treated cells of each group. Similar results in 3 independent experiments. (h) Redox genes expression was measured in keratinocytes from K14.Glut1 and WT mice supplemented with hexose supplementation for 36 hours (n=2 mice per genotype). (i) Keratinocytes from K14.Glut1 and WT were supplemented with different concentrations of fatty acids (1:1 palmitate:oleate, FA) for 36 hours, cell number (j) and ATP production (k) were assessed. ATP production was normalized by protein levels (n=3 mice per genotype). Data are shown as mean±s.d. P values (indicated above relevant comparison) were calculated by two-tailed t-test (a–c), two-way ANOVA with Dunnett tests (e, f, h, j). Results were confirmed in at least 2 independent experiments.
Metabolic profiling highlighted changes in galactose metabolism in the epidermis of K14.Glut1 mice. Intracellular galactose-1P is maintained at similar levels as glucose-6P/fructose-6P (G6P/F6P) and is depleted to similar levels as G6P/F6P in the epidermis of K14.Glut1 mice suggesting that it was actively metabolized in the epidermis (Fig. 4a). Moreover, keratinocytes express Glut5, SGLT1 (Fig. S1d–e), and ketohexokinase which should allow for fructose metabolism to occur. Therefore, we tested whether galactose and fructose could rescue the growth of Glut1 deficient keratinocytes. Both fructose and galactose significantly improved the growth of Glut1 deficient keratinocytes (Fig. 4e–f; S7a,b), ATP levels (Fig. 4g), the expression of redox homeostasis genes (Fig. 4h), and resistance to H2O2 (Fig. 4i) compared to keratinocytes from WT mice. Fructose almost fully rescued the growth and oxidative stress defects of keratinocytes from K14.Glut1 mice while galactose was less effective. Because fatty acid oxidation (FAO) contributes reducing equivalents and acetyl-CoA in endothelial cell metabolism10, we tested whether mixtures of oleate and palmitate could rescue the growth of Glut1 deficient keratinocytes. Adding up to 10µM of a fatty acid mixture partially rescued the growth of keratinocytes from K14.Glut1 mice and increased ATP production in both Glut1 deficient and WT keratinocytes (Fig. 4j–k). In contrast, a myriad of downstream metabolites and antioxidants including pyruvate, lactate, amino acids, GSH, NAC, glutamine, or ribose supplemented individually or in combinations (Fig. S7c–h) and growth under hypoxic conditions (Fig. S7i) were unable to rescue Glut1 deficient keratinocyte growth in vitro. In summary, keratinocytes can utilize galactose, fructose, and fatty acids as metabolic substrates to promote growth in the absence of Glut1 (Fig. S7j).
Glut1 Is Required for Stress Responses In vivo
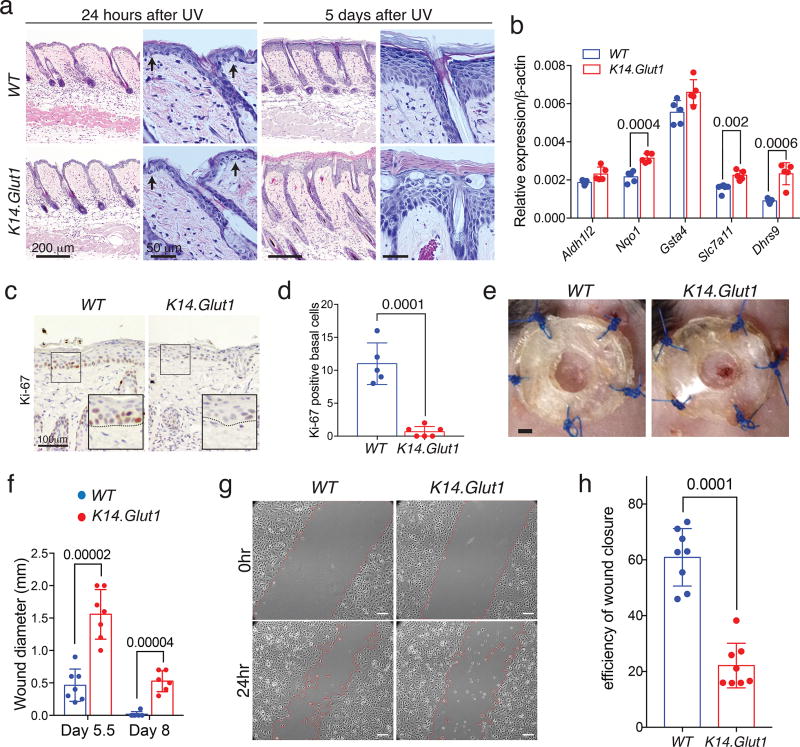
We next tested whether Glut1 was necessary for the epidermal responses to physiological stress. One critical function of human skin is protection from UV irradiation. Chronic irradiation has been reported to promote both Glut1 expression and acanthosis16. We shaved and Ultraviolet B irradiated (UVB) K14.Glut1 mice and WT littermates to determine the role of glucose transport in the acute and delayed response to UVB (Fig. S8a). There were no obvious macroscopic or histologic differences between the skin of K14.Glut1 mice and WT littermates 24 hours after UVB (Fig. 5a, S8a–b). UVB induced similar, significant increases in skin thickness for both WT and K14.Glut1 mice (Fig. S8c). TUNEL staining for apoptotic keratinocytes also showed no differences between the skin of WT and K14.Glut1 mice (Fig. S8d–e). Five days after UVB, WT mice had largely recovered and showed regular acanthosis while many areas of skin from K14.Glut1 mice showed areas of abnormal stratification with areas of keratinocyte necrosis (Fig. 5a, S8b). While non-irradiated epidermis did not show differences in redox homeostasis genes, 6 hours after UVB, Glut1 deficient epidermis showed a modest, but significant, upregulation of oxidative stress response genes compared to epidermis from WT controls. These changes were no longer significant 24 hours after UVB (Fig. 5b, S5g, S8f). Significantly fewer Ki-67+ cells were present in the epidermis of K14.Glut1 mice than WT controls (Fig. 5c,d). These defects in proliferation were corroborated by elevated staining of p-ACC Ser79 and decreased staining of p-S6 Ser240/244 in the skin of Glut1 deficient mice compared to WT controls after UVB (Fig. S8e). Keratinocytes secrete inflammatory cytokines in response to UVB27, which contributes to the physiological response to irradiation. UVB-induced inflammation, as assessed by the expression of IL1β, TNFα, IL6, IFNγ, revealed no significant differences between littermates (Fig. S8g). In summary, compared to WT mice, K14.Glut1 mice show impairments in redox homeostasis, proliferation, and recovery after UVB..
Figure 5. Glut1 Is Required for Proliferation in Response to UVB Irradiation and Wounding.
(a) Histologic analyses of UVB (50mj/cm2) irradiated skin from WT and K14.Glut1 mice. Skin was harvested for analysis at 24 hours and 5 days after UVB. Arrows highlight the presence of apoptotic keratinocytes in both the skin of WT and K14.Glut1 mice. Similar results obtained in 5 independent experiments. (b) RT-PCR for redox homeostasis genes from the epidermis of one week old WT and K14.Glut1 (n=5 mice per genotype) mice 6 hours after 50mj/cm2 UVB irradiation. (c) Cell proliferation as assessed by Ki-67 immunostaining 3 days after UVB irradiation. Similar results obtained in 3 independent experiments. (d) Significantly fewer Ki-67 basal keratinocytes were identified in K14.Glut1 mouse skin (n=3 mice per genotype). (e) Photo of splinted excisional wounds on the dorsal back revealed that K14.Glut1 mice had not completely healed wounds compared to their wildtype littermates after 8 days. Similar results obtained in 7 independent experiments. (f) Wound diameters in WT and K14.Glut1 mice (n=7 mice per genotype) 5.5 and 8 days after wounding. (g) Live cell imaging reveals that keratinocytes from K14.Glut1 mice show defects in closure of a scratch assay in vitro. Red lines indicate keratinocytes that have migrated from the wound edge. Scale bar, 50 µm. Similar results obtained in 3 independent experiments. (h) Quantitation of wound closure as a percentage of the initial wound reveals that keratinocytes from K14.Glut1 mice are significantly impaired in their ability to migrate and close wounds in vitro. (n=3 mice per genotype). Data are presented as mean±s.d.; P values (indicated above relevant comparison) were calculated by two-tailed t-test. Results were confirmed in at least 2 independent experiments.
The epidermis also undergoes rapid proliferation in response to wounds. To understand the role of Glut1 in wound repair, we assessed the recovery of mice from splinted 3-mm excisional wounds (Fig. S9a). Re-epithelialization was assessed after 5.5 and 8 days. Both K14.Glut1 and WT mice exhibited lymphohistiocytic infiltrates around the wound edge (Fig. S9b). However, healing in the K14.Glut1 mice was significantly impaired at both time points compared to WT littermates (Fig. 5e–f). Specifically, WT mice had largely completed wound healing by day 8 while all K14.Glut1 mice still exhibited persistent epidermal defects (Fig. 5e, S9b). Keratinocyte migration and proliferation both contribute to wound healing.28 To study cell migration in Glut1 deficient keratinocytes, we analyzed keratinocytes migration after a scratch assay by time-lapse microscopy. Keratinocytes from K14.Glut1 mice showed significantly delayed scratch closure compared to cells from WT littermates (Fig. 5g–h, Movie S1–2). To address the role of proliferation in re-epithelialization, we assessed the histology of wound edge. Wounds in K14.Glut1 had significantly fewer Ki-67 positive cells within 500µm of the wound edge than WT controls (Fig. S9c). Consistent with these in vitro proliferative defects, the excisional wounds of K14.Glut1 mice also showed elevated staining of p-ACC Ser79 and decreased staining of p-S6 Ser240/244 (Fig. S9e).
Glucose Transport Inhibition Ameliorates Psoriasis Models
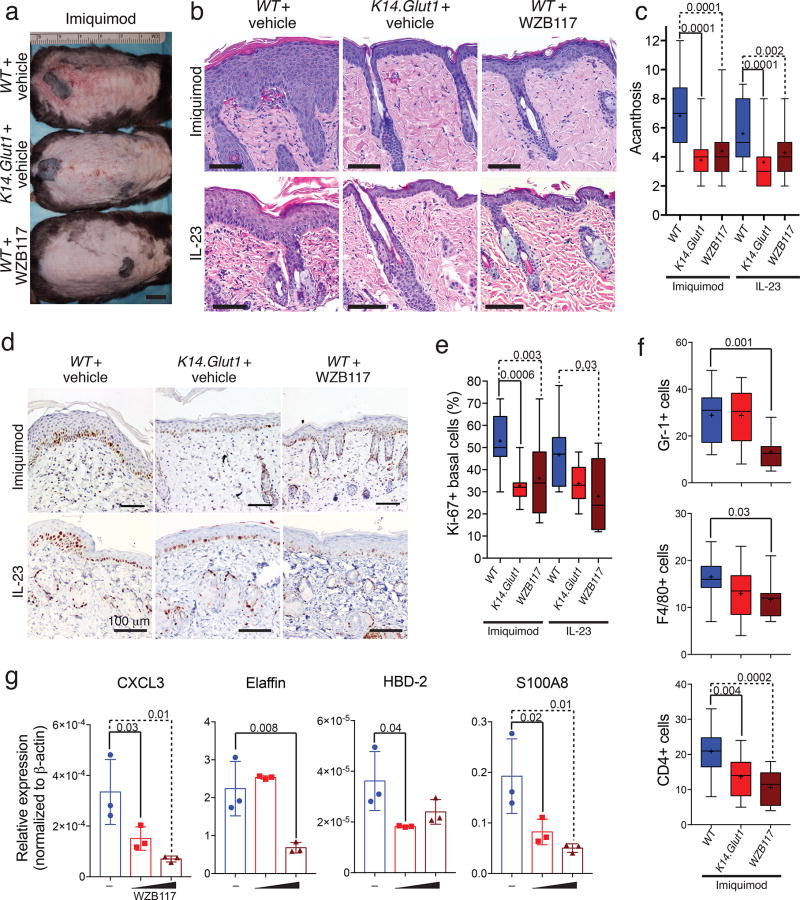
Although keratinocyte hyperproliferation can be triggered as a protective response against physiological insults, it can also be triggered pathologically. Psoriasis is a chronic cytokine-driven, inflammatory skin disease characterized by hyperplasia and abnormal differentiation of the epidermis that presents as thickened and scaly plaques. After confirming that GLUT1 transcription and expression were elevated in lesional biopsies from psoriasis patients (Fig. S10a–c), we tested whether Glut1 deletion might impact the development of pathological hyperplasia in diseases like psoriasis. We established psoriasiform hyperplasia in WT and K14.Glut1 mice through two models—topical application of imiquimod29,30 and intradermal injection of IL-2331. The skin of WT mice treated with imiquimod upregulated Glut1 expression (Fig. S10a, d, e) and showed acanthosis (Fig. S10a). In contrast, the epidermis of K14.Glut1 mice were markedly protected from imiquimod-induced psoriasiform hyperplasia (Fig. 6a, S10g). To extend the translational relevance of these findings, we tested whether the topical inhibition of glucose transport might also prevent the development of psoriasiform hyperplasia (Fig. S10f). In both mouse models of psoriasiform hyperplasia, treatment with a topical GLUT inhibitor, WZB117, decreased scale (Fig. 6a, S10g–h) and significantly inhibited skin thickening (Fig. 6b–c, S10h) compared to vehical treated mice. The skin of K14.Glut1 mice and WZB117 treated WT mice both showed decreased Ki-67 staining compared to vehicle treated WT controls (Fig. 6d–e). Western blots confirmed that both the skin of Glut1 deficient mice and WZB117 treated mice showed higher p-ACC and decreased p-S6 S240/S244 consistent with energetic stress (Fig. S10i).
Figure 6. Genetic or Topical Inhibition of Glucose Transport Decreases Psoriasiform Hyperplasia.
(a) K14.Glut1 have less scale and erythema compared to WT littermates after treatment with imiquimod. Similar results obtained in 3 independent experiments. Bar=1cm (b) Histologic sections that both genetic (K14.Glut1) or chemical (WZB117) inhibition of glucose transport decrease the acanthosis and parakeratosis induced by both imiquimod or IL-23 injections. Similar results obtained in 4 independent experiments. Bar=100 µm. (c) K14.Glut1 and WZB117 treated skin showed significantly less acanthosis after both imiquimod or IL-23 induced psoriasiform dermatitis. Acanthosis was scored blindly as the number of nucleated keratinocytes (n=6 mice per group). (d) Ki-67 staining (5 days) after imiquimod or IL-23 induced psoriasiform dermatitis treatment revealed significantly less proliferation in K14.Glut1 and WZB117 treated mice. Similar results obtained in 3 independent experiments. (e) The percentage of Ki-67 positive cells in epidermal basal cells (per 50 cells) was quantified in skin sections from control, K14.Glut1, or and WZB117 (n=6 mice per group). (f) WT, K14.Glut1, or WZB117 treated mice were treated with imiquimod for 6 days and skin section stained with the specified markers (n=4 mice per group). Treatment with WZB117 significantly decreases the number of leukocytes (CD45+), neutrophils (Gr-1+), macrophages (F4/80+), and T cells (CD4+) induced by imiquimod. (g) Human psoriatic skin organoids were treated with vehicle or WZB117 (6 or 30 mg/ml) for 24 hours. The epidermis of the skin organoids were harvested and psoriatic biomarkers analyzed by RT-PCR (n=3). Box shows 25th–75th percentiles, whiskers show min to max, crosses show means, and lines show medians. Bars show mean±s.d.. P values (indicated about relevant comparison) were calculated by one-way ANOVA with Holm-Sidak tests (c, e, f, g). Results were confirmed in at least 2 independent experiments.
In addition to keratinocyte proliferation, imiquimod also induces the accumulation of leukocytes in the skin, including neutrophils, macrophages, dendritic cells, and T cells30,32,33. We examined the effect of inhibiting glucose transport on inflammation through immunohistochemical staining. Topically applied WZB117 significantly reduced the number of leukocytes (CD45+), neutrophils (Gr-1+), macrophages (F4/80+), and T cells (CD4+), but not dendritic cells (CD11c) localized to the skin after imiquimod treatment compared to vehicle control. In contrast, the skin of K14.Glut1 mice only showed significant decreases in T cell number (CD4+) after imiquimod treatment (Fig. 6f, S11a–f). Similarly, several cytokines (Ccl3, Cxcl3, IL1b), which are induced in imiquimod induced psoriasiform dermatitis34, showed significant decreases after topical treatment with WZB117, but not in the K14.Glut1 mice, compared to vehicle control treated WT mice (Fig. S10j). Finally, we applied WZB117 topically to human psoriatic skin organoids35, WZB117 inhibited the expression of several psoriatic biomarkers (CXCL3, Elafin, HBD-2, S100A7, and S100A8) in a dose dependent fashion (Fig. 6g). Thus, while genetic Glut1 deletion rescued only the epidermal acanthosis and proliferation, the topical, chemical inhibition of glucose transport rescued both keratinocyte proliferation and prevented inflammation in animal and organoid models of psoriasiform inflammation.
DISCUSSION
The epidermis is a self-renewing organ that protects the body against environmental insults and excess water loss36. How glucose uptake and metabolism contribute to these essential processes has not been explored in vivo. Through the deletion of the Glut1 transporter in keratinocytes, we find that glucose uptake is critical for rapid keratinocyte proliferation and an efficient response to physiologically relevant stressors including full-thickness wounds and UVB irradiation. Despite evidence of stressed energy metabolism and oxidative stress in vitro, K14.Glut1 mice show normal epidermal development. In contrast to many other tissues, Glut1 is the only significant hexose transporter expressed in keratinocytes, and its deletion largely abolished glucose transport. Thus, our metabolic analyses of Glut1 deficient keratinocytes have broader implications on how other tissues and cancer cells might adapt to a general block of glucose metabolism.
Because glucose contributes to several metabolic pathways, cells induced multiple compensatory pathways in its absence. Our findings highlight the importance of the PPP in the skin, which had previously been demonstrated in vitro22,37. Transcriptional upregulation of many oxidative stress response genes was noted in the Glut1 deficient keratinocytes. In the face of decreased hexose availability, metabolic processes involving central carbon metabolism including glycolysis, the TCA cycle, and nucleotide synthesis were suppressed. Transcriptional and metabolomic analyses suggested that alterations in amino acid metabolism might help to maintain these biosynthetic cycles. Specifically, the pattern of alterations in amino acid metabolism could be explained by an increased catabolism of specific amino acids to generate ketoacids (e.g. α-ketoglutarate from glutamine and α–ketobutyrate form cystathionine) to compensate for the loss of glucose. In addition to alterations in amino acid metabolism, we also found that fatty acids could also partially rescue proliferative defects in Glut1 deficient keratinocytes. In vitro, alternative hexoses, especially fructose, could rescue the proliferative defects of Glut1 deficiency. Despite the low circulating levels of these hexoses38,39, galactose was detected intracellularly in both normal and Glut1 defecient epidermis, suggesting that these hexoses could contribute to normal skin development in the absence of glucose. Galactose rescued growth less efficiently than fructose, reflecting either the inefficient transport of galactose by non-Glut1 facilitative glucose transporters or limitations in the efficiency of the Leloir pathway in keratinocytes40,41. However, the supplementation of other downstream metabolites did not rescue the growth of keratinocytes from K14.Glut1 mice. We speculate that the inherent limitations of in vitro culture systems42 and the central role of glucose in diverse metabolic and signaling pathways limited our ability to rescue the growth of Glut1 deficient keratinocytes.
While the generation of mitochondria-derived ROS are necessary for skin differentiation43, the increased amounts of oxidative stress caused by loss of glucose transport does not impact skin differentiation. The glycosylation of both proteins and lipids plays an important biochemical role in the skin. Specifically, glycosylated ceramides have been found to be essential for the proper formation of an intact stratum corneum, as the deletion of glucosylceramide synthase (Ugcg) results in a disruption of normal ceramide metabolism, aberrant stratum corneum development, and perinatal lethality26. Despite defects in hexosylceramide synthesis in vitro, we found the skin barrier to be intact suggesting that the epidermis is able to maintain sufficient levels of ceramides for the maturation of epidermal ceramides even in the absence of Glut1.
Because glucose uptake is required for proliferating keratinocytes but not for normal skin development and function, our studies suggest that inhibiting glucose transport represents a promising therapy for skin diseases. Antimetabolites that target the heightened biosynthetic requirements of proliferating cells—including methotrexate44, 5-fluorouracil45, and mycophenolic acid46—are some of the most effective treatments for inflammatory and neoplastic diseases. While most antimetabolite therapies were discovered fortuitously, our analysis of Glut1 has uncovered glucose transport as a potential target. Psoriasis is characterized by excessive, cytokine-driven epidermal hyperplasia47. Psoriatic lesions have heightened requirements for glucose uptake and metabolism as evidence by increased Glut1 expression and PET scans48. Metabolomic studies have demonstrated increased levels of some amino acids in the serum patients with psoriasis49,50. Our metabolomic analysis of the epidermis of Glut1 deficient mice revealed decreases in a similar set of amino acids. Levels of sphingoid bases were increased in the serum and skin of patients consistent with our finding that these lipids were decreased in the skin of Glut1 deficient mice51. Comparing the metabolomics from patients to those of Glut1 deficient mice suggested that inhibiting glucose transport could impact metabolic pathways critical to the development of psoriasis. Consistent with this prediction, Glut1 deficient mice were protected from imiquimod-induced psoriasiform hyperplasia. While the systemic administration of a glucose transport inhibitor are predicted to cause neurologic sequelae, hyperglycemia, and lipodystrophy21,52, these toxicities could be avoided through the topical use of glucose transport inhibitors. Indeed, the topical application of a Glut1 inhibitor was also effective in protecting mice from imiquimod-induced psoriasiform hyperplasia. Similar to the deletion of Glut1 in keratinocytes, chemical inhibition of Glut1 inhibited the epidermal proliferation and acanthosis. Topical transport inhibitors had the added feature of suppressing the inflammatory infiltrates and cytokine secretion in animal models of psoriasis and human psoriatic skin organoids, respectively. Consistent with previously reported roles for Glut1 and glucose metabolism in T lymphocyte activation20,53, we speculate that the topical application of WZB117 might also inhibit glucose uptake in other cell types in the skin, including infiltrating lymphocytes, and thereby limit inflammation in vivo. In summary, our study provides insight into the metabolic reprogramming that allows cells to survive in the absence of glucose metabolism, highlights the role of glucose transport after physiological stressors, and identifies glucose transport as a viable target in hyperproliferative skin diseases.
Online Methods
All animal procedures were performed under protocols approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee (IACUC). Animal Protocol Number: 2015-101166. All efforts were made to follow the Replacement, Refinement and Reduction guidelines. To minimize discomfort, mice were anesthetized with Ketamine/Xylazine cocktail before being killed. Patients recruited from University of Texas Southwestern Medical Center outpatient clinics provided written informed consent to participate in a research study, which was approved by the UTSW institutional review board. IRB Protocol Number: STU 082010-241.
Animal Models
Glut1fl/fl mice were obtained from Dr. E. Dale Abel18, K14 driven Cre-recombinase mice were obtained from Jackson Laboratory, and all of them were maintained on the C57BL/6J background. We generated mice lacking Glut1 specifically in epidermal keratinocytes K14.Glut1 by crossing female Glut1fl/fl mice with male K14-Cre mice. Pilot experiments confirmed that previously reported sample sizes for UVB irradiation54, excisional wound splinting55, and imiquimod-induced psoriasiform hyperplasia32 studies would have sufficient power. For UVB lamp irradiation, we first anesthetized the mice with a Ketamine/Xylazine cocktail (Ketamine 120 mg/kg + Xylazine 16 mg/kg), shaved the dorsal hair of 2-month-old female mice, applied a thin layer of Nair on the shaved area for 50–60 seconds, and then washed off the Nair with wet cotton swabs. The dose of UVB lamp was measured by a IL700 Research Radiometer (International Light Technology). Cells or mice were placed at least 20 cm from the light source. For the excisional would splinting model, 2-month-old mice were shave and chemically depilated. The dorsal skin of the mouse, midline between the shoulder and neck of mice, was excised with a 3 mm biopsy punch (Miltex) to the level of the panniculus carnosus. A sterile, circular silicone splint was attached to the skin with Krazy glue, and then secured with 5-0 Prolene sutures. A topical antibiotic (Bacitracin + Polymyxin B + Neomycin) ointment was applied and wounds were dressed with Tegaderm transparent dressing daily. The size of wound was measured at indicated time points. For the imiquimod-induced psoriasiform hyperplasia model, 2-month-old mice were shaved and chemically depilated. The shaved dorsal skin samples were treated topically with 50 mg of Aldara cream (5% Imiquimod) (Aldara, 3M Pharmaceuticals) daily. For the intradermal IL-23 induced psoriasiform hyperplasia model, shaved and depilated 2-month-old mice were injected daily on a labeled location on the central back with 1µg rmIL-23 (ThermoFisher) in PBS/0.1% BSA. The appearance of the back skin were monitored daily and tissues were harvested, typically after 5 days of treatment. The appearance of the back skin were monitored daily and tissues were harvested, typically after 5 days of treatment. For WZB117 treatment, WT mice were randomly assigned to receive either WZB117 (100µl of 1mg/ml) or the acetone vehicle control. The WZB treatment was applied topically immediately prior to imiquimod treatment or IL-23 injection.
Keratinocyte Culture and Treatments
HEK001 (ATCC CRL-2404) and SCCT8 (obtained from Andrew South) were cultured according to instructions. Human and mouse primary keratinocytes were isolated by dispase digestion56. Before digestion, the subcutaneous fat was first removed from human skin. Abdominal human skin excisions or one-week-old mouse skin were floated on 1 U/ml dispase (Invitrogen, 17105-041) in HBSS (Gibco, 14170) for 18 hours at 4°C with dermis side down. The next day, the skin was placed in a new dish with the epidermis side down and the epidermis was peeled and placed into a new dish with HBSS. After washing, the cells were collected into a new 15 mL tube. The epidermis to cut into small pieces, resuspended in HBSS, gently pipetted up and down for several times, and combined them with the cells in the 15 ml tube. The cell solution was filtered the cells with a 70 µM cell strainer, centrifuged at 1000rpm for 4 min, and resuspended in KSFM medium (Invitrogen, 37010022) supplemented with 0.05 mM CaCl2 (Sigma, C7902), 0.05 µg/ml Hydrocortisone (Sigma, H0888), 5 ng/ml EGF (Invitrogen, 10450-013), 7.5 µg/ml Bovine Pituitary Extract (Invitrogen, 13028-014), 0.5 µg/ml insulin (Sigma, I9278), 100 U/mL penicillin, 100 µg/mL streptomycin, and 25 µg/mL of Amphotericin B (Gibco, 15240062). The cells were gently washed for once in media and seeded in 10 cm dishes with complete KSFM medium. The dishes were pre-coated with collagen (Advanced Biomatrix, 5005-B). keratinocytes from each mouse were seeded in one 10 cm dish and changed into fresh complete KSFM medium after 24 hours. After ~3 to 4 days, the primary keratinocytes to reach 80% confluence under normal culture conditions in 5% CO2, 37°C incubator. For hypoxia experiment, cells were cultured in a sealed chamber with pre-formulated gas mixture (1% O2, 5% CO2, and 94% N2). For keratinocyte differentiation, cells were either left confluent for 2 or 4 days (C2 or C4), or treated with 1 mM CaCl2 for 2 days. Undifferentiated cells were harvested before confluent (Pre-C). For treatment of the cells, H2O2 (Sigma, H1009), UVB irradiation, glutathione (GSH, Sigma, G6013), N-acetyl-Cysteine (NAC, Sigma, A7250), different concentrations of galactose/fructose (Sigma, G5388, F3510), or other substrates were supplemented to the KSFM complete cell culture medium as indicated. Human psoriatic skin equivalents (MatTek, SOR-300-FT) were cultured according to the manufacturers’ recommendation and treated topically with WZB117 (25µL; 6 or 30mg/ml in acetone) for 24 hours. The epidermal portions or the skin organoids were isolated and processed for RT-PCR.
In vitro Wound-healing Assay
For the wound-healing assay, cells were plated at a several densities ranging from 0.5–1×106 cells/well in a 6-well plate (3 wells per cell line) and cultured for 24 hours under standard culture conditions. The day of the assay, control and knockout cells showing comparable densities were selected for assessment. Three parallel lines per well were ‘drawn’ with gentle pressure, using a sterile 1000 µl pipette tip. Cells were washed twice with PBS to remove cell debris before adding pre-warmed standard culture medium. The plate was then placed into the temperature- and CO2-controlled environmental chamber (OKO lab) of a Nikon Ti Eclipse microscope with perfect focus, motorized stage and Zyla 4.2 sCMOS camera (Andor) driven by a NIS Elements V4.13 software package. Wound closure (1 region of interest per scratch) was recorded by phase contrast using a 10× air objective at a frame rate of 1 image/10 minute for 24 hours. For quantification in ImageJ, the wound area was measured manually in the first and last frame of each dataset (8 per cell line) and results were expressed as efficiency of wound closure (100−[(100/area first frame)*area last frame]).
3H-2-deoxyglucose (2-DG) Uptake Assay
2-DG uptake assays were performed as previously described49. Primary mouse or human keratinocytes were seeded in triplicate in 12-well plates overnight (50000), washed twice with PBS (Sigma, D8537), incubated in basic KSFM (without any supplementation) or serum free DMEM/F12 medium (Thermo, 11320033, for SCCT8 cells) for 2 hours, washed twice with KRHP buffer, and incubated in 0.45 ml KRHP buffer to each well, and starved for 30 minutes. For inhibition of glucose transporter, Cytochalasin B (10 µM, Sigma, C6762) and phloretin (100 µM, Sigma, P7912) were added to the KRHP medium for another 15 minutes. Uptake was initiated by adding 1µCi 3H 2-DG (25–30 Ci/mmol, PerkinElmer, NET549) and 0.1 mM unlabeled 2-DG (Sigma, D8375) in KRHP to each well for 10 or 20 min. Transport activity was terminated by the rapid removal of the uptake medium and washing 3 times with cold PBS with 25 mM glucose (Sigma, G7528). Cells were lysed with 0.5 ml of 0.5 M NaOH (Fisher Scientific, SS255-1) and neutralized with 0.5 ml or 0.5 M HCl (Sigma, 320331), mixed well. 250 µl of the lysate was transferred to a scintillation vial with scintillation solution, and the sample was quantitated by liquid scintillation counting. Protein concentrations were determined by BCA assay (Thermo, 23227).
Histological Analyses and Immunoblotting
For immunohistochemistry (IHC), tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (5 µm) were deparaffinized, heat retrieved (buffer with 10 mM Tris, 1.0 mM EDTA, PH=8.0, 94–96 °C for 30min, cool naturally), perforated (0.2% Triton × 100, 10 min), blocked in 3% BSA (Sigma, A9418) and then incubated with Glut1 (1:500, Thermo, RB-9052-P0), K10 (1:500, Santa Cruz, SC-70907), K14 (1:500, Santa Cruz, SC-53253), Loricrin (1:1000, BioLegend, 905104), p-ACC Ser79 (1:400, CST, 3661), p-S6 Ser240/244 (1:2000, CST, 5364), Ki-67 (1:400, Abcam, ab16667), CD45 (1:100, BioLegend 147701), F4/80 (1:50, BioLegend 122602), CD11c (1:100, BioLegend 117301), Gr-1 (1:20, BD Biosciences 550291), and CD4 (1:600, Thermo, 4SM95) primary antibodies. IHC and Hematoxylin (Vector, H3401) and Eosin Y (Thermo, 6766007) staining (HE staining) were performed using standard protocols or under the manufacturer’s instructions. Detection of IHC signal was performed with Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and DAB substrate kit for peroxidase (Vector Laboratories) followed by hematoxylin counterstaining (Vector Laboratories). For scoring of leukocytes, four sections from at least three mice per group from at least 2 independent experiments were stained, and photomicrographs were taken of representative 40× magnification fields. Positive cells per photomicrograph were quantified. For immunofluorescence of Glut1 and K14, after incubation with primary antibody, slides were washed and incubated with Alex Fluor 546 goat-anti-rabbit or Alex Fluor 488 goat-anti-mouse secondary antibody (Life Technology, A11001, A11010) at room temperature for 1 hour, then washed and sealed with ProLong Gold antifade reagent with DAPI (Life technology, P36935). TUNEL staining (ThermoFisher, C10617) was performed according to the manufacturer’s protocol. For all histological analyses, the rater, typically a dermatopathologist, was blinded to the genotype and/or treatment condition. For Western blotting, samples of cells or tissues were separated on SDS-PAGE gels, transferred to PVDF membranes and probed with primary antibodies as follows: Glut1 (1:2000, Thermo, RB-9052-P0), K10 (1:500, Santa Cruz, SC-70907), K14 (1:1000, Santa Cruz, SC-53253), HSP90 (1:1000, CST, 4877), Involucrin (1:1000, Thermo, MA5-11803), p-ACC Ser79 and ACC (1:1000, CST, 3661, 3662), p-S6 Ser240/244 (1:4000, CST, 5364), COX IV (1:2000, CST, 4844), and followed by secondary antibodies conjugated to HRP at a dilution of 1:5000 (Santa Cruz, Donkey anti-Rabbit: SC2077, Donkey anti-mouse: SC2096) and detection by the ECL System (PerkinElmer, NEL104001EA). Protein content was quantitated by the BCA protein assay kit (Thermo Pierce, 23227).
RNA extraction and qRT-PCR
Cells or tissues were lysed in Trizol reagents (Sigma, T9424), and mRNA was extracted following the standard protocols, and reversed transcribed using the Thermoscript RT system (Invitrogen, 11146-016) according to the manufacture’s instructions. qRT-PCR was performed using Real-Time System (Sybr-green, Applied Biosystems, A25741). Data were normalized to the internal control and presented as a relative expression level. All primers for qRT-PCR are described in Table S1.
Cytometry and Proliferation Measurements
For basal levels oxidative stress, cells were stained with 5 µM DCFDA (Thermo, D-399) for 30 min, cells were washed, trypsinized, collected on ice, centrifuged at 1500 rpm 4min, washed once with FACS buffer (DPBS with 2% FBS (Sigma, F2442) and 0.2 mM EDTA (Ambion, AM9260G)), and then resuspended in FACS buffer for analysis (FACScalibur, BD). For oxidative stress induced by H2O2, cells were supplemented with 5 µM DCFDA, then incubated in the indicated amount of H2O2, and harvested after 30 min of incubation. For oxidative stress induced by UVB irradiation, cells were incubated with 5 µM DCFDA for 10 minutes, took the medium to new tubes, washed the cells once with PBS, irradiated the cells for indicated doses, put the medium back to the cells, incubated for another 20 min, then harvested the for FACS analysis. For cultured cell proliferation, equal cells numbers were seeded, harvested at indicated times, and then counted in the presence of Trypan blue (Biorad, 145-0013). For staining, cells were stained with 0.25% Crystal Violet (Sigma, C0775) in 6.25% ethanol (Pharmco-AAPER, 111000200) for 30min at room temperature, washed 3 times with ddH2O, dried at room temperature, and photographed. For the MTT assay, cells were incubated with 0.5 mg/ml MTT (Thermo, M6494) for 2 hours, dissolved in DMSO (Sigma D4540), and measured the absorbance at 540 nm. For the keratinocytes proliferation in tissues, the tissues were stained with Ki-67 as described in histological analyses section.
ATP Measurements
Cellular ATP content was measured using the CellTiter-Glo Luminescent Assay Kit (Promega, G7570). Briefly, cells were seeded at least triplicate wells at a density of 10000 cells/well in two 96 well plate, and allowed for attachment overnight. One plate of cells was used for measuring the concentrations of protein by BCA method. Another plate of cells were put at room temperature for 30 min, added 100 µl of lysis buffer with CellTiter-Glo® Substrate, mixed well, and shake on an orbital shaker for 15 min at room temperature. Cellular ATP level was measured using a luminescent plate reader (BioTek). A standard curve of ATP was made to make sure the concentrations of cellular ATP were within the linear range.
Lipid Analyses
Epidermis from WT (n=4) and K14.Glut1 (n=4) mice were harvested for lipid analysis. Sphingolipids levels were quantitated using LC-MS/MS methodology57. Briefly, flash frozen epidermis scraps were homogenized in 500 µL of ice cold HPLC water using a sonic dismembrator probe, a 50 µL aliquot was reserved for protein determination. Immediately afterwards the aqueous homogenate was quenched by adding it to 2 mL of organic extraction solvent (isopropanol: ethyl acetate, 15:85; vol:vol) in a borosilicate glass culture tube. The original sample tube was rinsed twice with 500 µL of cold HPLC water and the aqueous emulsions were added to the organic extraction solvent. 20 µL of internal standard solution was added (Avanti Polar Lipids, AL Ceramide/Sphingoid Internal Standard Mixture II diluted 1:10 in ethanol). The mixture was vortexed and sonicated in ultrasonic bath for 10 minutes at 40 °C. Then, the samples were allowed to reach room temperature. Two-phase liquid extraction was performed, the supernatant was transferred to a new tube and the pellet was re-extracted. Supernatants were combined and evaporated under nitrogen. The dried residue was reconstituted in 2 mL of Folch solution, 500 µL was transferred to a new tube and reserved for organic phosphate determination. Both organic fractions were dried under nitrogen and stored at −80°C until analysis. Sphingolipids levels were quantitated using LC-MS/MS methodology using a Nexera X2 UHPLC system coupled to a Shimadzu LCMS-8050 triple quadrupole mass spectrometer operating the Dual Ion Source in Electrospray positive mode. Dried lipid extracts were reconstituted in 200 µL of HPLC solvent (methanol/ formic acid 99:1; vol:vol containing 5 mM ammonium formate) for LC-MS/MS analysis. Lipid separation was achieved on a 2.1 (i.d.) × 150 mm Kinetex C8, 2.6 micron core-shell particle (Phenomenex, Torrance, CA) column. Sphingolipids species were identified based on exact mass and fragmentation patterns, and verified by lipid standards. The concentration of each metabolite was determined according to calibration curves using peak-area ratio of analyte vs. corresponding internal standard. Calibration curves were generated using serial dilutions of each target analyte. Sphingolipid true standards were purchased from Avanti Polar Lipids (Alabaster, Al). For total phosphorous determination, total phosphorous content in the organic extracts was determined as described58.
Metabolite Profiling
Epidermis was harvested from the dorsal skin from WT and K14.Glut1 mice, each epidermal sample was from one mice. 8 pairs of samples were used for metabolomic analysis as described59. The harvested epidermis was snap frozen and metabolites extracted with 80% ice-cold methanol. Metabolite profiling was performed using a liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). The peak area for each detected metabolite was normalized against the total ion count (TIC). The pre-processed datasets were mean-centered and unit-variance scaled. Principal component analysis (PCA) and hierarchical clustering of metabolites in different samples were analyzed using the MetaboAnalyst 3.0.
Microarray Analyses
Primary keratinocytes derived from WT (n=8) and K14.Glut1 (n=8) mice were harvested. Two plates of cells of the same genotype were pooled into 2 groups for each genotype for microarray analysis. Cells were put into the Trizol solution (Sigma, T9424) for extraction of total RNA according to the manufacturer's instructions. 1 µg RNA were reverse transcribed to double stranded cDNA, then transcribed to biotin-labeled cRNA according to the standard Affymetrix protocol. Following fragmentation, 15 µg cRNA of each sample was hybridized for 16 hours at 45°C on GeneChip Mouse Transcriptome Assay 1.0, and scanned at the UTSW Microarray Core Facility. Array scanning was performed according to the manufacturer's instruction (Affymetrix). Raw data were processed with Affymetrix Expression console software for background correction and normalization.
Data Availability
All data supporting the findings of this study are available from the corresponding author upon request. Microarray data from this study have been deposited in GenBank with the accession codes GSE102955.
Supplementary Material
Acknowledgments
We thank the Ruth Gordillo for help with lipidomic studies; Chendong Yang, Jessica Sudderth, Lauren Zacharias, and Jorge Galvan Resendiz and the Children’s Research Institute Metabolomics Facility for help with metabolomic studies; Lin-chiang Tseng for help with patient sample collection; and Pedram Gerami for help with psoriasis models. This work was supported by the following grants: NCI R35 CA220449-01 and Welch Foundation (I-1733-06) to R.J.D.; NIAMS K23AR061441 to B.F.C.; NIDDK DK10550 to J.C.R.; NIAMS 1R01AR072655, Burroughs Wellcome Fund CAMS (1010978), and American Cancer Society/Simmons Cancer Center (ACS-IRG-02-196) to R.C.W.
Footnotes
Contributions
Z.Zh., R.J.D., and R.C.W. designed the experiments. Z.Zh., E.E.L., J.Z., M.M., and R.C.W. performed experiments. E.D.A. provided Glut1fl/fl mice. A.P.S. provided SCCT8 squamous cell carcinoma cells. B.F.C. enrolled control and psoriasis patients. Z.Zh., Z.Zi, E.E.L., J.Z., D.C.C., M.M., G.A.H., T.V., J.C.R., P.E.S., R.J.D., and R.C.W. analyzed data; R.C.W. and Z.Zh. wrote the manuscript with contributions from all authors.
Competing interests
The authors declare no competing financial interests.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palm W, et al. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259–270. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comerford SA, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley CT, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faubert B, et al. Lactate Metabolism in Human Lung Tumors. Cell. 2017;171:358–371. e359. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui S, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bock K, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Schoors S, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cura AJ, Carruthers A. Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis. Comprehensive Physiology. 2012;2:863–914. doi: 10.1002/cphy.c110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gherzi R, et al. "HepG2/erythroid/brain" type glucose transporter (GLUT1) is highly expressed in human epidermis: keratinocyte differentiation affects GLUT1 levels in reconstituted epidermis. J Cell Physiol. 1992;150:463–474. doi: 10.1002/jcp.1041500306. [DOI] [PubMed] [Google Scholar]
- 14.Elson DA, Ryan HE, Snow JW, Johnson R, Arbeit JM. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res. 2000;60:6189–6195. [PubMed] [Google Scholar]
- 15.Tao J, et al. Expression of GLUT-1 in psoriasis and the relationship between GLUT-1 upregulation induced by hypoxia and proliferation of keratinocyte growth. J Dermatol Sci. 2008;51:203–207. doi: 10.1016/j.jdermsci.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Tochio T, Tanaka H, Nakata S. Glucose transporter member 1 is involved in UVB-induced epidermal hyperplasia by enhancing proliferation in epidermal keratinocytes. Int J Dermatol. 2013;52:300–308. doi: 10.1111/j.1365-4632.2011.05299.x. [DOI] [PubMed] [Google Scholar]
- 17.Watt SA, et al. Integrative mRNA profiling comparing cultured primary cells with clinical samples reveals PLK1 and C20orf20 as therapeutic targets in cutaneous squamous cell carcinoma. Oncogene. 2011;30:4666–4677. doi: 10.1038/onc.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young CD, et al. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. doi: 10.1371/journal.pone.0023205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellberg EA, et al. The glucose transporter GLUT1 is required for ErbB2-induced mammary tumorigenesis. Breast Cancer Res. 2016;18:131. doi: 10.1186/s13058-016-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macintyre AN, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20:61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 22.Kuehne A, et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell. 2015;59:359–371. doi: 10.1016/j.molcel.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZZ, et al. Glutathione Depletion, Pentose Phosphate Pathway Activation, and Hemolysis in Erythrocytes Protecting Cancer Cells from Vitamin C-induced Oxidative Stress. J Biol Chem. 2016;291:22861–22867. doi: 10.1074/jbc.C116.748848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer M, Werner S. Nrf2--A regulator of keratinocyte redox signaling. Free Radic Biol Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Amen N, et al. Differentiation of epidermal keratinocytes is dependent on glucosylceramide:ceramide processing. Human molecular genetics. 2013;22:4164–4179. doi: 10.1093/hmg/ddt264. [DOI] [PubMed] [Google Scholar]
- 26.Jennemann R, et al. Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J Biol Chem. 2007;282:3083–3094. doi: 10.1074/jbc.M610304200. [DOI] [PubMed] [Google Scholar]
- 27.Takashima A, Bergstresser PR. Impact of UVB radiation on the epidermal cytokine network. Photochem Photobiol. 1996;63:397–400. doi: 10.1111/j.1751-1097.1996.tb03054.x. [DOI] [PubMed] [Google Scholar]
- 28.Raja, Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- 29.Hawkes JE, Gudjonsson JE, Ward NL. The Snowballing Literature on Imiquimod-Induced Skin Inflammation in Mice: A Critical Appraisal. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Fits L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 31.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flutter B, Nestle FO. TLRs to cytokines: mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol. 2013;43:3138–3146. doi: 10.1002/eji.201343801. [DOI] [PubMed] [Google Scholar]
- 33.Tortola L, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Belle AB, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 35.Sa SM, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 36.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freinkel RK. Metabolism of glucose-C-14 by human skin in vitro. J Invest Dermatol. 1960;34:37–42. [PubMed] [Google Scholar]
- 38.Sparks JW, Avery GB, Fletcher AB, Simmons MA, Glinsmann WH. Parenteral galactose therapy in the glucose-intolerant premature infant. J Pediatr. 1982;100:255–259. doi: 10.1016/s0022-3476(82)80651-3. [DOI] [PubMed] [Google Scholar]
- 39.Barone S, et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem. 2009;284:5056–5066. doi: 10.1074/jbc.M808128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–128. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 2003;278:43885–43888. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 42.Cantor JR, et al. Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell. 2017;169:258–272. e217. doi: 10.1016/j.cell.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamanaka RB, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6:ra8. doi: 10.1126/scisignal.2003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787–793. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- 45.Heidelberger C, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 46.Eugui EM, Almquist SJ, Muller CD, Allison AC. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol. 1991;33:161–173. doi: 10.1111/j.1365-3083.1991.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 47.Greb JE, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 48.Mehta NN, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–1039. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamleh MA, et al. LC-MS metabolomics of psoriasis patients reveals disease severity-dependent increases in circulating amino acids that are ameliorated by anti-TNFalpha treatment. J Proteome Res. 2015;14:557–566. doi: 10.1021/pr500782g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang H, et al. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br J Dermatol. 2016 doi: 10.1111/bjd.15008. [DOI] [PubMed] [Google Scholar]
- 51.Checa A, et al. Circulating levels of sphingosine-1-phosphate are elevated in severe, but not mild psoriasis and are unresponsive to anti-TNF-alpha treatment. Sci Rep. 2015;5:12017. doi: 10.1038/srep12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee EE, et al. A Protein Kinase C Phosphorylation Motif in GLUT1 Affects Glucose Transport and is Mutated in GLUT1 Deficiency Syndrome. Mol Cell. 2015;58:845–853. doi: 10.1016/j.molcel.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Telang S, et al. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J Transl Med. 2012;10:95. doi: 10.1186/1479-5876-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koo SW, Hirakawa S, Fujii S, Kawasumi M, Nghiem P. Protection from photodamage by topical application of caffeine after ultraviolet irradiation. Br J Dermatol. 2007;156:957–964. doi: 10.1111/j.1365-2133.2007.07812.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Ge J, Tredget EE, Wu Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc. 2013;8:302–309. doi: 10.1038/nprot.2013.002. [DOI] [PubMed] [Google Scholar]
- 56.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holland WL, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carles J. Colorimetric microdetermination of phosphorus. Bull Soc Chim Biol (Paris) 1956;38:255–257. [PubMed] [Google Scholar]
- 59.Mullen AR, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7:1679–1690. doi: 10.1016/j.celrep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available from the corresponding author upon request. Microarray data from this study have been deposited in GenBank with the accession codes GSE102955.