Abstract
Purpose
ASF participation rates in the United States are highest among high school (HS) athletes. This study sought to compare the cardiovascular response to HS versus collegiate ASF participation.
Methods
ASF participants (HS, N=61; Collegiate, N=87) were studied at pre- and post-season time points with echocardiography and applanation tonometry. Primary outcome variables included: left ventricular (LV) mass index, LV diastolic function (early relaxation velocity [E′]), and arterial stiffness (pulse wave velocity [PWV]).
Results
HS (17.1±0.4 years old) and collegiate ASF participants (18±0.4 years old) experienced similar LV hypertrophy (ΔLV mass HS=10.5±10 vs. Collegiate=11.2±13.6 g/m2, P=0.97). Among HS participants, increases in LV mass were associated with stable diastolic tissue velocities (ΔE′=−0.3±2.9 cm/s, P=0.40) and vascular function (ΔPWV=−0.1±0.6 m/s, P = 0.13). In contrast, collegiate participants demonstrated a higher burden of concentric LV hypertrophy (21/87, 24% vs. 7/61, 11%, P=0.026) with concomitant reductions in diastolic tissue velocities (ΔE′: −2.0±2.7 cm/s, P<0.001) and increased arterial stiffness (ΔPWV: Δ0.2±0.6 m/s, P=0.003), changes that were influenced by linemen who had the highest post-season weight (124±10 kg) and systolic blood pressure [(SBP) 138.8±11 mmHg]. In multivariable analyses adjusting for age and ethnicity, body mass was an independent predictor of post-season PWV (β estimate=0.01, P=0.04) and E′ (β estimate=−0.04, P=0.05) while SBP was an independent predictor of post-season LV mass index (β estimate=0.18, P=0.01) and PWV (β estimate=0.01, P=0.007).
Conclusion
The transition from HS to college represents an important physiologic temporal data point after which differential ASF cardiovascular phenotypes manifest. Future work aimed to clarify underlying mechanisms and the long-term clinical implications of these findings is warranted.
Keywords: cardiac remodeling, football, diastolic function, arterial stiffness
INTRODUCTION
American-style football (ASF) athletes appear to be at risk for the development of sub-clinical cardiovascular pathology during sport participation (1–5). Specifically, prior studies document a high prevalence of hypertension among both collegiate (1,2,4,5) and professional (3) ASF athletes and associations between increased blood pressure and sub-clinical pathologic cardiovascular remodeling, particularly among lineman (LM) position players, have been reported (1,2,5). However, ASF participation rates in the United States are highest at the high school (HS) school level with an estimated 1.1 million athletes each year (6). To date, data characterizing the cardiovascular impact of ASF participation at the HS level are lacking.
To address this knowledge gap, we sought to examine changes in cardiovascular phenotypes among HS ASF participants and to compare them with collegiate ASF participants. We hypothesized that cardiovascular remodeling patterns would differ as a function of participation level and that HS and collegiate ASF participants would demonstrate longitudinal differences in cardiovascular remodeling after competitive ASF training. To address this hypothesis, we conducted an observational study of HS and collegiate ASF participants with 2-D echocardiography and vascular applanation tonometry before and after competitive ASF participation.
METHODS
ASF participants from two NCAA Division-I programs [Georgia Institute of Technology (Atlanta, GA) and Furman University (Greenville, SC)] and three metropolitan high schools [Marist School, Woodward Academy, and St. Pius X (Atlanta, GA)] were recruited for this study between 2014–16. The Emory Institutional Review Board approved all aspects of the study and subjects provided written informed consent (subject assent with parental consent for participants <18 years old).
Study Population
Senior HS and freshman collegiate ASF participants were studied longitudinally at two predefined time points. Time point one was immediately prior to pre-season training and time point two was 5–6 months later at a program-specific date corresponding with the immediate conclusion of the ASF season (post-season). We chose to confine the HS cohort to seniors (4th year students) to minimize the impact of pubertal development and to maximize capture of the cardiovascular phenotype associated with complete HS football exposure. In addition, because HS rosters were limited in number, the seniors enrolled in this study were all experienced multi-year participants who participated in the majority of practice repetitions. We confined the collegiate cohort to freshmen students, a time period we have previously shown to be marked by significant cardiovascular plasticity in response to the hemodynamic stress of athletic training (4). Anthropometric and clinical data collected included age (years), height (cm), weight (kg), current medication use, family history of hypertension, systolic (SBP) and diastolic blood pressure [(mmHg), measured using a manual aneroid sphygmomanometer and an appropriately sized cuff], 2-D echocardiography, and vascular applanation tonometry. Participants were required to abstain from exercise for ≥24 hours prior to both data collection time points.
Field position for each ASF participant was classified as either LM or non-lineman (NLM) as previously proposed (7). In-season practice schedules, including strength training, differed between HS and collegiate ASF participants and were characterized as follows. HS practice sessions occurred, on average, 2 hours/weekday (10 hours/week) and collegiate practice sessions occurred, on average, 3 hours/weekday (maximum 20 hours/week of practice, weight training, film study, meetings, etc. per NCAA guidelines). Collegiate ASF participants were subject to testing for performance-enhancing drugs as dictated by NCAA standards.
Vascular Applanation Tonometry
Indices of arterial stiffness and wave reflection were measured using high fidelity applanation tonometry (SphygmoCor®, Atcor Medical, Australia), which records sequential high-quality pressure waveforms at peripheral pulse sites. Full details of tonometer technology and measurement algorithms have been previously detailed (8). Vascular function was characterized using validated surrogates of arterial stiffness and cardiovascular disease risk (9–12). The primary outcome variable for vascular function was pulse wave velocity (PWV), the gold standard index of arterial stiffness (8). PWV was measured by acquisition of pressure waveforms within the carotid and femoral arteries. The distance between measurement sites was recorded manually using the “foot-to-foot” method (13). Additional validated measures of vascular stiffness originating from the pulse wave analysis included the augmentation index (14) and subendocardial viability ratio (15). Pulse wave analysis derivation >80% of the operator index and PWV with <10% standard deviation were required for quality control.
2-D Echocardiography
Trans-thoracic echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI). 2-D, tissue-Doppler, and speckle-tracking imaging from standard parasternal and apical positions was performed by experienced sonographers. All data were stored digitally, and post-study offline data analysis (EchoPAC version 7, GE Healthcare) was performed (J.H.K). Definitions of normality for cardiac structure and function were adopted from the most recent guidelines (16). The primary outcome variables for left ventricular (LV) structure and diastolic function were LV mass (calculated using the area-length method) indexed for body surface area and early relaxation velocity (E´). LV ejection fraction, end-diastolic volume, and end-systolic volume were calculated using the modified biplane technique. Relative wall thickness was calculated as: [interventricular septum thickness+posterior wall thickness (mm)]/LV end-diastolic diameter (mm)]. Because LV mass was estimated using 2-D (area-length) instead of linear measurements, concentric LV hypertrophy was defined as a relative wall thickness of >0.42 with an LV mass index of >102 g/m2; concentric LV remodeling was defined as a relative wall thickness of >0.42 with an LV mass index of ≤102 g/m2; and eccentric LV hypertrophy was defined as a relative wall thickness of ≤0.42 with an LV mass index of >102 g/m2 (16). Measurements were adjusted for body surface area when appropriate (17). Comprehensive assessment of regional myocardial function using speckle-tracking and tissue-Doppler imaging was performed. Tissue velocities (E′, A′, and S′) were measured from color-coded images at the lateral and septal mitral annulus. E′ was then reported as the average value between the 2 measurements. Global LV longitudinal strain was measured in the apical four-chamber view.
Statistical Analysis
Categorical variables are presented as percentages and continuous variables as mean±SD. Repeated longitudinal measurements were assessed with the paired t-test for normally distributed variables. Comparison of post-season measurements between groups, HS vs. college, was assessed with a 2-sample t-test. Similarly, the comparison of the longitudinal change in select variables between groups was assessed with the 2-sample t-test. Comparisons between categorical variables were performed with the Chi-square test of homogeneity. The collegiate and HS cohorts were also stratified by LM versus NLM. Select continuous variable post-season outcomes (weight, SBP, indexed LV end-diastolic diameter, LV mass index, relative wall thickness, E′, and PWV) for these four groups were compared using one-way ANOVA with Bonferroni correction using the collegiate LM as the reference group (adjusted P-values presented). In addition, paired longitudinal analyses of the subset of HS and collegiate LM were performed. Multivariable linear regression was used to identify factors associated with the predefined primary end-points: post-season E′, PWV, and LV mass index in the total cohort. Models were adjusted for the preseason values of each respective dependent variable and for age, ethnicity, field position (LM versus NLM), family history hypertension, height, and post-season weight and SBP. Analyses were performed with SAS software (version 9.4, Cary, North Carolina). A P-value of ≤0.05 was considered significant.
RESULTS
Baseline characteristics
Of the158 ASF participants initially enrolled, 148 (HS seniors, N=61; collegiate freshman, N=87) completed the full season without significant breaks for musculoskeletal injury or concussion and were thus analyzed at both study time points. At baseline, collegiate ASF participants were older (18±0.4 vs. 17.1±0.4 years, P<0.001), taller (184±7 vs. 181±6 cm, P=0.006), and heavier (97±18 vs. 86.7±17 kg, P<0.001) compared to HS ASF participants. While pre-season SBP was similar between groups (collegiate: 131±12 vs. HS: 129±12 mmHg, P=0.59), diastolic blood pressures were higher in the collegiate ASF participants (75±10 vs. 70±8 mmHg, P=0.005). Caucasians accounted for the majority of HS participants (89%) while ethnicity was evenly distributed between Caucasians (53%) and African Americans (47%) in the collegiate cohort. The distribution of NLM versus LM was similar between groups (HS: 62% NLM/38% LM vs. collegiate: 69% NLM/31% LM, P=0.40). For the three primary outcomes variables, baseline values for HS and collegiate participants at the pre-season time point were similar (Table 1): LV mass: HS=89±11 vs. collegiate=90±16 g/m2, P=0.82; E′: HS=15.2±2 vs. collegiate=16.2±2.5 cm/s, P=0.01; and PWV: HS=4.6±0.6 vs. collegiate=4.7±0.7 m/s, P=0.31.
Table 1.
Comparison of the longitudinal impact of high school versus collegiate ASF participation
| Longitudinal ASF Data N = 148 |
||||||
|---|---|---|---|---|---|---|
| High School N = 61 |
Collegiate N = 87 |
|||||
| Anthropometrics and Blood Pressure |
Pre-Season | Post-Season | P-value* | Pre-Season | Post-Season | P-value* |
| Weight, Mean (SD), kg | 87 (16) | 86.6 (16)§ | 0.66 | 98 (18) | 100 (19)§ | <0.001 |
| Body Mass Index, Mean (SD), kg/m2 | 26.5 (4.2) | 26.4 (4.2)§ | 0.62 | 28.9 (4.4) | 29.5 (4.4)§ | <0.001 |
| Systolic Blood Pressure, Mean (SD), mmHg | 129 (12) | 131 (14)‖ | 0.29 | 131 (12) | 136 (12)‖ | 0.01 |
| Diastolic Blood Pressure, Mean (SD), mmHg | 70 (8) | 74 (9) | 0.003 | 75 (10) | 76 (10) | 0.44 |
| Cardiac Structure and Function | ||||||
| Averaged LV Wall Thickness†, Mean (SD), mm | 8.5 (0.7) | 8.9 (0.7)§ | 0.002 | 9.0 (0.9) | 10.0 (1.0)§ | <0.001 |
| LV Internal Diameter End-Diastole / Body Surface Area, Mean (SD), mm/m2 | 24.1 (2) | 24.6 (2)§ | 0.02 | 22.8 (3) | 23.2 (2)§ | 0.16 |
| LV Mass / Body Surface Area, Mean (SD), gm/m2 | 89 (11) | 100 (12) | <0.001 | 90 (16) | 102 (13) | <0.001 |
| Relative Wall Thickness, Mean (SD) | 0.34 (0.04) | 0.35 (0.04)§ | 0.045 | 0.35 (0.04) | 0.39 (0.05)§ | <0.001 |
| EF, Mean (SD), % | 59.4 (4) | 59.0 (4) | 0.37 | 62 (5) | 59 (5) | <0.001 |
| Global Longitudinal Strain, Mean (SD), % | 19.6 (2) | 19.8 (2) | 0.69 | 20.0 (2) | 19.7 (2) | 0.41 |
| Trans-Mitral E, Mean (SD), cm/s | 94 (17) | 87 (13) | 0.003 | 89 (15) | 83 (15) | <0.001 |
| Trans-Mitral A, Mean (SD), cm/s | 37 (11) | 37.4 (9)‖ | 0.66 | 43 (10) | 41 (8)‖ | 0.31 |
| Trans-Mitral E/A Ratio, Mean (SD) | 2.7 (0.9) | 2.4 (0.7)§ | 0.03 | 2.2 (0.7) | 2.0 (0.5)§ | 0.03 |
| Tissue-Doppler LV E′‡, Mean (SD), cm/s | 15.2 (2) | 14.9 (2.6) | 0.40 | 16.2 (2.5) | 14.2 (2.4) | <0.001 |
| Vascular Function | ||||||
| Pulse Wave Velocity, Mean (SD), m/s | 4.6 (0.6) | 4.5 (0.6)§ | 0.13 | 4.7 (0.7) | 5 (0.7)§ | 0.003 |
| Augmentation Index, Mean (SD), % | 3.5 (17) | −2 (14)‖ | 0.03 | 1.7 (13) | 3.0 (17)‖ | 0.62 |
| Subendocardial Viability Ratio, Mean (SD) | 147 (37) | 160 (34)‖ | 0.004 | 157 (31) | 151 (28)‖ | 0.11 |
ASF American-style football; EF ejection fraction; LV left ventricle
Paired t-test (pre- to post-season measurement) derived p-value
Averaged LV Wall Thickness = Interventricular Septum + Posterior Wall Thickness / 2
Averaged E′ (cm/s) = lateral E′ velocity + septal E′ velocity / 2
2-sample t-test comparison of post-season high school vs. collegiate measurement, P<0.001
2-sample t-test comparison of post-season high school vs. collegiate measurement, P<0.05
Longitudinal impact of HS versus collegiate ASF participation
At the post-season study time point, only collegiate ASF participants demonstrated significant increases in body mass and SBP (Table 1). While both HS and collegiate participants demonstrated similar and significant increases in LV mass index and wall thickness during the study period, there were differences in LV geometric remodeling patterns observed at post-season. Specifically, although there was no difference in the change in indexed LV diameter between HS and collegiate ASF participants (HS: 0.51±1.6 vs. collegiate: 0.17±1.6 mm/m2, P=0.18), there were significantly higher rates of concentric LV hypertrophy observed among collegiate (21/87, 24%) versus HS participants (7/61, 11%, P=0.026). LV systolic function, as assessed by ejection fraction and global longitudinal strain, was unchanged among HS participants but showed a non-significant trend towards decline among collegiate participants. Our primary diastolic function outcome variable, E′ (Table 1), significantly declined during the season among collegiate participants but was unchanged in the HS ASF cohort (ΔE′ comparison: −2.0±2.7 (college) vs. −0.3±2.9 (HS) cm/s, P=0.001). Similarly for vascular function (Table 1), HS ASF participants demonstrated stable vascular compliance while collegiate participants demonstrated arterial stiffening as evidenced by significant increases in PWV at the post-season time point. (ΔPWV comparison: 0.2±0.6 (college) vs. =−0.1±0.6 (HS) m/s, P=0.001).
The Impact of Player Position (LM versus NLM)
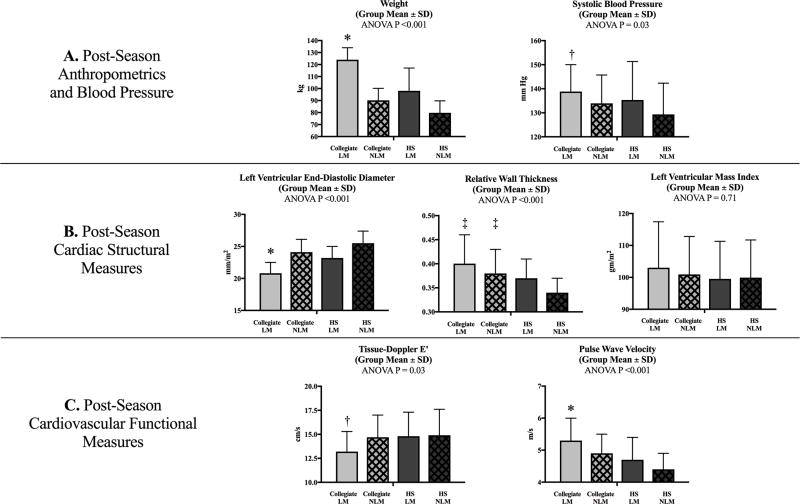
Post-season data comparing collegiate and HS ASF participants stratified by player field position are shown in Figure 1. Differences in weight, SBP, cardiac structural geometry, diastolic function, and vascular function were most pronounced for the collegiate LM (N=27) when compared to collegiate NLM (N=60) and HS athletes at both field positions (HS NLM: N=38; HS LM: N=23). Specifically, collegiate LM demonstrated the highest weight (124±10 kg) and SBP (138.8±11 mmHg) among groups. Differences in training-related LV remodeling, specifically increased concentric-type LV remodeling, were also more pronounced for the collegiate LM compared to the other groups as evidenced by significantly lower indexed LV end-diastolic diameter (collegiate LM=20.8±2 vs. collegiate NLM=24.1±2 vs. HS LM=23.2±2 vs. HS NLM=25.5±2 mm/m2, P<0.001) without concomitant differences in LV mass index. Finally, collegiate LM demonstrated relative reductions in E′ (collegiate LM E′=13.2±2.1 vs. collegiate NLM E′=14.7±2.3 vs. HS LM E′=14.8±2.5 vs. HS NLM E′=14.9±2.7 cm/s, P=0.03) and vascular compliance (collegiate LM PWV=5.3±0.7 vs. collegiate NLM PWV=4.9±0.6 vs. HS LM PWV=4.7±0.7 vs. HS NLM PWV=4.4±0.5 m/s, P<0.001) compared to the other groups.
Figure 1.
Comparison of collegiate and high school American-style football participants stratified by player position, LM and NLM
One-way ANOVA comparison of post-season weight and systolic blood pressure (row A), cardiac structural measures (row B), and cardiovascular functional measures (row C) by player position (Collegiate LM, Collegiate NLM, HS LM, and HS NLM).
HS: high school; LM: linemen; NLM: non-linemen
* Adjusted P <0.001 (Bonferroni correction) collegiate LM versus all other groups
† Adjusted P <0.05 (Bonferroni correction) collegiate LM versus HS NLM
‡ Adjusted P <0.05 (Bonferroni correction) collegiate LM and collegiate NLM versus HS NLM
Longitudinal comparison of the HS versus collegiate LM is reported in Table 2. While both groups developed concentric LV remodeling, only the collegiate LM gained significant weight and demonstrated reduced E′ and vascular function.
Table 2.
Longitudinal comparison of high school versus collegiate linemen
| Longitudinal ASF Linemen | ||||||
|---|---|---|---|---|---|---|
| High School N=23 |
Collegiate N=27 |
|||||
| Pre-Season | Post-Season | P-value* | Pre-Season | Post-Season | P-value* | |
| Body Mass Index, Mean (SD), kg/m2 | 29.5 (4.5) | 29.3 (4.5)§ | 0.32 | 34.2 (3.1) | 34.7 (2.9)§ | 0.02 |
| Systolic Blood Pressure, Mean (SD), mmHg | 133.2 (11) | 133.3 (16) | 0.79 | 134.8 (8) | 138.8 (11) | 0.06 |
| Diastolic Blood Pressure, Mean (SD), mmHg | 72.0 (8.7) | 75.6 (10) | 0.16 | 78.2 (10) | 78.2 (11) | 0.80 |
| Averaged LV Wall Thickness†, Mean (SD), mm | 8.8 (0.6) | 9.4 (0.6)§ | <0.001 | 9.4 (0.9) | 10.5 (1)§ | <0.001 |
| LV Internal Diameter End-Diastole / Body Surface Area, Mean (SD), mm/m2 | 23.5 (2) | 23.2 (2)§ | 0.34 | 20.0 (4) | 20.8 (2)§ | 0.30 |
| LV Mass / Body Surface Area, Mean (SD), gm/m2 | 88.7 (11) | 99.5 (12) | <0.001 | 93 (13) | 103 (14) | <0.001 |
| Tissue-Doppler LV E′‡, Mean (SD), cm/s | 14.5 (1.8) | 14.8 (2.5)‖ | 0.47 | 15.8 (2.5) | 13.2 (2.1)‖ | <0.001 |
| Pulse Wave Velocity, Mean (SD), cm/s | 4.8 (0.7) | 4.7 (0.7)‖ | 0.49 | 5.0 (0.6) | 5.3 (0.7)‖ | 0.003 |
ASF American-style football; LV left ventricle
Paired t-test (pre- to post-season measurement) derived p-value
Averaged LV Wall Thickness = Interventricular Septum + Posterior Wall Thickness / 2
Averaged E′ (cm/s) = lateral E′ velocity + septal E′ velocity / 2
2-sample t-test comparison of post-season high school vs. collegiate measurement, P<0.001
2-sample t-test comparison of post-season high school vs. collegiate measurement, P<0.05
Univariate/multivariate analyses
Univariate and multivariable analyses were performed to determine predictors of post-season E′, PWV, and LV mass index (Table 3). In the adjusted multivariable analyses, increased weight was a modest, but independent predictor of increased arterial stiffness (β=0.01, P=0.04) and reduced E′ (β=−0.04, P=0.05). Increased SBP was an independent predictor of both increased arterial stiffness (β=0.01, P=0.007) and increased LV mass index (β=0.18, P=0.01). Finally, strong independent associations between declining E′ and increasing arterial stiffness suggest an important element of ventriculo-arterial coupling.
Table 3.
Univariate and multivariable predictors of post-season cardiac and vascular indices
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Post-Season tissue-Doppler E′ Velocity* | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value |
| Pre-TDI E′ | 0.29 (0.13, 0.45) | <0.001 | 0.31 (0.15, 0.47) | <0.001 |
| Age | −0.42 (−1.1, 0.23) | 0.21 | − | − |
| Ethnicity | −0.58 (−1.28, 0.12) | 0.10 | − | − |
| Non-Linemen vs. Linemen | −0.80 (−1.6, 0.02) | 0.06 | − | − |
| Family History Hypertension | −0.86 (−1.67, −0.05) | 0.04 | − | − |
| Height | −0.03 (−0.09, 0.03) | 0.35 | − | − |
| Post-Season Weight | −0.03 (−0.05, −0.01) | 0.001 | −0.04 (−0.07, 0) | 0.05 |
| Post-Season Systolic Blood Pressure | −0.01 (−0.04, 0.02) | 0.47 | − | − |
| Post-Season Pulse Wave Velocity | −1.03 (−1.58, −0.48) | <0.001 | −1.07 (−1.74, −0.40) | 0.002 |
| Post-Season LV Mass Index | −0.01 (−0.05, 0.02) | 0.38 | − | − |
| Post-Season Pulse Wave Velocity | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value |
| Pre-Pulse Wave Velocity | 0.54 (0.38, 0.70) | <0.001 | 0.31 (0.15, 0.48) | <0.001 |
| Age | 0.36 (0.18, 0.53) | <0.001 | 0.24 (0.07, 0.40) | 0.01 |
| Ethnicity | −0.02 (−0.22, 0.18) | 0.84 | − | − |
| Non-Linemen vs. Linemen | 0.37 (0.14, 0.60) | 0.002 | − | − |
| Family History Hypertension | 0.20 (−0.03, 0.43) | 0.08 | − | − |
| Height | 0.04 (0.02, 0.05) | <0.001 | − | − |
| Post-Season Weight | 0.02 (0.01, 0.02) | <0.001 | 0.01 (0, 0.02) | 0.04 |
| Post-Season Systolic Blood Pressure | 0.02 (0.01, 0.03) | <0.001 | 0.01 (0.003, 0.02) | 0.007 |
| Post-Season TDI E′* | −0.08 (−0.13, −0.04) | <0.001 | −0.04 (−0.08, −0.01) | 0.02 |
| Post-Season LV Mass Index | 0.00 (−0.01, 0.01) | 0.75 | − | − |
| Post-SeasonLV Mass Index | Estimate (95% CI) | P-Value | Estimate (95% CI) | P-Value |
| Pre-LV Mass Index | 0.60 (0.47, 0.73) | <0.001 | 0.59 (0.45, 0.72) | <0.001 |
| Age | 2.49 (−0.8, 5.7) | 0.13 | − | − |
| Ethnicity | 0.35 (−3.2, 3.9) | 0.85 | − | − |
| Non-Linemen vs. Linemen | 0.91 (−3.3, 5.1) | 0.67 | − | − |
| Family History Hypertension | 0.94 (−3.2, 5.1) | 0.65 | − | − |
| Height | −0.07 (−0.37, 0.22) | 0.62 | − | − |
| Post-Season Weight | 0.04 (−0.1, 0.1) | 0.48 | − | − |
| Post-Season Systolic Blood Pressure | 0.19 (0.04, 0.35) | 0.01 | 0.18 (0.04, 0.32) | 0.01 |
| Post-Season TDI E′* | −0.36 (−1.2, 0.44) | 0.38 | − | − |
| Post-Season Pulse Wave Velocity | 0.46 (−2.5, 3.4) | 0.76 | − | − |
ASF American-style football; LV left ventricle, TDI tissue-Doppler velocity
Averaged E′ (cm/s) = lateral E′ velocity + septal E′ velocity / 2
DISCUSSION
This study, designed to characterize the temporal progression of cardiovascular phenotypes among competitive ASF athletes, generated the following key findings. First, longitudinal cardiovascular remodeling patterns differed between HS versus collegiate ASF participants. Specifically, collegiate ASF participants were more likely to develop concentric LV hypertrophy, to experience relative reductions in LV diastolic tissue velocities, and to develop relative arterial stiffening than HS ASF participants. Importantly, similar to prior studies, our data suggest that the collegiate LM position is most associated with the development of this characteristic cardiovascular remodeling pattern (1,2,5). Second, this dataset indicates that the initiation of the collegiate ASF experience represents the temporal data point after which differential ASF cardiovascular phenotypes begin to manifest. While player position has previously been shown to be a key determinant of cardiovascular alterations (1,2,5), our data suggest that HS LM do not experience similar magnitudes of change in cardiac and vascular function as the collegiate LM. Finally, our analyses suggest that intra-season weight gain and increased SBP represent synergistic mechanisms underlying the development of the differential cardiovascular remodeling observed more commonly among the collegiate ASF participants. In aggregate, our findings suggest that sport-specific cardiovascular remodeling differs between collegiate and HS ASF athletes, and that seasonal weight gain and increased SBP are associated with cardiovascular structural and functional alterations that may be maladaptive in occurrence.
Despite the expansive growth of ASF participation at the HS level (6), cardiovascular health profiles among HS ASF participants have not been rigorously detailed. The rationale for the current study arises from a growing body of literature that documents evidence of hypertension, LV hypertrophy, and premature mortality among ASF participants, particularly LM, who participate in collegiate and professional ASF (1–5,18). Our data suggest that, in general, ASF athletes at the HS level gain minimal weight, experience no significant increases in SBP, and are less likely to develop concentric LV hypertrophy compared to collegiate ASF athletes. As such, practitioners charged with the clinical care of this pediatric and young adult athletic population may better council athletes and parents who present in the clinics with concerns regarding their cardiovascular health. In contrast, differential cardiovascular phenotypes, including relative reductions in vascular function and LV tissue relaxation velocities, manifest more commonly among collegiate ASF participants, particularly the LM. While underlying mechanisms and the long-term clinical implications of these sub-clinical alterations remain unknown, it seems prudent for ASF professional team, university, and general sports medicine practitioners to consider the development of cardiovascular monitoring algorithms for certain ASF athletes, specifically LM and the athletes who develop hypertension or gain significant weight at any level of ASF participation. Moreover, our data add further compelling rationale for the emphasis on cardiac prevention vigilance in the primary care setting for these athletes as they age and after their ASF playing career concludes.
Findings from this study may best be considered in the context of relevant long-term clinical outcomes data derived from non-athletic but otherwise youthful populations (9,10,12,19–24). For example, hypertension and pre-hypertension present among ostensibly healthy young individuals, as measured at the time of college matriculation during the Harvard Alumni Health Study, predicted incident risk of later life cardiovascular disease (21). In the MESA cohort, concentric LV hypertrophy, more common among collegiate ASF participants in this study, at young ages was shown to increase the risk of later life attendant coronary heart disease and stroke (20). Finally, both increasing PWV and declining early diastolic relaxation velocity independently predict adverse cardiovascular outcomes when added to standard risk prediction models (12,19). While it is acknowledged that ASF athletes are not representative of the youthful populations previously studied in prior epidemiologic work, the emergence of hypertension and substantial increases in body weight, coupled with the cardiovascular phenotypes observed in this study, is concerning and underscores the critical need for future studies aimed to clarify the long-term clinical significance of these findings and clinical outcomes in this population.
While exact causal mechanisms underlying ASF-associated cardiovascular remodeling remain speculative, data from this study provide some novel advances. We observed 3 major factors differentiating HS from collegiate ASF participants in the current study: 1) intra-season weight gain, 2) intra-season increases in SBP and 3) ASF “dose” exposure. In addition, our data suggest that the impact of the LM player position on cardiovascular structure and function is most significant at the collegiate level. Our analyses suggest that increases in SBP and body weight may represent independent, yet synergistic determinants of differential ASF-associated cardiovascular remodeling. Specifically, weight gain is associated with relative reductions in E′ and vascular function while rising SBP is associated with relative reductions in vascular function and LV hypertrophy. It is intriguing that exercise/training load, particularly exposure to high intensity static exercise, appears to generally differ as a function of competition level with collegiate ASF athletes experiencing a significantly higher level of training exposure. To what degree the amount of isometric activity (i.e. tackling drills, weight lifting), in combination with the absence of concomitant isotonic activity and other important ASF-related environmental factors (ex. diet, pharmaceuticals, etc.), impact cardiovascular phenotypes in ASF athletes is uncertain and represents an important area of future work. Future study addressing the impact of intense isometric physiology will require rigorously controlled and multi-season longitudinal study designs of ASF athletes at the collegiate level and beyond that include multiple variables (such as oxidative stress biomarkers) (25), invasive physiologic data collection, and detailed analyses of non-ASF strength athletes.
Several limitations of this study are noteworthy. First, overall subject numbers in this analysis were limited and thus introduce the possibility of Type II error in our analyses. Assessing the impact of player position in the comparison between the collegiate and HS ASF athletes was further limited by the relatively small effect size in the change in measurements and the marked inter-individual variability for these measures in our subjects. Rather than a cross-sectional post-season comparison which was utilized in this analysis, future work addressing the differential impact of player position on cardiovascular phenotypes will require longitudinal analyses inclusive of large cohorts of athletes stratified by player position and should also account for the random effects present within subjects. Second, the possibility of unrecognized confounders, present during the season and off season, is acknowledged and may have limited our ability to precisely identify the causal mechanisms underlying our findings. Third, significant differences in baseline anthropometric measurements between HS and collegiate ASF athletes introduced the possibility of selection bias for the collegiate ASF participants. In addition, we acknowledge that the physical capabilities of collegiate ASF athletes are also different compared to the majority of HS ASF participants who are unable to compete at higher levels of competition and represent another example of potential selection bias. However, multi-center recruitment coupled with the use of a longitudinal repeated measures study design ensured that overall ASF participation at the HS and collegiate level, likely through numerous and discrete mechanisms, had a causal role in our findings. Fourth, we recognize the possibility of false positive findings because of the large number of repeated measures tests. However, we only chose 3 a priori key outcome variables as our primary measurements of interest, each with documented biologic relevance and established reproducibility in ASF athletes (1,2,4,5). Fifth, this study did not assess temporal trends in cardiac remodeling and vascular function beyond first year collegiate ASF participants. Long term repeated measures follow-up studies over the course of an entire collegiate and ultimately professional ASF career are warranted. Finally, we acknowledge that the HS and collegiate ASF cohorts were not matched for ethnicity and that results of our analyses may have incompletely captured the impact of race.
In summary, compared to HS ASF participants, collegiate ASF athletes develop differential sport-specific cardiovascular remodeling patterns as evidenced by a higher degree of concentric LV hypertrophy associated with relative reductions in LV relaxation velocities and concomitant relative arterial stiffening. These findings establish an important temporal data point along the continuum of ASF participation at which differential cardiovascular remodeling patterns are more likely to emerge. Future study designed to identify underlying causal mechanisms for these phenotypic changes and to identify the implications of these remodeling patterns on long-term cardiovascular health outcomes in this athletic population are warranted.
Acknowledgments
This work was supported by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute research grant K23 HL128795 (to Dr. Kim). We thank the Athletic Department and the student-athletes at Georgia Institute of Technology, Furman University, Marist School, Woodward Academy, and St. Pius X for ongoing support of this research. We also acknowledge Digirad® for providing all echocardiographic imaging services.
Footnotes
CONFLICTS OF INTEREST: No conflicts. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Weiner RB, Wang F, Isaacs SK, et al. Blood pressure and left ventricular hypertrophy during American-style football participation. Circulation. 2013;128(5):524–31. doi: 10.1161/CIRCULATIONAHA.113.003522. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Sher S, Wang F, et al. Impact of American-style football participation on vascular function. Am J Cardiol. 2015;115(2):262–7. doi: 10.1016/j.amjcard.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker AM, Vogel RA, Lincoln AE, et al. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301(20):2111–9. doi: 10.1001/jama.2009.716. [DOI] [PubMed] [Google Scholar]
- 4.Baggish AL, Wang F, Weiner RB, et al. Training-specific Changes in Cardiac Structire and Function: A Prospective and Longitudinal Assessment of Competitive Athletes. J Appl Physiol. 2008;104(4):1121–28. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Wang F, Weiner RB, et al. Blood Pressure and LV Remodeling Among American-Style Football Players. JACC Cardiovasc Imaging. 2016;9(12):1367–76. doi: 10.1016/j.jcmg.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Date accessed, September 15, 2017];NFHS Participation Statistics. http://www.nfhs.org/ParticipationStatistics/ParticipationStatistics.
- 7.Croft LB, Belanger A, Miller MA, Roberts A, Goldman ME. Comparison of National Football League linemen versus nonlinemen of left ventricular mass and left atrial size. Am J Cardiol. 2008;102(3):343–7. doi: 10.1016/j.amjcard.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–46. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92(10):595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 14.Woodman RJ, Kingwell BA, Beilin LJ, Hamilton SE, Dart AM, Watts GF. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18(2 Pt 1):249–60. doi: 10.1016/j.amjhyper.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Tsiachris D, Tsioufis C, Syrseloudis D, et al. Subendocardial viability ratio as an index of impaired coronary flow reserve in hypertensives without significant coronary artery stenoses. J Hum Hypertens. 2012;26(1):64–70. doi: 10.1038/jhh.2010.127. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 17.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 18.Baron SL, Hein MJ, Lehman E, Gersic CM. Body mass index, playing position, race, and the cardiovascular mortality of retired professional football players. Am J Cardiol. 2012;109(6):889–96. doi: 10.1016/j.amjcard.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Yip GW, Wang AY, et al. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens. 2005;23(1):183–91. doi: 10.1097/00004872-200501000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52(25):2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study) J Am Coll Cardiol. 2011;58(23):2396–403. doi: 10.1016/j.jacc.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171(12):1082–7. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 23.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105(11):1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 24.Abhayaratna WP, Barnes ME, O'Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006;98(10):1387–92. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Patel RS, Al Mheid I, Morris AA, et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218(1):90–5. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]