Abstract
Objective
Prophylactic levetiracetam is currently used in ~40% of patients with intracerebral hemorrhage (ICH), and the potential impact of levetircetam on health-related quality of life (HRQoL) is unknown. We tested the hypothesis that prophylactic levetiracetam is independently associated with differences in cognitive function HRQoL.
Design
Patients with ICH were enrolled in a prospective cohort study. We performed mixed models for T Scores of HRQoL, referenced to the US population at 50 ± 10, accounting for severity of injury and time to follow-up.
Setting
Academic medical center.
Patients
142 survivors of ICH.
Interventions
None
Measurements and Main Results
T Scores of Neuro-QOL Cognitive Function v2.0 was the primary outcome, while Neuro-QOL Mobility v1.0 and modified Rankin Scale (a global functional scale) were secondary measures. We prospectively documented if prophylactic levetiracetam was administered, and retrieved administration data from the electronic health record. Patients who received prophylactic levetiracetam had worse cognitive function HRQoL (T Score 5.1 points lower, P=0.01) after adjustment for age (P=0.3), NIH Stroke Scale (P<0.000001), lobar hematoma (P=0.9), and time of assessment; statistical models controlling for prophylactic levetiracetam and the ICH Score, a global measure of ICH severity, yielded similar results. Lower T Scores of cognitive function HRQoL at three months were correlated with more total levetiracetam dosage (P=0.01) and more administered doses of levetiracetam in the hospital (P=0.03). Patients who received prophylactic levetiracetam were more likely to have a lobar hematoma (27 of 38 versus 19 of 104, P<0.001), undergo electroencephalography monitoring (15 of 38 versus 21 of 104, P=0.02), but not more likely to have clinical seizures (4 of 38 versus 7 of 104, P=0.5). Levetiracetam was not independently associated with the modified Rankin Scale scores or mobility HRQoL (P>0.1).
Conclusions
Prophylactic levetiracetam was independently associated with lower cognitive function HRQoL at follow-up after ICH.
Keywords: Intracerebral hemorrhage, quality of life, seizure medications
Introduction
Seizures are a feared complication of intracerebral hemorrhage (ICH), occurring in 10-25% of patients acutely,(1) and in the years after ICH.(2) Seizures may lead to midline shift,(3) cerebral ischemia and worse patient outcomes. Thus, treatment with prophylactic seizure medications is a reasonable therapeutic strategy if the treatment carries a low risk of complications and the patient has an elevated risk of seizures (e.g., elevated CAVE Score,(2) a composite with one point each for lobar hematoma location, hematoma volume >10 mL, age < 65 years and a seizure within seven days of ICH onset).
Prophylactic phenytoin was recommended by guidelines in 1999 and 2007.(4, 5) However, in 2009, two independent reports noted that phenytoin use was independently associated with significant complications, particularly fever, and worse functional outcomes at follow-up.(6, 7) This led to a revised guideline recommendation in 2010 to avoid prophylactic seizure medications.(8)
Since the revised guideline recommendation, clinicians have switched from administering phenytoin to levetiracetam,(9, 10) which has fewer acute adverse events.(11) While likely to be safer than phenytoin, levetiracetam may also have side effects that impair health-related quality of life (HRQoL), such as altered cognitive function (e.g., trouble with memory),(12) and as noted in its package insert. Reductions in HRQoL, even if meaningful to patients, might not be detected by global, ordinal outcome scales, such as the modified Rankin Scale (mRS), a commonly utilized outcome measure for patients with ICH; a recent publication found no effect of prophylactic levetiracetam on the mRS.(9) Further, cognitive side effects may manifest as delirium symptoms, which is predictive of worse cognitive function HRQoL after ICH.(13–16) We hypothesized that prophylactic levetiracetam would be associated with lower cognitive function HRQoL after ICH.
Materials and Methods
Patients
Patients with spontaneous ICH were prospectively identified on hospital admission as previously noted.(17) Demographic information, medical history, home medications, standardized clinical assessments of severity (e.g., NIH Stroke Scale, a standardized neurological exam, and the ICH Score, a composite of age, hematoma volume, location and level of consciousness), imaging data, medical management variables, and medical complications, including the number of days with a core temperature at least 100.4F within 14 days of ICH and ventilator-free days within 14 days of ICH onset were prospectively recorded in a study database.
Severity of Injury
Apache IV scores(18) are automatically calculated from data in the electronic health record, and retrieved for analysis.
EEG Monitoring
The ICU protocol stipulated that all patients with ICH and reduced consciousness (e.g., not arousable to voice and following commands) undergo continuous EEG monitoring for at least 24 hours, which is reviewed at least twice daily by a certified electroencephalographer. Consideration of discontinuing EEG monitoring after 24 hours is determined by mutual agreement by the electroencephalographer and neurointensivist based upon the likelihood of discovering subclinical seizures.
Delirium
Delirium symptoms were assessed with the Confusion Assessment Method for the ICU (CAM-ICU) as documented in the electronic health record, as previously described.(15) Methods for identifying delirium in patients with neurologic disease have been previously described as new alterations in consciousness compared to the baseline of consciousness on hospital admission.(16)
Medication Dosing
We electronically retrieved each individual administered dose of levetiracetam, as previously described for phenytoin.(6) We did not determine levetiracetam use at follow-up as it was not feasible to perform medication reconciliation comparable to admission, and patients may not wish to divulge changes in seizure medication because this may affect their legal status to operate a motor vehicle.(19) We retrieved data on each administered dose of benzodiazepines.
Ethical Approval
The proposal was approved by the Institutional Review Board. Patients provided consent for data acquisition. If the patient could not be consented due to neurologic injury, a legally authorized representative was asked to provide consent.
HRQoL Assessment
As previously reported,(15, 20) we assessed HRQoL outcomes with Neuro-QOL, which are validated, standardized tests in specific domains of HRQoL, such as cognitive function v2.0 (e.g., ability to manage finances, concentration, memory), and mobility v1.0 (e.g., ability to get around, walking, daily activities). Neuro-QOL is similar to the NIH Patient Reported Outcomes Measurement Information System (PROMIS), and was validated with proxy report as part of its development.(21) We included data from patients from May 2011 through October 2016 who survived to post-hospitalization follow-up. We obtained HRQoL at one, three and twelve months after ICH onset, as previously described.(15) Results are reported in T Scores normalized to the US general population at 50 ± 10, i.e., the average person in the US will have a score about 50. As noted elsewhere, 0.5 SD, 5 points on the T Score, one-half a standard deviation, is considered a meaningful difference.(22) We also obtained outcomes with the mRS as previously described, using a validated questionnaire.(23)
Statistical Analysis
Normally distributed data (e.g., age) are reported as mean ± standard deviation (SD). Non-normally distributed data (e.g., days with fever) are reported as median [Q1 – Q3] and were compared with Kolmogorov-Smirnov test. Categorical data (e.g., categorical use of levetiracetam) are reported as proportions and were compared using a Chi-square or Fisher’s Exact Test as appropriate. Correlations of duration of levetiracetam use and T Scores of cognitive function HRQoL were performed with Spearman’s coefficient. We performed mixed models, as previously described, controlling for time of assessment (one, three and twelve months after ICH), age and NIH Stroke Scale score, (as previously reported)(15) with the use of prophylactic levetiracetam as the variable of interest. Alternatively, controlled for ICH Score instead of age and NIH Stroke Scale, because age and severity of injury are incorporated into the ICH Score. Statistical tests were two-sided. Statistical analyses were calculated with standard commercial software (NCSS 9, NCSS Inc., Kaysville, UT). The analysis was overseen and directed by Neuro-QOL, and NIH PROMIS Statistical Center, which were not involved in data collection (JB, DC).
Results
Of 394 patients with ICH, 122 died, 91 were lost to follow-up, 24 declined permission, and 15 did not provide HRQoL, leaving 142 in whom we assessed HRQoL data. Demographics for enrolled patients are shown in Table 1.
Table 1.
Demographics
| Variable | N(%), Mean ± SD, Median [Q1 – Q3] |
|---|---|
|
| |
| Age, years | 62.3 ± 14 |
|
| |
| NIH Stroke Scale on admission | 6 [2 – 12] |
|
| |
| International Normalized Ratio | 1.1 [1 – 1.1] |
|
| |
| Women | 66 (46) |
|
| |
| Race, White | 85 (59) |
| Black | 49 (35) |
| Asian | 4 (3) |
| Other | 4 (3) |
|
| |
| Hispanic or Latino | 17 (12) |
|
| |
| ICH Score, 0 | 51 (36) |
| 1 | 54 (38) |
| 2 | 26 (18) |
| 3 | 9 (6) |
| 4 | 2 (1) |
|
| |
| Previous ICH | 5 (4) |
|
| |
| History of hypertension | 111 (78) |
|
| |
| Warfarin prior to ICH | 7 (5) |
|
| |
| Novel oral anticoagulant prior to ICH | 2 (1) |
|
| |
| Moderate disability or worse prior to ICH | 14 (9) |
Characteristics of patients who received prophylactic levetiracetam or not are shown in Table 2. APACHE IV scores were not different between the groups of patients who received prophylactic levetiracetam or not. Patients who received prophylactic levetiracetam had a median of one more point on the one more point on the CAVE Score (2 [2 – 2] versus 1 [1 – 1], P<0.0001, typically from lobar hematoma location), suggesting a higher risk of seizures. Patients who received prophylactic levetiracetam were more likely to have a lobar hematoma (27 of 38 [71%] versus 19 of 104 [18%]) but less likely to have an infratentorial hematoma (0 of 38 versus 14 of 104 [10]%, P<0.001), older (66.9 ± 14.2 versus 60.6 ± 13.6 years, P=0.02) and had a higher NIH Stroke Scale (9 [6 – 12] versus 6 [4 – 7], P=0.01), indicating a moderately severe neurologic deficit.
Table 2.
History, Severity of Injury and Treatment Stratified by Levetiracetam Prophylaxis
| Variable | Prophylaxis | No Prophylaxis | P |
|---|---|---|---|
|
| |||
| N | 38 | 104 | |
|
| |||
| History of Intracerebral Hemorrhage | 2 (5) | 3 (3) | 0.6 |
|
| |||
| History of Diabetes | 8 (21) | 18 (17) | 0.6 |
|
| |||
| History of Ischemic Stroke | 6 (16) | 15 (14) | 0.8 |
|
| |||
| History of Coronary Artery Disease | 3 (8) | 7 (7) | 0.8 |
|
| |||
| History of Atrial Fibrillation | 4 (10) | 5 (5) | 0.2 |
|
| |||
| History of Hypertension | 30 (79) | 81 (78) | 0.9 |
|
| |||
| History of Dementia | 0 | 2 (2) | 0.4 |
|
| |||
| Lobar Hematoma | 27 (71) | 19 (18) | <0.001 |
|
| |||
| APACHE Predicted Length of Stay, days | 4.9 [4.0 – 6.7] | 4.7 [4.3 – 5.9] | 0.5 |
|
| |||
| APACHE Acute Physiology Score Points | 27 [19.5 – 56] | 25 [18.5 – 36.5] | 0.4 |
|
| |||
| APACHE Predicted Mortality (%) | 13 [4.2 – 25.6] | 10 [4.6 – 20.1] | 0.6 |
|
| |||
| Lorazepam, 0 mg | 32 (84) | 80 (77) | 0.2 |
| 1 mg | 3 (8) | 15 (14) | |
| 2 mg | 1 (3) | 8 (8) | |
| 4 – 8 mg | 2 (5) | 1 (1) | |
|
| |||
| Midazolam, 0 mg | 21 (55) | 78 (75) | 0.2 |
| 0.5 - 1 mg | 6 (16) | 12 (15) | |
| 1.5 - 2 mg | 7 (18) | 9 (12) | |
| 2.5 - 5.5 mg | 4 (11) | 5 (6) | |
|
| |||
| Ventilator Free Days in first 14 days | 14 [7 – 14] | 14 [12 – 14] | 0.2 |
|
| |||
| Days with Fever at least 100.4 in first 14 days | 0 [0 – 2] | 0 [0 – 0] | 0.3 |
APACHE, Acute Physiology and Chronic Health Evaluation IV.
Data are median [Q1 – Q3] or N(%) as appropriate
Delirium was uncommon in the cohort. Patients who received prophylactic levetiracetam were not more likely to have delirium compared to patients who did not (3 of 38 [8%] versus 6 of 104 [6%], P>0.2).
Prophylactic levetiracetam, on average, was given for about a week. Patients treated with levetiracetam received a median of 11 [7.25 – 26] doses during hospitalization (i.e. 5.5 days), with a median of 500 mg in each dose, typically administered every twelve hours. There was a correlation between the total dosage of levetiracetam and T Scores of cognitive function HRQoL, with more levetiracetam administered associated with lower (worse) T Scores (correlation -0.53, P=0.01) at three months. There was a similar relationship betweeen the number of administered doses of levetiracetam and T Scores of cognitive function HRQoL (correlation -0.46, P=0.03).
Clinical and subclinical seizures in this cohort were uncommon despite protocolized EEG monitoring. Eleven patients had a clinical seizure, and there was no association with receiving prophylactic levetiracetam (4 of 38 [11%] versus 7 of 104 [7%], P=0.5). Patients who received prophylactic levetiracetam were more likely to undergo EEG monitoring (15 of 38 [39%] versus 21 of 104 [21%], P=0.05), however, the association of prophylactic levetiracetam with EEG montioring was no longer significant after correcting for lobar hematoma. There were six (4%) patients with non-convulsive (subclinical) seizures discovered on EEG monitoring, five of whom received levetiracetam prophylaxis (P=0.006).
In mixed models, levetiracetam prophylaxis was independently associated with lower T Scores in cognitive function HRQoL (5.1 points, P=0.01) after correction for age (P=0.3) and NIH Stroke Scale score (P<0.000001). There were similar results (levetiracetam prophylaxis associated with a worse T score by 5.3 points, P=0.009) after correcting for the ICH Score (P=0.000001, Figure). Additionally, controlling for lobar hematoma location or non-convulsive seizures did not significantly add to the model (P>0.2) and did not meaningfully change the results. In unadjusted analyses, T Scores of cognitive function HRQoL were more different at one month (32.1 ± 14 versus 43.7 ± 12.7, P=0.04), but were not different at three months (36.8 ± 10.9 versus 37.8 ± 8.1, P>0.2) or twelve months (37.6 ± 12.6 versus 26.3 ± 13.5, P>0.2).
Figure.
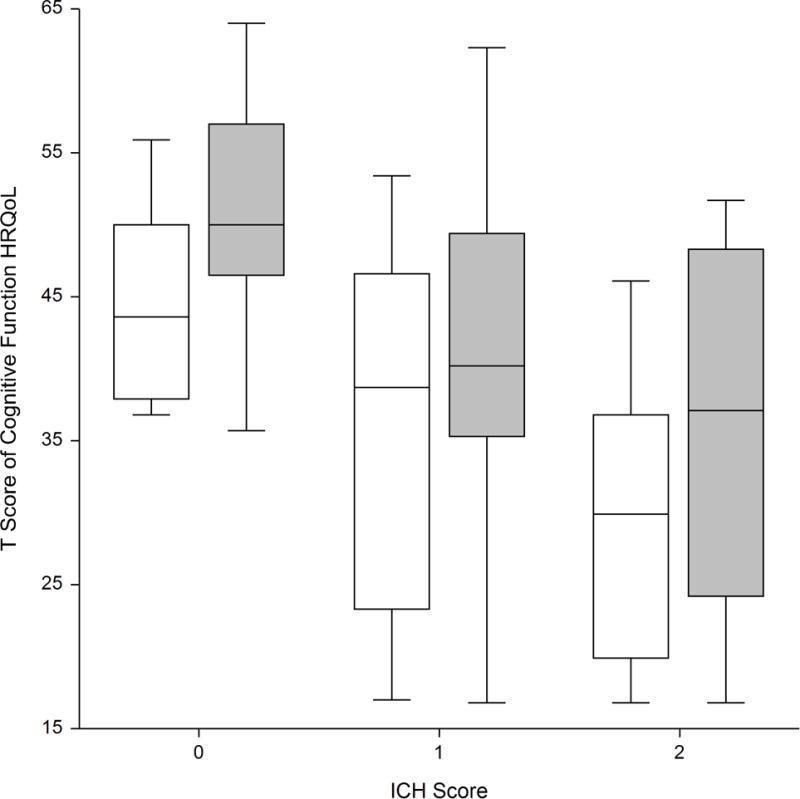
Box plot of T Score for Cognitive Function health-related quality of life (HRQoL) at three months, stratified by prophylactic levetiracetam (white) or no prophylactic levetiracetam (shaded). Prophylactic levetiracetam was associated with lower HRQoL, regardless of severity of injury (ICH Score). There were too few patients with ICH Scores of >2 to construct boxes.
Prior to admission for ICH, there were two patients who had a history of clinical seizure and two additional patients with a clinical history of dementia. Removing these patients from the analysis, or patients who had a clinical seizure in the hospital, did not meaningfully affect the results.
There were no associations between the administration of levetiracetam and mobility HRQoL (P>0.1), or the mRS (P>0.1).
Discussion
We confirmed the hypothesis that prophylactic levetiracetam, administered in the hospitalization for ICH, was independently associated with lower cognitive function HRQoL at follow-up after ICH. These data may be important for the management of ICH, because ~40% of ICH patients in the US receive prophylactic levetiracetam,(9, 10) higher than the incidence of clinical seizures. If prophylactic levetiracetam, even if potentially efficacious for preventing seizures, may worsen cognitive function HRQoL, these findings potentially impact many patients with ICH.
Patients with ICH do not indiscriminately receive levetiracetam, rather, treatment is generally given to patients at elevated risk for seizures due to lobar hematoma location,(3, 24) and a correspondingly higher CAVE Score. Controlling for lobar hematoma in statistical models was not statistically significant and did not change the association of prophylactic levetiracetam with worse cognitive function HRQoL. In other words, levetiracetam is already administered to patients who have known risk factors for seizures, and levetiracetam may worsen HRQoL in this subgroup.
Levetiracetam was administered at a relatively low dose of 500 mg twice daily in this cohort for a median of approximately one week after ICH, and even this relatively modest dose and duration of treatment was associated with lower cognitive function HRQoL. These data do not support the assertion that only long-standing therapy is responsible for lower cognitive function HRQoL; the positive correlation between the duration of treatment and lower cognitive function HRQoL suggests that longer treatment might lead to worse cognitive function HRQoL. The association between administration of seizure medication and lower subsequent measures of cognitive function is consistent with levetiracetam’s package insert and the known association of prophylactic phenytoin administration and lower subsequent measures of cognitive function after subarachnoid hemorrhage (ruptured brain aneurysm),(25) and stroke generally.(26) Conversely, levetiracetam has been associated with neuroprotection, particularly in animal models of traumatic brain injury.(27–29) The effects of levetiracetam on outcomes require further study, and may vary between neurologic conditions.
It is not clear from these data if levetiracetam is effective for preventing clinical seizures after ICH. A separate investigation suggested that levetiracetam is not effective for preventing seizures in patients with aneurysmal subarachnoid hemorrhage.(30) Levetiracetam prophylaxis was more common in patients who were subsequently discovered to have non-convulsive (subclinical) seizures in this cohort, likely representing a correct assessment of high seizure risk by clinicians and the known predictions of the CAVE score.
Delirium is common in patients with ischemic stroke,(31) as well as ICH,(16) and predicts worse cognitive function HRQoL.(13, 15) However, we did not find an association between prophylactic levetiracetam and delirium. These data suggest that the association of levetiracetam and worse cognitive function HRQoL is unlikely to be mediated by delirium.
Previous investigations have found that levetiracetam is better tolerated than phenytoin,(11) which was associated with more fever and worse outcomes after ICH.(6) Following the publication of data on the adverse drug effects on phenytoin after ICH,(6, 7) clinicians switched from phenytoin to levetiracetam,(9, 10) rather than abstaining from seizure medications generally. These data do not support switching from levetiracetam to another seizure medication. Indeed, levetiracetam is likely to be safer and better tolerated than other seizure medications available for parenteral use (e.g., valproate, which can lead to hepatic toxicity and coagulopathy, or lacosamide, which may lead to heart block).
There are limitations to this study. We did not have an adjudicated process for determining whether a seizure occurred after hospital discharge and patients may not be forthcoming in reporting a seizure for fear of losing the privilege of driving a motor vehicle for several months.(19) One would expect that patients who did not receive prophylactic levetiracetam would be more likely to have a seizure after hospital discharge and have lower HRQoL from a seizure,(32) which would bias our results toward finding no effect of levetiracetam, rather than the association of prophylactic levetiracetam with lower HRQoL. We have previously noted that HRQoL outcomes are less likely to be obtained in patients who are neurologically devastated or dead,(33) so these results are most applicable to patients with ICH who are likely to survive (ICH Score 0 through 2) with no deficit to a moderate neurologic deficit (e.g., modified Rankin Scale 0 through 4) and a CAVE score of one or greater. The importance of this bias in obtaining HRQoL may be attenuated because levetiracetam is not plausibly associated with mortality. We measured patient-reported HRQoL, not objective cognitive function, which would require in-person follow-up, and might make results less generalizable. However, patient-reported deficits in cognitive are typically accurate for identifying objective cognitive deficits.(34) Objective cognitive function assessments provide an opportunity for future research. While medication reconciliation is performed on hospital admission,(35) we do not have a validated medication reconciliation performed at follow-up, such as pill counts. Uncertainty of later levetiracetam use would be likely to bias our results toward the null hypothesis of no association between levetiracetam and HRQoL at follow-up.
Conclusions
Prophylactic administration of levetiracetam during hospitalization for ICH was independently associated with lower cognitive function HRQoL at follow-up. These data suggest that prophylactic levetiracetam may have persistent effects on cognitive function HRQoL. Further research may be appropriate to evaluate the benefits of prophylactic seizure medications in patients with ICH, and if the trade-off of potentially preventing seizures in patients at high risk justifies potential reductions in HRQoL from prophylactic seizure medications.
Acknowledgments
There are no additional acknowledgements.
All those to contributed to manuscripts are included as an author.
Funding
Dr. Naidech received support from Agency for Healthcare Research and Quality K18 HS023437.
Dr. Liotta receives support from the National Institutes of Health National Center for Advancing Translational Sciences grant KL2TR001424 and the National Institute of Health grant L30 NS098427.
Dr. Maas receives support from National Institutes of Health grants K23 NS092975 and L30 NS080176, and a Dixon Translational Research Grant from the Northwestern Memorial Foundation.
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences grant UL1 TR000150.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Copyright form disclosure: Dr. Naidech’s institution received funding from the Agency for Healthcare Research and Quality (AHRQ), and he received support for article research from the AHRQ and the National Institutes of Health (NIH). Dr. Liotta’s institution received funding from NIH National Center for Advancing Translational Sciences grant KL2TR001424, and he received funding from the NIH Division of Loan Repayment grant L30NS098427. Dr. Maas’ institution received funding from the NIH, and he received support for article research from the NIH. Dr. Cella’s institution received funding from the National Institutes of Neurological Disorders and Stroke U01 NS 056 975 02 and NIH/NIAMS, U5 AR057951, and he received support for article research from the NIH.
Footnotes
We do not expect to order reprints.
Dr. Naidech performed the statistical analysis under guidance from the NIH PROMIS Statistical Center (Dr. Cella, Ms. Beaumont).
Disclosures:
Dr. Eric M. Liotta reports no disclosures.
Dr. Matthew B. Potts reports no disclosures.
Dr. Babak S. Jahromi reports no disclosures.
Dr. Matthew B. Maas reports no disclosures.
Ms. Beaumont reports no disclosures.
Dr. David Cella reports no disclosures
Dr. Shyam Prabhakaran reports no disclosures.
Dr. Jane Holl reports no disclosures.
Dr. Andrew M. Naidech reports
Author Contributions:
Eric M. Liotta, MD: revised the manuscript for important intellectual content
Kathryn Muldoon, MPH: collected study data, revised the manuscript for important intellectual content
Jane Holl, MD, MPH: revised the manuscript for important intellectual content
Shyam Prabhakaran, MD, MS: revised the manuscript for important intellectual content
Jennifer Beaumont, MD: Oversaw the statistical analysis
Matthew B. Potts, MD: revised the manuscript for important intellectual content
Babak S. Jahromi, MD, PhD: revised the manuscript for important intellectual content
David Cella, PhD: revised the manuscript for important intellectual content
Matthew B. Maas, MD: designed and conceptualized the study, collected study data
Andrew M. Naidech, MD, MSPH: designed and conceptualized the study, analyzed and interpreted the data, collected study data, drafted the manuscript and revised the manuscript for important intellectual content
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Neshige S, Kuriyama M, Yoshimoto T, et al. Seizures after intracerebral hemorrhage; risk factor, recurrence, efficacy of antiepileptic drug. J Neurol Sci. 2015;359(1-2):318–322. doi: 10.1016/j.jns.2015.09.358. [DOI] [PubMed] [Google Scholar]
- 2.Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke. 2014;45(7):1971–1976. doi: 10.1161/STROKEAHA.114.004686. [DOI] [PubMed] [Google Scholar]
- 3.Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60(9):1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 4.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage in Adults: 2007 Update: A Guideline From the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(6):2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Adams HP, Barsan W, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1999;30(4):905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 6.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant Use and Outcomes After Intracerebral Hemorrhage. Stroke. 2009;40:3810–3815. doi: 10.1161/STROKEAHA.109.559948. [DOI] [PubMed] [Google Scholar]
- 7.Messe SR, Sansing LH, Cucchiara BL, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care. 2009;11(1):38–44. doi: 10.1007/s12028-009-9207-y. [DOI] [PubMed] [Google Scholar]
- 8.Morgenstern LB, Hemphill JC, 3rd, Anderson C, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheth KN, Martini SR, Moomaw CJ, et al. Prophylactic Antiepileptic Drug Use and Outcome in the Ethnic/Racial Variations of Intracerebral Hemorrhage Study. Stroke. 2015;46(12):3532–3535. doi: 10.1161/STROKEAHA.115.010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naidech AM, Beaumont J, Prabhakaran S, et al. Evolving Use of Seizure Medications After Intracerebral Hemorrhage: A Multi-Center Study. Neurology. 2017;88(1):52–56. doi: 10.1212/WNL.0000000000003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szaflarski JP, Sangha KS, Lindsell CJ, et al. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12(2):165–172. doi: 10.1007/s12028-009-9304-y. [DOI] [PubMed] [Google Scholar]
- 12.Verrotti A, Prezioso G, Di Sabatino F, et al. The adverse event profile of levetiracetam: A meta-analysis on children and adults. Seizure. 2015;31:49–55. doi: 10.1016/j.seizure.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal LJ, Francis BA, Beaumont JL, et al. Agitation, Delirium, and Cognitive Outcomes in Intracerebral Hemorrhage. Psychosomatics. 2017;58(1):19–27. doi: 10.1016/j.psym.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naidech AM, Polnaszek KL, Berman MD, et al. Hematoma Locations Predicting Delirium Symptoms After Intracerebral Hemorrhage. Neurocrit Care. 2016;24(3):397–403. doi: 10.1007/s12028-015-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naidech AM, Beaumont JL, Rosenberg NF, et al. Intracerebral Hemorrhage and Delirium Symptoms: length of stay, function and quality of life in a 114-patient cohort. Am J Respir Crit Care Med. 2013;188(11):1331–1337. doi: 10.1164/rccm.201307-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2012;40(2):484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 17.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81(2):107–112. doi: 10.1212/WNL.0b013e31829a33e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandenburg R, Brinkman S, de Keizer NF, et al. In-hospital mortality and long-term survival of patients with acute intoxication admitted to the ICU. Crit Care Med. 2014;42(6):1471–1479. doi: 10.1097/CCM.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 19.Chadwick DW. Driving restrictions and people with epilepsy. Neurology. 2001;57(10):1749–1750. doi: 10.1212/wnl.57.10.1749. [DOI] [PubMed] [Google Scholar]
- 20.Sangha RS, Caprio FZ, Askew R, et al. Quality of life in patients with TIA and minor ischemic stroke. Neurology. 2015;85(22):1957–1963. doi: 10.1212/WNL.0000000000002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bath PM, Lees KR, Schellinger PD, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43(4):1171–1178. doi: 10.1161/STROKEAHA.111.641456. [DOI] [PubMed] [Google Scholar]
- 23.Saver JL, Filip B, Hamilton S, et al. Improving the Reliability of Stroke Disability Grading in Clinical Trials and Clinical Practice: The Rankin Focused Assessment (RFA) Stroke. 2010;41(5):992–995. doi: 10.1161/STROKEAHA.109.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naidech AM, Toledo P, Prabhakaran S, et al. Disparities in the Use of Seizure Medications After Intracerebral Hemorrhage. Stroke: a Journal of the American Heart Association. 2017;48(3):802–804. doi: 10.1161/STROKEAHA.116.015779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidech AM, Kreiter KT, Janjua N, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36(3):583–587. doi: 10.1161/01.STR.0000141936.36596.1e. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LB. Common drugs may influence motor recovery after stroke. The Sygen In Acute Stroke Study Investigators. Neurology. 1995;45(5):865–871. doi: 10.1212/wnl.45.5.865. [DOI] [PubMed] [Google Scholar]
- 27.Browning M, Shear DA, Bramlett HM, et al. Levetiracetam Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J Neurotrauma. 2016;33(6):581–594. doi: 10.1089/neu.2015.4131. [DOI] [PubMed] [Google Scholar]
- 28.Zou H, Brayer SW, Hurwitz M, et al. Neuroprotective, neuroplastic, and neurobehavioral effects of daily treatment with levetiracetam in experimental traumatic brain injury. Neurorehabil Neural Repair. 2013;27(9):878–888. doi: 10.1177/1545968313491007. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Gao J, Lassiter TF, et al. Levetiracetam is neuroprotective in murine models of closed head injury and subarachnoid hemorrhage. Neurocrit Care. 2006;5(1):71–78. doi: 10.1385/NCC:5:1:71. [DOI] [PubMed] [Google Scholar]
- 30.Panczykowski D, Pease M, Zhao Y, et al. Prophylactic Antiepileptics and Seizure Incidence Following Subarachnoid Hemorrhage: A Propensity Score-Matched Analysis. Stroke. 2016;47(7):1754–1760. doi: 10.1161/STROKEAHA.116.013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldenbeuving AW, de Kort PL, Jansen BP, et al. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011;76(11):993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 32.Ridsdale L, Wojewodka G, Robinson E, et al. Characteristics associated with quality of life among people with drug-resistant epilepsy. J Neurol. 2017;264(6):1174–1184. doi: 10.1007/s00415-017-8512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naidech AM, Beaumont JL, Berman M, et al. Web-Based Assessment of Outcomes After Subarachnoid and Intracerebral Hemorrhage: A New Patient Centered Option for Outcomes Assessment. Neurocrit Care. 2014 doi: 10.1007/s12028-014-0098-1. [DOI] [PubMed] [Google Scholar]
- 34.Juan E, De Lucia M, Beaud V, et al. How Do You Feel? Subjective Perception of Recovery as a Reliable Surrogate of Cognitive and Functional Outcome in Cardiac Arrest Survivors. Crit Care Med. 2018;46(4):e286–e293. doi: 10.1097/CCM.0000000000002946. [DOI] [PubMed] [Google Scholar]
- 35.The Joint Commission. Using medication reconciliation to prevent errors. [cited 2008 June 16]Using medication reconciliation to prevent errors.] Available from: http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_35.htm.


