Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) use has consistently been associated with lower risk of colorectal cancer (CRC); however, studies showed inconsistent results on which cohort of individuals may benefit most. We performed multivariable logistic regression analysis to systematically test for the interaction between regular use of NSAIDs and other lifestyle and dietary factors on CRC risk among 11,894 cases and 15,999 controls. Fixed-effects meta-analyses were used for stratified analyses across studies for each risk factor and to summarize the estimates from interactions. Regular use of any NSAID, aspirin, or non-aspirin NSAIDs was significantly associated with a lower risk of CRC within almost all subgroups. However, smoking status and BMI were found to modify the NSAID-CRC association. Aspirin use was associated with a 29% lower CRC risk among never-smokers (OR = 0.71; 95% CI: 0.64, 0.79), compared to 19% and 17% lower CRC risk among smokers of pack-years below median (OR = 0.81; 95% CI: 0.71, 0.92) and above median (OR = 0.83; 95% CI: 0.74, 0.94), respectively (p-interaction = 0.048). The association between any NSAID use and CRC risk was also attenuated with increasing BMI (p-interaction = 0.075). Collectively, these results suggest that obese individuals and heavy smokers are unlikely to benefit as much as other groups from the prophylactic effect of aspirin against CRC.
Keywords: Aspirin, Colorectal cancer, Effect modification, Meta-analysis, Non-steroidal anti-inflammatory drugs
Introduction
Colorectal cancer (CRC) is one of the most common and fatal cancers in the world. Non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin and non-aspirin NSAIDs, are consistently observed to be protective against CRC (1,2). Long-term use of aspirin was found to significantly reduce the incidence of CRC by 24%, and the benefit increased with longer duration of treatment based on 20-year follow up of five randomized trials (1). A similar association was also reported in a meta-analysis of observational studies (2). Despite its promising chemopreventive effects, aspirin is recommended only to prevent cardiovascular disease and CRC in those who are at high risk of cardiovascular disease; no broad recommendation from national organization is in place due to concerns about gastrointestinal bleeding (3).
The main chemopreventive mechanism of NSAIDs is the inhibition of cyclooxygenase-2 (COX-2) activity and subsequent formation of prostaglandin E2 (PGE2) (4). Aspirin also inhibits the oncogenic Wnt/β-catenin pathway (5,6) and the extracellular-signal-regulated kinase (ERK) signaling pathway (7). In addition, NSAIDs may function partially through NFкB-signaling pathway (8) and PI3K signaling pathway (9) in colorectal carcinogenesis. Other pathways related to transcription factors, cell proliferation and apoptosis have also been suggested (10).
It is suspected that the association of NSAID use and CRC risk may be modified by other risk factors that are also related to inflammation, but the results have been inconsistent. Regular use of aspirin was associated with a larger decrease in CRC risk in men than in women in cohort studies (11,12), but meta-analyses did not find this difference to be statistically significant (2,13). Non-aspirin NSAID use was associated with a lower risk of CRC among individuals with body mass index (BMI) >25, but not with BMI ≤25, in a cohort study (14), but it was not reported in other studies (15–18). A case-control study found current use of NSAIDs was associated with larger reduction of CRC risk among individuals who had smoked for >40 years, compared to non-smokers (19). However, cohort studies found no interaction between NSAID use and smoking on CRC risk (16,17). In contrast, recent clinical trials found that aspirin was statistically significantly associated with lower risk of colorectal adenomas among non-smokers, but not among current smokers (20–22). A case-control study found that NSAID use was associated with lower colon cancer risk among postmenopausal hormone therapy (PMH) non-users, but not among PMH users (23). In addition, a randomized clinical trial reported synergistic effects of calcium and any NSAID use in lowering the risk of advanced colorectal neoplastic polyps (24); but the interaction between NSAID use and calcium on CRC risk was not found in a cohort study (17).
To our knowledge, no subgroups of the population stratified by lifestyle or dietary risk factors have been consistently identified who have a clearly larger benefit from use of aspirin or non-aspirin NSAIDs. Most previous studies did not have sufficient power to detect statistically significant differences between population subgroups. Thus, we aimed to evaluate the potential effect modification of other CRC risk factors on the associations of regular use of any NSAID, aspirin, and non-aspirin NSAIDs with CRC risk using studies from a large, international consortium.
Materials and Methods
Study participants
Study participants were from the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) and the Colon Cancer Family Registry (CCFR), an international collaboration that involves 12 case-control and cohort studies from North America, Australia and Europe (25). The studies included are listed in Table 1, and details have been described previously (9). In brief, we used data from 7 nested case-control studies in prospective cohorts [Health Professionals Follow-up Study (HPFS); Multiethnic Cohort Study (MEC); Nurses’ Health Study (NHS); Physician’s Health Study (PHS); Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO); VITamins And Lifestyle Study (VITAL); Women’s Health Initiative (WHI)] and 5 case-control studies [Assessment of Risk for Colorectal Tumors in Canada (ARCTIC); Hawai’i Colorectal Cancer Studies 2 & 3 (Colo2&3); Darmkrebs: Chancen der Verhutüng durch Screening (DACHS); Diet, Activity and Lifestyle Survey (DALS); Postmenopausal Hormone Study (PMH)]. Written informed consent was given by all participants, and studies were approved by their respective Institutional Review Boards. Studies were conducted in accordance with the Declaration of Helsinki.
Table 1.
Definition of Regular Use of NSAIDs Among Participating Studies
| Study Design | Study | Country | Case N | Control N | Male, N (%) | Age, mean (sd), year | Definition of regular use of aspirin and/or non-aspirin NSAIDsa |
|---|---|---|---|---|---|---|---|
| Cohort (nested case-control) | PLCO | United States | 1,096 | 2,719 | 2,597 (68.1) | 69.0 (6.1) | ≥2 times/week in the last 12 months |
| WHI | United States | 1,740 | 2,962 | 0 | 73.1 (7.3) | ≥1 time/week for at least the last 2 weeks | |
| HPFS | United States | 646 | 1,164 | 1,810 (100) | 69.6 (9.1) | Currently taking ≥2 times/week | |
| MEC | United States | 356 | 366 | 381 (52.8) | 70.0 (8.3) | ≥2 times/week for ≥1 month | |
| NHS | United States | 1,001 | 1,817 | 0 | 66.5 (8.2) | Currently using ≥15 days/month | |
| PHS | United States | 309 | 455 | 764 (100) | 69.2 (9.6) | Currently using ≥1 time/week | |
| VITAL | United States | 333 | 337 | 365 (54.3) | 70.5 (6.6) | ≥4 days/week for 1 year | |
| Case-control | ARCTIC | Canada | 1,066 | 1,204 | 1,098 (48.4) | 62.1 (8.7) | ≥2 times/week for >1 month about 2 years ago |
| DALS | United States | 1,451 | 1,474 | 1,644 (56.2) | 65.0 (9.9) | ≥3 times/week for ≥1 month within the last 2 years | |
| DACHS | Germany | 2,859 | 2,355 | 3,136 (60.2) | 68.6 (10.5) | Currently using for ≥2 time/week for ≥1 years | |
| Colo2&3 | United States | 94 | 131 | 128 (56.8) | 64.7 (11.4) | Currently using | |
| PMH | United States | 943 | 1,015 | 0 | 64.5 (7.2) | ≥2 times/week for >1 month | |
| Overall | 11,894 | 15,999 | 11,922 (42.7) | 68.2 (9.1) | |||
Abbreviations: ARCTIC: Assessment of Risk for Colorectal Tumors in Canada; DALS: Diet, Activity and Lifestyle Study; PLCO: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; WHI: Women’s Health Initiative; DACHS: Darmkrebs: Chancen der Verhutüng durch Screening Study; Colon 2&3: a case-control study from the University of Hawai’i; HPFS: Health Professionals Follow-up Study; MEC: Multiethnic Cohort; NHS: Nurses’ Health Study; PHS: Physicians’ Health Study; VITAL: Vitamins and Lifestyle Study; PMH: Postmenopausal Hormone Study – Colon Cancer Family Registry.
Definition of regular use of aspirin and/or NSAIDs was assessed at corresponding referent period: in cohort studies, baseline; case-control studies, at the time of diagnosis for cases, and at analogue time for controls.
Each study identified incident, invasive CRC cases (International Classification of Disease for Oncology Code 18.0–18.9, 19.9 and 20.9), confirmed by medical record, pathology report, or death certificate. Age at diagnosis, cancer subsites and stages were obtained from medical records and registries. Controls were individuals without history of CRC at the time of selection, and were selected based on study-specific eligibility and matching criteria (mostly sex and age; as well as smoking status for PHS).
Participants reported as members of racial/ethnic groups other than White were excluded, and European ancestry was confirmed using principal components analysis (26). Participants with missing information on both aspirin and non-aspirin NSAID use were excluded. A total of 11,894 colorectal cases and 15,999 controls were included in the analysis.
Assessment of NSAID use and covariates
Demographics and environmental exposures were self-reported at either in-person interview or via structured self-administered questionnaires, based on each participating study. A multistep, iterative data harmonization procedure was applied, reconciling each study’s unique protocols and data collection instruments (27). Numerous quality-control checks were performed, and outlying values of variables were truncated to the minimum or maximum value of an established range for each variable. Variables were combined into a single dataset with common definition, standardized coding, and standardized permissible values.
For the main exposure variables (regular use of any NSAID, aspirin, and non-aspirin NSAIDs), we attempt to capture both frequency and duration of use in defining regular use. Study-specific definitions of regular use of aspirin and/or non-aspirin NSAIDs were used instead of an identical definition due to variability in questions across studies (Table 1). Use of aspirin included both low-dose aspirin (81 mg), and regular or extra-strength aspirin (≥325 mg). Use of non-aspirin NSAIDs included ibuprofen, naproxen or other pain relievers, based on each study. Regular use of any NSAID was defined as regular use of either aspirin or non-aspirin NSAIDs.
An a priori list of potential confounders were also ascertained and harmonized, including study, age, sex, education, BMI, smoking, physical activity, first-degree family history of CRC, history of endoscopy, diabetes, and postmenopausal hormone (PMH) use in women. Age was defined as age at diagnosis for cases and age at selection for controls. Dietary covariates were ascertained using food frequency questionnaires (FFQ), including intakes of alcohol (non-drinker, 1–28 g/day and >28 g/day), fruit, vegetables, dietary fiber, red meat, processed meat and total energy, and total (diet plus supplemental) intakes of calcium and folate. Sex- and study-specific quartiles were created for smoking, physical activity, and all dietary variables except alcohol. For studies with dietary information in categories that did not allow conversion into quartiles, binary variables defined by sex-study-specific medians were used. The binary variable was coded as quartile 2 and 3 for these studies.
Statistical analyses
Statistical analyses were conducted using individual-level data. For each study, logistic regression was used to estimate odds ratios (OR) and corresponding 95% confidence intervals (CI) for each NSAID variable (any NSAID use, aspirin use, and non-aspirin NSAID use) by comparing regular users and non-regular users after adjusting for covariates (as specified in footnotes to tables). Indicators were used for missing covariates. Regular use of non-aspirin NSAIDs was also adjusted for in the analyses for aspirin, and vice versa. Study-specific estimates were combined, using a fixed-effects model, into summary ORs and corresponding 95% CIs. Heterogeneity across studies was assessed using percentage of variance (I2) and tested using Cochran’s Q test(28).
To assess factors that may modify the association between NSAID use and CRC risk, we computed stratum-specific estimates in each study, using logistic regression within each stratum of each factor adjusting for all other covariates, which were then combined into summary stratum-specific ORs and corresponding 95% CIs. Interaction was tested as the significance of the cross product of the NSAID variable and the effect modifier in the multivariable model that also included the main associations of the NSAID variable and the potential effect modifier. Demographic characteristics and lifestyle factors were evaluated including age (<70 and ≥70 years old), sex, BMI (kg/m2; normal [18.5–24.9], overweight [25–29.9] and obese [≥30]), smoking (pack-years; non-smoker, ≤median and >median), moderate/vigorous physical activity (quartiles; hours/week), first-degree family history of CRC, history of endoscopy (colonoscopy or sigmoidoscopy), diabetes and PMH use in women, Dietary factors were also tested for potential effect modification, including alcohol intake (non-drinker, 1–28 g/day and >28 g/day), fruit intake (quartiles), vegetable intake (quartiles), red meat intake (quartiles), processed meat intake (quartiles), dietary fiber intake (quartiles), total calcium intake (quartiles) and total folate intake (quartiles). The potential effect modifiers with more than two categories were modeled as group linear (trend) in multiplicative interaction terms. The study-specific estimates for cross products were combined into summary estimates for a single two-sided p-value for interaction, using a fixed-effect meta-analysis. Most interaction analyses did not show significant heterogeneity across studies. Therefore, we did not use a random-effects meta-analysis. For each potential effect modifier, studies with constant values were excluded from corresponding interaction analyses: specifically, WHI, NHS, HPFS and PHS, were excluded in the interaction analysis of NSAID use and sex; PHS was excluded in the interaction analysis of NSAID use and smoking; and HPFS and PHS were excluded in the interaction analysis of NSAID use and PMH use. For statistically significant effect modifiers, we further tested whether the observed interactions differed by sex or study type (case-control and cohort). In addition, we also performed sensitivity analyses for statistically significant effect modifiers using multiple imputation methods to impute missing values in the adjusted covariates in the interaction analysis.
Stratified analyses by cancer subsites (proximal colon, distal colon and rectal) and stages (local, regional and distant) were also performed for the association between regular NSAID use and CRC risk. Site-specific or stage-specific cases were compared to the same control group in stratified analyses; logistic regression limited to cases was used to test for heterogeneity. A p-value<0.05 were considered statistical significance in all analyses. All analyses were performed in Stata v.14 (StataCorp).
Results
Descriptions of the study populations and the definitions of regular NSAID use in each participating study are shown in Table 1. The main associations of NSAID use on CRC risk were examined for all studies (Figure 1). For each type of NSAID use (any NSAID, aspirin use, and non-aspirin NSAID use), regular NSAID use was statistically significantly associated with lower risk of CRC after adjusting for all the covariates, compared to non-regular users (P<0.001). Any NSAID use was associated with 25% lower risk of CRC, compared to non-regular NSAID use (OR=0.75, 95% CI: 0.71, 0.79; P<0.001; P-heterogeneity<0.001). The association was stronger among case-control studies.
Figure 1. Estimated Associations Between Regular Use of Aspirin and/or NSAIDs and Colorectal Cancer Riska, b, c .
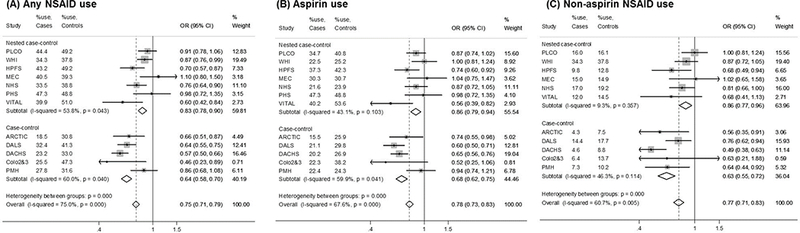
Abbreviations: OR: odds ratio; 95% CI: 95% confidence interval.
a The size of the data markers is proportional to the precision of the estimate, which is the inverse of the variance.
b Study-specific ORs and 95% CIs are estimated using logistic regression models, adjusting for age, sex, education (less than high school, high school graduate or GED, some college, college graduate, graduate degree), first-degree family history of colorectal cancer (yes/no), history of endoscopy (yes/no), postmenopausal hormone use among women (yes/no), history of diabetes(yes/no), body mass index (kg/m2), moderate/vigorous activity (hours/week), smoking (non-smokers and quartiles of pack-years), alcohol intake (none, 1–28g/day, >28g/day), dietary intakes (quartiles) of fruit, vegetables, red meat, processed meat and fiber, total energy intake (quartiles), total (dietary and supplemental) intakes of calcium and folate (quartiles). Covariates in quartiles are adjusted as group linear variables in the model. For aspirin or non-aspirin NSAID use only, the other type was also adjusted for.
c Subtotal and overall ORs and 95% CIs are estimated using fixed-effect meta-analysis. The estimates using random-effect are: (A) Any aspirin or NSAID use: OR=0.75 (0.67, 0.85) (B) Aspirin use: OR=0.79 (0.70, 0.89) (C) Non-aspirin NSAID use: OR=0.74 (0.64, 0.86).
Regular use of any NSAID, aspirin, or non-aspirin NSAIDs was statistically significantly associated with a lower risk of CRC across almost all subgroups, stratified by demographic and lifestyle factors (Table 2) and by dietary factors (Table 3). There was minimal heterogeneity by study in the test for interaction for all analyses, except for age and processed meat. The association between aspirin and CRC risk statistically significantly differed by smoking status after adjusting for other risk factors in the meta-analysis (P-interaction=0.048). Regular use of aspirin was associated with a 29% lower risk of CRC among non-smokers (OR=0.71; 95% CI: 0.64, 0.79), whereas it was associated with 19% and 17% lower risk of CRC among individuals with below the median of pack-years of smoking (OR=0.81; 95% CI: 0.71, 0.92) and above the median of pack-years (OR=0.83; 95% CI: 0.74, 0.94), respectively. There was a suggestive interaction between regular use of any NSAID and BMI (P-interaction=0.075), where the association between any NSAID use and CRC risk was attenuated with increasing BMI (normal: OR=0.69, 95% CI: 0.63, 0.77; overweight: OR=0.76, 95% CI: 0.70, 0.83; obese: OR=0.85, 95% CI: 0.75, 0.96). This possible interaction was primarily driven by aspirin (P-interaction=0.074). The association of regular use of aspirin on CRC risk was stronger among individuals with normal BMI (OR=0.75; 95% CI: 0.67, 0.84) and overweight (OR=0.75; 95% CI: 0.68, 0.83), and statistically non-significant among the obese (OR=0.93; 95% CI: 0.80, 1.08). No other interactions between NSAIDs and other risk factors of CRC were observed in meta-analyses. Similar results were observed using multiple imputation for missing values in covariates for both BMI and smoking (Supplemental Table S1). The interaction between regular use of aspirin and smoking remained statistically significant after multiple imputation (p-interaction=0.021). There was still suggestive interaction between regular use of any NSAIDs and BMI on CRC risk (p-interaction=0.078). We examined the effect modification of smoking and BMI on the association between NSAID use and CRC, stratified by sex (Table 4). Results for interactions were stronger among men for interaction between aspirin use and BMI (P-interaction=0.024), and between use of aspirin and smoking (P-interaction=0.097). While the direction of effect modifications was similar in women as men, the tests for interaction were non-significant.
Table 2.
Interactions Between Regular Use of NSAIDs and Demographic and Lifestyle Factors in Relation to Colorectal Cancer Risk
| Any NSAID | Aspirin | Non-aspirin NSAIDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI)a | P value | Cases | Controls | OR (95% CI) a | P value | Cases | Controls | OR (95% CI) a | P value | |||
| Age, years | ||||||||||||||
| <70 | 6,518 | 8,213 | 0.74 (0.68, 0.80) | <0.001 | 6,467 | 8,181 | 0.79 (0.72, 0.87) | <0.001 | 6,337 | 7,910 | 0.75 (0.67, 0.84) | <0.001 | ||
| >=70 | 5,376 | 7,786 | 0.76 (0.70, 0.82) | <0.001 | 5,321 | 7,733 | 0.76 (0.69, 0.83) | <0.001 | 5,195 | 7,593 | 0.79 (0.70, 0.90) | <0.001 | ||
| P value for interactionb | 0.672 | 0.320 | 0.954 | |||||||||||
| Sexc | ||||||||||||||
| Male | 3,993 | 5,355 | 0.68 (0.61, 0.75) | <0.001 | 3,943 | 5,314 | 0.72 (0.65, 0.80) | <0.001 | 3,970 | 5,337 | 0.70 (0.59, 0.83) | <0.001 | ||
| Female | 3,262 | 3,231 | 0.71 (0.63, 0.80) | <0.001 | 3,222 | 3,194 | 0.69 (0.60, 0.79) | <0.001 | 3,238 | 3,213 | 0.80 (0.68, 0.95) | 0.009 | ||
| P value for interactionb | 0.963 | 0.436 | 0.309 | |||||||||||
| BMI, kg/m2 | ||||||||||||||
| Normal (18.5–24.9) | 4,113 | 6,311 | 0.69 (0.63, 0.77) | <0.001 | 4,080 | 6,286 | 0.75 (0.67, 0.84) | <0.001 | 3,944 | 6,028 | 0.72 (0.61, 0.84) | <0.001 | ||
| Overweight (25–29.9) | 4,827 | 6,322 | 0.76 (0.70, 0.83) | <0.001 | 4,783 | 6,284 | 0.75 (0.68, 0.83) | <0.001 | 4,663 | 6,139 | 0.80 (0.70, 0.91) | 0.001 | ||
| Obese (≥30) | 2,647 | 2,957 | 0.85 (0.75, 0.96) | 0.006 | 2,623 | 2,939 | 0.93 (0.80, 1.08) | 0.361 | 2,621 | 2,928 | 0.79 (0.67, 0.93) | 0.005 | ||
| P value for interactionb | 0.075 | 0.074 | 0.967 | |||||||||||
| Smoking, pack-yearsd | ||||||||||||||
| Non-smoker | 4,902 | 6,930 | 0.71 (0.65, 0.77) | <0.001 | 4,854 | 6,889 | 0.71 (0.64, 0.79) | <0.001 | 4,882 | 6,911 | 0.74 (0.65, 0.84) | <0.001 | ||
| ≤ median | 2,934 | 4,211 | 0.79 (0.70, 0.88) | <0.001 | 2,913 | 4,192 | 0.81 (0.71, 0.92) | 0.002 | 2,915 | 4,204 | 0.79 (0.67, 0.93) | 0.005 | ||
| > median | 3,444 | 4,053 | 0.77 (0.69, 0.86) | <0.001 | 3,412 | 4,030 | 0.83 (0.74, 0.94) | 0.004 | 3,434 | 4,040 | 0.78 (0.66, 0.91) | 0.002 | ||
| P value for interactionb | 0.167 | 0.048 | 0.459 | |||||||||||
| Physical activity | ||||||||||||||
| Quartile 1 | 2,092 | 2,574 | 0.63 (0.54, 0.72) | <0.001 | 2,047 | 2,538 | 0.65 (0.55, 0.76) | <0.001 | 1,999 | 2,455 | 0.70 (0.57, 0.86) | 0.001 | ||
| Quartile 2 | 1,724 | 2,289 | 0.74 (0.63, 0.86) | <0.001 | 1,721 | 2,286 | 0.73 (0.61, 0.86) | <0.001 | 1,556 | 2,016 | 0.84 (0.66, 1.07) | 0.154 | ||
| Quartile 3 | 1,484 | 2,258 | 0.77 (0.65, 0.90) | 0.001 | 1,474 | 2,246 | 0.75 (0.63, 0.91) | 0.003 | 1,471 | 2,256 | 0.80 (0.62, 1.03) | 0.084 | ||
| Quartile 4 | 1,399 | 1,681 | 0.73 (0.61, 0.88) | 0.001 | 1,382 | 1,668 | 0.78 (0.64, 0.96) | 0.018 | 1,340 | 1,610 | 0.66 (0.50, 0.87) | 0.003 | ||
| P value for interactionb | 0.218 | 0.263 | 0.894 | |||||||||||
| CRC family history | ||||||||||||||
| Yes | 1,955 | 1,941 | 0.77 (0.66, 0.90) | 0.001 | 1,941 | 1,926 | 0.81 (0.67, 0.97) | 0.023 | 1,948 | 1,935 | 0.90 (0.71, 1.13) | 0.363 | ||
| No | 9,325 | 13,117 | 0.74 (0.69, 0.79) | <0.001 | 9,236 | 13,049 | 0.76 (0.70, 0.81) | <0.001 | 9,285 | 13,090 | 0.75 (0.69, 0.83) | <0.001 | ||
| P value for interactionb | 0.659 | 0.764 | 0.143 | |||||||||||
| History of endoscopy | ||||||||||||||
| Yes | 4,595 | 6,100 | 0.75 (0.69, 0.82) | <0.001 | 4,544 | 6,049 | 0.78 (0.70, 0.86) | <0.001 | 4,560 | 6,084 | 0.75 (0.65, 0.86) | <0.001 | ||
| No | 6,166 | 8,321 | 0.73 (0.67, 0.79) | <0.001 | 6,117 | 8,290 | 0.76 (0.69, 0.83) | <0.001 | 6,160 | 8,305 | 0.79 (0.70, 0.88) | <0.001 | ||
| P value for interactionb | 0.900 | 0.886 | 0.679 | |||||||||||
| Diabetes | ||||||||||||||
| Yes | 954 | 877 | 0.73 (0.59, 0.92) | 0.007 | 953 | 877 | 0.77 (0.60, 0.98) | 0.031 | 954 | 877 | 0.60 (0.42, 0.87) | 0.007 | ||
| No | 7,366 | 11,153 | 0.76 (0.71, 0.82) | <0.001 | 7,351 | 11,146 | 0.81 (0.75, 0.88) | <0.001 | 7,360 | 11,148 | 0.78 (0.71, 0.87) | <0.001 | ||
| P value for interactionb | 0.442 | 0.442 | 0.674 | |||||||||||
| PMH use in womene | ||||||||||||||
| Yes | 2,002 | 3,362 | 0.87 (0.77, 0.99) | 0.035 | 1,985 | 3,342 | 0.92 (0.78, 1.09) | 0.304 | 2,000 | 3,354 | 0.89 (0.76, 1.05) | 0.178 | ||
| No | 4,259 | 4,909 | 0.75 (0.68, 0.83) | <0.001 | 4,224 | 4,889 | 0.75 (0.66, 0.85) | <0.001 | 4,241 | 4,896 | 0.75 (0.65, 0.87) | 0.001 | ||
| P value for interactionb | 0.147 | 0.178 | 0.242 | |||||||||||
CRC: colorectal cancer; PMH: postmenopausal hormone; BMI: body mass index
Study-specific ORs and 95% CIs are estimated using logistic regression models, adjusting for age, sex, education (less than high school, high school graduate or GED, some college, college graduate, graduate degree), first-degree family history of colorectal cancer (yes/no), history of endoscopy (yes/no), postmenopausal hormone use among women (yes/no), history of diabetes(yes/no), body mass index (kg/m2), moderate/vigorous activity (hours/week), smoking (non-smokers and quartiles of pack-years), alcohol intake (none, 1–28g/day, >28g/day), dietary intakes (quartiles) of fruit, vegetables, red meat, processed meat and fiber, total energy intake (quartiles), total (dietary and supplemental) intakes of calcium and folate (quartiles). Covariates in quartiles are adjusted as group linear variables in the model. For aspirin or non-aspirin NSAID use only, the other type was also adjusted for.
P for interaction based on interaction of dichotomous NSAID variable and linear (trend) effect modifier variable, using fixed-effect meta-analysis. The p values for heterogeneity were all >0.05, except for age. More details are described in methods.
WHI, NHS, HPFS, PHS and PMH were excluded in subgroup and interaction analyses for sex since all participants have the same sex in each study.
PHS was excluded in subgroup and interaction analyses for smoking since cases and controls were matched on smoking status in PHS.
HPFS and PHS were excluded in subgroup and interaction analyses for PMH use in women since all participants were men.
Table 3.
Interactions Between Regular Use of NSAIDs and Dietary Factors in Relation to Colorectal Cancer Risk
| Any NSAID | Aspirin | Non-aspirin NSAIDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI)a | P value | Cases | Controls | OR (95% CI) a | P value | Cases | Controls | OR (95% CI) a | P value | |||
| Alcohol | ||||||||||||||
| Non-drinker | 3,785 | 5,364 | 0.77 (0.70, 0.85) | <0.001 | 3,759 | 5,328 | 0.77 (0.69, 0.87) | <0.001 | 3,720 | 3,720 | 0.78 (0.68, 0.90) | <0.001 | ||
| 1–28g/day | 4,131 | 6,219 | 0.72 (0.66, 0.79) | <0.001 | 4,097 | 6,195 | 0.80 (0.72, 0.88) | <0.001 | 3,890 | 3,890 | 0.74 (0.64, 0.85) | <0.001 | ||
| >28g/day | 1,204 | 1,377 | 0.65 (0.53, 0.80) | <0.001 | 1,188 | 1,373 | 0.66 (0.53, 0.82) | <0.001 | 1,367 | 1,186 | 0.84 (0.61, 1.15) | 0.265 | ||
| P value for interactionb | 0.572 | 0.790 | 0.540 | |||||||||||
| Fruit intake | ||||||||||||||
| Quartile 1 | 2,125 | 3,079 | 0.81 (0.71, 0.92) | 0.002 | 2,108 | 3,060 | 0.84 (0.71, 0.98) | 0.030 | 2,013 | 2,945 | 0.83 (0.69, 1.01) | 0.067 | ||
| Quartile 2 | 4,848 | 5,452 | 0.69 (0.63, 0.77) | <0.001 | 4,805 | 5,431 | 0.76 (0.68, 0.84) | <0.001 | 4,763 | 5,335 | 0.65 (0.56, 0.76) | <0.001 | ||
| Quartile 3 | 2,516 | 3,888 | 0.73 (0.64, 0.82) | <0.001 | 2,493 | 3,867 | 0.72 (0.63, 0.83) | <0.001 | 2,425 | 3,766 | 0.83 (0.70, 1.00) | 0.045 | ||
| Quartile 4 | 1,594 | 2,793 | 0.74 (0.64, 0.86) | <0.001 | 1,581 | 2,775 | 0.80 (0.67, 0.95) | 0.010 | 1,527 | 2,677 | 0.70 (0.56, 0.87) | 0.001 | ||
| P value for interactionb | 0.428 | 0.337 | 0.120 | |||||||||||
| Vegetable intake | ||||||||||||||
| Quartile 1 | 1,889 | 2,785 | 0.73 (0.63, 0.84) | <0.001 | 1,868 | 2,770 | 0.81 (0.69, 0.96) | 0.014 | 1,787 | 2,668 | 0.74 (0.60, 0.90) | 0.003 | ||
| Quartile 2 | 5,122 | 5,817 | 0.73 (0.66, 0.80) | <0.001 | 5,086 | 5,786 | 0.78 (0.70, 0.87) | <0.001 | 5,017 | 5,686 | 0.69 (0.59, 0.80) | <0.001 | ||
| Quartile 3 | 2,501 | 3,869 | 0.72 (0.63, 0.81) | <0.001 | 2,476 | 3,853 | 0.73 (0.63, 0.84) | <0.001 | 2,408 | 3,741 | 0.74 (0.62, 0.89) | 0.001 | ||
| Quartile 4 | 1,623 | 2,771 | 0.83 (0.71, 0.95) | 0.009 | 1,607 | 2,754 | 0.78 (0.66, 0.93) | 0.006 | 1,567 | 2,658 | 0.91 (0.74, 1.11) | 0.354 | ||
| P value for interactionb | 0.234 | 0.881 | 0.119 | |||||||||||
| Fiber intake | ||||||||||||||
| Quartile 1 | 1,516 | 2,356 | 0.69 (0.60, 0.81) | <0.001 | 1,500 | 2,342 | 0.71 (0.59, 0.86) | <0.001 | 1,509 | 2,353 | 0.81 (0.66, 1.00) | 0.048 | ||
| Quartile 2 | 1,532 | 2,411 | 0.82 (0.71, 0.95) | 0.009 | 1,516 | 2,395 | 0.82 (0.68, 0.98) | 0.027 | 1,529 | 2,407 | 0.87 (0.71, 1.07) | 0.173 | ||
| Quartile 3 | 1,337 | 2,407 | 0.79 (0.68, 0.92) | 0.002 | 1,323 | 2,390 | 0.84 (0.70, 1.01) | 0.063 | 1,334 | 2,404 | 0.78 (0.63, 0.97) | 0.027 | ||
| Quartile 4 | 1,405 | 2,415 | 0.83 (0.71, 0.96) | 0.015 | 1,390 | 2,403 | 0.76 (0.63, 0.91) | 0.003 | 1,403 | 2,413 | 0.90 (0.73, 1.11) | 0.328 | ||
| P value for interactionb | 0.142 | 0.495 | 0.559 | |||||||||||
| Red meat intake | ||||||||||||||
| Quartile 1 | 2,739 | 4,239 | 0.76 (0.67, 0.85) | <0.001 | 2,712 | 4,219 | 0.84 (0.73, 0.96) | 0.012 | 2,653 | 4,109 | 0.71 (0.58, 0.86) | <0.001 | ||
| Quartile 2 | 3,041 | 4,110 | 0.73 (0.65, 0.82) | <0.001 | 3,011 | 4,087 | 0.78 (0.68, 0.89) | <0.001 | 2,953 | 3,989 | 0.72 (0.61, 0.86) | <0.001 | ||
| Quartile 3 | 2,922 | 3,714 | 0.72 (0.64, 0.81) | <0.001 | 2,905 | 3,696 | 0.69 (0.60, 0.79) | <0.001 | 2,817 | 3,579 | 0.79 (0.67, 0.94) | 0.009 | ||
| Quartile 4 | 2,439 | 3,251 | 0.75 (0.66, 0.85) | <0.001 | 2,417 | 3,235 | 0.78 (0.67, 0.91) | 0.001 | 2,367 | 3,159 | 0.79 (0.66, 0.95) | 0.012 | ||
| P value for interactionb | 0.484 | 0.146 | 0.876 | |||||||||||
| Processed meat intake | ||||||||||||||
| Quartile 1 | 1,795 | 2,693 | 0.74 (0.64, 0.86) | <0.001 | 1,779 | 2,675 | 0.81 (0.68, 0.96) | 0.014 | 1,719 | 2,576 | 0.74 (0.58, 0.93) | 0.011 | ||
| Quartile 2 | 3,325 | 5,045 | 0.74 (0.67, 0.82) | <0.001 | 3,301 | 5,032 | 0.79 (0.70, 0.89) | <0.001 | 3,210 | 4,884 | 0.74 (0.64, 0.86) | <0.001 | ||
| Quartile 3 | 2,019 | 2,877 | 0.76 (0.67, 0.87) | <0.001 | 2,006 | 2,865 | 0.77 (0.65, 0.90) | 0.001 | 1,956 | 2,799 | 0.81 (0.66, 0.98) | 0.028 | ||
| Quartile 4 | 1,993 | 2,392 | 0.71 (0.61, 0.82) | <0.001 | 1,974 | 2,375 | 0.68 (0.57, 0.81) | <0.001 | 1,929 | 2,293 | 0.81 (0.66, 1.00) | 0.051 | ||
| P value for interactionb | 0.508 | 0.181 | 0.627 | |||||||||||
| Total calcium intake | ||||||||||||||
| Quartile 1 | 2,602 | 3,172 | 0.71 (0.63, 0.81) | <0.001 | 2,581 | 3,159 | 0.73 (0.63, 0.85) | <0.001 | 2,514 | 3,054 | 0.79 (0.65, 0.96) | 0.015 | ||
| Quartile 2 | 3,737 | 4,583 | 0.72 (0.65, 0.81) | <0.001 | 3,697 | 4,548 | 0.75 (0.66, 0.86) | <0.001 | 3,637 | 4,452 | 0.69 (0.58, 0.82) | <0.001 | ||
| Quartile 3 | 2,806 | 4,193 | 0.81 (0.72, 0.91) | <0.001 | 2,782 | 4,171 | 0.85 (0.74, 0.97) | 0.016 | 2,706 | 4,063 | 0.80 (0.67, 0.96) | 0.014 | ||
| Quartile 4 | 1,983 | 3,266 | 0.72 (0.63, 0.82) | <0.001 | 1,965 | 3,252 | 0.77 (0.66, 0.90) | 0.001 | 1,916 | 3,154 | 0.72 (0.60, 0.88) | 0.001 | ||
| P value for interactionb | 0.726 | 0.896 | 0.644 | |||||||||||
| Total folate intake | ||||||||||||||
| Quartile 1 | 1,608 | 2,540 | 0.72 (0.62, 0.84) | <0.001 | 1,584 | 2,530 | 0.74 (0.62, 0.89) | 0.001 | 1,603 | 2,535 | 0.82 (0.67, 1.01) | 0.061 | ||
| Quartile 2 | 3,375 | 4,679 | 0.82 (0.73, 0.91) | <0.001 | 3,342 | 4,640 | 0.88 (0.77, 1.00) | 0.044 | 3,096 | 4,295 | 0.73 (0.61, 0.87) | <0.001 | ||
| Quartile 3 | 1,851 | 3,086 | 0.79 (0.69, 0.91) | 0.001 | 1,833 | 3,067 | 0.80 (0.68, 0.94) | 0.007 | 1,786 | 2,989 | 0.82 (0.67, 1.00) | 0.051 | ||
| Quartile 4 | 1,467 | 2,589 | 0.79 (0.68, 0.91) | 0.001 | 1,452 | 2,574 | 0.79 (0.67, 0.95) | 0.009 | 1,463 | 2,586 | 0.83 (0.68, 1.01) | 0.058 | ||
| P value for interactionb | 0.679 | 0.848 | 0.703 | |||||||||||
Study-specific ORs and 95% CIs are estimated using logistic regression models, adjusting for age, sex, education (less than high school, high school graduate or GED, some college, college graduate, graduate degree), first-degree family history of colorectal cancer (yes/no), history of endoscopy (yes/no), postmenopausal hormone use among women (yes/no), history of diabetes(yes/no), body mass index (kg/m2), moderate/vigorous activity (hours/week), smoking (non-smokers and quartiles of pack-years), alcohol intake (none, 1–28g/day, >28g/day), dietary intakes (quartiles) of fruit, vegetables, red meat, processed meat and fiber, total energy intake (quartiles), total (dietary and supplemental) intakes of calcium and folate (quartiles). Covariates in quartiles are adjusted as group linear variables in the model. For aspirin or non-aspirin NSAID use only, the other type was also adjusted for.
P for interaction based on interaction of dichotomous NSAID variable and linear (trend) effect modifier variable, using fixed-effect meta-analysis. The p values for heterogeneity were all >0.05, except for processed meat. More details are described in methods.
Table 4.
Interaction Between Regular Use of NSAIDs and BMI/smoking in Relation to Colorectal Cancer by Sex
| Any NSAID | Aspirin | Non-aspirin NSAIDs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI)a | P value | Cases | Controls | OR (95% CI) a | P value | Cases | Controls | OR (95% CI) a | P value | |||
| Men | ||||||||||||||
| BMI, kg/m2 | ||||||||||||||
| Normal (18.5–24.9) | 1,403 | 2,413 | 0.65 (0.55, 0.77) | <0.001 | 1,390 | 2,412 | 0.68 (0.57, 0.81) | <0.001 | 1,245 | 2,150 | 0.73 (0.53, 1.01) | 0.061 | ||
| Overweight (25–29.9) | 2,473 | 3,302 | 0.68 (0.60, 0.77) | <0.001 | 2,446 | 3,284 | 0.71 (0.62, 0.81) | <0.001 | 2,323 | 3,125 | 0.68 (0.55, 0.85) | 0.001 | ||
| Obese (≥30) | 982 | 1,104 | 0.93 (0.74, 1.18) | 0.560 | 972 | 1,093 | 1.07 (0.84, 1.36) | 0.587 | 960 | 1,082 | 0.67 (0.48, 0.92) | 0.014 | ||
| P value for interactionb | 0.058 | 0.024 | 0.546 | |||||||||||
| Smoking, pack-yearsc | ||||||||||||||
| Non-smoker | 1,587 | 2,512 | 0.62 (0.53, 0.73) | <0.001 | 1,572 | 2,495 | 0.67 (0.56, 0.79) | <0.001 | 1,452 | 2,312 | 0.50 (0.38, 0.67) | <0.001 | ||
| ≤ median | 1,443 | 2,064 | 0.79 (0.66, 0.93) | 0.005 | 1,429 | 2,052 | 0.79 (0.66, 0.94) | 0.007 | 1,420 | 2,044 | 0.87 (0.67, 1.15) | 0.372 | ||
| > median | 1,658 | 2,012 | 0.69 (0.58, 0.81) | <0.001 | 1,638 | 2,002 | 0.77 (0.66, 0.92) | 0.003 | 1,636 | 1,988 | 0.71 (0.54, 0.93) | 0.012 | ||
| P value for interactionb | 0.143 | 0.097 | 0.075 | |||||||||||
| Women | ||||||||||||||
| BMI, kg/m2 | ||||||||||||||
| Normal (18.5–24.9) | 2,710 | 3,888 | 0.73 (0.64, 0.82) | <0.001 | 2,690 | 3,874 | 0.82 (0.70, 0.95) | 0.010 | 2,699 | 3,878 | 0.72 (0.60, 0.87) | 0.001 | ||
| Overweight (25–29.9) | 2,354 | 3,020 | 0.84 (0.74, 0.95) | 0.007 | 2,337 | 3,000 | 0.81 (0.69, 0.95) | 0.010 | 2,340 | 3,014 | 0.86 (0.72, 1.03) | 0.093 | ||
| Obese (≥30) | 1,665 | 1,853 | 0.83 (0.71, 0.98) | 0.024 | 1,651 | 1,846 | 0.88 (0.72, 1.08) | 0.217 | 1,661 | 1,846 | 0.86 (0.71, 1.06) | 0.161 | ||
| P value for interactionb | 0.458 | 0.852 | 0.631 | |||||||||||
| Smoking, pack-yearsc | ||||||||||||||
| Non-smoker | 3,443 | 4,612 | 0.76 (0.69, 0.85) | <0.001 | 3,410 | 4,588 | 0.76 (0.66, 0.87) | <0.001 | 3,430 | 4,599 | 0.82 (0.71, 0.95) | 0.010 | ||
| ≤ median | 1,504 | 2,165 | 0.79 (0.67, 0.93) | 0.004 | 1,497 | 2,158 | 0.85 (0.69, 1.04) | 0.119 | 1,495 | 2,160 | 0.75 (0.61, 0.93) | 0.008 | ||
| > median | 1,803 | 2,056 | 0.85 (0.73, 1.00) | 0.045 | 1,791 | 2,043 | 0.93 (0.77, 1.13) | 0.453 | 1,798 | 2,052 | 0.82 (0.66, 1.01) | 0.067 | ||
| P value for interactionb | 0.628 | 0.333 | 0.898 | |||||||||||
Study-specific ORs and 95% CIs are estimated using logistic regression models, adjusting for age, sex, education (less than high school, high school graduate or GED, some college, college graduate, graduate degree), first-degree family history of colorectal cancer (yes/no), history of endoscopy (yes/no), postmenopausal hormone use among women (yes/no), history of diabetes(yes/no), body mass index (kg/m2), moderate/vigorous activity (hours/week), smoking (non-smokers and quartiles of pack-years), alcohol intake (none, 1–28g/day, >28g/day), dietary intakes (quartiles) of fruit, vegetables, red meat, processed meat and fiber, total energy intake (quartiles), total (dietary and supplemental) intakes of calcium and folate (quartiles). Covariates in quartiles are adjusted as group linear variables in the model. For aspirin or non-aspirin NSAID use only, the other type was also adjusted for.
P for interaction based on interaction of dichotomous NSAID variable and linear (trend) effect modifier variable, using fixed-effect meta-analysis. All p values for heterogeneity were >0.05. More details are described in methods.
PHS was excluded in subgroup and interaction analyses for smoking since cases and controls were matched on smoking status in PHS.
Because there were significant differences in the main associations of NSAID use on CRC risk between case-control and cohort studies (Figure 1), we evaluated whether the effect modification of smoking and BMI differed by study type. The interaction terms for smoking and aspirin were almost identical for case-control (Interaction OR=1.08; 95% CI: 0.97, 1.21) and cohort studies (Interaction OR=1.07; 95% CI: 0.98, 1.18; between-group p-heterogeneity=0.95). Similarly, the interaction terms for BMI and any NSAIDs were similar for case-control (Interaction OR=1.12; 95% CI: 0.94, 1.34) and cohort studies (Interaction OR=1.09; 95% CI: 0.95, 1.26; between-study p-heterogeneity=0.82). However, the interaction terms for BMI and aspirin use appeared to differ between case-control (Interaction OR=1.17; 95% CI: 1.03, 1.33) and cohort studies (Interaction OR=1.02; 95% CI: 0.92, 1.12; between-group P-heterogeneity=0.085).No statistically significant differences in the associations between regular use of NSAIDs and CRC risk were observed between cancer subsites or stages (Supplemental Table S2).
Discussion
Consistent with evidence from randomized clinical trials and observational studies, regular use of aspirin and/or non-aspirin NSAIDs was statistically significantly associated with lower risk of CRC in this large consortium study. The association remained statistically significant among almost all the population subgroups stratified by other CRC risk factors.
We found a statistically significant interaction between regular use of aspirin and smoking, where regular use of aspirin was associated with a larger decrease in CRC risk among non-smokers, than among smokers. Similar to our findings, recent clinical trials among patients with colorectal adenomas suggested that aspirin was associated with lower risk of colorectal adenomas among non-smokers, but not among current smokers (20–22). In a large randomized trial of low-dose aspirin in combination with the calcium supplements among patients with colorectal adenomas, the treatment was suggested to be protective against adenoma recurrence among nonsmokers, but was associated with higher risk of recurrence among current smokers (20). Similar interactions were observed in two small trials of colorectal adenomas in Asian populations such that the protective effect of low-dose aspirin was abrogated among current smokers (21,22). A cross-sectional study of colonoscopy patients also found that daily NSAID use was associated with lower risk of colorectal polyps among non-smokers, but not among current smokers (29). However, a cohort study reported no statistically significant interaction between NSAID use and smoking on CRC risk (16). In contrast, a case-control study found that current NSAID use was associated with larger decrease in CRC risk among individuals who smoked for >40 years than among non-smokers (19).
The mechanisms by which smoking modifies the preventive effect of NSAIDs on CRC risk remain unclear. Cigarette smoking was found to be more strongly associated with colorectal tumors that arise from non-conventional pathways, such as serrated polyp pathway (30,31). Smoking status was found to be significantly associated with risk of advanced serrated polyps in a screening population (32). Smoking is also associated with CRC that are more likely to be microsatellite instability (MSI) positive (19), a hallmark of the serrated polyp pathway (33). Pooled analysis of three randomized trials to prevent serrated polyps found that aspirin use was only significantly associated with a lower risk of polyps in the right colon, whereas smoking was associated with an increased risk of polyps in the left colon (34), suggesting that aspirin and smoking may be associated with different tumor subsites. In addition, it was previously reported that smoking was strongly associated with increased risk of aspirin resistance (35), probably due to smoking-induced platelet hyper-reactivity (36). It is likely that the effect of aspirin is dependent on different carcinogenesis pathways of colorectal tumors among smokers and non-smokers.
Although the NSAID-CRC association was similar in men and women, we found that the interaction between aspirin and smoking status was statistically significant among men only. No previous study has reported this sex-difference. Men had higher cumulative levels of smoking than women (means: 29.7 pack-years among men; 24.1 pack-years among women), which allowed a larger window for interactions between aspirin and smoking. In addition, there were approximately 20% women that were PMH users in our study, and NSAIDs were previously shown to be associated with lower colon cancer risk among PMH non-users only, but not among PMH users (23), which was also suggested in our study (Table 2). Thus, the sex-difference of the interaction between aspirin and smoking may also be partially due to PMH use among women.
We also found a suggestion of interaction between NSAID use and BMI, by which regular use of any NSAID was associated with the lowest relative risk of CRC among individuals with normal BMI, followed by overweight, and the protective effect of NSAIDs was least among obese individuals. Consistently, a slightly more pronounced protection of regular NSAID use on the prevalence of left-sided colorectal adenomas was observed among individuals with normal BMI than among those who were overweight or obese (p-interaction=0.09), in a multi-center cancer screening trial (37). However, cohort studies observed no interaction between BMI and aspirin on colon cancer risk (14–16,18). This could be due to the fact that previous studies combined the overweight and obese subgroups or had imprecise estimates for three BMI categories due to small sample sizes. Individuals with higher BMI has higher chronic inflammation levels and it has been proposed that NSAIDs inhibit PGE2 synthesis and chronic inflammation to inhibit tumor development (38). High doses of salicylates were also shown to reverse insulin resistance in obese rodents (39), which could otherwise contribute to tumor development (40). In addition, NSAIDs and obesity may both act through the gut microbiome on colorectal tumorigenesis. NSAIDs inhibits inflammatory cytokines and mucin secretion (41), which may shape the composition of gut microbiota (42), whereas obesity was observed to disrupt microbial composition in the gut and promotes colorectal cancer in mice (43). However, our data suggested that the benefit of NSAIDs is attenuated, rather than enhanced as expected, among obese people. It is possible that larger dose, higher frequency, and longer duration of NSAID use are needed to reduce the elevated CRC risk among individuals with higher BMI.
Our study suggested that only aspirin, rather than non-aspirin NSAIDs, interacted with BMI or smoking on CRC risk, which may be partially explained by unique mechanisms of actions of aspirin that are not shared by other NSAIDs. Low-dose aspirin has shown to be associated with lower risk of CRC in randomized trials, suggesting the antiplatelet effect of aspirin may also play a role in the inhibition of colorectal tumor cells (1). In addition, aspirin can also acetylate COX-2 to synthesize anti-tumorigenic “aspirin-triggered lipoxin” (ATL), which is anti-inflammatory and inhibits carcinoma cell proliferation (44). The generation of ATL by aspirin was also observed at low, antiplatelet doses of aspirin in a small intervention study of healthy humans (45).
Our study has several strengths. First, we had larger sample size and therefore greater statistical power for interaction analyses than prior studies. Secondly, we had detailed assessment for most of the CRC risk factors from all participating studies, a characteristic not seen in previous meta-analyses, which allowed us to perform systematic analyses on potential effect modification. In addition, we were able to adjust for potential confounders in all the analyses, whereas meta-analyses using published data have had limited control for confounding. Furthermore, we used an iterative harmonization process on all the environmental variables across all participating studies to reduce the level of heterogeneity and the impact of outliers.
There are also limitations. As all the environmental factors were assessed via questionnaires and varied across studies, there may be measurement errors in the main NSAID exposures and the covariates. For example, the definition of “regular use of NSAIDs” varied across studies, ranging from current use to ≥4 days/week for ≥1 year. However, despite these differences, there was minimal evidence of heterogeneity across studies in the interaction analyses. Secondly, the main associations of NSAIDs on CRC were stronger in case-control studies than nested case-control studies from cohort studies. This stronger main effect in case-control studies could be due to more accurate exposure assessment of NSAID use in these studies, compared to that in cohort studies where NSAID use may have started or been discontinued in the long term period between exposure assessment and CRC diagnosis, leading to attenuation of the association.In addition, there might have been recall bias in the case-control studies, which could have spuriously exaggerated the effect of NSAIDs on CRC risk. Compared to the meta-analysis of randomized trials of aspirin and CRC risk (1), our results fro case-control studies were stronger and those from cohort studies were weaker, with an almost identical overall estimate. Case-control studies may also be more susceptible to selection bias in that the response of participants may be jointly influenced by NSAID use, CRC status and effect modifier status. However, the odds ratios for our results of interaction of smoking with aspirin use and of BMI with any NSAID use showed no evidence of heterogeneity between study types. We did not have information on the indicators for NSAIDs use nor the contraindications for use, such as ulcers, so the possibility for unadjusted confounding remains. Furthermore, all the participants were of European ancestry; therefore, our results may not be applicable to other race/ethnicity groups. Lastly, we acknowledge that the observed interactions were of borderline statistical significance, and we did not adjust for multiple testing in the analysis.
To our knowledge, this is the largest study to systematically analyze the interactions between NSAID use and other risk factors in relation to CRC risk. Regular use of NSAIDs, including both aspirin and non-aspirin NSAIDs was statistically significantly protective against CRC risk in almost all subgroups. We observed stronger associations between aspirin and CRC risk among non-smokers than among smokers. We also found a suggestive interaction between any NSAID use and BMI on CRC risk, primarily driven by aspirin. Our results suggested that aspirin may have different effects on CRC prevention within the general population, depending on other CRC risk factors. The beneficial effects of use aspirin on CRC risk appears to be attenuated, rather than enhanced, among those with greater CRC risk due to obesity and heavy smoking, making it unlikely that these groups would benefit from use aspirin for the prevention of CRC. Our results warrant further evaluation on both validation of observed interactions and risk-benefit evaluation of aspirin use in CRC prevention.
Supplementary Material
Significance:
Obesity and heavy smoking attenuate the benefit of aspirin use for colorectal cancer prevention.
Acknowledgements
GECCO (Genetics and Epidemiology of Colorectal Cancer Consortium) is supported by grants from the National Cancer Institute (NCI), National Institutes of Health (NIH), U.S. Department of Health and Human Services (U01 CA137088 and R01 CA059045 to U. Peters). COLO2&3 (Hawai’i Colorectal Cancer Studies 2 & 3) is supported by grant from the NIH (R01 CA60987 to L. Le Marchand). DACHS (Darmkrebs: Chancen der Verhutüng durch Screening) is supported by grants from the German Research Council (Deutsche Forschungsgemeinschaft, BR 1704/6–1, BR 1704/6–3, BR 1704/6–4 and CH 117/1–1 to M. Hoffmeister), and the German Federal Ministry of Education and Research (01KH0404 and 01ER0814 to M.Hoffmeister). DALS (Diet, Activity and Lifestyle Survey) is supported by grant from the NIH (R01 CA48998 to P.A. Slattery). HPFS (Health Professionals Follow-up Study) is supported by grants from the NIH (P01 CA055075 to G.A. Colditz; UM1 CA167552 to W.C. Willett; R01 CA137178 , and P50 CA127003 to A.T. Chan; R01 CA151993 and R35 CA197735 to S. Ogino; K07 CA190673 to R. Nishihara), NHS (Nurses’ Health Study) by grants from the NIH (R01 CA137178 and P50 CA127003 to A.T. Chan; P01 CA087969 to G.A. Colditz; UM1 CA186107 to M. Stampfer; R01 CA151993 and R35 CA197735 to S. Ogino; K07 CA190673 to R. Nishihara) and PHS (Physician’s Health Study) by grants from the NIH (R01 CA042182 to J. Ma). MEC (Multiethnic Cohort Study) is supported by grants from the NIH (R37 CA54281 and P01 CA033619 to L.N. Kolonel; and R01 CA63464 to B.E. Henderson). OFCCR is supported by grants from the NIH, through funding allocated to the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783 to S. Gallinger). PLCO (Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial) is supported by grants from the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. PMH-CCFR (Postmenopausal Hormone Study-Colon Cancer Family Registry) is supported by grant from the NIH (R01 CA076366 to P.A. Newcomb). VITAL (VITamins And Lifestyle) is supported by grant from the NIH (K05 CA154337 to E.White). WHI (Women’s Health Initiative) is supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. X.Wang and E. White are also supported by grant from the NCI (R25 CA094880).
The authors would also like to thank all those at the GECCO Coordinating Center for helping bring together the data and people that made this project possible. The authors also acknowledge Deanna Stelling, Mark Thornquist, Greg Warnick, Carolyn Hutter, and team members at COMPASS (Comprehensive Center for the Advancement of Scientific Strategies) at the Fred Hutchinson Cancer Research Center for their work harmonizing the GECCO epidemiological data set.
Footnotes
Conflicts of Interest: There is no conflict of interest.
References
- 1.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50 [DOI] [PubMed] [Google Scholar]
- 2.Flossmann E, Rothwell PM, British Doctors Aspirin T, the UKTIAAT. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603–13 [DOI] [PubMed] [Google Scholar]
- 3.Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, et al. Aspirin for the Prevention of Cancer Incidence and Mortality: Systematic Evidence Reviews for the U.S. Preventive Services Task Force. Annals of internal medicine 2016;164:814–25 [DOI] [PubMed] [Google Scholar]
- 4.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nature reviews Cancer 2006;6:130–40 [DOI] [PubMed] [Google Scholar]
- 5.Bos CL, Kodach LL, van den Brink GR, Diks SH, van Santen MM, Richel DJ , et al. Effect of aspirin on the Wnt/beta-catenin pathway is mediated via protein phosphatase 2A. Oncogene 2006;25:6447–56 [DOI] [PubMed] [Google Scholar]
- 6.Nan H, Morikawa T, Suuriniemi M, Imamura Y, Werner L, Kuchiba A, et al. Aspirin use, 8q24 single nucleotide polymorphism rs6983267, and colorectal cancer according to CTNNB1 alterations. Journal of the National Cancer Institute 2013;105:1852–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan MR, Chang HC, Hung WC. Non-steroidal anti-inflammatory drugs suppress the ERK signaling pathway via block of Ras/c-Raf interaction and activation of MAP kinase phosphatases. Cellular signalling 2008;20:1134–41 [DOI] [PubMed] [Google Scholar]
- 8.Seufert BL, Poole EM, Whitton J, Xiao L, Makar KW, Campbell PT, et al. IkappaBKbeta and NFkappaB1, NSAID use and risk of colorectal cancer in the Colon Cancer Family Registry. Carcinogenesis 2013;34:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich CM, White E, et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. Jama 2015;313:1133–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen V, Vogel U. Systematic review: interactions between aspirin, and other nonsteroidal anti-inflammatory drugs, and polymorphisms in relation to colorectal cancer. Alimentary pharmacology & therapeutics 2014;40:147–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brasky TM, Potter JD, Kristal AR, Patterson RE, Peters U, Asgari MM, et al. Non-steroidal anti-inflammatory drugs and cancer incidence by sex in the VITamins And Lifestyle (VITAL) cohort. Cancer causes & control : CCC 2012;23:431–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW, Jr. Aspirin use and risk of fatal cancer. Cancer research 1993;53:1322–7 [PubMed] [Google Scholar]
- 13.Ye X, Fu J, Yang Y, Chen S. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: a meta-analysis of published cohort studies. PloS one 2013;8:e57578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friis S, Poulsen AH, Sorensen HT, Tjonneland A, Overvad K, Vogel U, et al. Aspirin and other non-steroidal anti-inflammatory drugs and risk of colorectal cancer: a Danish cohort study. Cancer causes & control : CCC 2009;20:731–40 [DOI] [PubMed] [Google Scholar]
- 15.Allison M, Garland C, Chlebowski R, Criqui M, Langer R, Wu L, et al. The association between aspirin use and the incidence of colorectal cancer in women. American journal of epidemiology 2006;164:567–75 [DOI] [PubMed] [Google Scholar]
- 16.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. The American journal of gastroenterology 2011;106:1340–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Peters U, Potter JD, White E. Association of Nonsteroidal Anti-Inflammatory Drugs with Colorectal Cancer by Subgroups in the VITamins and Lifestyle (VITAL) Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2015;24:727–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Smith-Warner SA, Chan AT, Wu K, Spiegelman D, Fuchs CS , et al. Aspirin use, body mass index, physical activity, plasma C-peptide, and colon cancer risk in US health professionals. American journal of epidemiology 2011;174:459–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chia VM, Newcomb PA, Bigler J, Morimoto LM, Thibodeau SN, Potter JD. Risk of microsatellite-unstable colorectal cancer is associated jointly with smoking and nonsteroidal anti-inflammatory drug use. Cancer research 2006;66:6877–83 [DOI] [PubMed] [Google Scholar]
- 20.Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Aspirin, Calcitriol, and Calcium Do Not Prevent Adenoma Recurrence in a Randomized Controlled Trial. Gastroenterology 2016;150:114–22 e4 [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa H, Mutoh M, Suzuki S, Tokudome S, Saida Y, Abe T, et al. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut 2014;63:1755–9 [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura T, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med 2013;2:50–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slattery ML, Murtaugh MA, Quesenberry C, Caan BJ, Edwards S, Sweeney C. Changing population characteristics, effect-measure modification, and cancer risk factor identification. Epidemiologic perspectives & innovations : EP+I 2007;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grau MV, Baron JA, Barry EL, Sandler RS, Haile RW, Mandel JS, et al. Interaction of calcium supplementation and nonsteroidal anti-inflammatory drugs and the risk of colorectal adenomas. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005;14:2353–8 [DOI] [PubMed] [Google Scholar]
- 25.Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics 2006;38:904–9 [DOI] [PubMed] [Google Scholar]
- 27.Hutter CM, Chang-Claude J, Slattery ML, Pflugeisen BM, Lin Y, Duggan D, et al. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer research 2012;72:2036–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics 1954;10:101–29 [Google Scholar]
- 29.Drew DA, Goh G, Mo A, Grady JJ, Forouhar F, Egan G, et al. Colorectal polyp prevention by daily aspirin use is abrogated among active smokers. Cancer causes & control : CCC 2016;27:93–103 [DOI] [PubMed] [Google Scholar]
- 30.JE IJ, Vermeulen L, Meijer GA, Dekker E. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 2015;12:401–9 [DOI] [PubMed] [Google Scholar]
- 31.Figueiredo JC, Crockett SD, Snover DC, Morris CB, McKeown-Eyssen G, Sandler RS, et al. Smoking-associated risks of conventional adenomas and serrated polyps in the colorectum. Cancer Causes Control 2015;26:377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.JE IJ, Bossuyt PM, Kuipers EJ, Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Smoking status informs about the risk of advanced serrated polyps in a screening population. Endosc Int Open 2016;4:E73–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol 2005;124:380–91 [DOI] [PubMed] [Google Scholar]
- 34.Wallace K, Grau MV, Ahnen D, Snover DC, Robertson DJ, Mahnke D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009;18:2310–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirkhel A, Peyster E, Sundeen J, Greene L, Michelson AD, Hasan A, et al. Frequency of aspirin resistance in a community hospital. The American journal of cardiology 2006;98:577–9 [DOI] [PubMed] [Google Scholar]
- 36.Levine PH. An acute effect of cigarette smoking on platelet function. A possible link between smoking and arterial thrombosis. Circulation 1973;48:619–23 [DOI] [PubMed] [Google Scholar]
- 37.Johnson CC, Hayes RB, Schoen RE, Gunter MJ, Huang WY, Team PT. Non-steroidal anti-inflammatory drug use and colorectal polyps in the Prostate, Lung, Colorectal, And Ovarian Cancer Screening Trial. The American journal of gastroenterology 2010;105:2646–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez ME, Heddens D, Earnest DL, Bogert CL, Roe D, Einspahr J, et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. Journal of the National Cancer Institute 1999;91:950–3 [DOI] [PubMed] [Google Scholar]
- 39.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001;293:1673–7 [DOI] [PubMed] [Google Scholar]
- 40.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Archives of physiology and biochemistry 2009;115:86–96 [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K, Suemasu S, Ishihara T, Tasaka Y, Arai Y, Mizushima T. Inhibition of both COX-1 and COX-2 and resulting decrease in the level of prostaglandins E2 is responsible for non-steroidal anti-inflammatory drug (NSAID)-dependent exacerbation of colitis. European journal of pharmacology 2009;603:120–32 [DOI] [PubMed] [Google Scholar]
- 42.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfalzer AC, Nesbeth PD, Parnell LD, Iyer LK, Liu Z, Kane AV, et al. Diet- and Genetically-Induced Obesity Differentially Affect the Fecal Microbiome and Metabolome in Apc1638N Mice . PloS one 2015;10:e0135758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Molecular medicine 1996;2:583–96 [PMC free article] [PubMed] [Google Scholar]
- 45.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. Journal of immunology 2009;183:2089–96 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


