Supplemental digital content is available in the text.
Key Words: pancreatic adenocarcinoma, long-term survival, surgical resection, chemotherapy, differentiation, race/ethnicity
Abstract
Objectives
Pancreatic cancer continues to carry a poor prognosis with survival rates that have had minimal improvement over the past 4 decades. We report a population-based, comprehensive analysis of long-term survivors of pancreatic adenocarcinoma diagnosed in the diverse population of California.
Methods
Data from the California Cancer Registry were used to evaluate long-term survival. A total of 70,442 patients diagnosed with pancreatic adenocarcinoma between 1988 and 2009 were identified. Logistic regression was used to identify factors associated with achieving 5-year survival.
Results
The overall 5-year survival was 2.5%, with minimal incremental improvements throughout the 3 decades. Age, stage, degree of differentiation, and surgical resection were associated with 5-year survival. Furthermore, younger age and receiving care at a National Cancer Institute–designated cancer center were similarly correlated with 5-year survival regardless of surgical intervention. In addition, we identified stage, differentiation, and adjuvant chemotherapy as significant factors for long-term survival in surgically resected patients. In the unresectable patients, Asian/Pacific islanders and Hispanics were significantly more likely to reach the 5-year milestone than non-Hispanic whites.
Conclusions
Although pancreatic cancer mortality remains high, our study highlights baseline characteristics, treatment, biological factors, and ethnicity that are associated with long-term survival. These findings may serve as a springboard for further investigation.
Pancreatic cancer is the third leading cause of cancer-related deaths in the United States. More than 53,000 new cases and over 41,000 deaths occur annually.1 Moreover, it is estimated to become the second-leading cause of cancer death in the United States by 2020.2 Despite the various advances in multimodality treatment, long-term survival rates continue to range between 8% and 10%.1,3,4 Surgical resection remains the only potentially curative modality for patients with pancreatic cancer. However, only 15% to 20% of patients qualify for resection, because most tumors are locally advanced or metastatic at the time of diagnosis. Nevertheless, patients who undergo successful resection and adjuvant therapy have 5-year survival rates of approximately 20%, with a median survival time of 25 to 30 months.2,3,5,6
Therefore, there is a pressing need to decipher the underlying elements responsible for the long-term survival of patients with pancreatic cancer including genetic, immune and molecular mechanisms, as well as clinical outcome and racial-socioeconomic disparities. To date, there are very few population-based survival studies analyzing long-term survival of patients with pancreatic adenocarcinoma.7–9 However, these analyses are limited and do not include data from the most recent decade, nor they do investigate racial-socioeconomic disparities. Racial and socioeconomic disparities have been increasingly highlighted recently, due to growing evidence of these inequalities in various aspects of the US health care system.10,11 Therefore, we report a population-based, comprehensive analysis of long-term survivors of pancreatic adenocarcinoma diagnosed from 1988 to 2009 in the diverse population of California.
MATERIALS AND METHODS
The California Cancer Registry (CCR) is a state-mandated registry that was established in 1985 and routinely abstracts demographic, tumor, and treatment data on all cases diagnosed in the state. The 4 regional registries comprising the CCR are part of the national Surveillance, Epidemiology, and End Results (SEER) program and case ascertainment is estimated to be greater than 95% complete.12 We obtained data from the CCR on all cases diagnosed with invasive pancreatic cancer between January 1, 1988, and December 31, 2009 (n = 70,442), excluding cases diagnosed on death certificate or autopsy (n = 2067), without valid dates of diagnosis or follow-up (n = 1384), without microscopically confirmed tumors (n = 15,133), with histologies other than adenocarcinoma (n = 11,839), and who were not identified as non-Hispanic white, non-Hispanic black, Hispanic, or Asian/Pacific islander (n = 213).
The CCR routinely performs follow-up on patient vital status through hospital follow-up as well as regular linkages to other national and state databases, including state and national vital statistics, Social Security Administration, credit agencies, and the Department of Motor Vehicles. Vital status follow-up was complete through December 31, 2014. We calculated survival time in months from date of diagnosis to date of last contact or date of death, if deceased. Cases with less than 60 months survival time who were alive at the time of last follow-up, but whose vital status had not been confirmed as of December 31, 2014 (n = 257) were excluded as being lost to follow-up. Median follow-up for cases that were not deceased was 8.5 years (102.0 months; range, 60.1–315.5 months). The final cohort consisted of 39,460 patients.
For each patient, CCR abstracts age, sex, race/ethnicity, anatomic site of tumor, histology, extent of disease at diagnosis, first course of cancer-directed therapy (chemotherapy, radiation, and surgery), reporting hospital, and census-block group of residence at time of diagnosis. Anatomic subsite and histology were defined by specific ICD-O-3 topography and morphology codes, respectively. Adenocarcinomas were identified based on the following ICD-O-3 codes: 8140–8144, 8190, 8211, 8261–8263, 8290, 8310, 8440, 8500, 8503, 8560, 8570. Stage at diagnosis was defined using SEER summary stage (localized, regional, distant, unknown). SEER localized stage represents tumor confined to the pancreas, regional stage represents tumor with direct extension to adjacent organs or structures or spread to regional lymph nodes, and distant stage represents involvement of distant sites or lymph nodes. Because cancer registries generally do not collect individual-level information on patient socioeconomic status (SES), we determined the patient’s neighborhood SES using a previously described index13,14 that incorporates census measures of education, income, occupation, and cost of living. Each patient was assigned a quintile of neighborhood SES based on the neighborhood distribution of residents in California. Patients with a missing block group were randomly assigned to a block group within their county of residence.
The CCR identifies patients who have been diagnosed and/or treated at one of California’s National Cancer Institute (NCI)–designated cancer centers. National Cancer Institute cancer center designation can be viewed as a proxy for facilities with greater availability of specialized care and higher patient and procedure volumes.
We compared demographic and tumor characteristics between 5-year survivors and those surviving less than 5 years, using χ2 and t-tests, as appropriate. Kaplan-Meier curves of overall mortality were compared using log rank test. Logistic regression analysis was used to identify factors associated with the binary outcome of achieving the 5-year survival milestone. In this exploratory analysis, we modeled the effects with both univariate and multivariate regression of the following variables on the outcome: race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific islander), sex, age (continuous), SES quintile, anatomic tumor location (head, body, tail, overlapping/not otherwise specified), SEER summary stage (localized, regional, distant, unknown), receipt of care at an NCI-designated cancer center (yes/no), surgical resection with curative intent (yes/no), receipt of radiation (yes/no), receipt of chemotherapy (yes/no), and year of diagnosis (1988–1995, before any chemotherapy approval, 1996–2003, post approval of gemcitabine in 1996, and 2004–2009, post the final result of ESPAC-1 trial). We repeated the analysis stratified on surgical resection. All analyses were performed using SAS 9.4 (SAS Institute, Cary, Ind).
RESULTS
We identified 70,442 patients who were diagnosed with pancreatic adenocarcinoma between 1988 and 2009 in the CCR. Of those patients, 39,460 meet our inclusion criteria, and 987 (2.5%) survived at least 5 years (Table 1). For all patients, the median age at diagnosis was 69 years, with a similar distribution by sex. The majority of patients were non-Hispanic white (70%), followed by Hispanics (14.1%), Asian/Pacific islanders (8.4%), and non-Hispanic black (7.9%). Approximately half of the cases (48.7%) did not have pathological differentiation documented; of the documented cases, 23.3% were poorly differentiated or anaplastic tumors and 21.1% were moderately differentiated. As defined by SEER data, 7.2% of patients had localized disease, 34.9% had regional disease, and 50.4% of patients had distant disease, the remainder 7.5% had unknown SEER stage. Sixteen percent of all cases underwent surgical resection, 18% had radiation, and 41% received chemotherapy. Most patients (85%) did not receive care at an NCI designated cancer center. Yearly analysis of treatment modalities from 1988 to 2009 stratified by stage demonstrated interesting trends. The percentage of patients with regional disease who received surgery and systemic therapy increased since 1988, and the proportion of untreated patients decreased. Furthermore, the percentage of patients with distant disease who received chemotherapy and/or radiation treatment increased after the year 1996, whereas the percentage of nontreated patients decreased (Supplemental Fig. 1, http://links.lww.com/MPA/A664).
TABLE 1.
Characteristics of Cases With Microscopically Confirmed Pancreatic Adenocarcinoma Diagnosed in California, 1988–2009
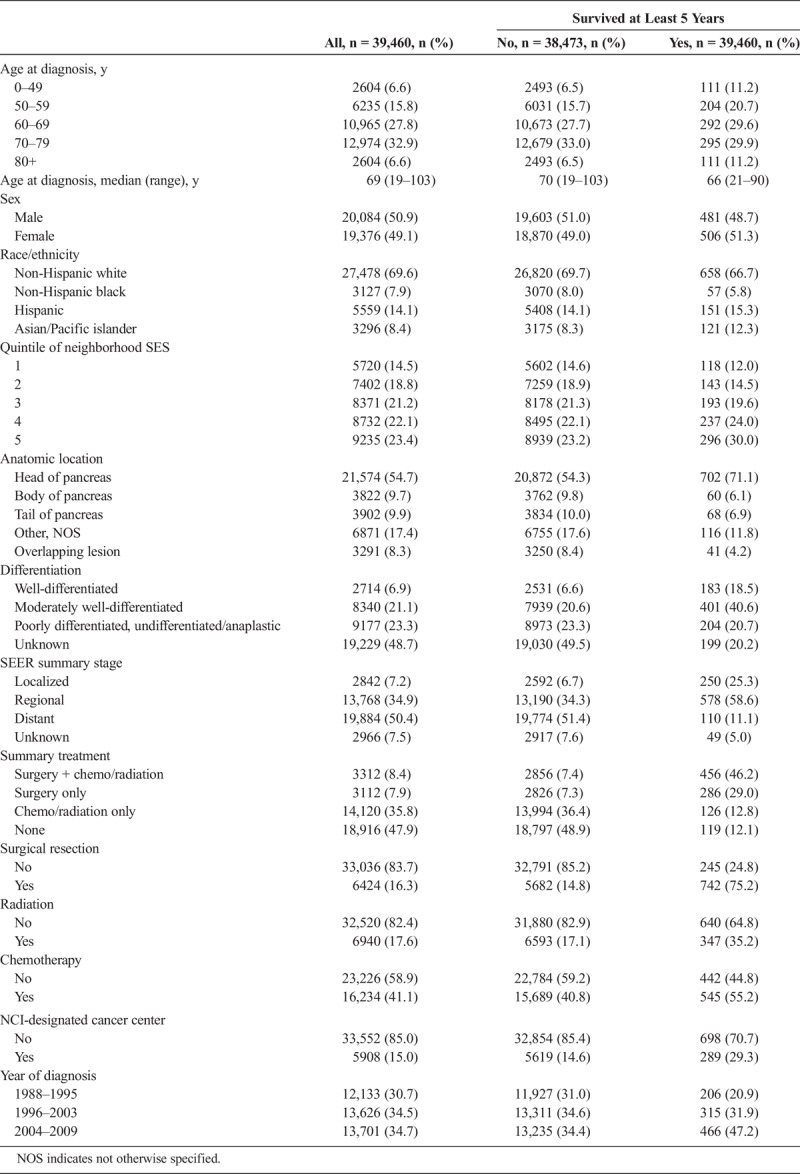
Long-term survivors (defined as alive after 5 years) were significantly younger at diagnosis, median age of 66 years, as compared with those who died before 5 years (median age of 70 years), and a greater proportion were of an Asian/Pacific islander or a Hispanic ethnic background. Furthermore, tumors of long-term survivors were more likely to be well or moderately differentiated, and the majority were located at the head of the pancreas. As expected, a greater proportion of long-term survivors had localized disease at diagnosis. Interestingly though, distant disease did not preclude long-term survival, as approximately 11% of long-term survivors presented with distant disease.
The majority of long-term survivors (75.2%) underwent surgical resection or resection combined with chemotherapy and radiation, as compared with those patients who did not survive 5 years (15%). Long-term survivors were twice as likely to have had radiation as a combined modality with chemotherapy with or without surgery (35% vs 17%) and were more likely to have received chemotherapy (55% vs 41%). In addition, long-term survivors were roughly twice as likely to have been seen at an NCI-designated cancer center (29% vs 15%) and have been diagnosed in recent years. Approximately half of patients who died within 5 years received no cancer-directed therapy. This may reflect the era of treatment as no treatment was available before 1996 when gemcitabine was the first chemotherapy to be approved (Supplemental Table 1, http://links.lww.com/MPA/A665).
On further analysis using univariate logistic regression models, localized disease and surgical intervention were the most significant predictors of long-term survival (Table 2). Despite a reduction in effect size after adjustment for other covariates in the multivariate model, both stage and surgery remained the strongest predictors of long-term survival. Surgically resected patients were over 10 times more likely to reach the 5-year milestone than unresected patients (odds ratio [OR], 10.7; 95% confidence interval [CI], 8.83–13.01), although the effect of localized disease (OR, 6.41; 95% CI, 4.95–8.30) was smaller than that of surgery after the adjustment. Median survival of our cohort stratified by surgical resection was 13.7 months (95% CI, 13.1–14.0), compared with a mere 3.7 months (95% CI, 3.7–3.8) for unresectable patients (Fig. 1). Furthermore, chemotherapy and radiation were also associated with 5-year survival in a univariate analysis. However, after adjustment for other tumor and sociodemographic factors in a multivariate analysis, only chemotherapy maintained a significant association (OR, 1.33; 95% CI, 1.12–1.57) with long-term survival.
TABLE 2.
Associations of Patient, Tumor, and Facility Characteristics With 5-Year Survival
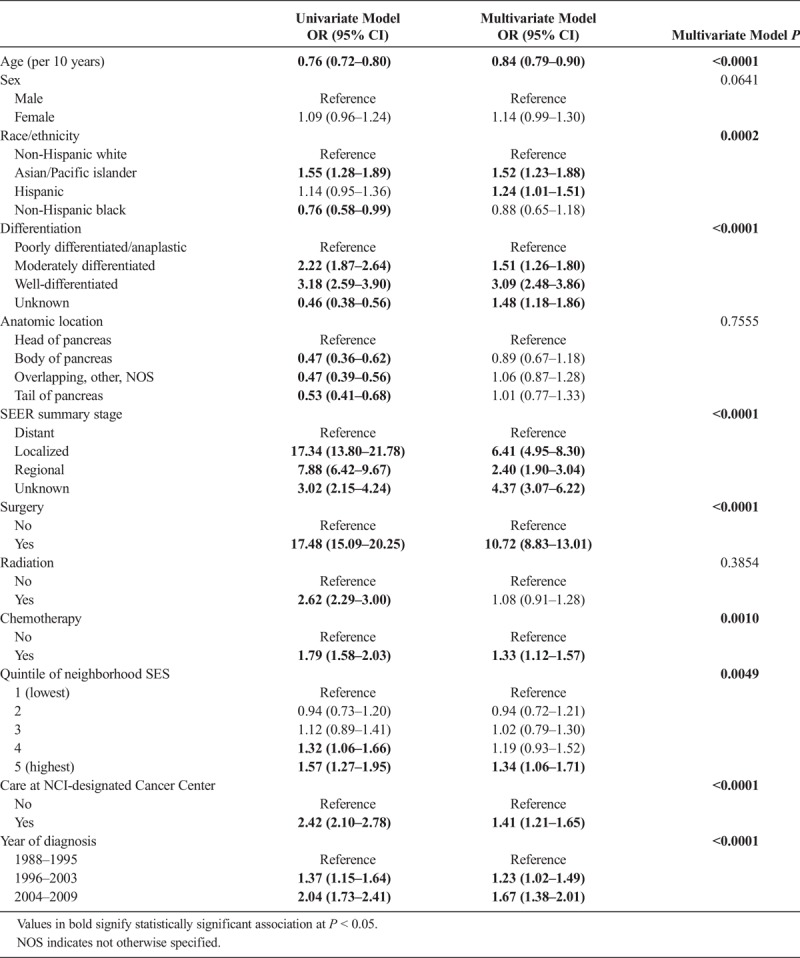
FIGURE 1.
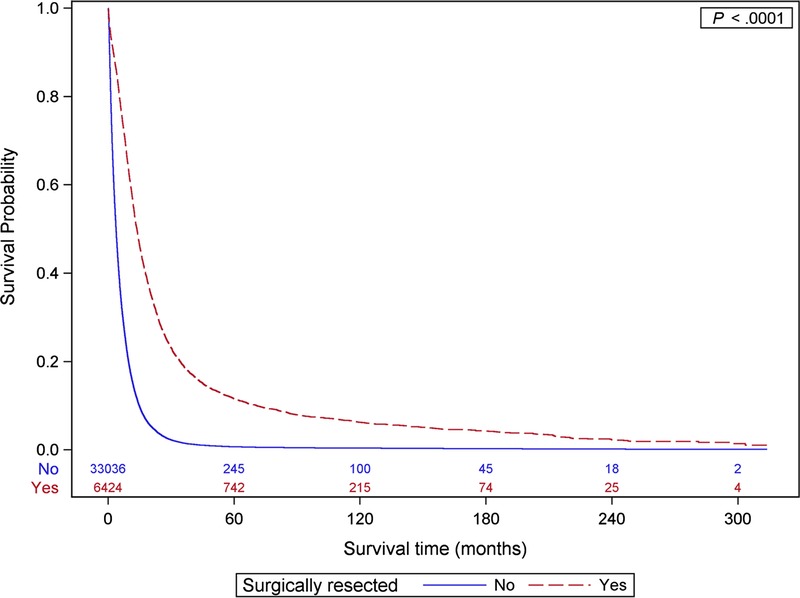
Overall survival by surgical resection.
Well-differentiated tumors were also highly associated with long-term survival (OR, 3.09; 95% CI, 2.48–3.86); however, anatomic location of the tumor was not significantly associated with long-term survival after adjusting for stage. Later year of diagnosis was associated with long-term survival (OR, 1.23; 95% CI, 1.02–1.49 for diagnoses in 1996–2003 vs 1988–1995 and OR, 1.67; 95% CI, 1.38–2.01 for patients diagnosed in 2004–2009 vs those diagnosed in 1988–1995), as was receipt of care at an NCI-designated cancer center (OR, 1.41; 95% CI, 1.21–1.65).
Asian/Pacific islanders and Hispanics were more likely than non-Hispanic whites to achieve long-term survival (OR, 1.52; 95% CI, 1.23–1.88; OR, 1.24; 95% CI, 1.01–1.51, respectively). Older age was correlated with a decreased likelihood of reaching 5-year survival (OR, 0.84 per 10 years; 95% CI, 0.79–0.90), but there was no significant effect of sex. Although the effect was slightly attenuated after adjustment for other factors, higher neighborhood SES was associated with a greater likelihood of being a 5-year survivor (highest vs lowest neighborhood SES, OR, 1.34; 95% CI, 1.06–1.71).
Given that surgery is one of the strongest predictors of survival, and the fact that patients with resectable disease differ clinically from those of unresectable disease, having significantly higher median survival (Fig. 1), we performed a stratified logistic regression analysis, revealing some interesting differences in the associations with long-term survival between the surgical and nonsurgical groups (Table 3). One of the most significant associations noted was for stage, which was over 3 times as large for localized resectable patients (OR, 12.19; 95% CI, 7.68–19.36) than localized unresectable patients (OR, 3.73; 95% CI, 2.39–5.80). Similarly, but to a lesser extent, differentiation was also associated with long-term survival. The effect of well versus poorly differentiated tumors was larger in resected versus unresected cases (OR, 3.29; 95% CI, 2.56–4.25 in resected patients and OR, 2.53; 95% CI, 1.62–3.95 in unresected patients). Chemotherapy was also associated with 5-year survival, but only in the adjuvant setting (OR, 1.46; 95% CI, 1.19–1.80); however, radiation was not associated with long-term survival in resected patients (OR, 1.04; 95% CI, 0.85–1.28).
TABLE 3.
Associations of Patient, Tumor, and Facility Characteristics With 5-Year Survival, Stratified by Surgical Resection
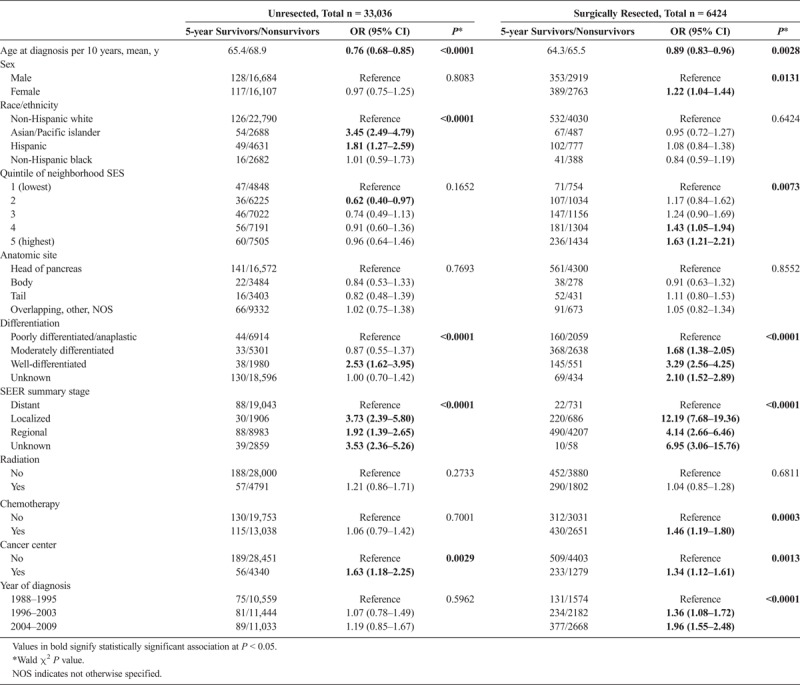
Similarly, the later year of diagnosis (OR, 1.36; 95% CI, 1.08–1.72 for 1996–2003 vs 1998–1995 and OR, 1.96; 95% CI, 1.55–2.48 for 2004–2009 vs 1988–1995), neighborhood SES (OR, 1.63; 95% CI, 1.21–2.21 for highest vs lowest quintile), and female sex (OR, 1.22; 95% CI, 1.04–1.44) were only associated with long-term survival in surgically resected cases.
Although race/ethnicity was not associated with 5-year survival in surgically resected cases, it proved to be one of the most significant factors associated with 5-year survival in unresectable disease. Asian/Pacific islanders with unresectable disease were more than 3 times (OR, 3.45; 95% CI, 2.49–4.79) as likely as non-Hispanic whites to survive over 5 years after diagnosis, and Hispanics were almost twice (OR, 1.81; 95% CI, 1.27–2.59) as likely. The magnitude of the effect of Asian race/ethnicity was on par with that of localized stage. Lastly, receiving care at an NCI-designated cancer center (OR, 1.34; 95% CI, 1.12–1.61 resected and OR, 1.63; 95% CI, 1.18–2.25 unresected) and younger age (OR per 10 years, 0.89; 95% CI, 0.83–0.96 resected and OR, 0.76; 95% CI, 0.68–0.85 unresected) were similarly associated with greater likelihood of 5-year survival for both groups.
DISCUSSION
In our study, the crude overall long-term survival, defined as 5 years or longer, of patients diagnosed with pancreatic adenocarcinoma in California between 1988–2009 was 2.5%. This is similar to the percentage reported by Lambe et al15 using population-based data from a Swedish registry and Zijlstra et al16 from the Netherlands cancer registry; however, lower than the 6% to 10% noted in other SEER and European databases.3,17 These differences may be explained by differences in methodology between the studies. Although we, Lambe et al, and Zijstra et al measured long-term survival as the proportion of patients confirmed to be alive 5 years after diagnosis, Bouvier et al and Sirri et al reported 5-year relative survival, which is a theoretical measure that estimates the probability of survival at 5 years in the absence of other causes of death. Regardless of the actual percentage of 5-year survival, the fact remains that in the general population, long-term survival of pancreatic adenocarcinoma has remained discouragingly low. Therefore, understanding the characteristics these individuals share may help lead to improved outcomes in the population.
Similar to previous results from the United States and international population-based cancer registries,1,3,18,19 we found that age, stage, degree of differentiation, and surgical resection were associated with 5-year survival. Surgery is regarded as the only potentially curative option for pancreatic adenocarcinoma, and in our analysis, it was the factor most strongly associated with long-term survival. However, we also identified a subset of long-term survivors that did not undergo resection with curative intent. Because the clear majority of pancreatic cancer patients do not undergo surgery, we were particularly interested in uncovering unique associations with survivorship in this rare and understudied group of patients.
Using stratified analysis, we found that specific factors such as younger age and receiving care at an NCI-designated cancer center were similarly associated with 5-year survival regardless of surgical intervention, whereas the effect of other prognostic factors differed between the 2 groups. In contrast to the findings in the surgically resected cohort, where clinicopathologic and treatment-related factors were more strongly associated with long-term survival, in unresected patients, race/ethnicity was almost as strongly associated with 5-year survival as stage. In the unresected cohort, Asian/Pacific islanders and Hispanics were significantly more likely to reach the 5-year milestone than non-Hispanic whites.
The improved overall survival associated with Asian race has inconsistently been reported in the literature. Using CCR data, Zell et al20 found that after adjusting for treatment and SES, non-Chinese Asian race was associated with a decreased hazard of death. Gong et al21 reported that Asian/Pacific islander race and receiving any active treatment at the time of diagnosis as independent factors for longer survival in SEER identified residents of San Francisco Bay Area counties. However, other SEER population-based studies have reported comparable overall survival between Asians and whites.22–24 This inconsistency may relate to the lack of adjustment for SES or treatment in the latter studies. In addition, the Asian populations of California and those of other geographic areas within the US may differ in the proportion of foreign-born individuals or in the representation of the various Asian ethnic groups, which may also contribute to the inconsistent associations reported by different studies.
In contrast to Asians, previous studies have reported similar20 or worse25 survival outcomes for pancreatic adenocarcinoma in Hispanics compared with non-Hispanic whites. To our knowledge, we are the first to report an association between Hispanic ethnicity and improved 5-year survival outcomes. This novel finding should be confirmed by future studies.
Both the Asian and Hispanic communities in California have large immigrant populations, and it has been suggested that the survival benefit seen in these populations may be artifactual, the result of loss to follow-up due to return migration of terminally ill patients. To minimize this potential bias, we required confirmation of vital status at 5 years for all nondeceased cases. Therefore, it is unlikely that return migration could explain the improved survival we observed.
It is noteworthy that neither we nor others9,26 found an association with race/ethnicity after controlling for SES in surgically resected patients. Although differences in long-term survival might be attributed to differential access and utilization of care among racial/ethnic groups, it appears unlikely that compared with non-Hispanic whites, Asian, and Hispanic minority patients would have improved access to care, resulting in a survival advantage. Instead, it may reflect biological differences that exist among races, which only become apparent in situations where the disease is unchecked by surgical intervention.
Dong et al27 previously reported differences in K-ras point mutations and p53 expression between Asian and Western populations with pancreatic carcinomas. It is possible that our finding of a positive association of long-term survival with race, but not SES, in patients with unresectable disease reflects racial differences in biological or immunological factors or genetic-immune-environment interactions that influence the disease’s aggressiveness or progression. Such differences may offer clues to the biology of the disease and warrant further investigation.
The need for a better understanding of the biology of this disease is highlighted by the finding that almost 12% of long-term survivors in our study were diagnosed with distant disease. We acknowledge that because of the limited diagnostic workup performed in some cases, especially those that did not undergo surgical resection, some of these patients may have been inaccurately staged, misdiagnosed, or had a more favorable diagnosis such as ampullary, periampullary, or even neuroendocrine tumors. Our inability to conduct histopathologic review is one of the study’s major limitations. To reduce the possibility of biasing our results by including other types of tumors or benign pancreatic masses, we required that all cases have microscopic confirmation of their pancreatic cancer diagnosis.
Although stage and tumor characteristics showed the expected associations with survival in regression analysis, we recognize that the lack of histopathologic review likely resulted in some degree of misclassification, which could have potentially inflated our survival estimates. Nevertheless, it is important to note that the finding of long-term survival in the face of adverse prognostic features is not unique to our study. It is well documented that Asian/Pacific islanders carry the lowest overall cancer incidence and death rates.1 And it has previously been described by several other investigators in surgical case series, including those with pathologic review of specimens,28–30 underscoring the fact that even within the group of long-term survivors there is significant heterogeneity in tumor biology that we do not understand.
Our study has some additional limitations. The registry records limited personal and clinical information, and we could not assess the effects of other potential confounders such as comorbidity, tobacco and alcohol use, family history, performance status, perioperative factors, or tumor molecular characteristics. Further, this study includes only residents of California, who may not be representative of patients in other regions.
Despite these limitations, our study has several key strengths. Ours is the largest cohort of 5-year survivors of pancreatic adenocarcinoma reported to date and is the first to address long-term survival in a surgically unresected population. It is based on cancer registry data that is subject to rigorous follow-up and quality control standards. Most importantly, the population-based nature of our analysis makes our findings generalizable to a much larger population than any prior studies.
Although better patient selection, surgical techniques, and postoperative treatments have benefitted patients with resectable pancreatic adenocarcinoma, it has not translated into a meaningful improvement in outcomes at the population level because too few patients present with disease amenable to resection. Until we develop strategies that lead to earlier diagnosis or therapies that are effective enough to convert the unresectable to resectable, we are unlikely to improve cure rates. The promise of targeting molecular vulnerabilities has been unfulfilled with the exception of rare patients with deficient mismatch repair (~1%–2%) treated with PD-1 blockade31,32 and BRCA mutations (~4%–5%) treated with platinum agents and experimental PARP inhibitors.33,34 Nevertheless, the impressive responses in these small subgroups offer proof of concept that there remain subgroups susceptible to targeted therapy and that the immune system is potent enough to be effective if there are neoantigens to recognize. The key to these developments is achieving a better understanding of the biology and immunology of the disease, and studying this unique cohort of long-term survivors may bring us a step closer to achieving this goal.
Supplementary Material
Footnotes
C.A.C. is now with GRAIL, Inc, Menlo Park, CA.
A.K. and D.Y.L. are co-first authors.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors. Also, this work was supported by the Stanford Cancer Institute, a National Cancer Institute–designated comprehensive cancer center.
The authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Muniraj T, Jamidar PA, Aslanian HR. Pancreatic cancer: a comprehensive review and update. Dis Mon. 2013;59:368–402. [DOI] [PubMed] [Google Scholar]
- 3.Sirri E, Castro FA, Kieschke J, et al. Recent trends in survival of patients with pancreatic cancer in Germany and the United States. Pancreas. 2016;45:908–914. [DOI] [PubMed] [Google Scholar]
- 4.Sun H, Ma H, Hong G, et al. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981–2010. Sci Rep. 2014;4:6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner J, Combs SE, Springfeld C, et al. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10:323–333. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. [DOI] [PubMed] [Google Scholar]
- 7.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14:1320–1326. [DOI] [PubMed] [Google Scholar]
- 8.Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. [DOI] [PubMed] [Google Scholar]
- 9.Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control. 2006;17:403–409. [DOI] [PubMed] [Google Scholar]
- 10.Bradley EH, Sipsma H, Taylor LA. American health care paradox-high spending on health care and poor health. QJM. 2017;110:61–65. [DOI] [PubMed] [Google Scholar]
- 11.de Souza JA, Hunt B, Asirwa FC, et al. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol. 2016;34:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.California Cancer Registry. California Cancer Registry FAQ. http://www.ccrcal.org/Inside_CCR/FAQ.shtml. Accessed June 14, 2013. [Google Scholar]
- 13.Yang J, Schupp CW, Harrati A, et al. Developing an area-based socioeconomic measure from American Community Survey data. Available at: https://cancerregistry.ucsf.edu/sites/cancerregistry.ucsf.edu/files/wysiwyg/Yang%20et%20al.%202014_CPIC_ACS_SES_Index_Documentation_3-10-2014.pdf. Accessed August 2, 2018. [Google Scholar]
- 14.Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. [DOI] [PubMed] [Google Scholar]
- 15.Lambe M, Eloranta S, Wigertz A, et al. Pancreatic cancer; reporting and long-term survival in Sweden. Acta Oncol. 2011;50:1220–1227. [DOI] [PubMed] [Google Scholar]
- 16.Zijlstra M, Bernards N, de Hingh IH, et al. Does long-term survival exist in pancreatic adenocarcinoma? Acta Oncol. 2016;55:259–264. [DOI] [PubMed] [Google Scholar]
- 17.Bouvier AM, Bossard N, Colonna M, et al. Trends in net survival from pancreatic cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev. 2017;26 Trends in cancer net survival in six European Latin Countries: the SUDCAN study: S63–S69. [DOI] [PubMed] [Google Scholar]
- 18.Ferrone CR, Brennan MF, Gonen M, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–706. [DOI] [PubMed] [Google Scholar]
- 19.Han SS, Jang JY, Kim SW, et al. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas. 2006;32:271–275. [DOI] [PubMed] [Google Scholar]
- 20.Zell JA, Rhee JM, Ziogas A, et al. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev. 2007;16:546–552. [DOI] [PubMed] [Google Scholar]
- 21.Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay area, 1995–1999. Am J Epidemiol. 2011;174:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worni M, Guller U, White RR, et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas. 2013;42:1157–1163. [DOI] [PubMed] [Google Scholar]
- 23.Fesinmeyer MD, Austin MA, Li CI, et al. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–1773. [DOI] [PubMed] [Google Scholar]
- 24.Longnecker DS, Karagas MR, Tosteson TD, et al. Racial differences in pancreatic cancer: comparison of survival and histologic types of pancreatic carcinoma in Asians, blacks, and whites in the United States. Pancreas. 2000;21:338–343. [DOI] [PubMed] [Google Scholar]
- 25.Bathe OF, Caldera H, Hamilton-Nelson K, et al. Influence of Hispanic ethnicity on outcome after resection of carcinoma of the head of the pancreas. Cancer. 2001;91:1177–1184. [DOI] [PubMed] [Google Scholar]
- 26.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong M, Nio Y, Tamura K, et al. Ki-ras point mutation and p53 expression in human pancreatic cancer: a comparative study among Chinese, Japanese and Western patients. Cancer Epidemiol Biomarkers Prev. 2000;9:279–284. [PubMed] [Google Scholar]
- 28.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. [DOI] [PubMed] [Google Scholar]
- 31.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Mestier L, Danset JB, Neuzillet C, et al. Pancreatic ductal adenocarcinoma in BRCA2 mutation carriers. Endocr Relat Cancer. 2016;23:T57–T67. [DOI] [PubMed] [Google Scholar]
- 34.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


