Abstract
Obesity associated insulin resistance (IR) is a major risk factor for developing type 2 diabetes (T2D) and an array of other metabolic disorders. In particular, hepatic IR contributes to the increase in hepatic glucose production and consequently the development of fasting hyperglycemia. In this study, we explored whether kaempferol, a flavonoid isolated from Ginko biloba, is able to regulate hepatic gluconeogenesis and blood glucose homeostasis in high-fat diet (HFD) -fed obese mice and further explored the underlying mechanism by which it elicits such effects. Oral administration of kaempferol (50 mg/kg/day), which is the human equivalent dose of 240 mg/day for an average 60 kg human, significantly improved blood glucose control in obese mice, which was associated with reduced hepatic glucose production and improved whole body insulin sensitivity without altering body weight gain, food consumption, or adiposity. In addition, kaempferol treatment increased Akt and hexokinase activity, but decreased pyruvate carboxylase (PC) and glucose-6 phosphatase activity in the liver without altering their protein expression. Consistently, kaempferol decreased PC activity and suppressed gluconeogenesis in HepG2 cells as well as primary hepatocytes isolated from the livers of obese mice. Further, we found that kaempferol is a direct inhibitor of PC. These findings suggest that kaempferol may be a naturally occurring anti-diabetic compound that acts by suppressing glucose production and improving insulin sensitivity. Kaempferol suppression of hepatic gluconeogenesis is due to its direct inhibitory action on the enzymatic activity of PC.
Keywords: Kaempferol, flavonoid, insulin resistance, type 2 diabetes, gluconeogenesis, pyruvate carboxylase
1. Introduction
Type-2 diabetes (T2D) is one of the leading causes of mortality in the United States [1]. In 2015 diabetes prevalence reached 9.4% of the American population [2], and this number is anticipated to double by the year 2050 [3]. There is also a great fiscal burden imposed by diabetes-associated health-care as the estimated annual cost of diagnosed diabetes, in the U.S., is nearly $250 billion [4]. Insulin resistance and β-cell dysfunction are two central components of T2D pathogenesis [5–7]. It is well recognized that T2D is typically prefaced by obesity, although the sequence of events leading to its development remains a matter of debate [5, 6].
Obesity-induced hepatic insulin resistance (IR) is a significant contributor to fasting hyperglycemia [8] as altered regulation of hepatic glucose metabolism results in decreased glycogen synthesis and concurrently increased gluconeogenesis and glycogenolysis [9]. Because of the significant contribution of the liver to whole body glucose homeostasis, the liver is a major target for preventing and treating chronic hyperglycemia and its resultant diabetes. As diabetes continues to develop into a greater fiscal and public health concern [10, 11], investigation into the potential of novel, low-cost, naturally occurring agents that promote insulin sensitivity may be an effective strategy to prevent diabetes and improve the overall health of those already suffering from the disease [12].
Recently, naturally occurring polyphenolic compounds have drawn great interest due to their pharmacological implications, with considerable attention being devoted to their use in diabetes management [13, 14]. Polyphenols exist naturally as secondary plant metabolites, and are the largest source of human dietary antioxidants with a typical daily intake of roughly 1 g/day [15]. One of the most common polyphenolic subclasses is the flavonols [16]. Kaempferol (3,5,7- trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one), a flavonol found in many traditional medicines and edible plants, may possess anti-diabetic activities [17]. We recently showed that dietary supplementation of kaempferol (0.05%) in a high fat diet (HFD) prevented the development of hyperglycemia and improved insulin sensitivity in middle-aged obese mice [18]. However, how kaempferol exerts such an antidiabetic effect are still unclear. In this study, we explored the mechanism underlying the anti-diabetic effect and/or insulin sensitizing effects of kaempferol.
2. Materials and Methods
2.1. Animals and experimental design
C57BL/6 male mice (4 mo-old, Envigo, Indianapolis, IN) were maintained on a 12-h light/dark cycle at constant temperature (22–25 °C) in an animal room with ad libitum access to a standard chow diet (SD) and water. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Virginia Tech. Mice were initially divided into 2 groups (18 mice/group) and fed either a SD, with 10% of calories from fat, or a HFD, with 58% of calories from fat (Research Diets Inc., New Brunswick, NJ), for 8 wks, when animals gradually became obese with significant IR, as determined by measuring their body composition and insulin tolerance. Mice were then divided into 4 groups (9 mice/group) with blood glucose, body weight (BW), and body composition balanced for the same dietary treatment groups, and then received either kaempferol (50 mg/kg/d dissolved in 2% 2-methyl cellulose) or vehicle (2% 2-methyl cellulose) via oral gavage for 6 wks.
2.2. Metabolic studies
Body weight (BW) and food intake were recorded weekly. Non-fasting and fasting blood glucose (15 h fasting) levels were measured weekly in tail vein blood samples using a glucometer (Kroger, Cincinnati, OH). Body composition was evaluated using an LF-90 instrument (Bruker Optics, Inc., Billerica, MA) at 0 and 4 wks after treatment. At 4 and 5 wks after kaempferol treatment, mice were fasted for 15 h and then injected intraperitoneally (IP) with a single dose of pyruvate (2 g/kg BW) or glucose (1.5 g/kg BW) for pyruvate and glucose tolerance tests, respectively. Blood glucose levels were then measured at 0, 30, 60, 90, and 180 min after the administration of pyruvate and at 0, 15, 30, 60, and 120 min post-injection of glucose. For insulin tolerance test (ITT), mice were fasted for 4 h followed by IP injection of insulin (0.75 units/kg BW). Blood glucose levels were then measured at 0, 15, 30, 60, and 120 min after injection of insulin. The area under the curve (AUC) for all tests was calculated using the trapezoidal rule. At the end of the study, the mice were fasted for 15 h and then euthanized. Blood was immediately collected, and multiple organs were isolated, weighed, snap-frozen in liquid nitrogen, and stored at −80 °C for further analyses. Plasma insulin and glucagon levels were measured using an ultrasensitive mouse insulin ELISA kit (Mercodia, Inc., Uppsala, Sweden) and a mouse glucagon ELISA kit (Crystal Chem, Downers Grove, IL), respectively. Liver triglyceride was extracted as described [19], and then measured using an assay kit (Teco Diagnostics, Anaheim, CA). The liver triglyceride contents are expressed as mg of triglyceride per gram of the liver sample.
2.3. Pyruvate and glucose oxidation
Fresh mouse liver samples were used to analyze pyruvate and glucose oxidation as previously described [20, 21] with modifications. Briefly, liver tissues were homogenized in a buffer containing 0.25 M Sucrose, 1 mM EDTA, 0.01 M Tris-HCl, and 2 mM ATP, pH = 7.4. The tissue homogenates were then incubated with either14C-labeled pyruvate for pyruvate oxidation, or14C-labeled glucose (American Radiolabeled Chemicals, St. Louis, MO) for glucose oxidation in a trapping device at 37 °C for 1 h. The14CO2 produced was trapped with 70% perchloric acid, and the resulting sodium hydroxide was collected to assess CO2 production.
2.4 Glycogen content
Mouse liver tissue was weighed, minced, and homogenized in phosphate buffer, pH 7.0. The homogenates were centrifuged (9,500 rpm for 10 min at 4°C) and the supernatant was used to determine glycogen concentration using a glycogen assay kit (Cayman, Ann Arbor, MI).
2.5. Glucose production
Primary mouse hepatocytes or HepG2 cells were maintained in DMEM containing 5 mM glucose supplemented with 1% pen-strep and 10% FBS. To measure glucose production, the cells were treated with kaempferol (0.1, 1, 10, 50 μM) or vehicle in FBS-free, low glucose DMEM for 5 h, washed, and then incubated in glucose production media (glucose- and phenol-free DMEM containing 20 mM sodium lactate and 2 mM sodium pyruvate) in the continued presence or absence of either the vehicle or kaempferol for 3 h. Glucose released in the media was measured using Amplex Red glucose assay kit (Life Technologies, Carlsbad, CA) and normalized to protein content of the same samples.
2.6. Enzyme activity assays
Liver and muscle tissues were homogenized in ice-cold lysis solution and then centrifuged (13,000 rpm for 3 min at 4 °C). Hexokinase activity in the cell lysates was measured using an assay kit (Biomedical Research Services Center, Buffalo, NY) according to manufacturer’s protocol. The test is based on the NADH-coupled reaction that leads to the reduction of tetrazolium salt INT to INT-formazan, which exhibits maximum absorbance at 492 nm. Briefly, the samples were treated with control solution and a reaction solution containing the substrate (20 mM glucose) for 30 min, and the reaction was stopped using 3% acetic acid. Absorbance at 492 nm was measured using a microplate reader. The activity of hexokinase in the cell lysates was calculated using the following equation: IU/L unit= μM/(L.min) = (O.D. × 1000 × 110 μL/ (30 min × 0.6 cm × 18 × 10 μL). Total hexokinase concentrations in the samples were determined by using a mouse hexokinase ELISA kit (Elabscience, Beijing, China).
The enzymatic activity of glucose-6 phosphatase (G6Pase) was measured as described with modifications [22]. This test quantifies liberated inorganic phosphate according to the Taussky-Shorr method [23]. Briefly, the assay buffer and substrate (200 mM glucose-6 phosphate) were mixed and allowed to equilibrate for 5 min followed by the addition of 5 μg of tissue homogenates. This mixture was incubated for 5 min at 37°C and the reaction was then stopped with 20% trichloracetic acid, followed by the addition of 5 μg of tissue homogenate to the blank. The mixtures were incubated at 25°C for 5 min and then centrifuged at 4,000 rpm for 10 min. The supernatant was collected and mixed with Taussky-Shoor color reagent. Following a 6-min incubation at 25°C, the A340nm was determined. The μmoles of inorganic phosphate was calculated according to the following equation: μmoles Pi = ΔA340nm (Test) – b/m.
The units per mg of enzymes was calculated according to the following equation:
Pyruvate carboxylase (PC) activity was measured essentially as described [24]. This coupled assay uses PC in the tissue sample to convert pyruvate to oxaloacetate and then malate dehydrogenase to convert oxaloacetate to malate, with the simultaneous oxidation of NADH, which can be measured spectrophotometrically at 340 nm. The activity of PC was calculated using the following equation:
2.7. Western blot analysis
Tissues or cultured cells were homogenized in cell lysis buffer (Cell Signaling, Danvers, MA) supplemented with protease and phosphatase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA). After centrifugation, equal amounts of protein from cell lysates were resolved using stain-free SDS-PAGE and transferred onto nitrocellulose membranes. Blots were blocked with 5% (w/v) milk protein or BSA/Tris-buffered saline plus 0.1% Tween-20 and then probed with antibodies against phospho-Akt (Ser473) (#9271), Akt (#9272) (Cell Signaling Technology, Inc, Danvers, MA), PEPCK (H-300), G6Pase (H-60), PC (H-300), glucokinase (H-88), and glucokinase regulatory protein (N-19) (Santa Cruz Biotechnology, Inc, Dallas, TX). The total protein content and immunoreactive proteins on the blot were detected and digitally imaged using ChemiDoc™ Touch Imaging System (Bio-Rad, Hercules, CA). Proteins of interest were normalized to total protein content of the same lane on the blot.
2.8. Statistical analysis
The data were analyzed by one-way ANOVA using SigmaPlot® (Version 11 Systate Software Inc., San Jose, California). If significant differences between treatments (P < 0.05) were observed, Duncan’s multiple range test was then performed for pairwise comparisons. Values are expressed as mean ± standard error of mean (SEM).
3. Results
3.1. Kaempferol improved blood glucose control in obese mice
To determine whether kaempferol can reverse or ameliorate IR and further prevent the development of T2D, mice were first fed HFD for 8 wks to induce obesity and IR [25] before kaempferol treatment. Non-fasting blood glucose levels in HF-fed mice with or without kaempferol treatment for 6 wks were not significantly different as comparted to SD-fed mice (Fig. 1A). Obese mice had significantly higher fasting blood glucose levels than those of lean mice (Fig. 1B). However, oral treatment with kaempferol gradually reduced fasting blood glucose levels over time in obese mice. After 4 wks of treatment, kaempferol-treated obese mice displayed lower (P < 0.05) fasting blood glucose levels as compared with the control obese mice (155.6 ± 7.5 mg/dl vs. 183.7 ± 5.6 mg/dl). After 6 wks of treatment, kaempferol reversed fasting blood glucose levels in obese mice comparable to those in SD-fed mice (Fig. 1B). Importantly, kaempferol had no effect on fasting blood glucose of SD-fed lean mice. IR contributes to the development of impaired glucose tolerance (IGT) [26, 27]. Obese mice had IGT as compared to lean mice. Consistently, kaempferol treatment significantly improved glucose tolerance in obese mice but had no effect on glucose tolerance in lean mice (Fig. C, D). IGT is known to be associated with defects in insulin secretion and/or IR [26]. We thus examined whether the better glycemic control in kaempferol treated obese mice was due to improved insulin sensitivity or insulin secretion [26]. We found that obese mice had significantly higher plasma insulin levels after 30 and 60 min of glucose injection than those detected in lean mice, suggesting that HFD-fed mice developed IR. Kaempferol did not modulate either basal insulin levels or glucose-stimulated insulin secretion (Fig. E), indicating that the anti-diabetic effect of kaempferol was not due to altering insulin secretory function of the islets Next, we assessed whole body insulin sensitivity in mice by performing ITT. Obese mice were insulin resistant as compared with lean mice, but kaempferol treatment improved insulin sensitivity in obese mice to the extent that did not significantly differ from that of lean mice (Fig. 1F, G).
Fig. 1.
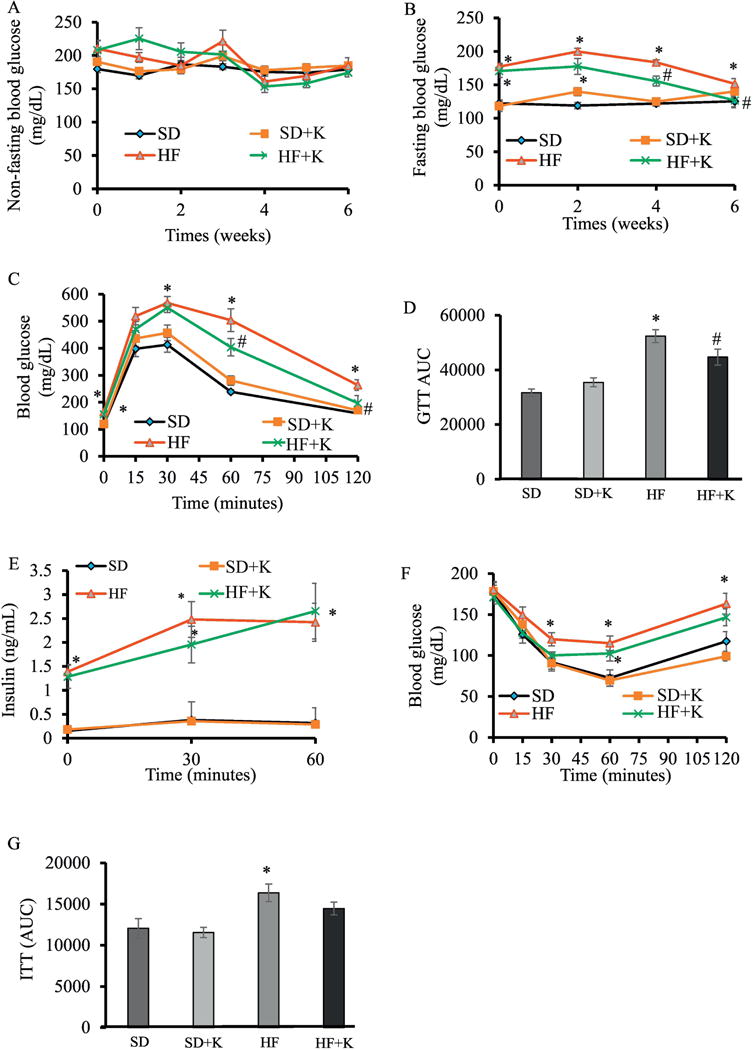
Kaempferol treatment reduced fasting blood glucose, improved glucose tolerance, and improved insulin sensitivity in HF diet-fed mice. Mice (4 mo old, male) were fed either a SD or a HFD for 8 wks, followed by dietary treatment with either kaempferol (50 mg/kg/d) or vehicle via oral gavage for 6 wks. (A) Non-Fasting and (B) fasting blood glucose levels were measured at indicated time points of dietary treatment. Data of intraperitoneal GTT (C) performed as described in the Method section and the area under the curve (AUC) calculated using the trapezoidal rule (D). (E) Blood glucose was withdrawn at time 0, 30, and 60 min after IP glucose injection to measure plasma insulin levels. Results from performing intraperitoneal ITT (F) and the AUC (G). Data are shown as Mean ± SEM (n=9). *, P < 0.05 vs. SD-fed mice (SD); #, P < 0.05 vs. HF-fed mice (HF). SD: SD diet; SD + K: SD diet with kaempferol treatment; HF: HF diet; HF + K: HF diet with kaempferol treatment.
3.2. Kaempferol had no effect on BW, food intake, body composition, or liver triglyceride metabolism of lean or obese mice
The average BW of experimental mice fed HFD (45.4 ± 1.14 g) was higher (P < 0.05) than mice fed SD (32.0 ± 0.89 g) throughout the study. Kaempferol had no effect on BW (Fig. 2A), BW gain (Fig. 2B), or food intake (Fig. 2C) of obese and lean mice. The percentage of body fat for obese mice was significantly higher while lean mass was lower than those of lean mice (Fig. D, E). Kaempferol had no effect on the degree of adiposity in either obese or lean mice. At the end of feeding study, the inguinal and visceral fat were significantly heavier in the obese mice than lean mice (Fig. 2F). Kaempferol had no effect on either fat type. As a compensatory mechanism for IR, prior to the development of overt hyperglycemia and T2D, the overall weight of the pancreas and pancreatic β-cell mass has been shown to increase [28, 29]. Although obese mice in the control group had heavier pancreas than that in lean mice, the difference was not significant, and kaempferol treatment did not alter pancreas weight (Fig. 2G). Similarly, HFD feeding non-significantly increased liver weight in obese mice, which was not affected by kaempferol treatment (Fig. 2H). As expected, HFD treatment significantly increased liver triglyceride contents in obese mice as compared in lean mice. However, kaempferol treatment for 6 wks did not modulate hepatic triglyceride levels (Fig. 2I), suggesting that kaempferol improvement of blood glucose control is not due to a secondary action whereby it affects hepatic triglyceride metabolism in obese mice.
Fig. 2.
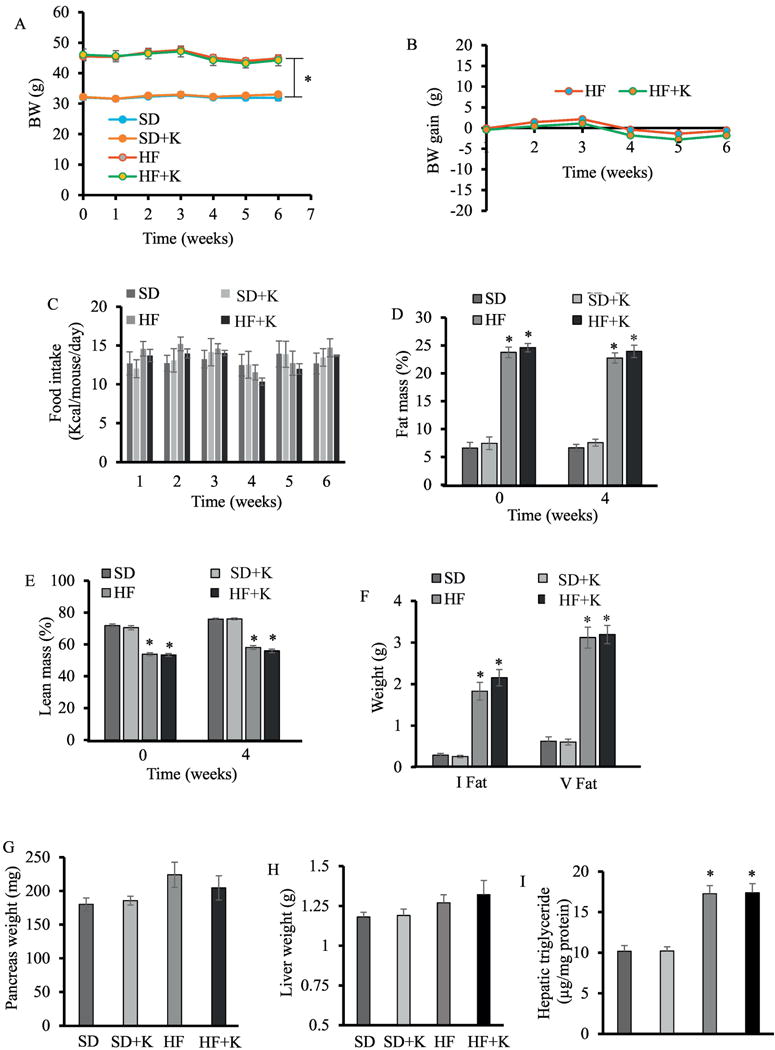
Kaempferol treatment had no significant effect on body weight, food consumption, body composition, pancreas weight, and liver triglyceride metabolism in HF diet-fed mice. (A) Body weight (BW) and BW gain (B) of the individual mouse was measured and calculated weekly. (C) Food intake was recorded each week, and the average daily food intake was calculated. Fat mass (D) and lean mass (E) at 0 and 4 wks after treatment were measured and expressed as percent of BW. At the end of the experiment, inguinal (I fat) and visceral fat (V fat) (F), pancreas (G), and livers (H) were isolated and weighed. (I). Hepatic triglyceride contents were measured by an assay kit. Data are shown as Mean ± SEM (n=9). *, P < 0.05 vs. standard diet-fed mice (SD). SD: SD diet; SD + K: SD with kaempferol treatment; HF: HF diet; HF + K: HF diet with kaempferol treatment.
3.3. Kaempferol increased hepatic Akt and hexokinase activity and glycogen contents but had no effect on pyruvate and glucose oxidation in obese mice
Glucagon regulates glucose homeostasis and counteracts insulin action by increasing glycogenolysis and gluconeogenesis, leading to increased hepatic glucose output [30]. However, circulating glucagon concentrations in fasted mice did not differ between groups (Fig. 3A), suggesting that kaempferol improvement in glucose homeostasis is not due to modulation of circulating glucagon levels. We then evaluated Akt phosphorylation in the liver, as the activation of Akt mediates insulin suppression of gluconeogenesis while increasing glycogen synthesis [30]. We observed that Akt protein levels in the liver of obese mice were higher (P < 0.05) than those of lean mice (Fig. 3B, H). Oral administration of kaempferol increased Akt phosphorylation in the liver of obese mice (Fig. 3C, H). In addition, the activity of hepatic hexokinase, which plays a primary role in glucose disposition in the liver by catalyzing the first step in glycolytic process and inducing glycogen synthesis [31], was significantly increased (P < 0.05) in kaempferol-treated obese mice (Fig. 3D). The increase in hepatic hexokinase activity was not associated with a change in protein expression of total hexokinases (Figure 3 (g)) or glucokinase (GCK) (Fig. 3E, H), which accounts for 90 and 95% of the total activity of glucose phosphorylation in rat and human liver, respectively [33]. Further, GCK regulatory protein (GCKRP) contents were similar between all groups (Fig. 3F, H). An increase in hexokinase activity can divert glucose into glycogen synthesis and indirectly suppresses the overall glucose output in the liver [32, 33]. Consistently, treatment with kaempferol increased glycogen contents (P<0.05) in obese mice (Fig. 4A). However, kaempferol did not modulate glucose or pyruvate oxidation in the liver (Fig. B, C), suggesting that the reduction of hepatic glucose production observed in kaempferol-treated mice was not due to increased glucose glycolysis or oxidation.
Fig. 3.
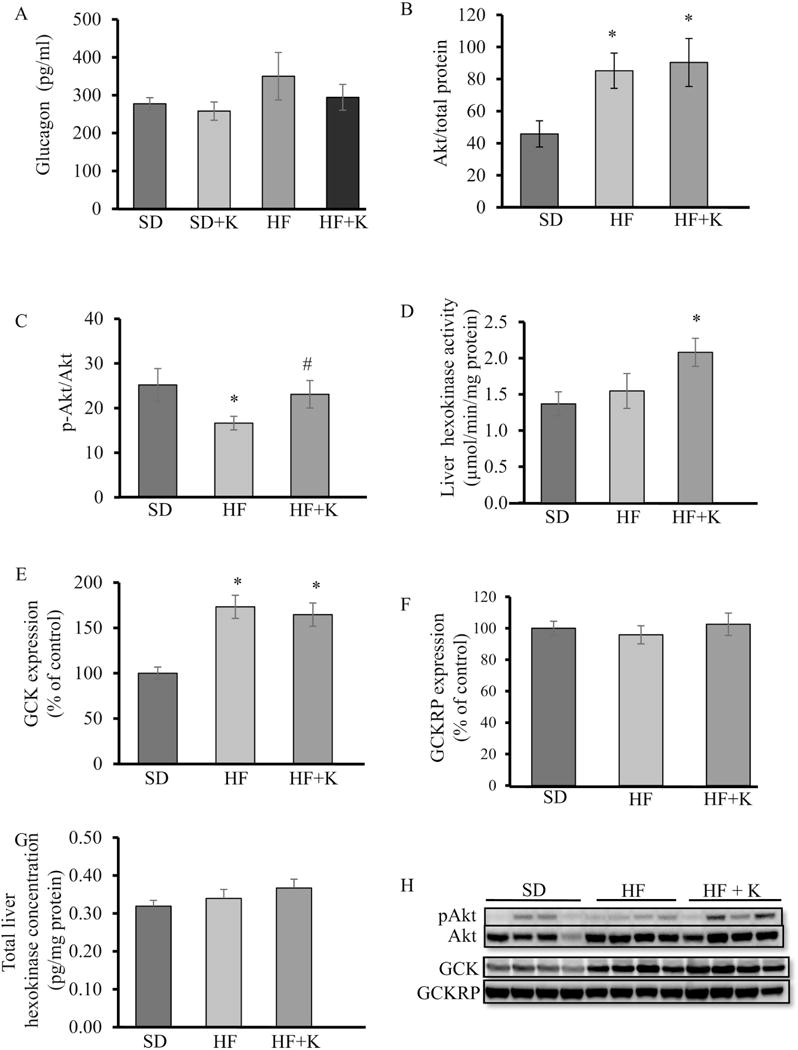
Kaempferol treatment increased liver Akt and GCK activity in HF-fed mice. At the end of the feeding experiment, (A) fasting plasma glucagon levels were measured using ELISA kit. (B, H) Akt, (C, H) pAkt, (E, H) GCK, and (F, H) GCKRP protein levels in whole cell lysates of liver tissue of mice were measured by immunoblotting and normalized to total protein. (D) Hexokinase activity and (G) total hexokinase contents in the liver were measured as described in the Method section. Values are Mean ± SEM from 8-9 mice per group. *, P < 0.05 vs. standard diet-fed mice (SD); #, P < 0.05 vs. HF-fed mice (HF). SD: SD diet; SD + K; SD diet with kaempferol treatment; HF: HF diet; HF + K: HF diet with kaempferol treatment.
Fig. 4.
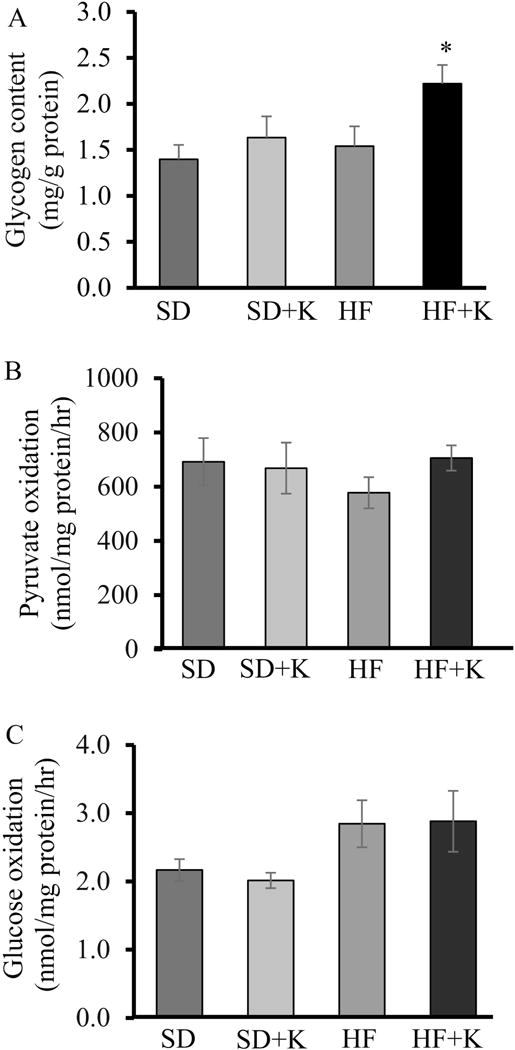
Kaempferol increased glycogen contents but had no effect on glucose and pyruvate oxidation in the liver of HF diet-fed mice. (A) Hepatic glycogen contents in the livers of fasted mice were measured using an assay kit. (B) Pyruvate and (C) glucose oxidation were measured in fresh mouse liver homogenates using either14C-labeled pyruvate for pyruvate oxidation or14C-labeled glucose as described in the Method section. Values are Mean ± SEM from 7 mice per group. SD: SD diet; SD + K; SD diet with kaempferol treatment; HF: HF diet; HF + K: HF diet with kaempferol treatment.
3.4. Kaempferol had no effects on Akt and hexokinase in skeletal muscle
As kaempferol increased Akt phosphorylation and GCK activity in the liver, we further assessed whether similar outcomes occurred in muscle tissue thereby contributing to blood glucose control. We measured skeletal muscle Akt and hexokinase protein levels and activities. Unlike kaempferol effect in the liver, neither proteins nor their activities were changed in skeletal muscle tissues (Fig. 5A-D).
Fig. 5.
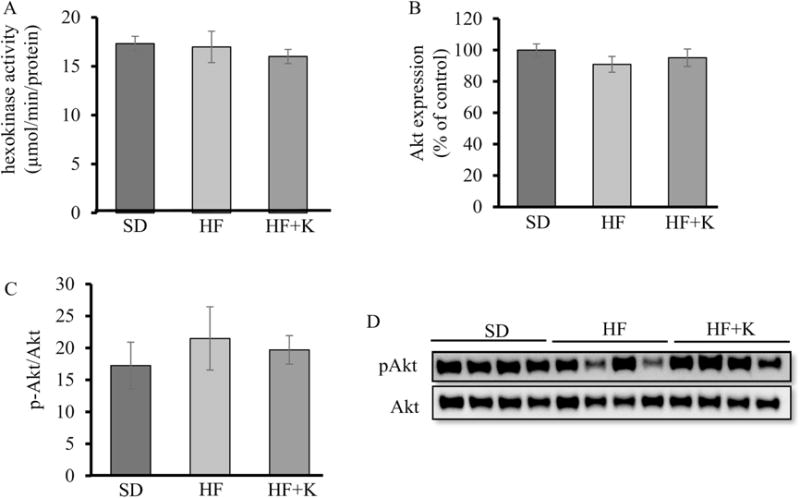
Kaempferol had no significant effect on Akt or hexokinase activities in skeletal muscle of HF diet-fed mice. At the end of the feeding experiment, (A) hexokinase activity was measured in the homogenates of red skeletal muscle using an assay kit. (B, D) Akt, and (C, D) pAkt protein levels in whole cell lysates of skeletal muscle tissue of mice were measured by immunoblotting and normalized to total protein contents. Values are Mean ± SEM from 8 mice per group. SD: SD diet; HF: HF diet; HF + K: HF diet with kaempferol treatment.
3.5. Kaempferol inhibits gluconeogenesis by suppressing PC activity
As the oral administration of kaempferol mitigated fasting hyperglycemia in obese mice, which is mainly caused by the increased hepatic glucose production in IR and T2D [34], we examined whether kaempferol affected hepatic gluconeogenesis, which appears to be predominantly responsible for the excessive hepatic glucose output in T2D [35]. As expected, glucose production from pyruvate was significantly increased in obese mice as compared to lean mice as determined by pyruvate tolerance test. Oral adminstraiton of kaempferol greatly attenuated elevated glucose production in obese mice (Fig. 6A, B). To determine whether kaempferol directly inhibits hepatic gluconeogenesis, we isolated and cultured mouse hepatocytes in the presence or absence of kaempferol for gluconeogenesis assay, and found that kaempferol treatment directly suppressed glucose production in hepatocytes (Fig. 6C), Consistently, cultured hepatocytes from obese mice displayed significantly higher gluconeogenic activity than that of hepatocytes from lean mice, which was attenuated by treatment with kaempferol (Fig. 6D).
Fig. 6.
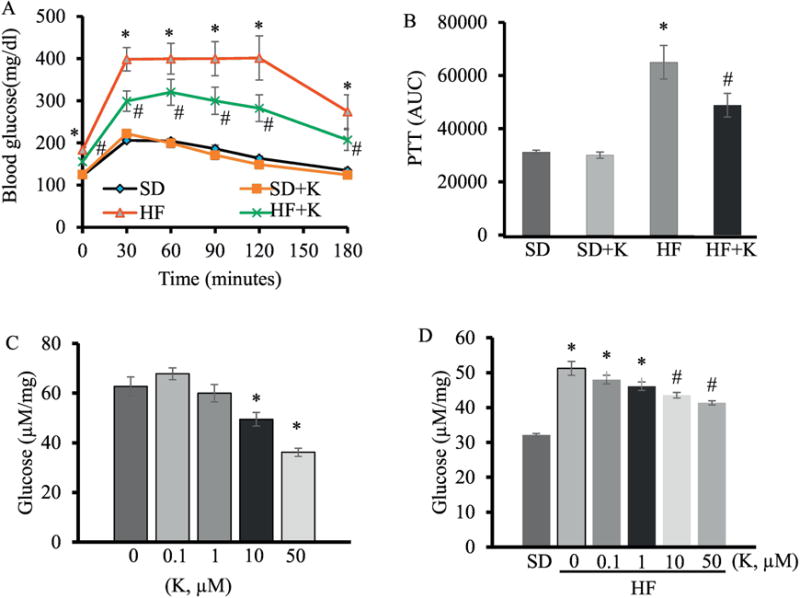
Kaempferol suppressed hepatic glucose production in HF diet-fed mice. (A) PTT was performed as described in in the Method section and the area under the curve (AUC) for PTT (B) was calculated. Primary hepatocytes were isolated from SD-fed mice (C) and HFD-fed mice (D). Cells were then cultured in gluconeogenic medium containing kaempferol, and its ability to suppress glucose production was assayed. Values are Mean ± SEM from 8-9 mice per group. Letter differences denote significant difference at P < 0.05, *, P < 0.05 vs. standard diet-fed mice (SD); #, P<0.05 vs. HF-fed mice (HF). Different letters denote P < 0.05. SD: SD diet; SD + K: SD with kaempferol treatment; HF: HF diet; HF + K: HF diet with kaempferol treatment.
To further determine how kaempferol inhibits glucose production, we studied some key enzymes for hepatic gluconeogenesis. PC (Fig. 7A, F) and G6Pase (Fig. 7B, F) protein levels were significantly higher in obese mice as compared with lean mice, whereas PEPCK protein expression did not differ between groups (Fig. 7C, F). Kaempferol treatment did not alter protein expression of these enzymes in the liver. Interestingly, it significantly reduced the elevated PC and G6Pase activity in the livers of obese mice (Fig. 7D, E). Similarly, kaempferol treatment inhibited glucose production (Fig. 8A), and PC activity (Fig. 8B) in HepG2 cells, further confirming a direct role for kaempferol in the control of hepatic glucose production. Lastly, we found that kaempferol at the same concentrations for inhibiting glucose production and PC activity in hepatocytes, directly inhibited the purified PC activity in a cell-free system (Fig. 8C), suggesting that kaempferol is an inhibitor of enzymatic activity of PC.
Fig. 7.
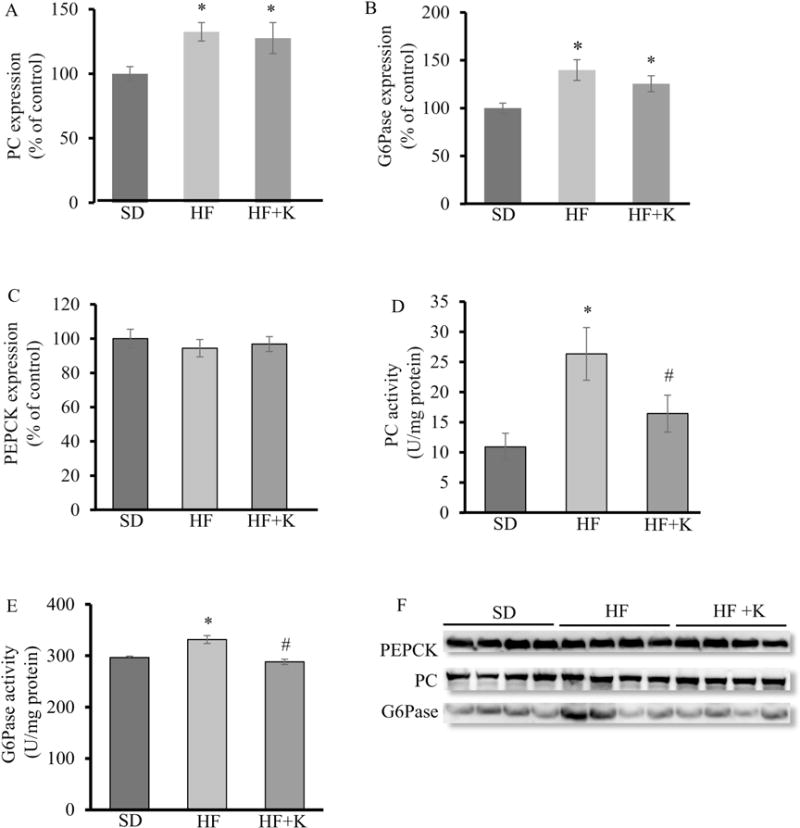
Kaempferol restored the impaired hepatic PC and G6Pase activity without altering their protein expression in HF diet-fed mice. At the end of feeding experiment, (A, F) PC, (B, F) G6Pase, and (C, F) PEPCK protein levels in whole cell lysates of liver tissue of mice were measured by immunoblotting and normalized to total protein, which were not altered by kaempferol. (D) PC and (E) G6Pase activity was measured as described in the Method section. Values are Mean ± SEM from 8-9 mice per group. Letter differences denote significant difference at P < 0.05. SD: SD diet; SD + K: SD with kaempferol treatment; HF: HF diet; HF + K: HF diet with kaempferol treatment.
Fig. 8.
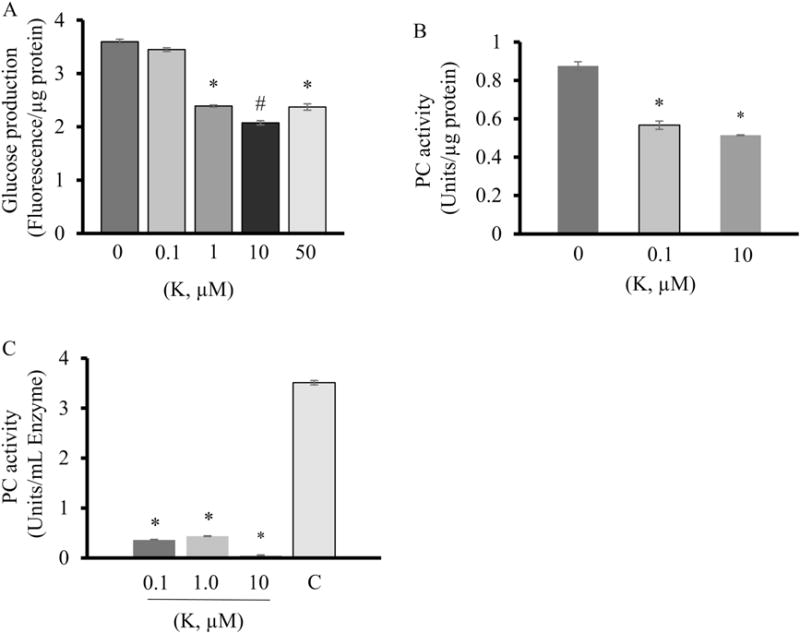
Kaempferol suppressed hepatic glucose production and pyruvate carboxylase activity in HepG2 cells. (A) Glucose production was determined using an assay kit. (B, C) Pyruvate carboxylase activity was determined as describe in the Methods section. Values are expressed as Mean ± SEM (n=3). *, P < 0.05 vs. control.
3. Discussion
In this study, we demonstrate that oral administration of kaempferol (50 mg/kg daily) for 4 wks restores glucose homeostasis and insulin sensitivity, independent of BW change or adiposity, in HFD-induced obese mice. Importantly, kaempferol did not exert an effect on the metabolic phenotypes of SD-fed lean mice, suggesting that the potential side effect of nutritional supplementation of kaempferol is minimal and that the health benefits of kaempferol depend on the metabolic state. The observed metabolic effects of kaempferol in obese mice were associated with a robust suppression of hepatic glucose production. Impaired IGT and fasting hyperglycemia may reflect the abnormal response of β-cells to circulating glucose and impaired insulin sensitivity. However, kaempferol had no effects on basal circulating insulin levels or glucose-stimulated insulin secretion, indicating that the improvement in IGT and fasting blood glucose observed in kaempferol-treated mice may be primarily due to the direct actions of kaempferol on hepatic gluconeogenesis and glucose metabolism. Our data from in vivo, in vitro, and pure enzyme-based assays consistently show that kaempferol is an inhibitor of hepatic PC activity. In addition, we observed that obese mice treated with kaempferol displayed higher hepatic Akt and GCK activities, which could contribute to improved glucose homeostasis through enhancing hepatic glucose uptake and metabolism. These results, together with our recent findings that long-term dietary kaempferol intake prevented diet-induced IGT and IR in middle-aged mice [18], suggest that kaempferol could potentially be a safe and inexpensive natural compound for preventing and treating T2D.
It is well recognized that obesity increases the risk for developing IR and T2D. In order to determine the potential therapeutic effect of kaempferol on obesity-related IR and glucose intolerance, C57BL/6 mice were fed a HFD for 8 wks to induce obesity, IR, and glucose intolerance prior to receiving kaempferol treatment. The diet-induced obese mouse models have been widely used due to the phenotypic similarities to those induced by a Western diet in humans [25], although IR can develop at any degree of adiposity in humans [36]. In the present study, we observed that kaempferol improved glucose tolerance and insulin sensitivity without altering insulin levels, BW gain, food intake, or the degree of adiposity, suggesting that the anti-diabetic action of kaempferol is not a secondary effect whereby it modulated these metabolic parameters.
The liver plays an integral role in maintaining glucose homeostasis [37]. Activation of GCK, the predominant hexokinase in the liver, is proposed to be a potential target for diabetes treatment due to its critical role in maintaining glucose homeostasis [31]. When activated, GCK phosphorylates glucose and increases its clearance by diverting glucose into glycolysis and inducing glycogen synthesis in the liver [38]. Although the factors that regulate GCK are not entirely clear, several studies demonstrated that insulin and GCKRP play a role for regulating GCK [39]. In T2D, hepatic glucose uptake and transport are blunted thus decreasing its flux into hepatocytes. It is suggested that these alterations associated with IR and diabetes may be due to reduced GCK activity [40]. Therefore, targeting GCK to promote its activity has been actively explored for better glycemic control. Indeed, several studies demonstrated that the use of GCK activators led to a significant increase in glucose uptake and metabolism, thereby reducing glycaemia and improving glucose tolerance in T2D mice [41], which was associated with reduced insulin secretion, suggesting improved insulin sensitivity [42]. Consistently, we found that kaempferol treatment increased GCK activity in HFD-fed mice, which may contribute to the improved glucose control and enhanced insulin sensitivity observed in these mice. It is possible that the increase in GCK activity might increase glycolysis and glycogen synthesis, however, in the present study the increase in GCK was not accompanied by an increase in hepatic glucose oxidation. Glycogen synthesis is primarily controlled at the level of GCK and its product, glucose-6-phosphate, whereas glycolysis is regulated by other enzymes such as phosphofructokinase, and to a lesser extent GCK [43, 44]. However, it is unclear whether this activation was a direct effect of kaempferol or a result of the alterations in other metabolic pathways, which warrants further investigation.
Hepatic Akt signaling plays a central role in suppressing hepatic glucose production and maintaining whole body glucose homeostasis [45] The activation of Akt phosphorylates and thereby inhibits PGC-1α, which is an important regulator of post-absorptive hepatic metabolism [46]. This inhibition of PGC-1α impedes its ability to activate the expression of gluconeogenic genes PEPCK and G6Pase [46, 47]. Akt also suppresses gluconeogenesis by phosphorylating and inactivating FOXO1 [48–51]. Moreover, induction of Akt signaling stimulates glycogen synthesis by phosphorylating and inactivating glycogen synthase kinase-3 (GSK-3), thereby resulting in glycogen synthesis [52, 53]. In this study, we found that kaempferol increased hepatic Akt activity in obese mice. Consistently, kaempferol treatment also increased hepatic glycogen contents in obese mice.
Hepatic IR is associated with upregulated expression and activity of gluconeogenic enzymes that control glucose production [54] including PC, PEPCK [55], and G6Pase [56], consequently leading to excessive hepatic gluconeogenesis and thus glucose output, which primarily contribute to fasting hyperglycemia [57–59]. Indeed, increased hepatic gluconeogenesis is considered one of the early pathological changes in newly diagnosed T2D subjects [57]. Our results show that G6Pase protein levels increased significantly in obese compared to lean mice, whereas PEPCK protein levels were not altered. This observation was consistent with the results of another study [60]. However, neither PEPCK nor G6Pase protein levels were altered by kaempferol treatment. It was demonstrated that neither the gene nor the protein expression of PEPCK or G6Pase was associated with fasting hyperglycemia in either rats or patients with T2D [47]. Consistently, another study showed that the elevated hepatic glucose production in morbidly obese diabetic patients was primarily associated with an increase in hepatic G6Pase activity [61], suggesting that the increase in gluconeogenesis in insulin resistant and diabetic subjects could be independent of changes in the expression of gluconeogenic enzymes.
While PEPCK and G6Pase play a vital role in gluconeogenesis, they display relatively weak control over gluconeogenic progression [62]. Instead, PC, which is responsible for the first step in gluconeogenesis, was found to be strongly associated with glycemia in humans [63], as its inhibition greatly reduced gluconeogenesis in vitro and in vivo [64]. To our surprise, we observed that kaempferol treatment had no effect on PC expression, but inhibited PC activity in the liver of obese mice, cultured primary mouse hepatocytes, and HepG2 cells. Therefore, it is conceivable that kaempferol may suppress gluconeogenesis and thus hepatic glucose output primarily via inhibition of this gluconeogenic enzyme. To determine whether kaempferol is a direct inhibitor of PC, we measured the enzymatic activity of pure PC in response to kaempferol treatment, and observed that kaempferol potently inhibited its activity. To that end, it is possible that increased hepatic Akt activity in kaempferol-treated obese mice may be secondary whereby it inhibited PC activity. Indeed, in a study where PC expression was specifically ablated in hepatic and adipose tissue by ip injection of a specific antisense oligonucleotide of PC in HFD-fed rats, it was shown that the inhibition of PC in liver and adipose tissue subsequently improved hepatic insulin sensitivity, which was reflected by the suppression of hepatic glucose production and an increase in hepatic Akt activity [63].
Metabolically inflexible individuals typically have higher levels of blood glucose in the post-absorptive state when compared to metabolically flexible individuals as has been demonstrated by elevated respiratory quotients in skeletal muscle from obese insulin-resistant [65] and T2D adults [66]. This is consistent with findings that endogenous glucose production is higher in T2D compared to healthy subjects [67]. This increased blood glucose, partially due to elevated gluconeogenesis, along with blunted insulin sensitivity, results in increased insulin secretion as a compensatory mechanism for elevated blood glucose and blunted insulin sensitivity [68]. Conversely, increased insulin sensitivity reduces pancreatic insulin release [69], as the amount of insulin that is required to maintain euglycemia is decreased [70]. Although we did not measure fasting-blood insulin levels in this study, data from our previous studies showed that kaempferol decreased circulating insulin levels in obese mice [18], consistent with the improved insulin sensitivity and glucose homeostasis [71].
In summary, hepatic IR increases the risk of T2D development due to increased hepatic glucose production and the subsequent development of fasting hyperglycemia. Here we show that oral administration of kaempferol ameliorated fasting hyperglycemia, glucose intolerance, and IR in diet-induced obese mice. These effects may largely due to the ability of kaempferol to inhibit hepatic gluconeogenesis by inhibiting PC and G6Pase activity. In addition, kaempferol treatment also improved hepatic glucose metabolism by increasing Akt and GCK activity. Collectively, these findings suggest that kaempferol could be an effective anti-diabetic natural compound by regulating hepatic gluconeogenesis and improving insulin sensitivity.
Acknowledgments
Funding sources: The work was supported by grants from National Center for Complementary and Integrated Health of National Institutes of Health (1R01AT007077 to D. Liu). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no Conflict of Interest.
Author contributions
H. A., and W. M., conceived and designed the study, conducted research, collected and analyzed data, performed statistical analysis, and wrote the manuscript; A. W., J. L., R. P. M., Y. W., and W. Z., performed research; D. L. conceived and designed the study, participated in the interpretation of data, and revised the manuscript; M. W. H. contributed to the design of in vivo experiments and discussion.
References
- 1.Heron M. Deaths: Leading Causes for 2011. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64:1–96. [PubMed] [Google Scholar]
- 2.National Diabetes Statistics Report | Data & Statistics | Diabetes. CDC; 2017. [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocrine Reviews. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 6.Michael DJ, Ritzel RA, Haataja L, Chow RH. Pancreatic beta-cells secrete insulin in fast- and slow-release forms. Diabetes. 2006;55:600–7. doi: 10.2337/diabetes.55.03.06.db05-1054. [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 8.Halter JB, Ward WK, Porte D, Best JD, Pfeifer MA. Glucose regulation in non-insulin-dependent diabetes mellitus. Interaction between pancreatic islets and the liver. The American Journal of Medicine. 1985;79:6–12. doi: 10.1016/0002-9343(85)90579-0. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. Journal of Hepatology. 2007;47:142–56. doi: 10.1016/j.jhep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet (London, England) 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. Journal of Diabetes and Its Complications. 2007;21:363–70. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, et al. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. American Journal of Physiology Endocrinology and Metabolism. 2002;283:E745–52. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-Y, Cheon Y-H, Oh HM, Rho MC, Erkhembaatar M, Kim MS, et al. Oleanolic acid acetate inhibits osteoclast differentiation by downregulating PLCγ2-Ca(2+)-NFATc1 signaling, and suppresses bone loss in mice. Bone. 2014;60:104–11. doi: 10.1016/j.bone.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhen W, Maechler P, Liu D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. The Journal of Nutritional Biochemistry. 2013;24:638–46. doi: 10.1016/j.jnutbio.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamora-Ros R, Knaze V, Romieu I, Scalbert A, Slimani N, Clavel-Chapelon F, et al. Impact of thearubigins on the estimation of total dietary flavonoids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. European Journal of Clinical Nutrition. 2013;67:779–82. doi: 10.1038/ejcn.2013.89. [DOI] [PubMed] [Google Scholar]
- 16.Ovaskainen M-L, Törrönen R, Koponen JM, Sinkko H, Hellström J, Reinivuo H, et al. Dietary intake and major food sources of polyphenols in Finnish adults. The Journal of Nutrition. 2008;138:562–6. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 17.Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Reviews in Medicinal Chemistry. 2011;11:298–344. doi: 10.2174/138955711795305335. [DOI] [PubMed] [Google Scholar]
- 18.Alkhalidy H, Moore W, Zhang Y, McMillan R, Wang A, Ali M, et al. Small Molecule Kaempferol Promotes Insulin Sensitivity and Preserved Pancreatic β -Cell Mass in Middle-Aged Obese Diabetic Mice. Journal of Diabetes Research. 2015;2015:532984. doi: 10.1155/2015/532984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–18. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortright RN, Sandhoff KM, Basilio JL, Berggren JR, Hickner RC, Hulver MW, et al. Skeletal Muscle Fat Oxidation Is Increased in African-American and White Women after 10 days of Endurance Exercise Training*. Obesity. 2006;14:1201–10. doi: 10.1038/oby.2006.137. [DOI] [PubMed] [Google Scholar]
- 21.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metabolism. 2005;2:251–61. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enzymatic Activity of Glucose-6-Phosphatase [EC 3.1.3.9]. Sigma-Aldrich.
- 23.Taussky HH, Shorr E, Kurzmann WttaoG. A Microcolorimetric Method for the Determination of Inorganic Phosphorus. Journal of Biological Chemistry. 1953;202:675–85. [PubMed] [Google Scholar]
- 24.Enzymatic Activity Assay of Pyruvate Carboxylase [EC 6.4.1.1].
- 25.Wang C-Y, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods in Molecular Biology (Clifton, NJ) 2012;821:421–33. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, et al. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–80. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108(Suppl 6a):2S–8S. doi: 10.1016/s0002-9343(00)00336-3. [DOI] [PubMed] [Google Scholar]
- 28.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. American Journal of Physiology Endocrinology and Metabolism. 2013;305:E149–59. doi: 10.1152/ajpendo.00040.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldassano S, Rappa F, Amato A, Cappello F, Mule F. GLP-2 as Beneficial Factor in the Glucose Homeostasis in Mice Fed a High Fat Diet. J Cell Physiol. 2015;230:3029–36. doi: 10.1002/jcp.25039. [DOI] [PubMed] [Google Scholar]
- 30.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, Han Y, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55:1–12. [PubMed] [Google Scholar]
- 32.Pagliassotti MJ, Cherrington AD. Regulation of net hepatic glucose uptake in vivo. Annu Rev Physiol. 1992;54:847–60. doi: 10.1146/annurev.ph.54.030192.004215. [DOI] [PubMed] [Google Scholar]
- 33.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. The Biochemical journal. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 34.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697–707. doi: 10.2337/db10-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbasi F, Brown BW, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. Journal of the American College of Cardiology. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 37.Triplitt CL. Examining the mechanisms of glucose regulation. The American Journal of Managed Care. 2012;18:S4–10. [PubMed] [Google Scholar]
- 38.Agius L. The physiological role of glucokinase binding and translocation in hepatocytes. Advances in Enzyme Regulation. 1998;38:303–31. doi: 10.1016/s0065-2571(97)00001-0. [DOI] [PubMed] [Google Scholar]
- 39.Massa ML, Gagliardino JJ, Francini F. Liver glucokinase: An overview on the regulatory mechanisms of its activity. IUBMB life. 2011;63:1–6. doi: 10.1002/iub.411. [DOI] [PubMed] [Google Scholar]
- 40.Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, et al. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–83. doi: 10.2337/diabetes.49.2.272. [DOI] [PubMed] [Google Scholar]
- 41.Grimsby J, Sarabu R, Corbett WL, Haynes N-E, Bizzarro FT, Coffey JW, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science (New York, NY) 2003;301:370–3. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 42.Winzell MS, Coghlan M, Leighton B, Frangioudakis G, Smith DM, Storlien LH, et al. Chronic glucokinase activation reduces glycaemia and improves glucose tolerance in high-fat diet fed mice. European Journal of Pharmacology. 2011;663:80–6. doi: 10.1016/j.ejphar.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Agius L, Peak M, Newgard CB, Gomez-Foix AM, Guinovart JJ. Evidence for a Role of Glucose-induced Translocation of Glucokinase in the Control of Hepatic Glycogen Synthesis. Journal of Biological Chemistry. 1996;271:30479–86. doi: 10.1074/jbc.271.48.30479. [DOI] [PubMed] [Google Scholar]
- 44.de la Iglesia N, Mukhtar M, Seoane J, Guinovart JJ, Agius L. The role of the regulatory protein of glucokinase in the glucose sensory mechanism of the hepatocyte. The Journal of Biological Chemistry. 2000;275:10597–603. doi: 10.1074/jbc.275.14.10597. [DOI] [PubMed] [Google Scholar]
- 45.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–95. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–6. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 47.Samuel VT, Beddow SA, Iwasaki T, Zhang X-M, Chu X, Still CD, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12121–6. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. The Journal of Biological Chemistry. 2010;285:35245–8. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metabolism. 2007;6:208–16. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. The Journal of Clinical Investigation. 2001;108:1359–67. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–5. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 52.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 53.Weeren PC, Bruyn KMT, Vries-Smits AMM, Lint J, Burgering BMT. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation Characterization of dominant-negative mutant of PKB. Journal of Biological Chemistry. 1998;273:13150–6. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 54.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 55.Granner D, Andreone T, Sasaki K, Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983;305:549–51. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- 56.Dickens M, Svitek CA, Culbert AA, O’Brien RM, Tavaré JM. Central Role for Phosphatidylinositide 3-Kinase in the Repression of Glucose-6-phosphatase Gene Transcription by Insulin. Journal of Biological Chemistry. 1998;273:20144–9. doi: 10.1074/jbc.273.32.20144. [DOI] [PubMed] [Google Scholar]
- 57.Chung ST, Hsia DS, Chacko SK, Rodriguez LM, Haymond MW. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia. 2015;58:596–603. doi: 10.1007/s00125-014-3455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703–11. doi: 10.2337/db06-1776. [DOI] [PubMed] [Google Scholar]
- 59.Basu R, Barosa C, Jones J, Dube S, Carter R, Basu A, et al. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. The Journal of Clinical Endocrinology and Metabolism. 2013;98:E409–17. doi: 10.1210/jc.2012-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ha BG, Shin EJ, Park J-E, Shon YH. Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice. Marine Drugs. 2013;11:4193–212. doi: 10.3390/md11114193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clore JN, Stillman J, Sugerman H. Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes. 2000;49:969–74. doi: 10.2337/diabetes.49.6.969. [DOI] [PubMed] [Google Scholar]
- 62.Ramnanan CJ, Edgerton DS, Rivera N, Irimia-Dominguez J, Farmer B, Neal DW, et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes. 2010;59:1302–11. doi: 10.2337/db09-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumashiro N, Beddow SA, Vatner DF, Majumdar SK, Cantley JL, Guebre-Egziabher F, et al. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes. 2013;62:2183–94. doi: 10.2337/db12-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahl JJ, Matsuda M, DeFronzo RA, Bressler R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochemical Pharmacology. 1997;53:67–74. doi: 10.1016/s0006-2952(96)00660-0. [DOI] [PubMed] [Google Scholar]
- 65.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. The American Journal of Physiology. 1999;277:E1130–41. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 66.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. Journal of Clinical Investigation. 1994;94:2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by Which Metformin Reduces Glucose Production in Type 2 Diabetes. Diabetes. 2000;49:2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weir GC, Bonner-Weir S. A dominant role for glucose in beta cell compensation of insulin resistance. The Journal of Clinical Investigation. 2007;117:81–3. doi: 10.1172/JCI30862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Utsugi T, Ohno T, Ohyama Y, Uchiyama T, Saito Y, Matsumura Y, et al. Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism: Clinical and Experimental. 2000;49:1118–23. doi: 10.1053/meta.2000.8606. [DOI] [PubMed] [Google Scholar]
- 70.Koranyi LI, Bourey RE, Slentz CA, Holloszy JO, Permutt MA. Coordinate reduction of rat pancreatic islet glucokinase and proinsulin mRNA by exercise training. Diabetes. 1991;40:401–4. doi: 10.2337/diab.40.3.401. [DOI] [PubMed] [Google Scholar]
- 71.Vukovich MD, Arciero PJ, Kohrt WM, Racette SB, Hansen PA, Holloszy JO. Changes in insulin action and GLUT-4 with 6 days of inactivity in endurance runners. Journal of Applied Physiology (Bethesda, Md: 1985) 1996;80:240–4. doi: 10.1152/jappl.1996.80.1.240. [DOI] [PubMed] [Google Scholar]


