Abstract
Introduction/Purpose
Inflammation contributes to heart failure (HF) progression and the interleukin (IL)-1 cytokine IL-1β is implicated in this process. The adaptor protein ASC is necessary for inflammasome activation of IL-1β. Lower ASC methylation is associated with worse outcomes in HF. The purpose of this study was to examine the effects of exercise on changes in ASC methylation and activation of the interleukin-1 family cytokine IL-1β in persons with HF.
Methods
Participants (N=54) were randomized to receive exercise intervention (n=38) or attention control (n=16) for 3 months. Percent methylation of the ASC gene, plasma IL-1β, and ASC mRNA and were obtained at baseline, 3 months, and 6 months.
Results
ASC methylation was higher in the exercise group as compared to control at 3 months (6.10±0.5% vs. 5.80±0.4%; p=.04) and 6 months (6.07±0.4 vs. 5.82±0.4; p=.04). Plasma IL-1β was lower in the exercise group at 3 months (1.43±0.5 pg/mL vs. 2.09±1.3 pg/mL; p=.02) and 6 months (1.49±0.5 pg/mL vs. 2.13±1.4 pg/mL; p=.004). ASC mRNA expression was negatively associated with ASC methylation at baseline (r=−.97, p=.001), 3 months (r=−.90, p=.001), and 6 months (r=−.81, p=.001). ASC mRNA was lower than baseline at 3 months (p=.004) and 6 months (p=.002) among those in the exercise group. ASC methylation was positively associated with six-minute walk test (6MWT) at baseline (r=.517, p<.001), 3 months (r=.464, p=.004), and 6 months (r=497, p=.05).
Conclusions
Exercise was related to increased mean percent ASC methylation and decreased IL-1β and ASC mRNA gene expression in HF. Epigenetic regulation of ASC can be a biological mechanism by which exercise can promote better outcomes in HF.
Keywords: epigenetics, inflammation, cytokines, walking
Introduction
Heart failure (HF) is a progressive, terminal illness with frequent decompensation related to ventricular remodeling. Because HF remains a leading cause of morbidity and mortality in the United States (1), identification of novel therapeutic targets that may slow disease progression are urgently needed. HF is associated with a chronic sterile inflammation characterized by the formation and activation of a protein complex, the inflammasome, which activates inflammatory cytokines that promote cardiac hypertrophy and myocardial apoptosis (2, 3). The inflammasome is composed of a NOD (nucleotide-binding oligomerization domain)-like receptor (NLRP3), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 (4, 5).
Interleukin-1β (IL-1β) and IL-18 are pleiotropic, inflammatory cytokines theorized to play a prognostic and mechanistic role in HF (6, 7); increased levels have been shown to significantly contribute to worsening HF severity and mortality (6, 8). IL-1β and IL-18 increase during acute HF decompensation, suggesting a role in myocardial dysfunction (8). IL-1β induces nitric oxide production (iNOS) (9), leading to worsening of ventricular remodeling in HF. IL-1β and IL-18 are activated via caspase-1 dependent proteolytic cleavage (5). Caspase-1 is recruited by an adaptor molecule, ASC, which leads to IL-1β and IL-18 activation (9). Unlike NLRP3 and caspase-1, ASC has no known independent activity outside of the inflammasome and is necessary for caspse-1 activation (10). Thus, ASC availability may be a limiting factor in inflammasome activation.
ASC gene expression is epigenetically controlled by DNA methylation (11, 12). Increased methylation of 7 CpG sites in the promoter region of exon 1 of the ASC gene is associated with decreased ASC mRNA and protein expression (12). ASC methylation is positively associated with six-minute walk test total distance and aerobic capacity in HF (12, 13). Increased ASC methylation has been shown to be higher in older adults who participated in an aerobic exercise program as compared to controls (11). However, no studies to date have examined changes in ASC methylation in response to an exercise intervention in persons with HF.
Although no intervention studies targeting epigenetic changes with exercise in HF have been reported to date, studies in healthy adults and adults with chronic diseases have demonstrated that short-term and long-term exercise interventions can result in genome-wide and gene-specific epigenetic changes (14–16). The 2013 American Heart Association recommendations for HF (1) include exercise as a safe and effective non-pharmacological therapy that improves physical and psychological function, reduces hospital readmission rates, lowers mortality in some studies, and improves quality of life (17, 18). Moderate exercise has been shown to reduce inflammation, and it has been suggested that changes in inflammation with exercise is due to epigenetic changes (19).
The purpose of this study was to examine the effects of exercise on changes in ASC methylation and activation of the interleukin-1 family cytokines IL-1β and IL-18 in persons with HF. We hypothesized that: 1) persons with HF who participate in a 3-month aerobic exercise intervention will have increased ASC methylation at completion of the intervention as compared to persons with HF in an attention-control group, and 2) increased ASC methylation will be related to decreased plasma IL-1β and IL-18 and to iNOS mRNA expression in persons with HF. Further, we investigated if changes in ASC methylation and plasma IL-1β and IL-18 were sustained 3 months after completing the aerobic exercise intervention.
Methods
Study Design
Fifty-four participants were enrolled in this pilot study from 1 of 4 large urban tertiary-care hospitals that had multidisciplinary outpatient HF clinics. Participants were randomized to receive an exercise intervention (n=38) or attention control (n=16) for 3 months. Participants were followed for an additional 3-month maintenance phase after the intervention. Both groups received two home baseline visits, weekly phone calls during the 3-month intervention phase, and bi-monthly phone calls during the maintenance phase, from 3 months to 6 months. The time points were chosen to examine physiological changes over a short-term (3 month) moderate intensity exercise program and if the changes persisted or diminished 3 months post exercise intervention (at 6 months).
The exercise group received the exercise prescription using a progressive, moderate intensity aerobic protocol. To ensure that participants achieve adequate training stimulus, dose-specific exercise was based on maximum heart rate obtained during symptom-limited, modified Balke treadmill test at baseline. Participants in the exercise group were instructed to walk for 30 minutes 3 times per week at 60% maximum heart rate for the first two weeks, 45 minutes 3 times per week at 60% maximum heart rate for weeks three and four, and 45 minutes 3 times per week at 70% maximum heart rate for the remaining eight weeks. The attention control group received education and flexibility and stretching exercises to control for the possible confounding variable of receiving attention from a healthcare professional.
All studies were performed under research protocols approved by the Institutional Review Boards of Emory University and participating institutions. Each subject was informed of testing protocols and the potential risks and benefits of participation. All participants provided written consent before participation.
Study Sample
Participants were screened for eligibility via medical record. Inclusion and exclusion criteria were as previously described (13). Severity of illness was controlled by limiting participants to NYHA class II and III, who are more similar in response to exercise than class I and IV. In addition, severity of illness was controlled by LVEF limitations (LVEF > 10%) and optimal medication therapy for HF. Participants below the age of 40 years are likely to have HF for other reasons than the majority of the general HF population. Older age (>75) is associated with reduced exercise capacity which may confound the physiological outcome measurements. Both resting and exercise heart rates are influenced by beta-blockers, which is considered optimal therapy for HF patients, and this was controlled for during analysis. For both exercise testing and training, the heart rate reserve method was used, which takes into account the patient’s resting heart rate, thereby reducing the effect of beta-blockade. Best efforts were made to schedule patients for their exercise testing and training a minimum of three hours after taking beta blockers. Patients with an ICD were enrolled if their heart rate limits were set to be higher than the target heart rate for the exercise regimen. Participants with recurrent angina, more severe symptoms, or have uncontrolled hypertension were excluded due to the higher risk for adverse cardiovascular events during exercise testing and the walking intervention. Because the benefit of exercise is being evaluated, participants who were currently or recently enrolled in an exercise program for the previous eight weeks or were exercising at regular intervals (more than twice per week for 30 minutes) were excluded from the study.
G*Power software was used to assess the power/effect size detectable given an estimated sample size of 54 for this pilot study. A sample size of 54 at 80% power can detect large effect sizes in the repeated measures analysis (f=0.37 for group main effect, f=0.41 for time main effect and f=0.41 for the group-x-time interaction effect) using an F-Test with a significance level (alpha) of 0.05.
Measurements
Demographic and Clinical Data
Sociodemographic and clinical variables included age, gender, medical history, and medications, and were obtained from medical records and a self-report questionnaire. The Charlson Comorbidity Index (CCI) (20) was used to assess for other chronic conditions. Height was measured with a standard stadiometer, without shoes and recorded in centimeters. Weight was measured in kilograms using a calibrated scale with the participant in light clothing, without shoes. Body mass index (BMI) was calculated by the formula: BMI = (weight in kg)/(height in cm)2. Participants with LVEF <40% were categorized as heart failure with reduced ejection fraction (HFrEF), and those with LVEF ≥40% were categorized as preserved ejection fraction (HFpEF).
Blood draws took place at BL, intervention completion (3 months) and after a 3-month maintenance period (6 months). Blood samples were collected in the morning after an overnight fast. Blood was collected in a vacutainer with EDTA, separated into plasma and buffy coat, and stored at −80°C until analysis.
Exercise Logs
Participants were provided 3 calendars to record exercise sessions completed during each month of the intervention. Participants who documented at least 12 exercise sessions per month were considered to have completed the exercise program. Participants who recorded less than 12 exercise sessions per month or did not turn in any completed exercise calendars were considered to have not completed the exercise program.
Six-Minute Walk Test
The six-minute walk test (6MWT) was performed at BL, intervention completion (3 months) and after a 3-month maintenance period (6 months). Participants were instructed to walk as far as possible for 6 minutes along a level walk way. Total distance walked in 6 minutes was measured in meters.
Cardiopulmonary Exercise Stress Test
Exercise stress testing was performed at was performed at BL and intervention completion (3 months). The dose-specific exercise was based on maximum heart rate obtained during a modified Balke maximal symptom-limited treadmill test. The test protocol and parameters have been described previously (13). In brief, heart rate, continuous gas exchange, telemetry, blood pressure, rating of perceived exertion, and oxygen saturation were assessed for each patient 1 minute before, during, and 4 minutes after the exercise test according to American Heart Association guidelines (21). The test protocol began at 0% incline at 2.0 mph on a motorized treadmill for 3 minutes with an increase in incline of 3.5% every 3 minutes for 18 minutes. All participants completed the treadmill test regardless of group assignment.
ASC Methylation
Percent methylation of 7 CpG sites in exon I of ASC was measured as previously reported (12, 13). In brief, genomic DNA (see Table, SDC 1, genomic DNA concentrations for each sample) from peripheral blood mononuclear cells (PBMCs) was bisulfite treated and amplified by PCR, followed by pyrosequencing for methylation quantification (11). Methylation of 7 CpG sites in exon 1 were measured (12) and analyzed as mean percent methylation. The mean percent methylation of the 7 CpG sites for each individual was calculated as the sum of percent methylation of the CpG sites divided by 7. All results are presented as mean percent methylation.
Cytokines
IL-1β and IL-18 were analyzed from plasma that has been separated from collected whole blood and stored at −80°C immediately after collection. Plasma cytokines were measured in duplicate using commercially available ELISA kits (eBioscience). Plates were read on a BioTek microplate reader and analyzed using Gen5 software. Curve fitting was selected among linear, quadratic and 4-point based on the best regression coefficient.
mRNA
mRNA was extracted using a commercial kit (mRNA Catcher™ Plus, Invitrogen) and converted to cDNA using reverse transcriptase PCR (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems). IL-18, ASC, and iNOS mRNA were quantified via quantitative real-time PCR (RT-PCR). GAPDH was used as the reference gene. Primers used for RT-PCR PCR [GAPDH: 5′-GCTTGAATCTAAATTATCAGTC-3′, 5′-GAAGATTCAAATTGCATCTTAT-3′; ASC: 5′-GCCGAGCTCACCGCTAACG-3′, 5′-CATCCAGCAGCCACTCAACG-3′; IL-18: 5′-CAAGGAAATCGGCCTCTATT-3′, 5′-TCCTGGGACACTTCTCTGAA-3′; iNOS: 5′-CAAGCCTACCCCTCCAGATG-3′, 5′-CATCTCCCGTCAGTTGGTAGGT-3′] were developed using Primer Express® software. To normalize gene expression relative to a non-HF reference sample, mRNA quantification results were calculated using the 2−ΔΔCT method (22).
Data Analysis
Descriptive statistics were analyzed for all study variables and data were reviewed for normality assumptions and outliers, in preparation for analysis. IL-18 did not met criteria for normality and was log transformed (LN) for statistical analysis. All data were analyzed using SAS version 9.4 with an alpha set at 0.05. Student t-tests were used to compare group differences in demographic and clinical variables at baseline. PROC MIXED with Bonferroni adjusted least squares mean analysis was used to compare within GROUP and between GROUP changes across TIME. For hypothesis testing, data was analyzed according to intention to treat principles. The primary analysis used to test the hypotheses employed a general linear mixed model for repeated measures data. For most variables, the model has one between-subjects variable GROUP with two levels (control and exercise) and one within-subjects variable TIME with three levels (BL, 3 and 6 months). The test of the hypotheses hinged on the GROUP by TIME interaction as an indication of group differences in the variable of interest over time. A given hypothesis was supported by the finding of a statistically significant GROUP by TIME interaction, and the finding that the means are in the hypothesized direction. Multilevel modeling, using PROC MIXED, provided separate estimates of the means for each variable by time and treatment groups. This method allowed to control for attrition over time. The linear model was fit using restricted maximum likelihood estimation with an appropriate form for the variance-covariance among the repeated measures. Covariates for each hypothesis were selected based on literature documentation of relationships and performance of the data and relationships within this study. PROC MIXED allowed for the handling of missing data through the use of restricted maximum likelihood estimation, retaining participants in the analysis and preserving sample size, and accommodated covariates as defined in the model. Effect sizes were calculated using Hedges’ g, which adjusts the calculation of the pooled standard deviation with weights for the sample sizes (23). Mediation models to examine the indirect effects of ASC methylation on downstream variables via IL-1 cytokines were performed using PROC IML with PROCESS version 2.15 (24).
Results
Patient Characteristics
Demographic and clinical data are presented in Table 1. No significant group differences at baseline were found. Age varied widely, ranging from 40 to 75 years. LVEF ranged from 15 to 65%, and 65.0% of the participants had LVEF <40%. The most common comorbidities were hypertension (n=36, 66.7%), dyslipidemia (n=30, 55.6%), depression (n=20, 37.0%), and diabetes (n=19, 35.2%). One-third of participants (n=16, 29.6%) had a previous MI. Older participants had higher LVEF (r=.281, p=.044), more comorbidities (CCI, r=.75, p<.001), and had lower total distance walked on 6MWT (r=−.463, p<.001). Females had higher LVEF as compared to males (38.23 ± 14.9 vs. 29.44 ± 13.6, respectively; t=2.26, p=.03).
Table 1.
Characteristics for the Total Sample and by Group
| Measure | Control Group (n=16) | Exercise Group (n=38) | Differences (p-value)1 |
|---|---|---|---|
| Age (years) mean (SD) | 58.19 (12.8) | 60.0 (8.7) | .54 |
| Gender, n (%) | |||
| Male | 5 (31%) | 21 (55%) | .11 |
| Female | 11 (69%) | 17 (45%) | |
| Race, n (%) | |||
| AA/Black | 11 (69%) | 21 (55%) | .36 |
| Non-black | 5 (31%) | 17 (45%) | |
| LVEF, %, mean (SD) | 35.53 (12.7) | 32.85 (15.9) | .55 |
| BMI (kg/m2), mean (SD) | 31.03 (6.1) | 31.51 (7.1) | .81 |
| CCI, mean (SD) | 3.47 (2.1) | 4.0 (1.7) | .35 |
| Education, n (%) | |||
| ≤ HS | 8 (50%) | 18 (47%) | .90 |
| ≥ College | 8 (50%) | 20 (53%) | |
| β-Blocker, n (%) | |||
| No | 4 (25%) | 6 (16%) | .43 |
| Yes | 12 (75%) | 32 (84%) | |
| Type of HF | |||
| HFpEF | 6 (38%) | 10 (26%) | .92 |
| HFrEF | 13 (62%) | 23 (74%) | |
| Total days exercised | |||
| Compliant | -- | 51.58 (14.2) | -- |
| Non-compliant | -- | 9.87 (11.46) | |
p-value of group differences from Student’s t-test for continuous variables and chi-square for categorical variables.
AA – African American
LVEF – Left ventricular ejection fraction
BMI – Body mass index
CCI – Charlson Comorbidity Index
HS – High school
HFpEF – Heart failure with preserved ejection fraction
HFrEF – Heart failure with reduced ejection fraction
Among participants in the exercise group, 60% completed at least 12 days of exercise per month for the 3-month intervention (Table 1). Total number of reported days exercised ranged from 0 to 84.
Outcome Measures by Group and over Time
Outcome data are presented by group and time in Table 2. No significant group differences at baseline were found. Mean percent ASC methylation was higher in the exercise group as compared to the control group at 3 months and 6 months, with medium to large effects sizes of .72 and .81, respectively (Table 2). Plasma IL-1β was lower among the exercise group as compared to the control group at 3 months and 6 months, with medium to large effect sizes of 0.83 and 0.79, respectively (Table 2). Among those in the exercise group mean percent ASC methylation was higher at 3 months as compared to baseline, and IL-1β was significantly lower than baseline at both 3 months and 6 months (Table 2). Total distance walked during the 6MWT was higher in the exercise group as compared to the control group at 3 months and 6 months, with medium effect sizes of 0.61 and 0.70, respectively (Table 2). Among those in the exercise group, 6MWT total distance was higher at 6 months as compared to baseline. No group differences or changes from baseline were found for peak V̇O2 (Table 2).
Table 2.
Outcome Measures by Group over Timea
| % ASC methylation Mean (SD) |
IL-1β (pg/mL) Mean (SD) |
IL-18 (pg/mL) Mean (SD) |
6MWT (meters) Mean (SD) |
Peak V̇O2 (ml/kg/min) Mean (SD) |
|
|---|---|---|---|---|---|
| Baseline | |||||
| Control | 5.75 (0.6) | 1.88 (1.0) | 162.67 (62.1) | 330.97 (84.8) | 15.75 (4.3) |
| Exercise | 5.88 (0.9) | 1.65 (0.8) | 149.90 (33.8) | 351.97 (73.5) | 17.31 (5.2) |
| 3 Months | |||||
| Control | 5.80 (0.4) | 2.09 (1.3) | 162.86 (66.8) | 324.31 (137.3) | 16.16 (6.3) |
| Exercise | 6.10 (0.5)*† | 1.43 (0.5)*† | 138.18 (56.0) | 372.22 (64.5)* | 16.61 (4.2) |
| 6 Months | |||||
| Control | 5.82 (0.4) | 2.13 (1.4) | 160.58 (34.7) | 324.29 (128.3) | -- |
| Exercise | 6.07 (0.4)* † | 1.49 (0.5)*‡§ | 130.15 (52.9) | 387.33 (56.2)*‡ | -- |
PROC MIXED with least squares mean analysis adjusting with Bonferroni was used to compare within GROUP and between GROUP changes across TIME.
Group differences significant at p<.05.
Difference from baseline is significant at p<.01.
Difference from baseline significant at p<.05.
Difference from 3 months significant at p<.05.
ASC – Apoptosis associated speck-like protein containing a caspase recruitment domain
IL - Interleukin
6MWT – Six-minute walk test
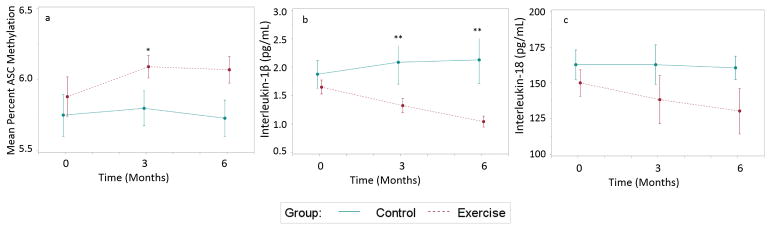
Significant group differences in change scores from baseline to 3 months (Figure 1) were found for IL-1β (control 0.18 pg/mL vs exercise −0.33 pg/mL, t=3.73, p=.001) and mean percent ASC methylation (control −0.04% vs exercise 0.04%, t=−2.71, p=.01). In addition, group differences in change scores from baseline to 6 months (Figure 1) were found for IL-1β (control 0.19 pg/mL vs exercise −0.62 pg/mL, t=2.65, p=.013). Mean percent ASC methylation was negatively associated with IL-1β at baseline (r=−.443, p=.001), 3 months (r=−.533, p=.01), and 6 months (r=−.477, p=.009). Mean percent ASC methylation was positively associated with peak V̇O2 at baseline (r=.455, p=.002) and 3 months (r=.355, p>.05) and with 6MWT at baseline (r=.517, p<.001), 3 months (r=.464, p=.004), and 6 months (r=497, p=.05). IL-1β was negatively associated with peak V̇O2 at baseline (r=−.357, p=.060) and with 6MWT at baseline (r=−.389, p=.023), 3 months (r=−.335, p=.049), and 6 months (r=−.353, p=.05). Among those in the exercise group, total number of days exercised during the intervention was negatively associated with 6 month measures of IL-1β (r=−.498, p=.035).
Figure 1. Changes in mean percent ASC methylation, IL-1β, and IL-18 over time by group.
a. Group differences in change scores for ASC methylation were found from baseline to 3 months (control 0.04% vs. exercise −0.04%, t=−2.71, p=.011). These changes were sustained at 6 months for the exercise group. b. Group differences in change scores for IL-1β were found from baseline to 3 months (control 0.18 pg/mL vs exercise −0.33 pg/mL, t=3.73, p=.001). These changes were sustained at 6 months for the exercise group. c. No group differences in change scores over time were found for IL-18.
*Significant group differences in change from baseline at p<.05
**Significant group differences in change from baseline at p<.01
IL-18, ASC, and iNOS mRNA
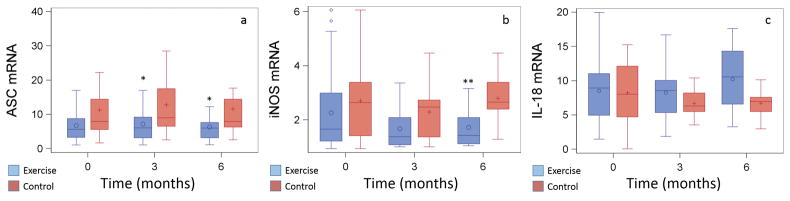
There were no significant intra-individual changes over time or inter-individual group differences over time in IL-18 mRNA (Figure 2). There were significant differences in ASC mRNA expression between control group and exercise group (Figure 2) at 3 months (14.95 ± 2.5 vs. 8.46 ± 1.3, respectively; t=2.27; p=.027) and at 6 months (11.60 ± 3.1 vs. 6.32 ± 1.0, respectively; t=2.24, p=.029), but not at baseline (10.87 ± 2.2 vs. 9.00 ± 2.0, respectively; t=0.55, p=.58). For the exercise group, there were within-group changes in ASC mRNA from baseline to 3 months (t=−3.01; p=.004), from baseline to 6 months (t=−3.21; p=.002), and from 3 months to 6 months (t=4.82; p=.001). There were significant group differences in iNOS mRNA expression between control group at exercise group at 6 months (2.78 ± 0.2 vs. 1.72 ± 0.2; t=3.51, p=.001), but not at baseline or 3 months (Figure 2). Within the control group, there was a significant drop in iNOS mRNA expression from baseline to 3 months (t=2.33, p=.02), but the values returned to baseline levels at 6 months (baseline to 6 months: t=−0.45, p=.65; 3 months to 6 months: t=−0.31, p=.003). There were significant within-group iNOS mRNA changes for the exercise group from baseline to 3 months (t=3.32, p=.002), which remained significant at 6 months (t=2.65, p=.011).
Figure 2. Mean ASC, IL-18, and iNOS mRNA Expression by Group over Time1.
PROC MIXED with least squares mean analysis adjusting with Bonferroni was used to compare within GROUP and between GROUP changes across TIME. To normalize gene expression relative to a non-HF reference sample, mRNA quantification results were calculated using the 2−ΔΔCT method.22 Values are presented as mean ± SD. a. There were significant group differences in relative ASC mRNA expression at 3 months (t=2.27, p=.027) and 6 months (t=2.24, p=.029). There were within-group decreases in relative ASC mRNA expression from baseline to 3 months (t=3.01, p=.004) and from 3 months to 6 months (t=4.82, p=.001) for the exercise group. b. There were no significant inter- or intra-individual changes in relative IL-18 mRNA expression over time. c. There were significant group differences in relative iNOS mRNA expression at 6 months (t=3.51, p=.009). There were within-group decreases in relative iNOS mRNA expression from baseline to 3 months (t=3.32, p=.002), and the decrease was sustained at 6 months (t=2.65, p=.01) in the exercise group and from baseline to 3 months in the control group (t=2.33, p=.02). Relative iNOS mRNA expression returned to baseline levels in the control group by 6 months.
1mRNA gene expression was normalized relative to a reference sample (set at a value of 1).
*Significant group differences at p=.03
**Significant group differences at p=.01
ASC mRNA was negatively associated with mean percent ASC methylation at baseline (r=−.97, p=.001), 3 months (r=−.90, p=.01), and 6 months (r=−.89, p=.001) and was positively associated with plasma IL-1β levels at baseline (r=.39, p=.003), 3 months (r=.48, p=.004), and 6 months (r=.42, p=.02). iNOS mRNA was positively related to plasma IL-1β levels at baseline (r=.72, p=.001) and 6 months (r=.84, p=.001) and was negatively associated with mean percent ASC methylation at baseline (r=−.34, p=.011) and 6 months (r=−.45, p=.015). We hypothesized that increased ASC methylation would be related to decreased iNOS expression caused by a decrease in IL-1β. Due to the multicollinearity between ASC methylation, iNOS mRNA, and IL-1β, we tested a mediation model in which IL-1β mediates the relationship between ASC methylation and iNOS mRNA expression. There was a significant indirect effect of ASC methylation on iNOS mRNA expression via IL-1β at baseline (effect −0.62, 95% CI [−1.15, −0.41]) and 6 months (effect −0.23, 95% CI [−0.80, −0.04].
Multilevel Models for Change
To examine factors related to ASC methylation and cytokine levels and to factors related to rate of change over time, clinical and demographic variables theorized to be related to inflammation were used as predictors in the multilevel model for change. Models were created for the combined sample to examine effects of the exercise intervention and for the exercise only group to examine factors related to changes over time within the exercise intervention.
Combined Sample
Mean percent ASC methylation at baseline was positively associated with LVEF and the interaction effect of peak V̇O2 and gender. Group assignment was associated with rate of change over time such that participants in the intervention group had a higher rate of change than the control group (Table 3). Baseline plasma IL-1β levels were negatively associated with LVEF and the interaction effect of peak V̇O2 and gender. While group assignment to the exercise group was associated with a negative rate of change in IL-1β (β=−0.11), the rate of change was higher among those who reported they completed the prescribed exercise program (β=−0.6). Although peak V̇O2 was positively associated with mean percent ASC methylation (r=.47, p=.001) and negatively associated with IL-1β (r=−.38, p=.007) at baseline, the interaction of peak V̇O2 and gender was used in the models due to the significant differences in peak V̇O2 between males and females (18.85 ± 4.8 vs 14.59 ± 3.6, respectively; t=−3.54, p=.001). No group by time effects using multilevel modeling were found for IL-18, however significant fixed effects were found for race (β=46.34, SE=13.8, p=.003) and BMI (β=2.48, SE=1.0, p=.02). Group by time effects were found for 6MWT (β=7.9, SE=2.68, p=.03).
Table 3.
Multilevel Modeling for Entire Sample (N=54)
| Variable | Coefficient | SE | p-value |
|---|---|---|---|
| ASC Methylation | |||
| Fixed Effects | |||
| Intercept | 5.64 | 0.13 | <.001 |
| LVEFa | 0.2 | 0.01 | .05 |
| Peak V̇O2a × Genderb | 0.02 | 0.01 | .01 |
| Rate of Change | |||
| Intercept | −0.10 | 0.01 | .06 |
| Groupc | 0.3 | 0.01 | .006 |
|
| |||
| Interleukin-1β | |||
| Fixed Effects | |||
| Intercept | 1.96 | 0.21 | <.001 |
| LVEFa | −0.1 | 0.004 | .05 |
| Peak V̇O2a × Genderb | −.04 | 0.01 | .006 |
| Rate of Change | |||
| Intercept | −0.11 | 0.04 | 0.02 |
| Groupc | −0.11 | 0.04 | .02 |
| Completed Interventiond | −0.63 | 0.02 | .006 |
LVEF (%) and peak V̇O2 (ml/kg/min) were mean-centered for analysis
0=Female, 1=Male
0=Control Group, 1=Exercise Group
0=Did not complete 3-month intervention, 1=Exercised for 3 months
LVEF – Left ventricular ejection fraction
V̇O2 – Oxygen uptake
Exercise Group
In the exercise group analysis, mean percent ASC methylation at baseline remained positively associated with LVEF and the interaction effect of peak V̇O2 and gender (Table 4). Rate of change for participants in the exercise intervention was associated with gender, race, and level of education. Male gender (β=0.04) and having attended college (β=0.04) were associated with a higher rate of change in ASC methylation. The rate of change in ASC methylation was lower for those who identified as non-black (β=−0.06). The exercise group analysis for IL-1β produced results that were similar to the whole group analysis (Table 4). LVEF and the interaction effect of peak V̇O2 and gender remained negatively associated with IL-1β at baseline. Within in the exercise group, those who reported they had completed the exercise program had a negative rate of change (β=−0.06)
Table 4.
Multilevel Modeling for Exercise Only Group (n=38)
| Variable | Coefficient | SE | p-value |
|---|---|---|---|
| ASC Methylation | |||
| Fixed Effects | |||
| Intercept | 3.99 | 0.56 | <.001 |
| LVEFa | 0.13 | 0.09 | .049 |
| Peak V̇O2a × Genderb | 0.11 | 0.03 | .002 |
| Rate of Change | |||
| Intercept | −0.02 | .001 | .051 |
| Genderb | 0.04 | 0.01 | .008 |
| Racee | −0.06 | 0.01 | .004 |
| Collegef | 0.04 | 0.01 | .021 |
|
| |||
| Interleukin-1β | |||
| Fixed Effects | |||
| Intercept | 1.96 | 0.21 | <.001 |
| LVEFa | −.01 | 0.004 | .050 |
| Peak V̇O2a × Genderb | −.04 | 0.01 | .006 |
| Rate of Change | |||
| Intercept | −0.11 | 0.04 | .016 |
| Completed Interventiond | −0.64 | 0.02 | .010 |
LVEF (%) and peak V̇O2 (ml/kg/min) were mean-centered for analysis
0=Female, 1=Male
0=Control Group, 1=Exercise Group
0=Did not complete 3-month intervention, 1=Exercised for 3 months
0=African American/Black 1=Caucasian
0=Did not attend college 1=Attended college
LVEF – Left ventricular ejection fraction
V̇O2 – Oxygen uptake
Discussion
This is the first study, to our knowledge, to examine the effects of an exercise intervention on epigenetic changes in persons with HF. We hypothesized that persons with HF who participated in a 3-month aerobic exercise intervention would have increased ASC methylation at the completion of the intervention as compared to persons with HF in an attention-control group. A 3-month exercise intervention in persons with HF was associated with increased ASC methylation and decreased IL-1β at 3 months with medium to large effect sizes, supporting this hypothesis. The increase in ASC methylation after exercise was associated with a decrease in ASC mRNA expression, suggesting that exercise may decrease inflammation in HF via epigenetic control of inflammasome formation due to decreased bioavailability of ASC. The increase in ASC methylation after the exercise intervention was also associated with an increase in 6MWT total distance by 6 months.
A meta-analysis of changes in DNA methylation associated with exercise found that effect size of DNA methylation change across 16 different publications and 1580 people was large, with a Cohen’s d of 1.20 (25). Further, analyses indicated that the effect size of DNA methylation change with exercise was greater for participants over 40 years of age as compared to participants under 40 years, particularly with regard to studies measuring increases in DNA methylation (25). Genome-wide DNA methylation has been shown to decrease with age (26, 27), and thus, the epigenetic protective effects of exercise are likely more apparent in the face of the age-related changes in DNA methylation of older adults as compared to the younger population. In this study, we only included participants who were at least 40 years of age, and our hypothesis was to find an increase in DNA methylation of ASC after the exercise intervention. Combined with the previous evidence demonstrating higher ASC methylation among older adults (≥ 40 years of age) who exercised as compared to a control group (11), the medium to large effect sizes related to changes in DNA methylation in this study were not surprising.
ASC methylation and IL-1β were related to 6MWT total distance but not peak V̇O2 over time. This corresponds with improvements over time in 6MWT but not peak V̇O2 among those in the exercise group. The clinically significant change (28, 29) from baseline in 6MWT distance at 6 months (35.36 meters) was positively associated with ASC methylation and negatively associated with IL-1β.
Secondly, we hypothesized that increased ASC methylation would be related to decreased plasma IL-1β and IL-18 and to iNOS mRNA expression in persons with HF. We also investigated if changes in ASC methylation and plasma IL-1β and IL-18 were sustained 3 months after completing the aerobic exercise intervention. Increased ASC methylation was related to decreased IL-1β at baseline, 3 months, and 6 months but not to IL-18. ASC methylation was also negatively related to iNOS mRNA expression over time, and this relationship was mediated by IL-1β. Changes in IL-1β over time were related to changes in IL-18 from baseline to 3 months and from baseline to 6 months. These data suggest that the reduction in inflammatory cytokines after an exercise intervention may occur via decreased activation of IL-1β and that this reduction in IL-1β may be related to epigenetic control of ASC production.
IL-1β is one of the most important and potent inflammatory mediators in the acute phase response and in the pathophysiology of chronic diseases, such as heart failure (19, 30); activation of IL-1β is tightly regulated by the inflammasome (2, 19). IL-1β stimulates nitric oxide production, as evidenced by a corresponding increase in iNOS, leading to further activation of inflammatory mediators (30). IL-1β is also associated with patient report of sickness symptoms (31), perhaps contributing to the common symptoms of fatigue and depression also associated with HF, and, in other studies, reduced by exercise (32, 33). Combined with the associated changes in ASC methylation and expression, these results suggest that exercise may decrease inflammation, at least in part, via decreased inflammasome formation.
We expected to see between group and within group changes in IL-18 over time, but none were found. The few studies examining changes in IL-18 after an exercise intervention have had conflicting results, where some studies demonstrated a decrease in IL-18 (34, 35) and other studies (36, 37) did not. There have been no studies to date examining changes in IL-18 after an exercise intervention in persons with HF. While the values of IL-18 and IL-1β were not associated at any time point, changes in these two cytokines from baseline to 3 months and from 3 months to 6 months were related.
IL-1β is produced as a precursor protein in response to an inflammatory stimulus and inflammasome activation (5). IL-18 is constitutively expressed as a biologically inactive precursor molecule that lacks a signal peptide (38). IL-18 mRNA has a long half-life, thus contributing to steady state production of IL-18 (9). Although both IL-1β and IL-18 require caspase-1 dependent proteolytic cleavage for activation (7), IL-1β is more tightly regulated by the inflammasome than IL-18. Additionally, IL-18 is regulated by an endogenous inhibitor, IL-18 binding protein (IL-18BP), which inactivates IL-18 when bound (39), and thus has a more complex mechanism of function than IL-1β. There is some evidence that IL-1β can also induce IL-18 (38, 40), while other studies have shown that IL-18 induces IL-1β (7, 9). The changes in these two cytokines over time suggest that they are changing together even if the actual levels are not related at time points. Further studies are needed to untangle the relationships between these IL-1 family cytokines.
Our second hypothesis was partially supported in that increased ASC methylation was associated with decreased IL-1β and iNOS, but not with IL-18. Inflammation in HF can be initiated by danger-associated molecular patterns (DAMP), which are host-derived molecules indicative of cellular damage (3). It is possible that the effects of exercise led to decreased DAMP formation, the impetus for inflammasome formation and activation, independent of changes in ASC methylation. Further, the changes in IL-1β with exercise may occur in a non-inflammasome dependent manner. However, the associations between ASC methylation, ASC mRNA expression, and IL-1β before and after the exercise intervention suggest the changes in ASC methylation may decrease inflammasome formation and function, at least, to some extent.
No changes over time in IL-18 mRNA expression were found. IL-18 is most abundant in a constitutively expression form (7), and these results are likely reflective the steady-state levels of IL-18 mRNA in peripheral blood mononuclear cells. There were significant changes in ASC mRNA expression over time. ASC mRNA levels were negatively associated with ASC methylation at each time point. While we cannot determine causation using this model, this evidence, in addition to our findings in a study with a different HF population, is suggestive of epigenetic control of ASC gene expression in persons with HF (12). Further mechanistic work is warranted.
In our multilevel modeling analysis, baseline ASC methylation and IL-1β levels were related to LVEF, gender, and peak V̇O2, which we previously reported (12, 13). The analysis of the entire sample again demonstrated that the exercise intervention was effective in increasing mean percent ASC methylation and in decreasing plasma IL-1β. Response to the exercise intervention was examined by analyzing factors related to rate of change within the exercise group only. We found that males who participated in the exercise intervention had a greater increase in ASC methylation. One study examining DNA methylation and gene expression in skeletal muscle of healthy persons after an exercise intervention found differential DNA methylation patterns between males and females (41), however no specific patterns or meaningful changes were revealed. No other studies examining gender differences in DNA methylation changes in response to exercise have been reported to date. In this study, the gender difference in the DNA methylation response to the exercise intervention could be related to gender differences in baseline aerobic capacity and body composition. Male participants may have exercised at higher intensities due to higher aerobic capacity or may have had a faster physiological response to exercise due to a higher proportion of muscle mass than female participants. Education could be a proxy measure for socioeconomic status and may be reflective of better access to care, a gym, or reliable exercise space. Income data were not collected, so we were unable to compare the measures for analysis. The IL-1β rate of change was related to self-reported adherence to the exercise program. This relationship suggests that the amount of exercise may be related to the level of decrease in IL-1β in HF, and further suggests that exercise may modulate inflammasome formation or activation. The exercise adherence measure was self-report, and we assumed in our analysis that those who did not submit the exercise calendar did not complete the exercise. Thus, the analysis may not have accurately captured the true level of adherence.
These results demonstrate that behavior may influence gene expression of inflammation via epigenetic regulation of a key inflammatory protein in persons with HF. We followed participants for an additional 3 months after the exercise intervention. Some participants may have continued the walking program, although this was not monitored. If they did, this could have contributed to the prolonged effect. A longer intervention time with longer follow-up may demonstrate the effects of long-term behavior change. Previous studies have demonstrated beneficial effects with walking exercises in persons with HF (18, 42), and here we support the evidence that walking is an appropriate and beneficial level of activity in HF. The possibility that exercise may be able to reverse epigenetic-induced changes in gene expression of inflammatory proteins and other markers associated with HF pathophysiology, thereby improving outcomes, is an area of research greatly in need of further exploration.
Limitations
This study used a short-term exercise program (12 weeks); longer interventions may provide more insight into the dynamics of changes in inflammatory proteins over time and examine the limits and rate of change of DNA methylation with exercise. We did not capture the level of exercise during the 3-month maintenance phase, so we were unable to identify if changes are sustained at 3 months were due to continued exercise or if there are short term lasting epigenetic and anti-inflammatory effects. Exercise adherence was self-report, and some participants did not provide a self-report exercise log, which we inferred to mean they did not adhere to the protocol as described. Thus, we may have over or underestimated actual adherence. Real-time activity trackers could provide more objective data to better identify the relationships between exercise intensity and endurance with changes in DNA methylation and cytokine expression. The results of this study are specific to the 3-month walking exercise program and may not be generalizable to all types and durations of exercise.
The lack of improvement in peak V̇O2 after the exercise intervention was unexpected, especially in light of the improvements over time in 6MWT distance. One possible explanation is the lack of volitional effort during exercise treadmill testing. For many participants anaerobic threshold was not met. The mean respiratory quotient (RQ) at both BL and 3 months was 0.94 ± 0.1, and only 31% of participants reached a RQ ≥ 1. The peak V̇O2 values may not accurately reflect true aerobic capacity. Secondly, the improvement in 6MWT but not peak VO2 after an exercise intervention could be reflective of more physiologic effects on peripheral mechanisms, such as decreased skeletal muscle inflammation, and less effects on central mechanisms. Further studies examining ASC methylation and expression changes at the tissue level with exercise are needed.
The measures of ASC methylation and mRNA were completed in peripheral blood mononuclear cells (PBMCs) and may not be reflective of changes that occur in other tissues important in HF function, such as myocardium or skeletal muscle. There may be meaningful tissue-specific epigenetic changes with exercise resulting in altered inflammatory profile in the myocardium and skeletal muscle, and these changes may more tightly align with changes in cytokine expression. Patterns of myocardial DNA methylation have been found to relate to cardiac function (43, 44), suggesting the myocardial epigenome may be an important regulator of cardiac function. However, DNA methylation of distinct genomic regions is conserved between heart tissue and peripheral blood (44), suggesting that PBMCs may be a potential biomarker in HF. Further research examining levels of ASC methylation in PBMCs, myocardial, and skeletal tissues is needed to better understand how changes in ASC methylation with exercise translate to clinical improvement for persons with HF. We used PBMCs collected from whole blood and did not control for leukocyte differential. Intra-individual and inter-individual differences over time may be related to different leukocyte composition that we were unable to capture.
This is a pilot study of a small cohort of persons with HF, providing a novel contribution to the field of exercise epigenetics. These results are suggestive of a possible epigenetic regulation of inflammation via exercise in persons with HF. However, considering the reproducibility crisis in biobehavioral research and the small sample size (N=54) in this study, further validation of epigenetic changes with exercise in a larger HF population and across multiple tissue types are needed to fully understand these effects.
Conclusions
We demonstrated that an exercise intervention in persons with HF is associated with changes in DNA methylation of a key component of the inflammasome, ASC, and that these changes are associated with decreased ASC gene expression. Further, changes in ASC methylation and expression were associated with decreased plasma IL-1β among participants in the exercise intervention. Epigenetic regulation of ASC may be a biological mechanism by which exercise can promote better outcomes in HF. Further research examining mechanisms of change can lead to improved understanding of physiological adaptations and more precise prediction of adverse outcomes in persons with HF.
Supplementary Material
Supplemental Table 1.docx—genomic DNA concentrations for each sample
Acknowledgments
Supported in part by National Institutes of Health National Institute of Nursing Research grant number 1P30NR014134-01 (Co-investigator – R. Gary), the Heart Failure Society of America Nursing Research Grant (PI – B. Butts), and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR000454.
Effort for B. Butts was funded in part by the National Institutes of Health National Institute of Nursing Research grant numbers T32NR012715 (PI – S. Dunbar) and 1F31NR015180-01 (PI – B. Butts).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. Results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflicts of Interest: None for BB, RG, EC, and SD. JB is a consultant to Amgen, Bayer, Cardiocell, Celladon, Novartis, Stealth Peptide, Relypsa, Z Pharma, Trevena, and Zensun.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. Epub 2013/06/12. [DOI] [PubMed] [Google Scholar]
- 2.Abbate A. The heart on fire: inflammasome and cardiomyopathy. Exp Physiol. 2013;98(2):385. doi: 10.1113/expphysiol.2012.069021. Epub 2013/01/26. [DOI] [PubMed] [Google Scholar]
- 3.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp Physiol. 2013;98(2):462–72. doi: 10.1113/expphysiol.2012.068338. Epub 2012/08/01. [DOI] [PubMed] [Google Scholar]
- 4.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathwayson ROS production? Nature Reviews Immunology. 2010;10:210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J. The inflammasome. Cell Adhes Commun. 2010;140(821–832) doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Eslick GD, Thampan BV, Nalos M, McLean AS, Sluyter R. Circulating interleukin-18 concentrations and a loss-of-function P2X7 polymorphism in heart failure. Int J Cardiol. 2009;137(1):81–3. doi: 10.1016/j.ijcard.2008.05.017. Epub 2008/08/06. [DOI] [PubMed] [Google Scholar]
- 7.Mallat Z, Heymes C, Corbaz A, Logeart D, Alouani S, Cohen-Solal A, et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004;18(14):1752–4. doi: 10.1096/fj.04-2426fje. Epub 2004/09/17. [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka-Tojo M, Tojo T, Inomata T, Machida Y, Osada K, Izumi T. Circulating levels of interleukin 18 reflect etiologies of heart failure: Th1/Th2 cytokine imbalance exaggerates the pathophysiology of advanced heart failure. J Card Fail. 2002;8(1):21–7. doi: 10.1054/jcaf.2002.31628. Epub 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 9.Lebel-Binay S, Berger A, Zinzindohoue F, Cugnenc P, Thiounn N, Fridman WH, et al. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw. 2000;11(1):15–26. Epub 2000/03/08. [PubMed] [Google Scholar]
- 10.Taniguchi S, Sagara J. Regulatory molecules involved in inflammasome formation with special reference to a key mediator protein, ASC. Semin Immunopathol. 2007;29(3):231–8. doi: 10.1007/s00281-007-0082-3. Epub 2007/09/07. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima K, Takeoka M, Mori M, Hashimoto S, Sakurai A, Nose H, et al. Exercise effects on methylation of ASC gene. Int J Sports Med. 2010;31(9):671–5. doi: 10.1055/s-0029-1246140. Epub 2010/03/05. [DOI] [PubMed] [Google Scholar]
- 12.Butts B, Gary RA, Dunbar SB, Butler J. Methylation of Apoptosis-Associated Speck-Like Protein With a Caspase Recruitment Domain and Outcomes in Heart Failure. J Card Fail. 2016;22(5):340–6. doi: 10.1016/j.cardfail.2015.12.004. Epub 2015/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butts B, Butler J, Dunbar SB, Corwin EJ, Gary RA. ASC Methylation and Interleukin-1beta Are Associated with Aerobic Capacity in Heart Failure. Med Sci Sports Exerc. 2017;49(6):1072–8. doi: 10.1249/mss.0000000000001200. Epub 2017/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denham J, O’Brien BJ, Marques FZ, Charchar FJ. Changes in the leukocyte methylome and its effect on cardiovascular-related genes after exercise. Journal of applied physiology (Bethesda, Md : 1985) 2015;118(4):475–88. doi: 10.1152/japplphysiol.00878.2014. Epub 2014/12/30. [DOI] [PubMed] [Google Scholar]
- 15.Ronn T, Volkov P, Davegardh C, Dayeh T, Hall E, Olsson AH, et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS genetics. 2013;9(6):e1003572. doi: 10.1371/journal.pgen.1003572. Epub 2013/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowlands DS, Page RA, Sukala WR, Giri M, Ghimbovschi SD, Hayat I, et al. Multi-omic integrated networks connect DNA methylation and miRNA with skeletal muscle plasticity to chronic exercise in Type 2 diabetic obesity. Physiological genomics. 2014;46(20):747–65. doi: 10.1152/physiolgenomics.00024.2014. Epub 2014/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMaeyer C, Beckers P, Vrints CJ, Conraads VM. Exercise training in chronic heart failure. Therapeutic Advances in Chronic Disease. 2013;4(3):105–17. doi: 10.1177/2040622313480382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. Epub 2009/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev. 2015;21:26–41. Epub 2015/04/01. [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. Epub 1994/11/01. [DOI] [PubMed] [Google Scholar]
- 21.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. Epub 2010/06/30. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. Epub 2002/02/16. [DOI] [PubMed] [Google Scholar]
- 23.Hedges LV. Distribution Theory for Glass’s Estimator of Effect size and Related Estimators. Journal of Educational and Behavioral Statistics. 1981;6(2):107–28. doi: 10.3102/10769986006002107. [DOI] [Google Scholar]
- 24.Hayes AF. Introduction to mediation, moderation, and conditional process analysis : a regression-based approach. New York: New York: The Guilford Press; 2013. [Google Scholar]
- 25.Brown WM. Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: a bioinformatics meta-analysis. Br J Sports Med. 2015;49(24):1567–78. doi: 10.1136/bjsports-2014-094073. Epub 2015/04/01. [DOI] [PubMed] [Google Scholar]
- 26.Zinovkina LA, Zinovkin RA. DNA Methylation, Mitochondria, and Programmed Aging. Biochemistry (Mosc) 2015;80(12):1571–7. doi: 10.1134/S0006297915120044. Epub 2015/12/08. [DOI] [PubMed] [Google Scholar]
- 27.Smith JA, Zagel AL, Sun YV, Dolinoy DC, Bielak LF, Peyser PA, et al. Epigenomic Indicators of Age in African Americans. Hereditary genetics : current research. 2014;3(3) doi: 10.4172/2161-1041.1000137. Epub 2016/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulmonary physical therapy journal. 2013;24(3):21–9. Epub 2013/09/03. [PMC free article] [PubMed] [Google Scholar]
- 29.Gremeaux V, Troisgros O, Benaim S, Hannequin A, Laurent Y, Casillas JM, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil. 2011;92(4):611–9. doi: 10.1016/j.apmr.2010.11.023. Epub 2011/03/29. [DOI] [PubMed] [Google Scholar]
- 30.Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7(3):e33438. doi: 10.1371/journal.pone.0033438. Epub 2012/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12(12):4447–56. Epub 2000/12/21. [PubMed] [Google Scholar]
- 32.Tu RH, Zeng ZY, Zhong GQ, Wu WF, Lu YJ, Bo ZD, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail. 2014;16(7):749–57. doi: 10.1002/ejhf.101. Epub 2014/05/07. [DOI] [PubMed] [Google Scholar]
- 33.Pozehl B, Duncan K, Hertzog M. The effects of exercise training on fatigue and dyspnea in heart failure. European journal of cardiovascular nursing : journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. 2008;7(2):127–32. doi: 10.1016/j.ejcnurse.2007.08.002. Epub 2007/09/29. [DOI] [PubMed] [Google Scholar]
- 34.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–9. doi: 10.1016/j.bbi.2005.12.002. Epub 2006/03/01. [DOI] [PubMed] [Google Scholar]
- 35.Stensvold D, Slordahl SA, Wisloff U. Effect of exercise training on inflammation status among people with metabolic syndrome. Metabolic syndrome and related disorders. 2012;10(4):267–72. doi: 10.1089/met.2011.0140. Epub 2012/03/30. [DOI] [PubMed] [Google Scholar]
- 36.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. American journal of physiology Endocrinology and metabolism. 2010;298(4):E824–31. doi: 10.1152/ajpendo.00574.2009. Epub 2010/01/21. [DOI] [PubMed] [Google Scholar]
- 37.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, et al. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4(7) doi: 10.1161/jaha.115.002014. Epub 2015/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toldo S, Mezzaroma E, O’Brien L, Marchetti C, Seropian IM, Voelkel NF, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. American journal of physiology Heart and circulatory physiology. 2014;306(7):H1025–31. doi: 10.1152/ajpheart.00795.2013. Epub 2014/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10(1):127–36. doi: 10.1016/s1074-7613(00)80013-8. Epub 1999/02/19. [DOI] [PubMed] [Google Scholar]
- 40.Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162(2):1096–100. Epub 1999/01/23. [PubMed] [Google Scholar]
- 41.Lindholm ME, Marabita F, Gomez-Cabrero D, Rundqvist H, Ekstrom TJ, Tegner J, et al. An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics. 2014;9(12):1557–69. doi: 10.4161/15592294.2014.982445. Epub 2014/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsarouhas K, Tsitsimpikou C, Haliassos A, Georgoulias P, Koutsioras I, Kouretas D, et al. Study of insulin resistance, TNF-alpha, total antioxidant capacity and lipid profile in patients with chronic heart failure under exercise. In Vivo. 2011;25(6):1031–7. Epub 2011/10/25. [PubMed] [Google Scholar]
- 43.Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S, Down T, et al. Distinct epigenomic features in end-stage failing human hearts. Circulation. 2011;124(22):2411–22. doi: 10.1161/circulationaha.111.040071. Epub 2011/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meder B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Frese K, Lai A, et al. Epigenome-Wide Association Study Identifies Cardiac Gene Patterning and a Novel Class of Biomarkers for Heart Failure. Circulation. 2017;136(16):1528–44. doi: 10.1161/circulationaha.117.027355. Epub 2017/08/26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1.docx—genomic DNA concentrations for each sample