Abstract
Monoclonal antibodies are very important in modern therapeutics and constitute a substantial percentage of newly approved drugs. Every therapeutic monoclonal antibody must be analyzed for structural and functional integrity, and all protein heterogeneities need to be identified and quantified. The conformational stabilities of the monoclonal antibodies are also important for antibody storage and handling stabilities. One of the first and simplest of the structural analysis techniques utilized is SDS-PAGE, which can be performed both with and without prior reduction to break disulfide bonds. This permits sizing of both heavy and light chains in the reduced condition, and sizing of the intact antibody and any disulfide aggregates in the non-reduced condition. Analyzing our human anti-cocaine monoclonal antibody, we noted unexpectedly larger apparent molecular weights and apparent protein size heterogeneities using non-reducing SDS-PAGE. These apparent molecular weight heterogeneities are not consistent with other analysis techniques. Heterogeneities were noted using several heating and pre-electrophoretic sample preparation protocols, and are modified by the inclusion of small concentrations of certain alcohols such as propanol and butanol. All of these unexpected results were also observed for a commercial human IgG1 antibody, suggesting that these observations are applicable to IgGs in general. Thus, careful attention must be paid to the interpretation of non-reducing SDS-PAGE results for IgGs. It is hypothesized that differential thermal unfolding of the Fab, CH2 and CH3 domains of the IgGs in SDS give rise to the stable, discreet bands observed using different heating protocols prior to non-reducing SDS-PAGE.
Keywords: Monoclonal antibody, non-reducing SDS-PAGE, alcohol additives, antibody analysis, electrophoretic migration, antibody domain unfolding
Graphical Abstract
Introduction
Monoclonal antibodies (mAbs) are important therapeutic agents, and both monoclonal and polyclonal antibodies are widely used in basic and translational research. However, it has been reported that a large percentage of commercially available antibodies for basic research are unable to accurately and selectively recognize their intended targets [1,2], and there are substantial ongoing efforts to systematically validate antibodies for their intended usages and targets. There are very well-developed protocols and methodologies used to characterize protein therapeutics, e.g., monoclonal antibodies, as well as fragments and derivatives of monoclonal and polyclonal antibodies. Our laboratory has developed a humanized mAb (h2E2) that has high affinity for cocaine and selectivity for cocaine over its inactive metabolites [3], which is intended for treatment of cocaine addiction. This recombinant mAb protein can be produced in gram quantities in Chinese hamster ovary (CHO) cells [4].
SDS-PAGE is one of the simplest, least expensive, and most commonly used techniques to analyze antibodies for purity. Reduced samples of the IgG class of antibodies give rise to glycosylated heavy chains of approximately 50 kDa and light chains of approximately 25 kDa on SDS-PAGE. When analyzed without reduction of disulfide bonds, these antibodies should give rise to a single band on SDS-PAGE, i.e., the intact antibody consisting of 2 heavy and 2 light chains, with a combined size of approximately 150 kDa.
In this work, we note apparent overestimated molecular weights and size heterogeneities for a recombinant humanized monoclonal anti-cocaine mAb (h2E2), as evidenced by multiple bands on non-reducing gels, some of which migrate with artificially low electrophoretic mobilities (high apparent molecular weights). These anomalies are dependent on the sample pre-electrophoretic heating temperature and duration. In addition, we noted that under some conditions, both the apparent band heterogeneity and the anomalously high molecular weights observed are modulated by the inclusion of a small concentration of certain short chain alcohols (e.g., propanol and butanol) in the sample buffer. These unexpected effects of heating and the addition of alcohols were similar for both the humanized h2E2 monoclonal antibody and a commercial human IgG1 polyclonal antibody, suggesting that these properties may be shared by many polyclonal and monoclonal antibodies in use for both research and clinical applications.
It is well known that, in the absence of SDS, IgGs have structural domains that unfold relatively independently, and exhibit differential thermal stabilities, as measured by the multiple transitions and melting temperatures observed for the native proteins in non-denaturing buffers, as assessed by differential scanning calorimetry (DSC, [5,6,7]). In this study, we hypothesize that the observed changes in electrophoretic mobilities and apparent size heterogeneities of these IgG antibodies in non-reducing SDS-PAGE are due to differential unfolding of the Fab, CH2, and CH3 domains of the immunoglobulins in the SDS sample buffer prior to non-reducing electrophoresis. Using such analyses could add to the existing therapeutic antibody characterization techniques, as well as possibly represent a simple means to analyze the effects of mutations on the stability of IgG protein domains in therapeutic antibodies.
Materials and Methods
Materials
The generation and production of the h2E2 anti-cocaine monoclonal antibody was previously described [8]. The recombinant h2E2 mAb was purified by protein A affinity chromatography and used as supplied by the manufacturer, Catalent. The purity, structure, and function of the resultant mAb protein has been well characterized in our laboratory [4,9,10,11]. The human polyclonal IgG1 protein used for comparative purposes was purchased from Sigma-Aldrich (catalogue # I5029, batch # SLBV3436, reported to be 96% pure by capillary electrophoresis, 1.06 mg/ml as supplied). Acrylamide, bisacrylamide, and all reagents used to pour, run, and stain SDS-PAGE gels were from BioRad. HPLC grade methanol, ethanol, 1-propanol, 2-propanol (isopropanol) and 1-butanol (butanol) were from Fisher. Pre-stained SDS-PAGE molecular weight standards (10 – 240 kDa) were purchased from SMOBIO Technology, Inc., catalogue # PM1700.
Methods
The 7% SDS-PAGE gels were poured, run, and stained with Coomassie Blue, basically as described by Laemmli [12]. Antibody samples were usually diluted to a final protein concentration of 0.2 mg/mL in non-reducing sample buffers (containing 5% SDS, and usually containing 25% glycerol to provide the needed density for loading, but sometimes replacing the glycerol with 8M urea). Identical results were obtained loading 2 ug IgG per well, after pre-electrophoretic heating of 0.1–1.0 mg/ml IgG concentrations in SDS sample buffer, indicating that the results are protein concentration-independent and not due to overloading of the gels (data not shown). Some samples also contained low concentrations (1–10%) of various short chain alcohols (methanol, ethanol, 1- and 2-propanol, or 1-butanol). After incubation at various temperatures for varying times (boiled (100°C) samples were always incubated in boiling water for 5 minutes) prior to electrophoresis, typically 10 μL (2 ug) of each sample was loaded into individual wells of 15 well, 1.5 mm thick 7% acrylamide gels. Experiments were designed so that comparisons of conditions and effects on electrophoretic mobility were done on the same gel. All gels were used at least 24 hours after they were poured (polymerized), and all gel sample wells were washed 3 times with Laemmli running buffer, prior to loading samples, to eliminate possible artefacts due to reaction of the protein samples with any residual unpolymerized acrylamide or residual free radicals. Electrophoresis was performed until the bromophenol blue tracking dye reached the bottom of the gel. Pre-stained molecular weight standards (10 μL) were also run on each gel. Gels were stained at room temperature for 30–45 minutes with Coomassie blue, and destained for 4–20 hours prior to photography. Each panel in each figure in this study depicts a single SDS-PAGE gel, to enable direct comparisons of bands. The vertical dashed lines in some figures were overlaid on the gel image to aid in alignment of lanes between panels, as well as to facilitate reader understanding and interpretation of the gels, and do not represent lanes from different gels that were aligned following cutting and pasting from different photographic images.
Results
We previously noted that when different laboratory personnel analyzed the h2E2 anti-cocaine mAb under development in our laboratory as a potential therapeutic for cocaine addiction, varying molecular weights were obtained using non-reducing SDS-PAGE. Specifically, larger than expected molecular weight(s) and size heterogeneity were often observed, which were not consistent with other analyses done on the same mAb. Therefore, a systematic analysis of the pre-electrophoresis heating and treatment of the mAb sample was initiated. Possible sources of artefacts due to reaction of the sample with incompletely polymerized acrylamide remaining in the gel and the gel wells and heating-induced inter-antibody disulfide formation were eliminated by using fully polymerized “aged” gels, by washing the sample gel wells with running buffer prior to sample loading, and by including a sulfhydryl-reactive alkylating agent (N-ethylmaleimide) in the non-reducing sample buffer. None of these treatments eliminated the size or size heterogeneity artifacts. We then evaluated various heating protocols and the use of water soluble and miscible short chain alcohols to determine if they were able to eliminate the presumed non-covalent aggregates or the possible incomplete denaturation in SDS, which might explain the unexpected results.
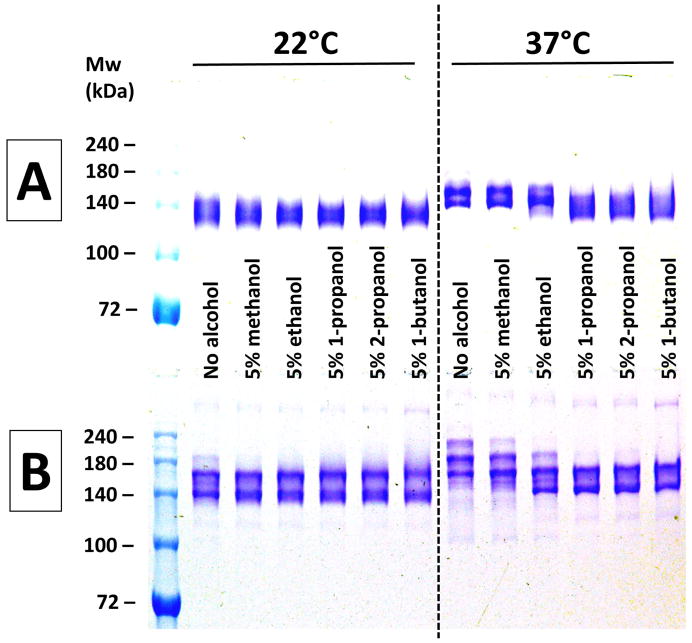
Fewer apparent size heterogeneities were evident when not heating (22°C) or only gently heating (37°C) the samples. Also shown in Figure 1 is the effect of pre-incubation for 10 minutes with 5% (vol/vol) of five short chain alcohols on the electrophoretic banding patterns of both h2E2 mAb and an IgG1 polyclonal antibody. As is evident from Figure 1, there are few anomalies seen with either the mAb (top Panel A) or the IgG1 (bottom Panel B) without heating (22°C). However, at 37°C a doublet is seen for the mAb, and multiple bands are noted for the polyclonal IgG1 without any added alcohol. Addition of some alcohols (1- and 2-propanol, as well as 1-butanol) eliminated this apparent size heterogeneity, and also reduced the apparent molecular weights. The actual molecular weight of the h2E2 mAb used in this study has been accurately determined by mass spectral analysis of the Fab and Fc fragments [7]: the total Mw = 145,036 = (45,868 × 2) (due to 2 Fab fragments) + 53,300 (due to the Fc fragment, having the most abundant (G0F) glycoform). In addition, mass spectral analysis of the intact mAb was performed, demonstrating a mass spectral peak from just above 145 kDa to just below 146 kDa, with a maximum at 145,412 Da (unpublished results). Thus, the small degree (approximately 1 kDa) of actual size heterogeneities revealed by mass spectral analyses are not consistent with the large changes in electrophoretic mobilities (apparent molecular weights) for this mAb, which are observed on non-reducing SDS-PAGE (e.g., Figures 2 and 3).
Figure 1. The effect pre-incubation with alcohols at 22°C and 37°C on non-reducing SDS-PAGE analyses of antibodies.
Panel A (top) – results using h2E2 mAb, Panel B (bottom) – results using human IgG1 polyclonal antibody. A 1.5 mm thick, 7% acrylamide gel was loaded with 2 μg (10 μL) of antibody per well, electrophoresed until the tracking dye reached the bottom of the gel, and stained with Coomassie blue. Only the top portion of the gels are shown – no bands were detected in the lower section of the gels. Panels A and B depict photographs of single gels. Pre-electrophoretic incubation in sample buffers under the indicated conditions was done for 10 minutes at either 22°C or 37°C, as indicated in the figure.
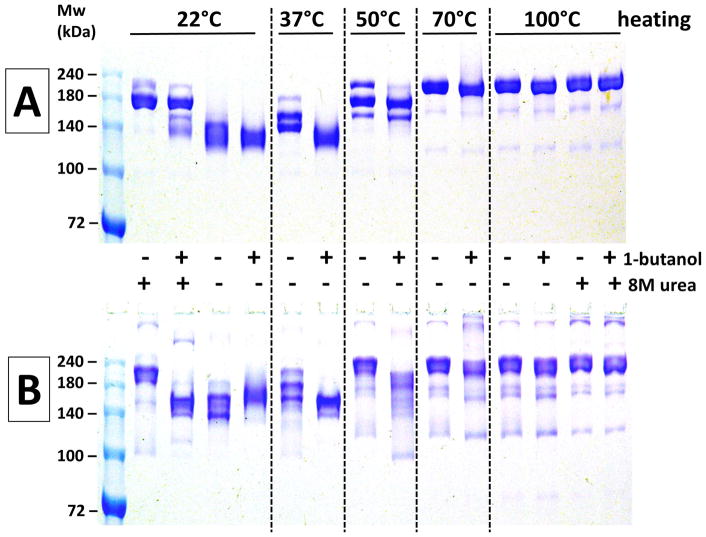
Figure 2. The effect of temperature, urea, and 5% 1-butanol on non-reducing SDS-PAGE analyses of antibodies.
Panel A (top) – results using h2E2 mAb, Panel B (bottom) – results using human IgG1 polyclonal antibody. A 1.5 mm thick, 7% acrylamide gel was loaded with 2 μg (10 μL) of antibody per well, electrophoresed until the tracking dye reached the bottom of the gel, and stained with Coomassie blue. Only the top portion of the gels are shown – no bands were detected in the lower section of the gels. Panels A and B depict photographs of single gels. Pre-electrophoretic incubation in sample buffers under the indicated conditions was done for 10 minutes at all temperatures except for 100°C – those samples were boiled for 5 minutes.
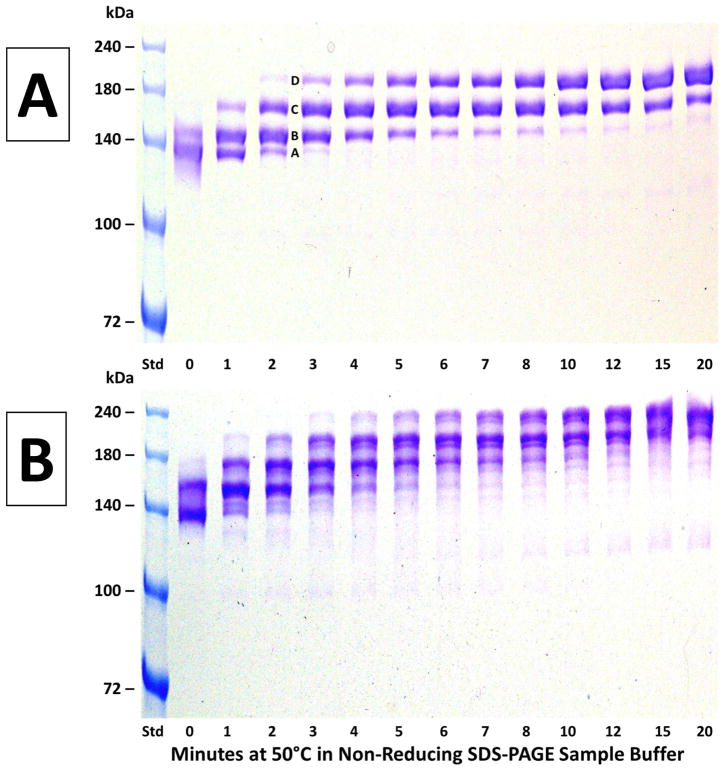
Figure 3. The time course of heating at 50°C on non-reducing SDS-PAGE analyses of antibodies.
Panel A (top) – results using h2E2 mAb, Panel B (bottom) – results using human IgG1 polyclonal antibody. A 1.5 mm thick, 7% acrylamide gel was loaded with 2 μg (10 μL) of antibody per well, electrophoresed until the tracking dye reached the bottom of the gel, and stained with Coomassie blue. Only the top portion of the gels are shown – no bands were detected in the lower section of the gels. After the heating times shown in the figure, samples were cooled in room temperature (21°C) water prior to electrophoresis, and loaded onto gels after all samples were heat treated and cooled.
As shown in Figure 2, the bands for both the monoclonal (Panel A) and polyclonal (Panel B) antibodies are shifted to higher molecular weights, and/or display more size heterogeneity after heating at 50°C, 70°C and 100°C. Inclusion of 5% 1-butanol in the sample buffer was effective at eliminating these size increases and apparent heterogeneities for both antibodies at 37°C, and had obvious effects on the electrophoretic band patterns at 50°C as well (inclusion of 5% 2-propanol resulted in data essentially identical to the 1-butanol data shown in Figure 2, data not shown). At 70°C and 100°C both antibodies appeared relatively homogenous by non-reducing SDS-PAGE, but the apparent size of the mAb (≈200 kDa) was substantially larger than the actual size (145 kDa by mass spectral analyses). Interestingly, inclusion of 8M urea in the non-reducing sample buffer decreased the artificial heterogeneity at both 22°C and 100°C, but also increased the apparent size of the mAb to about 180 kDa and 200 kDa at 22°C and 100°C, respectively. In addition, with urea present, the effect of 5% butanol was decreased at 22°C, and virtually eliminated at 100°C. Qualitatively very similar results were observed using the same protocols for the commercial polyclonal IgG1 antibody (Panel B).
Figure 3 shows the time course of the progression of observed bands upon heating at 50°C for 0–20 minutes prior to non-reducing SDS-PAGE for both the h2E2 mAb (top, Panel A) and the commercial IgG1 polyclonal antibody (bottom, Panel B). As seen in Panel A, the electrophoretic pattern changes from being dominated by the lowest apparent molecular weight band A at 0 minutes to being dominated by the highest molecular weight band D after 20 minutes at 50°C. The bands are observed at discrete and reproducible electrophoretic mobilities in all wells (i.e., no smearing of protein bands). A similar pattern of progression of bands is seen for the polyclonal IgG1 (Panel B), but that pattern is somewhat more complicated, likely due to the polyclonal nature of that antibody.
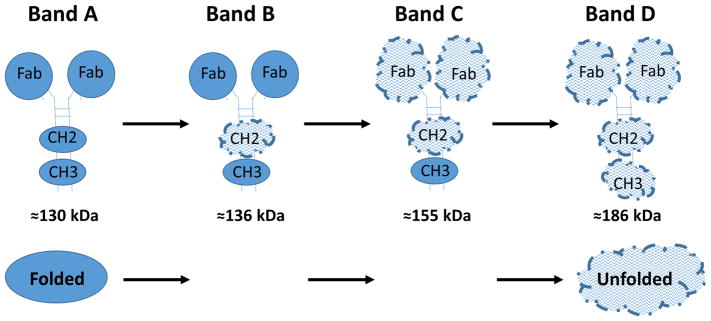
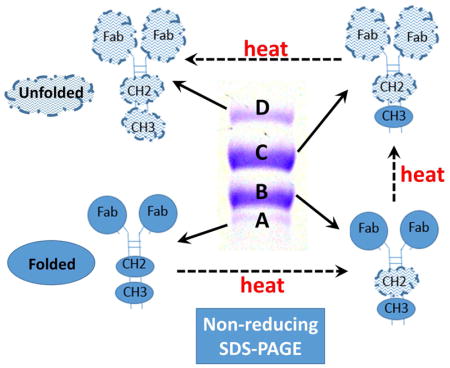
Figure 4 is a cartoon model explaining the 4 bands seen in Panel A of Figure 3 for the anti-cocaine mAb. This hypothetical explanation is based on the observations of multiple, relatively independently unfolding domains in IgGs observable by DSC (in non-SDS containing buffers), with a typical progression of stability for many antibodies in the absence of SDS being from the least stable CH2 domain, to the most stable CH3 domain, with the 2 Fab domains unfolding at the same temperature, with a stability intermediate between the CH2 and CH3 domains. The apparent molecular weights of Bands A–D shown in Figure 4 are calculated by fitting of the molecular weight standard bands plot of molecular weight vs electrophoretic mobility, using data from Figure 3A.
Figure 4. A cartoon model hypothesizing the conformational identity of the mAb bands observed in Figure 3A, based on differential stability of IgG domains.
Sequential unfolding in SDS of the CH3, Fab, and CH2 domains are shown to explain the 4 discreet molecular weight bands observed for the h2E2 anti-cocaine mAb. More compact “Folded” domains are shown as filled circles or ellipses, while more extended “Unfolded” domains are depicted as larger, uneven cloud-like structures.
Discussion
Variable results upon analysis by non-reducing SDS-PAGE have been observed in our laboratory when analyzing the h2E2 anti-cocaine mAb, which is under development for treatment of cocaine addiction. Specifically, apparent molecular weights measured by non-reducing SDS-PAGE were often much higher than the theoretically expected values, and multiple protein bands were sometimes evident, both of which observations are not consistent with mass spectral data demonstrating a high degree of purity and a lack of size heterogeneity that would be measurable on SDS-PAGE gels. Thus, although we have previously reported the small structural heterogeneities present in this monoclonal antibody (e.g., multiple glycoforms at the single heavy chain N-linked glycosylation site, differential cleavage of the heavy chain C-terminal lysine residue, and a light chain glutamine to pyro-glutamate N-terminal modification [10]), no modifications that would result in the observed large size heterogeneities or large changes in the molecular weight of the mAb were detected by any other methods, including multiple mass spectral analyses performed on the same samples [4,10,11].
Thus, we conducted studies to determine under what pre-electrophoretic sample preparation conditions these artificial results are evident. First, the possible covalent modifications of the antibody during sample preparation and running of the non-reducing gel were assessed. To eliminate the possibility of reaction with unpolymerized acrylamide left over from the gel polymerization process, gels were used from 1 day to several weeks after they were poured (polymerized), and all gel sample wells were washed three times with the running buffer, prior to loading samples. In addition, to eliminate the possibility of disulfide-linked oligomers forming, excess alkylating agents (e.g., N-ethylmaleimide) were included in the non-reducing sample buffer (with no effect on the resulting banding patterns, data not shown). This is consistent with the demonstrated lack of unpaired sulfhydryls (cysteine) present in this mAb [4].
In an attempt to disrupt putative hydrophobic interactions leading to the aberrant heterogeneity and sizing results, water soluble alcohols (methanol, ethanol, 1- and 2-propanol, and 1-butanol) were added to the samples prior to heating and loading onto the gels. At 37°C and 50°C, inclusion of several alcohols minimized or eliminated the artificial size and size heterogeneity (see Figure 2). Propanols and butanol were the most effective of the alcohols tested, and the concentration dependence of the effect for 2-propanol indicated that the full effect was attained at a 2-propanol concentration of about 4% (vol/vol, data not shown). At higher pre-electrophoresis heating temperatures (70°C and 100°C), neither butanol (Figure 2) nor isopropanol (data not shown) had significant effects on protein band electrophoretic mobilities for either the monoclonal or polyclonal antibody.
The protein denaturant urea, at 8M, was also added to some samples prior to heating and electrophoresis to determine if it could eliminate the anomalous apparent molecular weights or apparent size heterogeneities for the monoclonal and polyclonal antibodies (Figure 2). Little effect of urea was noted for the boiled (100°C) samples. At 22°C, inclusion of urea did change the apparent heterogeneity, but it also resulted in apparent molecular weights much higher than expected.
Accurate sizing by SDS-PAGE is dependent on all proteins being unfolded and converted to similar rod-shaped molecules, which are evenly coated with negatively charged SDS molecules. Often this is attained by heating or boiling samples in SDS prior to electrophoresis. It seems likely, in the case of the monoclonal and polyclonal antibodies examined in this study using non-reducing SDS-PAGE, that these proteins do not denature in SDS into uniform, rod-shaped molecules without heating, but instead form more compact, variable shapes that migrate through the polyacrylamide sieve faster. In contrast to the reason that they were added (to enhance unfolding and decrease chances for aggregation), the presence of certain short-chain alcohols apparently increases the resistance to thermal unfolding of the IgG structural domains remaining in the presence of SDS. The present study demonstrates that the heating and solvent conditions used to prepare antibodies prior to non-reducing electrophoresis are critical for the results obtained. This is true both for our anti-cocaine mAb, and for a commercially obtained human polyclonal IgG1, making the results of the present study likely to be applicable to the analysis of many polyclonal and monoclonal antibodies used for experimental, diagnostic, and therapeutic purposes. Therefore, we recommend that careful attention be paid to the sample heating protocols and that investigators use caution in the interpretation of the electrophoretic mobility of IgG molecules under non-reducing conditions.
In addition, the current study suggests that the simple, inexpensive, and ubiquitous method of non-reducing SDS-PAGE may be useful to assess the relative stability of the independently unfolding domains in IgGs, i.e., the CH2, CH3 and Fab domains. Analyses such as that shown in Figure 3 (the time course of heating at 50°C on the apparent unfolding of these domains in the presence of SDS) can be used to compare the relative stabilities of mutational variants of a mAb. This is currently most frequently done in the absence of SDS using differential scanning calorimetry (DSC), and deconvoluting the heat capacity trace into CH2, Fab and CH3 unfolding components. However, DSC is a relatively expensive and specialized method, not available to many smaller laboratories, and is subject to limitations due to protein aggregation which occurs in many proteins and antibodies when heating to high temperatures in the absence of SDS. Although obviously not done under native conditions, the data shown in Figure 3 can be easily quantified by gel densitometry to compare the kinetics of unfolding of domains of mutants, conjugates, or other derivatives of antibodies that are generated for therapeutic or experimental purposes. Further work will be needed to confirm the tentative hypothetical band assignments (Figure 4) made for the domain unfolding steps giving rise to different electrophoretic bands observed in Figure 3, but regardless, such simple, inexpensive, and available analyses should prove generally useful for the characterization of antibodies and their derivatives.
Highlights.
Aberrant artefactual mAb masses and size heterogeneity noted by non-reducing SDS-PAGE
This effect depends on pre-electrophoresis sample heating temperatures and conditions
These phenomena are also observed for a commercial human IgG1 polyclonal antibody
Multiple observed bands hypothesized to reflect IgG domain thermal unfolding in SDS
These results are likely relevant to analyses of many monoclonal and polyclonal Abs
Acknowledgments
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse Grant U01DA039550. We are grateful to Catalent PharmaSolutions, Inc. (Madison, WI) for providing the recombinant humanized h2E2 anti-cocaine mAb protein expressed using their GPex® technology.
Abbreviations
- mAb
monoclonal antibody
- h2E2
humanized anti-cocaine monoclonal antibody
- CHO
Chinese Hamster Ovary
- PBS
phosphate buffered saline
- ESI-Tof MS
electrospray ionization time-of-flight mass spectrometry
Footnotes
CONFLICT-OF-INTEREST AND FINANCIAL DISCLOSURE STATEMENT:
Dr. Norman is named as a co-inventor on a patent for the matter and use of the h2E2 humanized anti-cocaine monoclonal antibody.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker M. Reproducibility crisis: Blame it on the antibodies. Nature. 2015;521:274–276. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- 2.Baker M. Antibody anarchy: A call to order. Nature. 2015;527:545–551. doi: 10.1038/527545a. [DOI] [PubMed] [Google Scholar]
- 3.Paula S, Tabet MR, Farr CD, Norman AB, Ball WJ., Jr Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 4.Kirley TL, Norman AB. Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its Fab fragment. Hum Vaccin Immunother. 2015;11:458–467. doi: 10.4161/21645515.2014.990856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demarest SJ, Rogers J, Hansen G. Optimization of the antibody C(H)3 domain by residue frequency analysis of IgG sequences. J Mol Biol. 2004;335:41–48. doi: 10.1016/j.jmb.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Garber E, Demarest SJ. A broad range of Fab stabilities within a host of therapeutic IgGs. Biochem Biophys Res Commun. 2007;355:751–757. doi: 10.1016/j.bbrc.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CM. Differential scanning calorimetry as a tool for protein folding and stability. Arch Biochem Biophys. 2013;531:100–109. doi: 10.1016/j.abb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Norman AB, Gooden FC, Tabet MR, Ball WJ. A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats. Drug Metab Dispos. 2014;42:1125–1131. doi: 10.1124/dmd.114.057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetzel HN, Webster RP, Saeed FO, Kirley TL, Ball WJ, Norman AB. Characterization of a recombinant humanized anti-cocaine monoclonal antibody produced from multiple clones for the selection of a master cell bank candidate. Biochem Biophys Res Commun. 2017;487:690–694. doi: 10.1016/j.bbrc.2017.04.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirley TL, Greis KD, Norman AB. Structural characterization of expressed monoclonal antibodies by single sample mass spectral analysis after IdeS proteolysis. Biochem Biophys Res Commun. 2016;477:363–368. doi: 10.1016/j.bbrc.2016.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirley TL, Greis KD, Norman AB. Selective disulfide reduction for labeling and enhancement of Fab antibody fragments. Biochem Biophys Res Commun. 2016;480:752–757. doi: 10.1016/j.bbrc.2016.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli UK. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]