Abstract
Objective
To employ metabolomics-based pathway and network analyses to evaluate the cerebrospinal fluid (CSF) metabolome after severe traumatic brain injury (TBI) in children, and the capacity of combination therapy with probenecid and N-acetylcysteine (NAC) to impact glutathione-related and other pathways and networks, relative to placebo treatment.
Design
Analysis of CSF obtained from children enrolled in an IRB-approved, randomized, placebo-controlled trial of a combination of probenecid and NAC after severe TBI (Trial Registration NCT01322009).
Setting
36-bed pediatric intensive care unit in a university-affiliated children’s hospital.
Patients and Subjects
Twelve children 2 to 18 years-of-age after severe TBI and five age-matched control subjects.
Intervention
Probenecid (25mg/kg) and NAC (140mg/kg) or placebo administered via naso/orogastric tube.
Measurements and Main Results
The CSF metabolome was analyzed in samples from TBI patients 24h after the first dose of drugs or placebo, and control subjects. Feature detection, retention time, alignment, annotation, and principal component (PCA) and statistical analyses were conducted using XCMS-online. The software mummichog was used for pathway and network analyses. A two-component PCA revealed clustering of each of the groups, with distinct metabolomics signatures. Several novel pathways with plausible mechanistic involvement in TBI were identified. A combination of metabolomics and pathway/network analyses showed that seven glutathione-centered pathways and two networks were enriched in the CSF of TBI patients treated with probenecid and NAC vs. placebo-treated patients. Several additional pathways/networks consisting of components that are known substrates of probenecid-inhibitable transporters were also identified, providing additional mechanistic validation.
Conclusion
This proof-of-concept neuropharmacometabolomics assessment reveals alterations in known and previously unidentified metabolic pathways, and supports therapeutic target engagement of the combination of probenecid and NAC treatment after severe TBI in children.
Keywords: Cerebrospinal fluid, Head injury, N-acetylcysteine, Metabolomics, Neurocritical care, Probenecid
The biochemical milieu of the CNS is dynamic and tightly regulated, and can be explored using metabolomics (1). Metabolomics systematically identifies and quantifies the inventory of small molecule metabolites altered by biochemical and cellular processes at a specific time point, and has recently been used to evaluate biochemical responses and metabolic pathways in CNS disease (2). However, the metabolomics profile after traumatic brain injury (TBI)—an important cause of mortality and morbidity in humans (3)—has not been investigated (4). In TBI, the primary impact onto the brain and/or disruption of brain tissue is followed by secondary biochemical processes including a surge in excitatory amino acids, glycolysis, oxidative stress, and mitochondrial dysfunction (5). Given that CSF provides a window into the CNS, evaluation of metabolomics signatures in CSF after TBI and identification of biochemical pathways altered in response to injury could uncover new and validate suspected pathophysiologic mechanisms in humans, and define clinically relevant therapeutic targets. In addition, evaluation of CSF metabolites could also be used to determine the holistic effects of pharmacotherapies, including target engagement or potential toxicity.
While there are many regulators of the biochemical milieu of the brain at the blood-brain barrier (BBB) and blood-CSF barriers (BCSFB), membrane transporters such as ATP-binding cassette (ABC) transporters and solute carriers (SLC) such as organic anion transporters (OAT) are particularly important in terms of brain bioavailability of clinically used drugs, especially those that do not readily cross the BBB. Probenecid is a promiscuous SLC and ABC transporter inhibitor that has been used to identify CSF elimination pathways for endogenous organic acids and monoamines such as homovanillic acid and serotonin (6), and exogenous drugs such as methotrexate (7) and N-acetylcysteine (NAC) (8).
We recently reported the repurposed use of probenecid and NAC in a randomized, placebo-controlled phase I trial (Pro-NAC) after severe TBI in children to capitalize on this drug interaction (9). The goal of achieving measurable NAC in the CSF was achieved, and adverse events attributable to the drug (NAC)/drug adjuvant (probenecid) combination were not identified. While the Pro-NAC study was the first use of probenecid as an adjuvant for a potential neuroprotective drug, in this case NAC, and the first pharmacokinetic trial in severe pediatric TBI, it was a Phase I study and not powered to evaluate outcome or effects on intracranial pressure. CSF collected as part of the Pro-NAC trial did however provide an opportunity for a pharmacometabolomics interrogation of the CNS after TBI. Accordingly, the purpose of the present study was to evaluate metabolomics changes in human TBI vs. control subjects, and to evaluate pharmacometabolomic changes in TBI patients treated with probenecid and NAC vs. placebo.
MATERIALS AND METHODS
Parent Study
Samples for this study represent CSF obtained from patients who participated in a randomized, double-blind, Phase I study (Trial Registration NCT01322009) of the combination of probenecid and NAC (n = 7) versus placebo (n = 5) in children 2 to 18 years-of-age after severe TBI (Glasgow Coma Scale [GCS] score ≤ 8) recruited from November 2011–September 2013 at a single, tertiary children’s hospital (9). The study was approved by the University of Pittsburgh Institutional Review Board and informed consent was obtained from parents and/or legal guardians of all children enrolled in the study. Control CSF samples were obtained from five age-matched control subjects who underwent lumbar puncture to rule out meningitis.
Sample Collection
CSF samples for this study were collected 41.7 hours (range 34–47.25) after injury and 24 hours after the first dose of probenecid (25 mg/kg) and NAC (140 mg/kg). Drugs or placebo were administered via naso/orogastric tube. CSF was immediately centrifuged at 3000 ×g for 10 min and supernatant was aliquoted and stored at −80°C for batch analysis.
UPLC-QTOFMS Analysis
CSF samples were prepared by first precipitating protein with acetonitrile at a 3:1 ratio followed by centrifugation. Five μL of supernatant was analyzed by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-QTOFMS, Synapt G2-S Waters, Milford, MA). An Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm; Waters, Milford, MA) was used to separate metabolites. The flow rate of the mobile phase was 0.5 mL/min using a gradient ranging from 2 to 98% acetonitrile/water containing 0.1% formic acid over 12 min. The column temperature was maintained at 50°C. TOFMS was operated in positive and negative modes with electrospray ionization. The source and desolvation temperatures were set at 150°C and 500°C, respectively. Nitrogen was applied as cone and desolvation gas, and the gas flow rates were set as 50 L/h and 800 L/h, respectively. Capillary and cone voltages were set at 0.8 kV and 40 V, respectively. TOFMS was calibrated with sodium formate and monitored by the intermittent injection of lockspray leucine encephalin in real-time. MS data were acquired in centroid format over a range of 50–1000 Da with an acquisition time of 0.1 sec/scan.
Metabolomics Analysis
The XCMS-online metabolomics platform (Scripps Research Institute, San Diego, CA) was used for data management and analysis including feature detection, retention time correction, alignment, annotation, principal component analysis (PCA), statistical analysis, and data visualization (10–14). Raw data files were converted to mzData files using ms2mz, uploaded to XCMS-online and processed in positive and negative MS modes. Both positive and negative MS modes for ionization of sample molecules were used to capture and analyze metabolites which otherwise would have been ionized in only one of the two settings. A multi-group experiment was performed to compare all groups simultaneously. Pair-wise experiments were performed to compare control subjects vs. placebo-treated TBI groups, and placebo-treated TBI vs. probenecid and NAC-treated TBI groups.
For data analysis with XCMS-online, the centWave method was used for feature detection with 15 ppm m/z tolerance, minimum peak width of 2 sec and maximum peak width of 25 sec. The Obiwarp function was used for retention time correction. Chromatogram alignment was performed with mzwid (mz width) = 0.1, minfrac (minimum fraction) = 0.05, and bandwidth (bw) = 2. Isotopes and adducts were annotated using CAMRRA with an m/z absolute error of 0.015 and 5 ppm. Selection and filtering of metabolite features for inclusion in pathway/network analyses were done based on a fold-change ≥ 1.5 and p-value ≤ 0.01. Metabolites were identified by searching the Human Metabolome Database (HMDB) with 10 ppm error margin.
Pathway and Network Analysis
Network and pathway analyses were performed using Mummichog (Emory University, Atlanta, GA) which is incorporated within the XCMS-online platform (15). We conducted both pathway and network analyses because the methods are complimentary to each other and identify biochemical processes of interest independently. Pathways represent well-established biochemical processes that in general allow for intuitive interpretation. On the other hand, networks are derived from large-scale genomic, proteomic or other systems level biological data where interpretation is less intuitive. However, networks are less biased and provide information not attained in pathways (16, 17). Significant metabolite features in the pair-wise comparisons were used as input, and the total list of features were used as reference. For the pathway analysis, the enrichment of input metabolites against random data resampled from the reference list (in the background of known human metabolic pathways) provided adjusted p-values while accounting for the fact that single m/z values can map to different metabolites and different m/z features can represent a single metabolite. Network analysis was done in a similar way except that compounds are placed within the human metabolic network model. Modules are defined as subsets within the network that show more connectivity and interrelationship than what is expected randomly in the network as a whole, and are identified by their inter-connectivity. Output data from the network analysis were imported to Cytoscape 3.4.0 (18) to generate network visualization. Excel 2013 (Microsoft Corporation, WA) was used to generate cloud plots of the impacted pathways. R-Studio 1.0.136 was used to generate volcano plots of metabolite features.
Statistical Analysis
Statistical analysis of metabolomics data using the XCMS-online platform is described in Gowda, et al. (11). Briefly, multi-group differences used for PCA and box plots were determined by one-way ANOVA with Tukey’s post-hoc test. An unpaired Welsh t-test was used to assess differences between 1) control subjects vs. placebo-treated TBI groups, and 2) placebo-treated TBI vs. probenecid and NAC-treated TBI groups, visualized in cloud and volcano plots. A p-value ≤ 0.01 was considered significant. A fold-change ≥ 1.5 was also required for inclusion in pathway and network analyses.
RESULTS
Patient Characteristics
In the parent study, 14 patients were randomized to probenecid and NAC (n = 7) or placebo groups (n = 7) (9). Residual CSF was available from 12 patients collected 24 h after the first drug or placebo administration, seven in the probenecid and NAC and five in the placebo groups. Demographic characteristics are summarized in Supplemental Table S1.
Multi-group Metabolomics Analysis
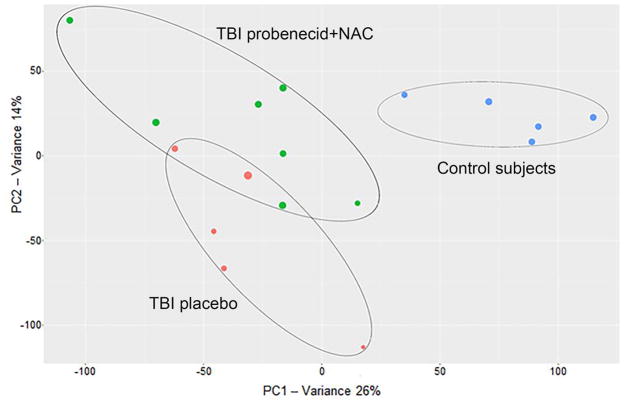
Multi-group analysis was performed to compare the CSF metabolomics profiles of control subjects, TBI patients administered placebo, and TBI patients treated with probenecid and NAC. Two-component PCA revealed clustering within each of the groups and separation between groups (Figure 1).
Figure 1.
PCA analysis based on the first two components shows clustering of the three study groups based on their difference in metabolic profile (blue = control subjects, red = TBI treated with placebo, green = TBI treated with probenecid+NAC). The diameter of each circle represents the DModX value, which indicates the observed distance of a particular individual to the principal components model. XCMS-online metabolomics platform (Scripps Research Institute, San Diego, CA)
Pair-wise Analysis of Control Subjects vs. Placebo-Treated TBI Patients
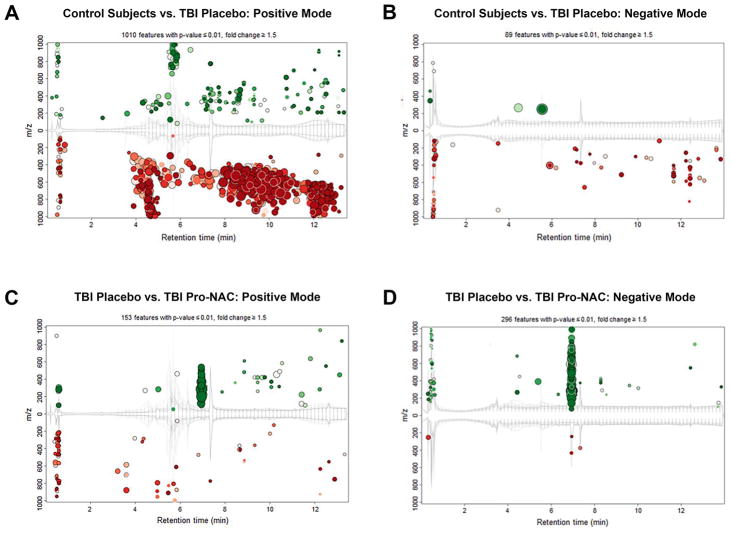
To detect metabolites that are altered in response to TBI, CSF from placebo-treated TBI patients and control subjects were compared. Using m/z-retention time plots, 1010 features in the positive MS mode (Figure 2A) and 89 features in the negative MS mode (Figure 2B) that were significantly increased or decreased were identified (p ≤ 0.01, fold-change ≥ 1.5). The majority of features appeared to decrease in TBI patients vs. control subjects.
Figure 2.
Cloud plots of features increased (green) or decreased (red) in placebo treated TBI patients vs. control subjects in positive MS mode (A) and negative MS mode (B). Cloud plots of features increased (green) or decreased (red) in TBI patients treated a combination of probenecid and NAC vs. placebo in positive MS mode (C) and negative MS mode (D). The size of the circle corresponds to the fold-change of the particular feature vs. the reference group and the intensity of the color corresponds inversely to the p-value, with more intense shades representing a smaller p-value. XCMS-online metabolomics platform (Scripps Research Institute, San Diego, CA).
We next sought to identify individual metabolites of interest altered in control subjects vs. placebo treated TBI patients. Metabolites with maximum absolute intensity ≥ 2,500, −log(p) > 3, and log2(fold-change) > 1 were selected for identification (Supplemental Figure S1). Excluding metabolites with poor chromatographic resolution yielded 41 significant features whose masses were searched in the HMDB (15, 19, 20). Excluding putative metabolites that were of dietary or exogenous drug source yielded endogenous metabolites with a previously known or biologically plausible association with TBI. Putative metabolites in 19 of these features corresponded to long-chain fatty acids; however, because of the difficulty distinguishing among analogues with the same m/z, were not further characterized. The final list of 10 features and their corresponding TBI-associated metabolites is shown in Supplemental Table S2A. Of potential interest are changes in multiple species of gangliosides, ceramide, and dipeptides in TBI patients vs. control subjects.
Pair-wise Analysis of TBI Patients Receiving Probenecid and NAC vs. Placebo
To elucidate the impact of probenecid and NAC treatment on the CSF metabolomics profile after TBI, placebo- and Pro-NAC-treated TBI patients were compared. Using m/z-retention time plots, 153 features in the positive MS mode (Figure 2C) and 296 features in the negative MS mode (Figure 2D) that were significantly increased or decreased were identified. In contrast to control subjects vs. TBI patients, the majority of features appeared to increase in patients treated with probenecid and NAC vs. placebo.
We next sought to identify individual metabolites of interest altered in TBI patients receiving probenecid and NAC vs. placebo. Metabolites with maximum absolute intensity ≥ 2,500, −log(p) > 2.3, and log2(fold-change) > 1 were selected for possible identification. (Supplemental Figure S1). Excluding metabolites with poor chromatographic resolution yielded 67 significant features whose masses were searched in the HMDB. Excluding metabolites that were of dietary or exogenous drug source yielded 25 metabolites that may be affected by probenecid and NAC treatment after TBI (Supplemental Table S2B). Probenecid was upregulated in the Pro-NAC group, validating XCMS-online platform’s capacity to discriminate patients based on their metabolomics signature. Glutathione was also increased in probenecid and NAC-treated patients vs. placebo, consistent with drug combination target engagement.
Internal Validity
As a means of internal validation, we evaluated the study drug probenecid and phenytoin (administered to 11/12 TBI patients for seizure prophylaxis). Searching for either the parent drugs or metabolites of probenecid and phenytoin among the putative metabolites shows matching features with expected differential abundance in the three groups (Supplemental Figure S1). In addition, the peak area of probenecid determined by UPLC-QTOFMS correlated with previously quantified probenecid concentrations (r2 = 0.76, p = 0.01) determined by UPLC-MS/MS (9).
Pathway Analysis of Control Subjects vs. Placebo-Treated TBI Patients
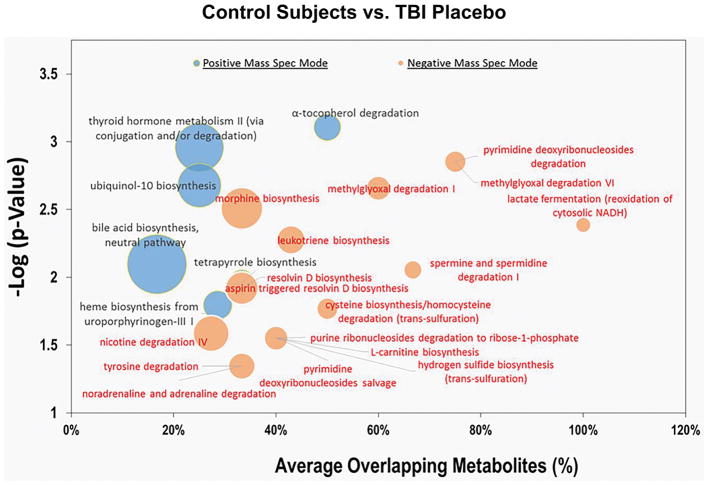
We conducted pathway analysis of CSF metabolites in placebo-treated TBI patients vs. control subjects. Metabolites that were significantly changed in response to TBI were enriched in six pathways in the positive MS mode and 17 pathways in the negative MS mode (Figure 3; Supplemental Tables S3 and S4).
Figure 3.
Cloud plot of select pathways affected by TBI vs. control subjects, identified by metabolomics analysis of CSF. The y-axis represents significance (p-value) and the x-axis the percentage of overlapping metabolites. The radius of each circle increases with the number of metabolites relative to the number of metabolites represented by other circles. Excel 2013 (Microsoft Corporation, WA) was used to generate cloud plots of the impacted pathways.
Pathway Analysis of TBI Patients Receiving Probenecid and NAC vs. Placebo
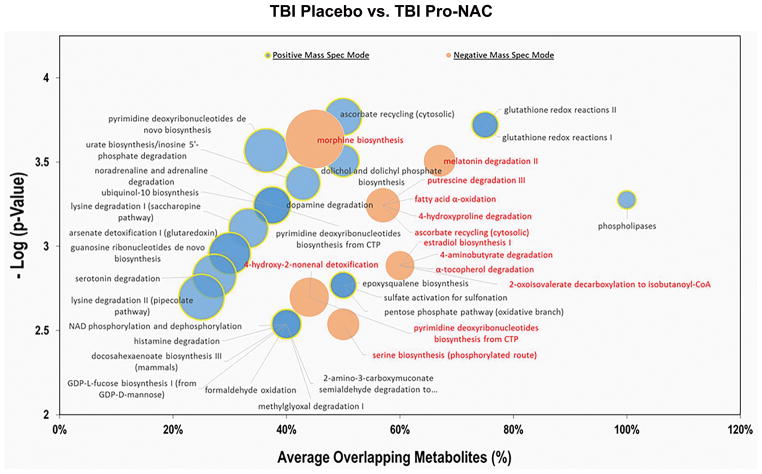
Pathway analysis was performed to identify biochemical processes modified by probenecid and NAC-treatment after TBI. In the positive MS mode, a total of 77 pathways were enriched in metabolites that were differentially dysregulated between the two groups, with 45 pathways showing ≥ 50% overlap between putative metabolites with a known component of the particular pathway. In the negative MS mode, a total of 47 pathways were found to be significantly dysregulated, with eight pathways showing ≥ 50% overlap between putative metabolites (Figure 4; Supplemental Tables S5 and S6).
Figure 4.
Cloud plot of select pathways affected by treatment with probenecid and NAC vs. placebo in TBI patients, identified by metabolomics analysis of CSF. The y-axis represents significance (p-value) and the x-axis the percentage of overlapping metabolites. The radius of each circle increases with the number of metabolites relative to the number of metabolites represented by other circles. Excel 2013 (Microsoft Corporation, WA) was used to generate cloud plots of the impacted pathways.
Network Analysis of TBI Patients Receiving Probenecid and NAC vs. Placebo
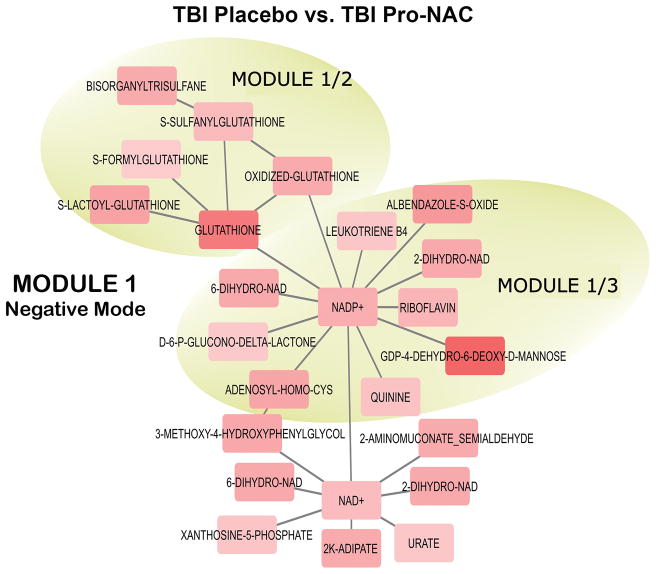
Network analysis identified five modules that are significantly affected by probenecid and NAC treatment in the positive MS mode and four modules in the negative MS mode. Modules represent internal connectivity greater than what is expected randomly in the network. Some modules identified are nested, i.e. bigger module consists of smaller, independent modules. The complete list putative metabolites that constitute the modules are in Supplemental Tables S7 and S8. Notable features of these modules include upregulation of glutathione and its conjugates (Figure 5); upregulation of donors in sulfate conjugation and metabolites with sulfate conjugation characteristics (Supplemental Figure 2A) that are confirmed substrates of probenecid-inhibitable transporters (21); a collective downregulation of prostaglandins (Supplemental Figure 2B) which are also known to be substrates of probenecid-inhibitable transporters (22); 2-ketoglutarate, which interacts with probenecid-inhibitable OAT1 (8, 23); kynurenate, 3-hydroxykynurenine and dipeptides that share substrates of OAT transporters (24–27); and up-regulation of UDP-conjugated monosaccharides and down-regulation of their interacting counterparts, acetylated sugar moieties. Transporters responsible for the translocation of these sugar nucleotides are known to be sensitive to inhibition by probenecid (25, 28).
Figure 5.
Network connectivity of representative modules (1/2 and 1/3) in negative MS mode affected by treatment with probenecid and NAC vs. placebo in TBI patients, identified by metabolomics analysis of CSF. Upregulation of glutathione and a number of its conjugates and connectivity between NADP-dependent pathways are highlighted. Red nodes are upregulated while the blue nodes are downregulated relative to other nodes within individual mode. The color intensity indicates the extent of upregulation or downregulation. Output data from the network analysis were imported to Cytoscape 3.4.0 (18) to generate network visualization.
DISCUSSION
To our knowledge, this is the first study to perform a comprehensive metabolomics analysis in TBI patients, and the first clinical application of “neuropharmacometabolomics” after administration of potentially therapeutic drugs, including probenecid which is well known to impact the biochemical milieu of the CSF (6, 7). We focused on pathway and network analysis of the metabolomics data, leveraging recent advances that enable exploration without exhaustive verification of individual metabolites (15). Pathway analysis (16) revealed novel biochemical processes associated with TBI, while also confirming others with a previously known role. Treatment with probenecid and NAC resulted in a significant alteration in the metabolomics signature of TBI, with the majority of features showing relative increases vs. placebo. Importantly, relative glutathione levels were increased and both pathway and network analyses showed that biochemical processes involving detoxification with glutathione and glutathione recycling were enriched in the probenecid and NAC-treated group, consistent with target engagement of at least one of the components of the combination therapy. Furthermore, modules and pathways with components known to be substrates of transporters inhibited by probenecid were identified.
Comparing the TBI placebo-treated patients with the control subjects revealed several biochemical pathways altered after TBI. Previous studies have reported TBI-related catabolism of numerous small molecules including amino acids, catecholamines, hormones, and polyamines. Components of a number of these molecular pathways were amplified after TBI, including: tyrosine, noradrenaline/adrenaline, spermine/spermidine, and nicotine degradation; thyroid hormone conjugation and/or degradation; and L-carnitine biosynthesis. Other pathways found to be enriched that are implicated in the pathophysiology of TBI include oxidative stress (alpha-tecopherol degradation, ubiquinol-10 biosynthesis), bioenergetics (lactate fermentation pathway), lipid peroxidation (leukotriene and resolvin-D biosynthesis), and heme metabolism (tetrapyrrole and heme biosynthesis). Cystathionine-β-synthase, which catalyzes the first step in the transulfuration of homocysteine to cystathionine, has been reported to mediate pathological processes after TBI (29). Cysteine biosynthesis/homocysteine degradation and hydrogen sulfide biosynthesis were also found to be upregulated.
Some of the enriched pathways uncovered had not been associated with TBI, e.g. morphine biosynthesis. Endogenous morphine biosynthesis by human neuroblastoma cells has been reported (30, 31), and the activation of this pathway in TBI patients may indicate a role in pain modulation after injury. Two methylglyoxal-related pathways were found to be upregulated in TBI patients. Methylglyoxal, a potentially toxic byproduct of glycolysis, threonine catabolism, and lipid peroxidation has been implicated in models of cerebral ischemia (32, 33). Also in the present study, pathways related to purine and pyrimidine metabolism were enriched, reported to mediate activation of astrocytes and other glia in acute and chronic neuroinflammation (34).
Treatment with a combination of probenecid and NAC resulted in significant changes in the metabolomics signature in CSF after TBI. Pathway and network analyses revealed over 80 pathways and nine modules, respectively, that were dysregulated in the treatment group vs. placebo. Seven pathways that involve glutathione as a component of detoxification processes were enhanced in the treatment group: glutathione redox reactions I and II, 4-hydroy-2-nonenal and arsenate detoxification, methylglyoxal degradation, ascorbate recycling, and formaldehyde oxidation. These observations were congruent with network analysis which featured glutathione-centered modules. These findings provide validity to the metabolomics-based pathway and network analyses, given that probenecid and NAC can increase glutathione via preventing cellular efflux of glutathione-disulfide and glutathionylated xenobiotics, and serving as a cysteine donor for glutathione synthesis, respectively. Furthermore, NAC is a substrate for probenecid-inhibitable OATs (8). Collectively, our findings are consistent with combination treatment with probenecid and NAC increasing glutathione and its conjugates, suggestive of target engagement.
Other prominent pathways/biochemical processes possibly altered by treatment include purine and pyrimidine metabolism; catabolism, salvage and biosynthesis of amino acids; catecholamines; hormones; peptides and polyamines; oxidation, degradation and biosynthesis of fatty acids and lipids; glycolysis; and aerobic cellular respiration. This suggests that many biochemical substrates, intermediates, and end products may be substrates of probenecid-inhibitable transporters. Some components of the enriched pathways and modules such as urate (35); kynurenate, xanthurenate, and indoxyl sulfate (24, 25); cyclic nucleotides (36); and prostaglandins (22) are known substrates. Additionally, a module centered around 2-ketoglutarate, which acts as a gradient exchanger in the transport of organic anions by OATs, was identified (37).
Many alterations in the biochemical milieu observed in probenecid and NAC-treated vs. placebo-treated TBI patients can be explained by the known properties of probenecid. Some of these changes, such as increased abundance of kynurenine may be beneficial after TBI. Kynurenine has been studied in combination with probenecid to attenuate seizures, quinolinic acid-induced neurotoxicity, and neuropathic pain (38–40). Other effects may be undesirable, e.g. increased indoxyl sulfate (a uremic toxin) which has been linked to CNS toxicity in patients with impaired kidney function (41).
Initial screening yielded ten features in CSF that distinguished TBI patients from control subjects. Subsequent querying of the HMDB showed that gangliosides appear as putative metabolites in four out of ten features, consistent with a role for gangliosides in TBI (42, 43). Comparing placebo and probenecid and NAC-treated TBI patients yielded 24 putative metabolites. Congruent with pathway and network analyses, glutathione and its conjugates were commonly featured. As these are exploratory findings, feature verification and further evaluation are necessary.
Limitations
Although this study does represent the first comprehensive metabolomics analysis of any kind in human TBI, sample sizes are limited. However, the number of subjects was sufficient to clearly discriminate between groups using PCA (Figure 1). We chose to study individual vs. pooled samples despite the risk of increased sample variation and cost of analysis, in order to take advantage of pathway and network analysis (16, 17). One advantage of using a multi-modal approach, supplementing individual feature identification with pathway and network analysis, is that the likelihood of false discovery when examining multiple feature enrichment within select pathways and/or networks is lower than when identifying individual features alone, an important consideration when large numbers of statistically significant features are detected. It was partially confirmatory that many individual features identified clustered into known biological pathways and/or networks. From a technical standpoint, the samples were precipitated with acetonitrile, thus the analytical approach did not identify proteins. Finally, other than probenecid measurements, which were tightly correlated with previously published concentrations determined by UPLC-MS/MS (9), individual metabolites/features and pathways were not confirmed independently.
Conclusion
Advanced analysis of metabolomics data revealed pathways and networks that are dysregulated after TBI. Some of these pathways have known involvement while others represent novel findings, potentially bringing new insights into the pathogenesis of TBI. Similarly, treatment with a combination of probenecid and NAC resulted in enrichment of numerous pathways and networks (including several glutathione-related pathways) supporting the a priori hypothesis for using this drug combination in TBI. Furthermore, several individual features, pathways, and networks with biologically plausible interactions consistent with probenecid and/or NAC therapeutic target engagement were identified. This proof-of-concept study demonstrates that neuropharmacometabolomics may represent a powerful tool for developing a comprehensive understanding of the multiple biochemical processes and mechanisms involved in the pathogenesis of TBI and the effects of pharmacological interventions.
Supplementary Material
Volcano plot of the CSF metabolome profiles for control subjects vs. TBI placebo treated patients (A) and TBI patients treated with probenecid and NAC vs. placebo (B) in both positive and negative MS modes. Metabolites that were upregulated or downregulated by ≥ two-fold with a p-value < 0.001 are shown in blue. R-Studio 1.0.136 was used to generate volcano plots of metabolite features. C, Relative intensity of the feature putatively identified to be phenytoin (administered as part of institutional standard-of-care after TBI to prevent seizures). Phenytoin increased in both TBI groups vs. control subjects. D, Relative intensity of the feature putatively identified to be probenecid. Probenecid increased in both TBI groups vs. control subjects. E, The peak area of probenecid determined by UPLC-QTOFMS correlated with previously quantified probenecid concentrations determined by UPLC-MS/MS (9) in individual patients treated with probenecid (r2 = 0.76, p = 0.01). Mean ± SEM; n = 5–7/group; *p < 0.01; ***p < 0.001; PB = probenecid; CS = control subjects. XCMS-online metabolomics platform (Scripps Research Institute, San Diego, CA).
Network connectivity of representative modules. (A) Module 4 in negative MS mode showing upregulation of metabolites relevant to sulfate conjugation that are confirmed substrates of probenecid-inhibitable transporters. (B) Module 1 in positive MS mode. Alterations in submodule 1/2 centered around 2-ketoglutarate (yellow circle), and pathways centered around the collective downregulation of prostaglandins, and upregulation of UDP-conjugated monosaccharides and downregulation of their acetylated sugar moieties (dashed ovals) are highlighted. Red nodes are upregulated while the blue nodes are downregulated relative to other nodes within individual mode. The color intensity indicates the extent of upregulation or downregulation. Output data from the network analysis were imported to Cytoscape 3.4.0 (18) to generate network visualization.
Acknowledgments
We thank Rachelle Bell, RN for expertly managing the parent Pro-NAC trial, and Dr. Alicia Au for providing control CSF.
Footnotes
Conflicts of Interest and Source of Funding: NIH grants R01 NS069247 (RSBC, PEE, MJB), 1TL1 TR001858-01 (FTH).
Copyright form disclosure: Mr. Hagos, Drs. Empey, Poloyac, Bayir, Bell, and Clark received support for article research from the National Institutes of Health (NIH). Mr. Hagos received funding from NIH and University of Pittsburgh CTSI. Mr. Hagos, Dr. Empey, and Dr. Bell disclosed off-label product use of probenecid and N-acetylcysteine for pediatric traumatic brain injury. Drs. Empey, Kochanek, and Bell’s institutions received funding from the NIH. Dr. Kochanek received funding from SCCM and WFPICCS (Editor-in-Chief of Pediatric Critical Care Medicine) and he has served as an expert witness on a number of cases and has given numerous lectures as a guest professor. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mo Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddurah-Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology. 2009;34(1):173–186. doi: 10.1038/npp.2008.174. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolahan SM, Hirt D, Braas D, Glenn TC. Role of Metabolomics in Traumatic Brain Injury Research. Neurosurg Clin N Am. 2016;27(4):465–472. doi: 10.1016/j.nec.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6:1307–1315. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garelis E, Young SN, Lal S, Sourkes TL. Monoamine metabolites in lumbar CSF: the question of their origin in relation to clinical studies. Brain Res. 1974;79(1):1–8. doi: 10.1016/0006-8993(74)90562-9. [DOI] [PubMed] [Google Scholar]
- 7.Bode U, Magrath IT, Bleyer WA, Poplack DG, Glaubiger DL. Active transport of methotrexate from cerebrospinal fluid in humans. Cancer Res. 1980;40(7):2184–2187. [PubMed] [Google Scholar]
- 8.Hagos FT, Daood MJ, Ocque JA, Nolin TD, Bayir H, Poloyac SM, Kochanek PM, Clark RS, Empey PE. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica. 2017;47(4):346–353. doi: 10.1080/00498254.2016.1187777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark RSB, Empey PE, Bayir H, Rosario BL, Poloyac SM, Kochanek PM, Nolin TD, Au AK, Horvat CM, Wisniewski SR, et al. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS One. 2017;12(7):e0180280. doi: 10.1371/journal.pone.0180280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benton HP, Ivanisevic J, Mahieu NG, Kurczy ME, Johnson CH, Franco L, Rinehart D, Valentine E, Gowda H, Ubhi BK, et al. Autonomous metabolomics for rapid metabolite identification in global profiling. Anal Chem. 2015;87:884–891. doi: 10.1021/ac5025649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowda H, Ivanisevic J, Johnson CH, Kurczy ME, Benton HP, Rinehart D, Nguyen T, Ray J, Kuehl J, Arevalo B, et al. Interactive XCMS online: Simplifying advanced metabolomic data processing and subsequent statistical analyses. Anal Chem. 2014;86:6931–6939. doi: 10.1021/ac500734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patti GJ, Tautenhahn R, Rinehart D, Cho K, Shriver LP, Manchester M, Nikolskiy I, Johnson CH, Mahieu NG, Siuzdak G. A view from above: Cloud plots to visualize global metabolomic data. Anal Chem. 2013;85:798–804. doi: 10.1021/ac3029745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS online: A web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84:5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Z-J, Schultz AW, Wang J, Johnson CH, Yannone SM, Patti GJ, Siuzdak G. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat Protoc. 2013;8:451–460. doi: 10.1038/nprot.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, et al. HMDB 3. 0--The Human Metabolome Database in 2013. Nucl Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creixell P, Reimand J, Haider S, Wu G, Shibata T, Vazquez M, Mustonen V, Gonzalez-Perez A, Pearson J, Sander C, et al. Pathway and network analysis of cancer genomes. Nat Methods. 2015;2:1–6. doi: 10.1038/nmeth.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deo RC, Hunter L, Lewis GD, Pare G, Vasan RS, Chasman D, Wang TJ, Gerszten RE, Roth FP. Interpreting metabolomic profiles using unbiased pathway models. PLoS Comput Biol. 2010;6(2):e1000692. doi: 10.1371/journal.pcbi.1000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes CT, Franz M, Kazi F, Donaldson SL, Morris Q, Bader GD. Cytoscape Web: an interactive web-based network browser. Bioinformatics. 2010;26:2347–2348. doi: 10.1093/bioinformatics/btq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, et al. HMDB: a knowledgebase for the human metabolome. Nucl Acids Res. 2009;37:D603–D610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, et al. HMDB: the Human Metabolome Database. Nucl Acids Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng KH, Lim BG, Wong KP. Sulfate conjugating and transport functions of MDCK distal tubular cells. Kidney Int. 2003;63:976–986. doi: 10.1046/j.1523-1755.2003.00818.x. [DOI] [PubMed] [Google Scholar]
- 22.Rennick BR. Renal tubular transport of prostaglandins: inhibition by probenecid and indomethacin. Am J Physiol. 1977;233:F133–137. doi: 10.1152/ajprenal.1977.233.2.F133. [DOI] [PubMed] [Google Scholar]
- 23.Ingraham L, Li M, Renfro JL, Parker S, Vapurcuyan A, Hanna I, Pelis RM. A plasma concentration of alpha-ketoglutarate influences the kinetic interaction of ligands with organic anion transporter 1. Mol Pharmacol. 2014;86(1):86–95. doi: 10.1124/mol.114.091777. [DOI] [PubMed] [Google Scholar]
- 24.Bahn A, Ljubojević M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolić I, Burckhardt G, Hagos Y. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol - Cell Physiol. 2005:289. doi: 10.1152/ajpcell.00619.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hadley B, Maggioni A, Ashikov A, Day CJ, Haselhorst T, Tiralongo J. Structure and function of nucleotide sugar transporters: Current progress. Comput Struct Biotechnol J. 2014;10:23–32. doi: 10.1016/j.csbj.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugawara M, Ogawa T, Kobayashi M, Miyazaki K. Uptake of dipeptide and β-lactam antibiotics by the basolateral membrane vesicles prepared from rat kidney. Biochim Biophys Acta - Biomembranes. 2003;1609:39–44. doi: 10.1016/s0005-2736(02)00634-x. [DOI] [PubMed] [Google Scholar]
- 27.Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res. 2012;65:254–260. doi: 10.1016/j.phrs.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia E, Nowell S, Drake RR, Mizeracka M, Berg CL, Magdalou J, Fournel-Gigleux S, Gollan JL, Lester R, Radominska A. Two kinetically-distinct components of UDP-glucuronic acid transport in rat liver endoplasmic reticulum. Biochim Biophys Acta. 1996;1283:223–231. doi: 10.1016/0005-2736(96)00098-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Shan H, Wang Y, Wang T, Liu W, Wang L, Zhang L, Chang P, Dong W, Chen X, et al. The Expression Changes of Cystathionine-β-synthase in Brain Cortex After Traumatic Brain Injury. J Mol Neurosci. 2013;51:57–67. doi: 10.1007/s12031-012-9948-5. [DOI] [PubMed] [Google Scholar]
- 30.Boettcher C, Fellermeier M, Boettcher C, Dräger B, Zenk MH. How human neuroblastoma cells make morphine. Proc Natl Acad Sci USA. 2005;102:8495–8500. doi: 10.1073/pnas.0503244102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poeaknapo C, Schmidt J, Brandsch M, Dräger B, Zenk MH. Endogenous formation of morphine in human cells. Proc Natl Acad Sci USA. 2004;101:14091–14096. doi: 10.1073/pnas.0405430101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalapos MP, Frye EB, Degenhardt TP, Thorpe SR, Baynes JW, Onorato J, et al. Where does plasma methylglyoxal originate from? Diabetes Res Clin Pract. 2013;99:260–271. doi: 10.1016/j.diabres.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Pieroh P, Koch M, Wagner D-C, Boltze J, Ehrlich A, Ghadban C, Hobusch C, Birkenmeier G, Dehghani F. Temporal Dynamics of Glyoxalase 1 in Secondary Neuronal Injury. PLoS ONE. 2014;9:e87364. doi: 10.1371/journal.pone.0087364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christjanson LJ, Middlemiss PJ, Rathbone MP. Stimulation of astrocyte proliferation by purine and pyrimidine nucleotides and nucleosides. Glia. 1993;7:176–182. doi: 10.1002/glia.440070207. [DOI] [PubMed] [Google Scholar]
- 35.Weiner IM. Urate transport in the nephron. Am J Physiol - Renal Physiol. 1979:237. doi: 10.1152/ajprenal.1979.237.2.F85. [DOI] [PubMed] [Google Scholar]
- 36.Angel C, Deluca DC, Murphree OD. Probenecid-induced accumulation of cyclic nucleotides, 5-hydroxyindoleacetic acid, and homovanillic acid in cisternal spinal fluid of genetically nervous dogs. Biol Psychiatry. 1976;11:743–753. [PubMed] [Google Scholar]
- 37.Nagai J, Yano I, Hashimoto Y, Takano M, Inui K. Efflux of intracellular alpha-ketoglutarate via p-aminohippurate/dicarboxylate exchange in OK kidney epithelial cells. J Pharmacol Exp Ther. 1998;285:422–427. [PubMed] [Google Scholar]
- 38.Pineda-Farias JB, Pérez-Severiano F, González-Esquivel DF, Barragán-Iglesias P, Bravo-Hernández M, Cervantes-Durán C, Aguilera P, Ríos C, Granados-Soto V. The l -kynurenine-probenecid combination reduces neuropathic pain in rats. Eur J Pain. 2013;17:1365–1373. doi: 10.1002/j.1532-2149.2013.00305.x. [DOI] [PubMed] [Google Scholar]
- 39.Santamaría A, Ríos C, Solís-Hernández F, Ordaz-Moreno J, González-Reynoso L, Altagracia M, Kravzov J. Systemic dl-Kynurenine and probenecid pretreatment attenuates quinolinic acid-induced neurotoxicity in rats. Neuropharmacology. 1996;35:23–28. doi: 10.1016/0028-3908(95)00145-x. [DOI] [PubMed] [Google Scholar]
- 40.Vécsei L, Miller J, MacGarvey U, Flint Beal M. Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res Bull. 1992;28:233–238. doi: 10.1016/0361-9230(92)90184-y. [DOI] [PubMed] [Google Scholar]
- 41.Leong S, Sirich T. Indoxyl Sulfate—Review of Toxicity and Therapeutic Strategies. Toxins. 2016;8:358. doi: 10.3390/toxins8120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carr KR, Shah M, Garvin R, Shakir A, Jackson C. Post-Traumatic brain injury (TBI) presenting with Guillain-Barre syndrome and elevated anti-ganglioside antibodies: a case report and review of the literature. Int J Neurosci. 2015;125:486–492. doi: 10.3109/00207454.2014.957760. [DOI] [PubMed] [Google Scholar]
- 43.Woods AS, Colsch B, Jackson SN, Post J, Baldwin K, Roux A, Hoffer B, Cox BM, Hoffer M, Rubovitch V, et al. Gangliosides and ceramides change in a mouse model of blast induced traumatic brain injury. ACS Chem Neurosci. 2013;4:594–600. doi: 10.1021/cn300216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Volcano plot of the CSF metabolome profiles for control subjects vs. TBI placebo treated patients (A) and TBI patients treated with probenecid and NAC vs. placebo (B) in both positive and negative MS modes. Metabolites that were upregulated or downregulated by ≥ two-fold with a p-value < 0.001 are shown in blue. R-Studio 1.0.136 was used to generate volcano plots of metabolite features. C, Relative intensity of the feature putatively identified to be phenytoin (administered as part of institutional standard-of-care after TBI to prevent seizures). Phenytoin increased in both TBI groups vs. control subjects. D, Relative intensity of the feature putatively identified to be probenecid. Probenecid increased in both TBI groups vs. control subjects. E, The peak area of probenecid determined by UPLC-QTOFMS correlated with previously quantified probenecid concentrations determined by UPLC-MS/MS (9) in individual patients treated with probenecid (r2 = 0.76, p = 0.01). Mean ± SEM; n = 5–7/group; *p < 0.01; ***p < 0.001; PB = probenecid; CS = control subjects. XCMS-online metabolomics platform (Scripps Research Institute, San Diego, CA).
Network connectivity of representative modules. (A) Module 4 in negative MS mode showing upregulation of metabolites relevant to sulfate conjugation that are confirmed substrates of probenecid-inhibitable transporters. (B) Module 1 in positive MS mode. Alterations in submodule 1/2 centered around 2-ketoglutarate (yellow circle), and pathways centered around the collective downregulation of prostaglandins, and upregulation of UDP-conjugated monosaccharides and downregulation of their acetylated sugar moieties (dashed ovals) are highlighted. Red nodes are upregulated while the blue nodes are downregulated relative to other nodes within individual mode. The color intensity indicates the extent of upregulation or downregulation. Output data from the network analysis were imported to Cytoscape 3.4.0 (18) to generate network visualization.