Abstract
We here reconsider current theories of neural ensembles in the context of recent discoveries about neuronal dendritic physiology. The key physiological observation is that the dendritic plateau potential produces sustained depolarization of the cell body (amplitude 10–20 mV, duration 200–500 ms). Our central hypothesis is that synaptically-evoked dendritic plateau potentials lead to a prepared state of a neuron that favors spike generation. The plateau both depolarizes the cell towards spike threshold, and provides faster response to inputs through a shortened membrane time constant. As a result, the speed of synaptic-to-action potential transfer is faster during the plateau phase. Our hypothesis relates the changes from “resting” to “depolarized” neuronal state to changes in ensemble dynamics and in network information flow. The plateau provides the Prepared state (sustained depolarization of the cell body) with a time window of 200-500 ms. During this time, a neuron can tune into ongoing network activity and synchronize spiking with other neurons to provide a coordinated Active state (robust firing of somatic action potentials), which would permit “binding” of signals through coordination of neural activity across a population. The transient Active ensemble of neurons is embedded in the longer-lasting Prepared ensemble of neurons. We hypothesize that “embedded ensemble encoding” may be an important organizing principle in networks of neurons.
Keywords: dendritic, plateau potential, glutamate, UP state, binding, rate code
Introduction
How do neurons and neural networks interact to solve complex tasks? Despite major advances in cellular and molecular neuroscience (Grosenick et al., 2015; Tao et al., 2015; Zhang et al., 2015), and in human brain structural and functional imaging (Lee et al., 2017), the basis of neural computation is not completely understood (Olshausen and Field, 2004; Vogels et al., 2005; Dayan et al., 2011; Sompolinsky, 2014). In some ways, these major experimental advances and new data sets produce major strains on theories and viewpoints that had seemed clearly delineated in a time of simpler data. A primary challenge for neuroscience in general, and for theoretical neuroscience in particular, is to fit together existing theories with new findings, reconciling top-down concepts, models, frameworks and theories (Sompolinsky, 2014; Palmer et al., 2015) with the changing bottom-up landscape of neuroscience discoveries (Bittner et al., 2015; Grosenick et al., 2015; Seibt et al., 2017). Here we review recent experimental evidence of glutamate-mediated dendritic plateau potentials, capable of bringing neurons into a sustained depolarized state, which favors action potential firing and formation of new neural ensembles. Our hypothesis is based on the idea that synaptically-evoked dendritic plateau potentials lead to a prepared state that favors spike generation. The plateau both depolarizes the cell towards spike threshold, and provides faster response to inputs through a shortened membrane time constant. The speed of synaptic-to-action potential transfer in individual cells is faster during the neuronal plateau depolarization. In this way, dendritic plateau potentials occurring in a fraction of cells are poised to affect the dynamic response of the whole network. The sustained depolarization of the cell body provides neurons with a time window of 200-500 ms, during which neurons can tune into ongoing network activity and synchronize spiking with other neurons. The group of actively spiking cells (ensemble “2”) is recruited from the group of cells (ensemble “1”) brought into sustained depolarized state by ongoing dendritic plateau potentials. The group of actively spiking cells is thus embedded in the group of prepared (depolarized) cells. The transient ensemble “2” of spiking neurons is embedded in the longer-lasting ensemble “1” of neurons in sustained depolarized state. Besides trying to fit together existing theories with new experimental findings, the “embedded ensemble encoding” hypothesis, also pulls together two concepts, rate coding vs. temporal coding, that are typically seen to be in opposition.
PART 1: ELECTRICAL SIGNALING
In this section, we argue that the theory of neural ensembles and the “embedded ensemble encoding” hypothesis are both critically dependent on electrical signaling (Grewe et al., 2017; Pinotsis et al., 2017). Researchers accept that electrical signaling is the key mechanism for information transmission and processing in the brain. Why are brain theory and computational models of brain function based on the electrical signal, and not on some other physical signal (optic or magnetic)? We identify six cardinal features of electrical signaling that are essential for a theory of the brain and cannot be replicated by other signaling mechanisms.
1. Electrical signaling - Cellular specificity
A humoral signal emitted from a blood vessel or a liver cell will indiscriminately activate a large set of neighboring cells in one region of a large organ like the liver (Fig. 1A1, Liver, red cells). The bottom line of the liver signaling scheme is that all activated cells are more or less grouped in one location (Fig. 1A1, red cells). The number of potential ensembles of activated liver cells is low, because these cells are activated in large sections of adjacent cells via body fluids. Also, the structural complexity of a potential liver cell ensemble (Fig. 1A1, red cells) is low for the same reason.
Fig. 1. Unique advantages of electrical signaling.
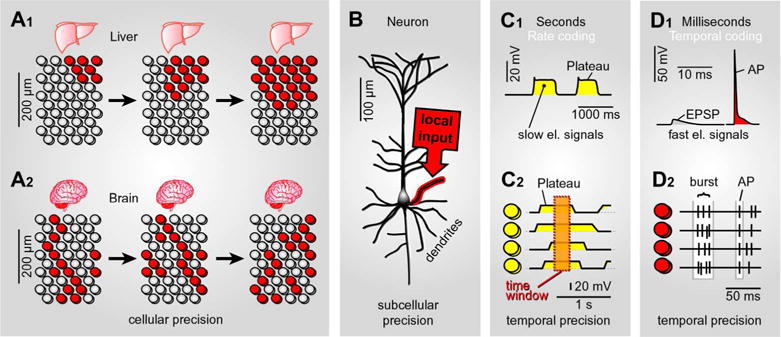
(A1) Schematic representation of a liver tissue. Each circle represents one hepatocyte. Color indicates “activated” cells. Cellular activity is synchronized among neighboring hepatocytes by diffusing chemical signals. (A2) Schematic representation of a brain tissue section. Each circle represents one neuron. Color indicates “activated” cells. Cellular activity is synchronized between particular neurons (not all neurons) by electrical signaling.
(B) Synaptic inputs impinge on one basal dendrite of a cortical pyramidal cell. Synaptically-induced depolarization is strongest in one cellular compartment (red halo).
(C1) Slow electrical signals in the form of sustained plateau depolarizations; amplitude ~20 mV, duration ~500 ms. (C2) Electrical activity is synchronized among 4 cells on a slow temporal scale (seconds). Dashed rectangle indicates a period of time when all 4 cells are in depolarized state.
(D1) Fast electrical signals, excitatory postsynaptic potential (EPSP) and action potential (AP), are shown on a fast time scale. (D2) Action potential firing is simultaneously recorded in 4 neurons. Each vertical tick represents one AP, as in “single-unit” recordings. Synchronization can be based on the temporal relation between between groups of APs (AP bursts) or individual APs (spikes). Synchronized bursts of APs or synchronized single APs are marked by white background rectangle.
The brain signals are a combination of bioelectrical and specialized humoral signals. Long-range brain signals are predominantly electrical, and many humoral signals are restricted by synapses so as to be point-to-point rather than broadcast. Nervous systems combine signal speed and signal specificity through precise targeting. Brain signals are carried great distances by electrical impulses without crosstalk via specific conduits – the axons. The enormous abundance and complexity of axonal paths allows for activation of specific individual cells separated by specific inactive cells (Fig. 1A2, red cells). The targeting mechanism based on axons and electrical impulses permits activation of distinct groups of selected cells in and among brain regions (neural ensemble). Brain signaling therefore permits activated cells to be scattered in space (Fig. 1A2, red cells). That is, any cell in the brain can potentially become a member of a functional ensemble. The number of distinct ensembles that can potentially be formed out of the total number of brain neurons (100 billion) is very large. If each neural ensemble is made of approximately 1,000 cells per functional ensemble, the number of distinct ensembles of this size that can potentially be formed out of the total number of living brain cells is astronomical, allowing for tremendous number of percepts and memory traces over the entire life span. In summary, electrical signaling permits large number of combinations and permutations of individually-activated cells (Fig. 1A2, red cells).
2. Electrical signaling - Subcellular specificity
Histo-anatomical and physiological data both show that electrical signals target specific sub-compartments of neurons (Feldmeyer et al., 2002; Markram et al., 2015); down to the level of individual dendritic branches; for example, thin dendrites of cortical pyramidal neurons (Fig. 1B). If synaptic inputs are restricted to one dendritic branch, then the resulting membrane depolarizations can be spatially restricted to that input site (Mel, 1993). At the same moment of time, one dendritic branch experiences strong membrane potential transient, while the neighboring dendritic branch is without voltage transients ((Milojkovic et al., 2004), their figure 7). Local dendritic depolarizations and local dendritic summation of synaptic inputs, assure that nerve cells process afferent synaptic inputs in two stages (two-layered model, (Poirazi et al., 2003; Polsky et al., 2004)). In the first processing stage, incoming synaptic inputs are summed within thin-dendrite compartments or subunits, each of which is governed by timing-based, regenerative thresholding, or other I/O functional forms - for example, the sigmoidal thresholding form proposed by Poirazi et al. 2003. “Thresholding nonlinearity” simply means that upon reaching a certain voltage threshold the dendrite is no longer behaving as a linear summator of potentials, but instead it is producing additional regenerative potential, disproportional to the received input. The examples of local regenerative potentials in thin branches of cortical pyramidal neurons (basilar, oblique and tuft) include dendritic sodium spikelet, dendritic Ca2+ spikes, dendritic NMDA spikes, and glutamate-mediated dendritic plateau potentials (Schiller et al., 2000; Ariav et al., 2003; Milojkovic et al., 2005b; Antic et al., 2010; Grienberger et al., 2015)). In a second stage of the synaptic input processing, the subunit outputs are combined linearly in the cell body to determine the overall response of the cell - the neuron generates an axonal action potential (AP), or spike which is selectively transmitted to the group of cells determined by the connectome. In other words, the neuron output signal, the final result of intracellular integration, is broadcast to all “prepaid” members of the neuronal network. A key prediction of the two-layer model, but not of the global summation model, is that summation should obey different rules for inputs delivered to the same versus different thin branches of the nerve cell (Rall et al., 1992; Koch and Segev, 2000). In essence, the action potential output and the computational task of a given brain cell, both depend on the exact location of the synaptic input on its dendritic tree (Branco and Hausser, 2011; Briggman et al., 2011; Smith et al., 2013; Vlasits et al., 2016). The final neuronal computations depend not only on the type of dendritic operation and the dendritic and axo-somatic thresholds, but also on the global mapping of input features throughout the dendritic tree (Tran-Van-Minh et al., 2015). The necessary level of subcellular input specificity is exclusively accomplished by axons and electrical signals flowing through neural circuits and arriving at a precise dendrite (Fig. 1B) or even dendritic segment (i.e. distal versus proximal dendritic segment) (Jadi et al., 2014). One exciting theme emerging in the field of dendrite physiology is the notion that dendritic regenerative potentials are essential for the organization of neural networks. According to this theme, glutamate-mediated dendritic spikes are the key determinants of synaptic plasticity (Golding et al., 2002; Gordon et al., 2006; Brandalise et al., 2016) and in the long run dendritic nonlinear responses build a high-performance connectome (Bono and Clopath, 2017; Legenstein and Maass, 2017). In summary, electrical signaling underlies not only function but also the development and structure of the complex brain.
3. Electrical signaling - Temporal specificity
Human brain areas, involved in complex behavioral responses, are separated by considerable path distances often exceeding 5 centimeters (~2,500 cell body diameters) and the flow of information in the brain is not unidirectional. Nearly all brain regions send signals into hierarchically higher brain areas and receive immediate feedback from these areas, in real time. For example, the cortical primary sensory areas (visual, auditory, somatosensory, etc.) receive feedback (top-down) projections from secondary and tertiary sensory areas, providing them with pre-processed pieces of information pertaining to the object in the perceptual field (Gilbert and Wiesel, 1983a; Shipp and Zeki, 2002; Larkum, 2012). In this way, the outcome of the sensory process at the primary sensory area (which is supposed to process the simplest fragments of the perceptual object such as color, orientation, etc.) is strongly influenced (Bayesian biased) by the higher cortical areas that have already been informed about the complex features of the perceptual object including size, class, emotional valence, etc. (Ro et al., 2003; Dura-Bernal et al., 2012). As the uninterrupted flow of information forth (from lower to higher) and back (from higher to lower) is critical for ongoing Bayesian expectation information processing and critical for the success of a complex information processing task, one can also assume that an exchange of just one forward and one feedback “message” would not suffice. Rather, several iterations may be required to achieve desirable results. If a psychophysical reaction takes ~200 ms (Wu et al., 2015), then a communication signal underlying this reaction should be capable of shuttling several times between two regions (couple of centimeters apart) in a period of time shorter than 200 ms. Among all known cellular and molecular signals operating in the brain, only electrical signals have sufficient speed to allow rapid exchanges between cells in the network. Very brief durations of electrical signals (Fig. 1D1) allow neural networks to operate at relatively high frequencies and accomplish tasks very quickly (Greene and Oliva, 2009). Fast network communication at high frequency could in principle be achieved between connected neurons through radiation of photons, fluctuations of magnetic field, and passage of electricity. Since photons and magnetic fields have not yet been documented as the means of neuron-to-neuron communication, there is only one type of biological signal suited for the rapid transfer of information within a neural network: electrical signal (Kopell and Ermentrout, 2004; Salkoff et al., 2015).
4. Electrical signaling – Arithmetic Integration
Electrical signals provide brain cells with a capacity to integrate signals - add and subtract signal intensities rapidly. In rough terms, the subthreshold depolarizing synaptic potentials add and hyperpolarizing synaptic potentials subtract from the “total score” in real time, and on a very fast time scale measured in milliseconds. Other signaling mechanisms that operate in the brain are also capable of positive and negative regulation, but these actions occur on a much slower time scale, seconds and minutes, and they do not follow precisely the rules of arithmetic summation and subtraction, such as linear summation of two EPSPs arriving into the cell body from two dendritic branches (Cash and Yuste, 1998).
5. Electrical signaling – Sustained Depolarization
Cortical and hippocampal pyramidal cells, and medium spiny neurons of the striatum, nucleus accumbens and amygdala, comprise approximately 85% of neurons in telencephalon, and are the principle neurons responsible for sensory perception, cognition, emotions and motor output. Interestingly, all of the aforementioned neuron types exhibit glutamate-mediated dendritic plateau potentials (Milojkovic et al., 2004; Milojkovic et al., 2005a; Plotkin et al., 2011; Oikonomou et al., 2014). Dendritic plateau potentials produce sustained depolarizations of the cell body, amplitude ~20 mV and duration ~ 300-500 ms (Fig. 1C1, Plateau). Upon the cessation of the dendritic plateau potential, the cell body returns to resting membrane potential or a hyperpolarized inactive state (Milojkovic et al., 2005a). Cortical and hippocampal pyramidal cells, medium spiny neurons of the striatum, nucleus accumbens and amygdala, all exhibit two distinct physiological states, depolarized (UP) state and hyperpolarized (DOWN) state (Wilson, 2008). Such slow oscillatory activity (Engel et al., 2001; Yu and Ferster, 2010; Neymotin et al., 2011; Petersson and Fransen, 2012) can facilitate synchronous interactions by biasing neurons to discharge within the same time frame. Coincident depolarized plateau-states may help the recruitment of neurons into the functional ensemble. The critical time window for the formation of a neural ensemble is the one over which the individual member cells are in depolarized states (Fig. 1C2, dashed rectangle). Principal cells of cortex and striatum are not spontaneously active. An oncoming barrage of glutamatergic projections can move a group of cortical or striatal cell from resting into sustained depolarized state (UP state). A second group of glutamatergic inputs can then initiate action potentials. Only cells which are already in the depolarized (UP) state will respond to the second set of inputs, because it is difficult to initiate firing in cortical and striatal cells during the hyperpolarized DOWN state (but see (Petersen et al., 2003)). In our view, a group of neurons in sustained depolarized state constitutes one neural ensemble. From this group, the second set of excitatory inputs can recruit neurons into an ensemble of a higher order; an ensemble comprised of cells engaged in the firing of action potentials. Thus, the group of spiking cells is embedded in the group of depolarized cells. We think that such “ensemble embedding” may be an important organizing principle in neural networks.
6. Electrical signaling – Synchronization and Binding
Representation of a physical or mental “object” during sensory perception, as well as sensorimotor coordination, requires binding together of many brain areas to produce coordinated activity. Binding requires some type of coordination of neural activity across populations of neurons on various temporal scales. Most popular in discussions of binding over the past decades, has been rapid binding through fast temporal coordination at rates corresponding to gamma oscillations in the range of 40 Hz (binding theory, (Singer and Gray, 1995)). Variations of this scheme include coding based on precise spike synchrony and coding based on timing relative to a slower wave envelope provided by theta or alpha oscillation. In the present account we will focus on one type of synchrony coding which implies multiple cells are firing within a short period of time (like Fig. 1D2). Synchronized spiking activity has been found in different species and different cortical areas (Bair, 1999; Salinas and Sejnowski, 2001; Buzsaki and Silva, 2012). For the same level of firing, a synchronous input is more effective on postsynaptic neurons than asynchronous input (Schneidman et al., 1998; London et al., 2002). Large-scale models predict that synchrony occurs due to the reciprocal connectivity and loops between clumps of neurons (Tononi et al., 1992; Durstewitz et al., 2000; Compte et al., 2003).
In summary, electrical signaling (Galvani, Helmholtz, Adrian, Eccles, etc.) endows neurons with the means for achieving communication between specific cells and avoiding their neighbors (Fig. 1A2, red cells), for delivering inputs on specific subcellular compartments, individual dendrites and avoiding neighboring dendrites, for rapid exchange of messages between two remote areas (a millisecond scale), and for arithmetic operations (additions & subtractions) using rapid afferent signals. Electrical signaling gives neurons the ability to shift quickly between hyperpolarized and depolarized states, back and forth, remain in depolarized state for several hundred milliseconds, and achieve binding through slow oscillations at ~1 Hz (UP states), or through synchronized or precise spike timing activity which can reach frequencies of up to 40 Hz (Singer and Gray, 1995)). There are no other biological signals capable of supporting the aforementioned features, and hence computational modeling of neuronal electrical signaling is currently the most promising approach towards understanding how the brain “walks” and “talks”.
PART 2: SINGLE NEURON SIGNIFICANCE
The classical point neuron has simple dynamics and no spatial extent. A more modern concept (alternative abstraction) endows neurons with large nonisopotential dendritic trees, where individual dendritic branches behave as semi-independent units (Koch et al., 1983; Mel, 1993). The dendritic tree effectively increases the total number of processing units in the network. The cost of not having good single-neuron models is potentially high. In studying a neural circuit, computational neuroscientists naturally draw their concept of the neuron from the example of transistors used in computers, leading to imperfect assumptions regarding the capabilities of the various neuron types that make up the circuit. The large investment of time and money required to design the integrated large-scale systems performing multiple computations may be wasted if critical capabilities of individual neurons are omitted from the design (Rall and Shepherd, 1968; Agmon-Snir et al., 1998; Euler et al., 2002; Lavzin et al., 2012; Legenstein and Maass, 2017).
Spiny Neurons
In vivo intracellular recordings have documented UP and DOWN transitions in cortical L5 pyramidal neurons, cortical L4 stellate cells, striatal medium spiny neurons and spiny neurons of the amygdala (Wilson and Kawaguchi, 1996; Steriade et al., 2001; Brecht and Sakmann, 2002; Volgushev et al., 2006; Padival et al., 2013). UP states in striatum and UP states in cortex use different network organizations to achieve the same thing: sustained somatic depolarization. Recurrent excitation between neighboring pyramidal cells and cortico-cortical projections are dominant in cortex. Thalamic and cortical efferents, on the other hand, supply excitation to the striatum. In some nomenclatures, UP and DOWN states are terms reserved for specific membrane polarization levels occurring during slow wave sleep, occurring in a rhythmic fashion (~1 Hz) and involving nearly all cells in a block of tissue (Wilson, 2008). In the present article we borrow the term “UP state” from the sleep studies to describe sustained somatic depolarization 200 – 500 ms in duration and ~20 mV in amplitude (“neuronal UP state”). There is a conceptual difference between “cortical UP state” and “neuronal UP state”. In cortical UP state occurring during slow wave sleep, almost all pyramidal cells in the area are synchronously depolarized. In the neuronal UP state (proposed here as a cellular mechanism for the recruitment of pyramidal cells into functional ensembles) only a selected group of neurons are depolarized, and all members of this depolarized group code for similar or related objects.
Neuronal UP State
Neuronal UP state in sleep and neuronal UP state in wake occur in the same cell: spiny telencephalic neuron. As a rule, spiny CNS neurons do not fire action potentials from a DOWN state. Spiny neurons only fire action potentials when their cell body is in a depolarized, UP state (Wilson and Kawaguchi, 1996; Steriade et al., 2001; Brecht and Sakmann, 2002; Volgushev et al., 2006; Padival et al., 2013). A successful synchronization of the firing activity among spiny neurons in cortex, striatum and amygdala would thus absolutely require that members of a neuronal ensemble have overlapping UP states (Fig. 1C2, dashed rectangle). Synchronization can be achieved at two levels of precision: [A] neurons burst within the same window of time (100 ms precision), or [B] there is individual spike synchrony (1 ms precision). To become eligible for inclusion into a functional neuronal ensemble, a spiny neuron must quickly and reliably switch from a DOWN to an UP state, remain in the depolarized UP state as long as necessary, and quickly abort the UP state when a percept is formed or expired.
Dendritic plateau potential may not be the only cellular mechanism of sustained electrical activity in individual CNS neurons. Persistent firing which outlasts the original stimulus can be sustained via two traditional mechanisms: [i] Recurrent network excitation within microcircuits of CNS neurons (Goldman-Rakic, 1995). [ii] Intrinsic membrane conductances of neurons which do not require fast synaptic transmission (Egorov et al., 2002). Activation of [iii] cholinergic, [iv] noradrenergic and [v] dopaminergic afferents, as well as [vi] metabotropic glutamate receptors have also been implicated with different forms of sustained neuronal activity (Zhang et al., 2013). It is important to emphasize that the majority of the abovementioned cellular mechanisms [i – vi] are fundamentally based on the glutamatergic transmission on spiny dendrites. Glutamate-mediated plateau potentials may occur in dendrites of neurons engaged in persistent activity via recurrent excitation (Goldman-Rakic, 1995). Glutamate-mediated plateau potentials may occur upon a norepinephrine surge causing activation of alpha-1 adrenoceptors facilitating glutamate release and subsequent activation of postsynaptic mGluR5 receptors (Zhang et al., 2013).
Neuronal UP states are known to play an important role in the consolidation of memory (Marshall et al., 2006), but the exact cellular mechanism by which UP states control storage of information in the brain is poorly understood. A recent study showed that UP state-induced synaptic weakening of subthreshold inputs, while preserving synaptic strength of strong inputs (Bartram et al., 2017). This cellular mechanism causes a near-global synaptic downscaling during slow wave sleep. Subthreshold inputs, which comprise a majority of inputs, are downsized but a group of selected inputs (strong enough to bring cell to firing) are preserved unaltered.
Dendritic UP State
Synaptically-evoked somatic plateau depolarization can be the consequence of glutamate-mediated dendritic plateau potential/spike, also known as “dendritic UP state” (Milojkovic et al., 2005a; Antic et al., 2010; Plotkin et al., 2011). The voltage waveforms of dendritic plateau potentials (Fig. 2A, basal) were characterized using voltage-sensitive dye imaging (Milojkovic et al., 2004; Milojkovic et al., 2005a). The somatic plateau rises a few milliseconds after the onset of the dendritic voltage transient and collapses with the breakdown of the dendritic plateau depolarization (Milojkovic et al., 2005a). In other words, the cell body shifts from DOWN to UP state after the generation of the dendritic plateau potential. It stays in the UP state as long as the dendritic plateau lasts, and it collapses with the collapse of the dendritic plateau potential. The slow component of the somatic depolarization accurately mirrors the duration of glutamate-evoked dendritic plateau potential (Fig. 2A, compare voltage signal in dendrite “P” and voltage signal in soma). This observation is most apparent in experiments in which a gradually increasing intensity of glutamatergic input was delivered onto a basilar dendritic branch. At subthreshold glutamate input intensities the dendritic and somatic depolarizations are both subthreshold. As soon as the dendritic membrane develops a regenerative dendritic plateau potential (threshold reached in one dendrite), the somatic compartment of this neuron reports a neuronal UP state (Oikonomou et al., 2012, their figure 3). In summary, the relation between dendritic plateau potential and somatic UP state is reliable (Milojkovic et al., 2005a; Milojkovic et al., 2007).
Fig. 2. Dendritic plateau potentials – somatic plateau depolarizations.
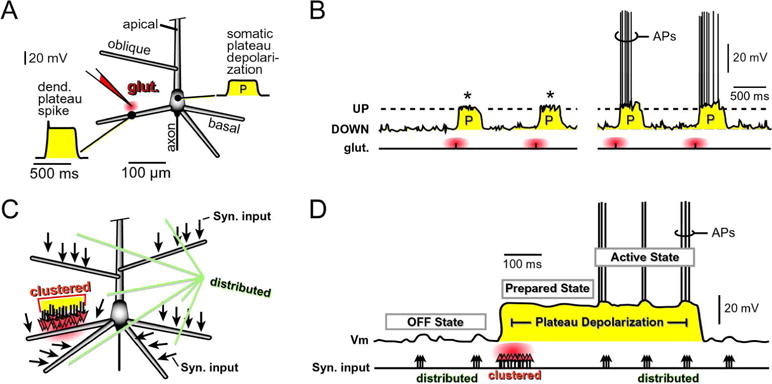
(A) Cartoon of a pyramidal neuron showing classes of dendrites: basal, oblique and apical trunk. Voltage waveform of dendritic plateau potential and the resulting somatic depolarization (“P”), in response to glutamatergic stimulation of one basal dendrite. “Glut.” marks the glutamate iontophoresis site.
(B) Cartoon of four glutamate iontophoresis pulses (each pulse = 5 ms) delivered on one basal dendrite. Glutamate stimuli trigger somatic plateau depolarizations (P), which resemble neuronal UP states. The somatic P without APs (*) is termed a “Prepared state” of a neuron (Pr). The somatic P accompanied by action potentials (APs) is termed “Active state” (Act). The somatic DOWN state is termed an “OFF state” of a neuron (off).
(C) Cartoon of a pyramidal neuron - black arrows mark glutamatergic inputs of approximately identical weight. Two spatial patterns of synaptic inputs are shown: distributed (everywhere) and clustered (on one basal dendrite). Clustered inputs produce glutamate spillover (red cloud).
(D) At the low levels of incoming synaptic inputs, the somatic membrane potential (Vm) is dwelling near resting potential (OFF state). Clustered glutamatergic inputs onto one basal dendrite (“clustered”) produce spillover glutamate (red cloud), which triggers dendritic plateau potential, which in turn brings the cell body into a plateau depolarization. During the plateau depolarization (Prepared state) the membrane potential is ~20 mV closer to the AP threshold and the membrane time constant is shorter due to a glutamate-mediated drop in membrane resistance. As a result, the neuron is more responsive to incoming synaptic inputs distributed across the dendritic tree (black arrows). The same-size synaptic input (3 arrows) fails to initiate AP in the OFF state, but successfully drives AP firing in the Prepared state.
Fig. 3. Object coding by neural ensembles.
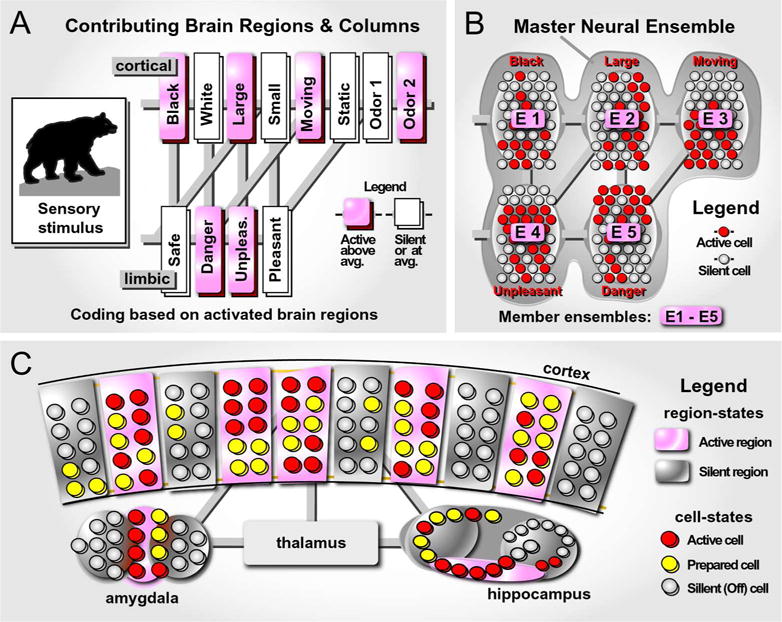
(A) An object in the perceptual field (bear) activates pockets of cells in many brain regions. Each rectangle represents a column, or a segment of a nucleus, devoted to one simple attribute of the object. Brain regions are connected with axonal projections (gray stripes). The overall activity of a column increases above average if an attribute were detected (pink) – the column is “net-active”. If an attribute was not detected, the net electrical activity remains at or below the average level (white) – the column is “net-silent”.
(B) Schematic depiction of net active brain regions at cellular resolution. The net silent brain regions (white rectangles in the previous panel) are omitted form this presentation, for simplicity. Active neurons = red circles. Inactive neurons = gray circles. Multiple neural ensembles (E1 – E5) are embedded in the master neural ensemble, which codes for a large, dark and dangerous moving object detected by sensory system. Member ensembles (E1 – E5) may reside in separate brain regions (e.g. cortex, striatum and amygdala). Together the member ensembles form a master neural ensemble (gray contour).
(C) Schematic depiction of 4 brain areas: cortex, hippocampus, thalamus and amygdala. Each brain area is divided into smaller functional units/segments/columns. Units can be either net-active (pink) or net-silent (gray). In respect to electrical activity at single-cell level, neurons can be found in 3 functional states: OFF (gray circle), Prepared (yellow circle) and Active (red circle).
Glutamate
Glutamate processing in neuronal and astrocytic processes (uptake, packaging and release) occurs rapidly and vigorously. Spiny neurons in cortex and striatum are exposed to confluent and repetitive excitatory drives during intense neural activity. For example, neurons experience strong barrages of glutamatergic inputs every other second of the slow-wave sleep period; 1 Hz oscillation (Wilson and Kawaguchi, 1996; Steriade et al., 2001; Brecht and Sakmann, 2002; Volgushev et al., 2006; Padival et al., 2013). Whole-cell somatic recordings show that dendritic membrane responds robustly to repetitive glutamatergic stimulations with no signs of glutamate receptor desensitization or fatigue (Fig. 2B), see also ((Milojkovic et al., 2004) their figure 1, (Oikonomou et al., 2012) their figure 7). Spiny neurons seem properly equipped to convert a surge of extracellular glutamate into a sustained depolarization episode, and to do so repeatedly (Fig. 2B). Dendritic voltage imaging showed that sustained somatic depolarizations (Fig. 2B, “P”) are caused by local dendritic plateau potentials (Milojkovic et al., 2005a; Milojkovic et al., 2007). Therefore, the glutamate-mediated plateau is a plausible membrane mechanism for placing CNS cells in a sustained depolarized state during intense cognitive operations.
Principal neurons of cortex, hippocampus, neostriatum and amygdala are highly branched structures hosting numerous glutamatergic synapses on every thin dendrite (Fig. 2C, black arrows). In respect to glutamatergic synapses, there is a one-to-one relation between donor (presynaptic axon terminal) and the acceptor (postsynaptic dendritic spine). This means that in order to map synaptic inputs on a spiny neuron, it is not necessary to label and visualize presynaptic terminals (axons). By simply counting and recording the locations of dendritic spines one can arrive at a precise density of excitatory glutamatergic synapses on a given neuron (Larkman, 1991). One distinguishing feature of all principal spiny CNS neurons involved with cognitive tasks (cortex, striatum, and hippocampus) is the tendency to make as many synapses as possible, and extract information from as many neural circuits as possible (Chen et al., 2013). The number of excitatory glutamatergic contacts on any given CNS spiny neuron is directly proportional to the complexity of its computational task. For example, layer 5 pyramidal neurons in the occipital lobe (primary visual cortex) have 2-3 times fewer synapses than the equivalent (L5) neurons in the prefrontal cortex (higher association cortex). The dendritic spine count per cell increases gradually from occipital to parietal, to prefrontal cortex, in the same way the number of the sensory modalities increases from primary, to secondary, to tertiary association cortex (Elston, 2003).
Prolonged depolarizations, long decay time-constants and efficient temporal summations of synaptic inputs on pyramidal neurons are attributed to glutamate activation of NMDA receptors (Lisman et al., 1998; Wang et al., 2008). Sustained depolarizations are in part driven by glutamate spillover (Fig. 2A, red cloud), which keeps dendritic NMDA receptors in the open state for several hundred milliseconds (Milojkovic et al., 2007; Chalifoux and Carter, 2011; Oikonomou et al., 2012).
We think that astrocytes are essential for triggering of dendritic plateau potentials. We envision three synaptic scenarios for dendritic EPSPs, NMDA Spikes and Plateau Potentials, respectively. [EPSPs] EPSPs are generated when glutamatergic inputs are sparse on spiny dendritic branches. Astrocytic processes, positioned in between dendritic spines, have ample capacity to clean up synaptic glutamate during sparse and scattered synaptic transmission (Oikonomou et al., 2012, their figure 1A). [NMDA Spikes] NMDA spikes are generated when inputs are clustered in a small dendritic segment (Fig. 2C, clustered). Input clustering facilitates summation of the spill-over glutamate, which can partially overcome the local glutamate uptake in astrocytic process, resulting in a dendritic NMDA spike (Oikonomou et al., 2012, their figure 1B). [Plateau Potentials] Plateau potentials are generated when glutamatergic inputs are both clustered and repetitive, which brings a lot of glutamate molecules into a restricted space, where synaptic inputs are functionally clustered. Facing the excessive amounts of glutamate, the astrocytic processes not only stop the glutamate uptake, but they reverse glutamate uptake and spill out glutamate into the space surrounding spiny dendritic segment, resulting in a “glutamate clamp” of the dendritic membrane potential. Recall that the plateau phase of a local dendritic plateau potential is ~50 mV above the resting potential (Milojkovic et al., 2004), and this voltage is at or near the glutamate reversal potential. In summary, repetitive synaptic stimulation overcomes the ability of astrocytic processes to clear glutamate from the extracellular space, allowing some dendritic segments to become submerged in a pool of glutamate, for a brief period of time. This dynamic arrangement activates extrasynaptic NMDA receptors located on dendritic shafts. Strong and sustained activation of AMPA and NMDA conductances keep the selected dendritic segments in a 50 mV depolarized dendritic UP state. The aforementioned synaptic scenarios are still in the domain of speculations. A solid experimental evidence for the astrocytic role in dendritic plateau potentials awaits methodological improvements in measuring glutamate transients inside astrocytic processes and in the extracellular matrix surrounding dendritic segments.
Two Maps of Glutamatergic Inputs
Glutamatergic inputs can be spatially segregated on one dendritic branch, one dendritic segment (Fig. 2C, Clustered), or they can be sparsely distributed across the dendritic tree (Fig. 2C, Distributed). Both spatial organizations, Clustered and Distributed, could play important roles within the neuron’s computational task.
Clustered
Theories based on the functional clustering of glutamatergic synapses on developing and mature dendrites, are gaining momentum (Koch et al., 1983; Poirazi et al., 2003; Larkum and Nevian, 2008; DeBello et al., 2014; Kastellakis et al., 2015; Bono and Clopath, 2017; Ujfalussy et al., 2017). In parallel with hypotheses and neuron-modeling, experimentalists try to address this issue in the laboratory. New experimental results mostly support the theory of synaptic clusters (Kleindienst et al., 2011; Makino and Malinow, 2011; Fu et al., 2012; Hill et al., 2013; Pinchas and Baranes, 2013; DeBello et al., 2014; Palmer et al., 2014; McBride and DeBello, 2015), but see (Chen et al., 2013) for an alternative perspective. We proceed by listing several studies in support of clustering.
-
*
Glutamate uncaging experiments determined that approximately 50 synaptic inputs clustered within 100 μm of a thin dendritic branch need to be activated within 3 ms in order to trigger a regenerative dendritic potential (Gasparini et al., 2004), with similar ionic composition to glutamate-mediated dendritic plateau potential. The exact size of synaptic cluster necessary for initiation of local dendritic plateau potential will vary depending on the branching order, distance from the cell body and proximity to large or complex dendritic bifurcations (Larkum et al., 2009).
-
*
Calcium imaging in neonatal brain slices during spontaneous bursts of activity show that synapses located near each other on the same dendritic branch exhibit a higher degree of temporal correlation than synaptic pairs on different dendrites (Kleindienst et al., 2011). This highly correlated activity of neighboring synapses could not be attributed to individual axons forming multiple synapses on short stretches of dendrite. Furthermore, Kleindienst et al. (2011) showed that repetitive spontaneous activity in developing neural networks causes spatiotemporal clustering of functional synapses on dendrites, and that this process is dependent on the intact function of glutamatergic NMDA receptors.
-
*
Experience-dependent formation of synaptic input clusters can occur in juvenile brains and synaptic clusters formed early in life can be preserved to adulthood (McBride and DeBello, 2015).
-
*
Anatomical studies demonstrate that excitatory connections in cortex are not uniformly distributed across a network but instead exhibit clustering into groups of highly connected neurons. Individual pyramidal and spiny stellate neurons in the cat primary visual cortex project their axons horizontally (up to 4 mm) but the axon collaterals are often distributed in repeating clusters, with an average periodicity of 1 mm. The clustering pattern is most apparent when the cells are viewed parallel to the cortical surface (Gilbert and Wiesel, 1983b).
-
*
While sensory deprivation causes homeostatic synaptic enhancement globally on all dendrites, meaningful sensory experiences preferentially produce synaptic potentiation onto nearby dendritic synapses. Such clustered synaptic potentiation is thought to bind behaviorally relevant information onto dendritic subcompartments (Makino and Malinow, 2011; Brandalise et al., 2016; Bono and Clopath, 2017).
-
*
Different motor tasks trigger calcium spikes in largely non-overlapping distal apical tuft branches of the same L5 pyramidal neurons (Cichon and Gan, 2015). The apparent specialization of individual branches for a particular motor task (Cichon and Gan, 2015), suggests that ensembles of neurons specific for one motor task cluster on one branch, while other task-specific ensembles cluster on other branches of the same neuron.
-
*
Computational modeling studies suggest that neurons with correlated activities will tend to form clusters of synapses close together on the dendrites of a target neuron, whereas neurons with unrelated activities will tend to form synapses that are further apart (Ujfalussy et al., 2017). Synaptic clustering may increases storage capacity of CNS neurons (Poirazi and Mel, 2001; Sheffield and Dombeck, 2015). Information storage in neural tissue could reside primarily in the selective addressing of synaptic contacts onto dendritic subunits (clustering), as opposed to the traditional view stating that memories are primarily encoded in the overall connection strengths between neurons.
Distributed
Excitatory synaptic inputs distributed on dendritic branches contribute little depolarization to the action potential initiation zone located in the axon initial segment (Gulledge et al., 2005). In our view, the most basic job of the distributed synaptic assortment (Fig. 2C) is to extract the background patterns of network activity. In the schematic representation, black arrows depict distributed glutamatergic inputs arriving onto three classes of dendrites in a pyramidal neuron (Fig. 2C, tuft, oblique and basal). On the time axis (Fig. 2D, synaptic input), black arrows indicate the timing of synaptic triplets arriving in regular time intervals, as being driven by a hypothetical rhythmic network activity. A triplet of synaptic inputs (triplet of black arrows) arriving during the neuronal OFF state is unable to bring the cell to fire an AP (Fig. 2D). An identical triplet of inputs occurring during a responsive, Prepared, state successfully drives the AP firing (Fig. 2D). Glutamate-mediated dendritic plateau potentials depolarize neuronal membrane potential (Vm), decrease neuronal membrane resistance (rm) and shorten the membrane time constant (τ). EPSPs with faster rising times have better chance of reaching the AP voltage threshold because voltage transients with faster rising times are more powerful activators of the voltage-gated sodium current (which constitutes AP) than slow voltage transients (Hodgkin and Huxley, 1990). Due to the voltage dependence of the sodium channel inactivation gate, a slow ramp potential may reach very depolarized levels without ever triggering an AP (Hodgkin and Huxley, 1990). This why faster rising EPSPs are more effective triggers of neuronal spiking. A second important factor that renders distributed synaptic inputs more efficient in the neuronal Prepared state is depolarization itself. The glutamate-mediated dendritic plateau potential effectively shifts the somatic membrane potential by approximately +20 mV (Fig. 2D). At this new depolarized membrane potential (Prepared state), small voltage transients from distributed synaptic inputs have a better chance of reaching the AP voltage threshold, compared to the OFF state, in which the somatic and axonal membranes were 20 mV more hyperpolarized (Fig. 2D). In summary, during Prepared state, small synaptic inputs, previously ineffective, now gain the ability to drive the initiation of action potentials. The Prepared state allows a pyramidal neuron to faithfully convert the incoming patterns of synaptic activity into the outgoing patterns of AP firing (McCormick et al., 2003), but see (Petersen et al., 2003).
The plateau phase of a glutamate-mediated dendritic plateau potential (Fig. 2C, Prepared state) may serve to provide spiny neurons with a window of opportunity, for tuning into the ongoing network activity more robustly (increased firing) and more accurately (faster membrane response; Fig. 2C, Activated state). For example, in hippocampal “place cells”, the generation of plateau potentials notably elevates AP firing frequency (Bittner et al., 2015). CA1 pyramidal neuron synapses are facilitating in nature, hence dendritic plateau potential initiation appears to be a powerful gain modulation of place cell’s output improving both the firing rate and short-term synaptic efficacy of its axon terminals. Dendritic plateau potentials effectively change the state of a CNS neuron. Dendritic plateau potentials create a context for a sharp increase in neuronal output (Lavzin et al., 2012; Grienberger et al., 2015; Schmidt-Hieber et al., 2017). In hippocampus, the context will be “place”. In cortex, depending on the area, the context may be a class of external objects (Fig. 3A). The cellular mechanism for bringing spiny neurons into receptive Prepared state is most likely based on synaptic clustering (Mel, 1993; Larkum and Nevian, 2008; Magee, 2011; Shai et al., 2014; Kastellakis et al., 2015). The job of a clustered synaptic assortment is to bring the target CNS neuron into a “receptive” Prepared state (Fig. 2D). This transition from the inert and silent OFF state to a depolarized and responsive Prepared state occurs when the neuronal network fulfils three requirements: [1] Glutamate-releasing axons converge anatomically onto the dendritic tree (Fig. 4A); [2] These axons become activated synchronously, within 10-20 milliseconds (Gasparini et al., 2004; Branco and Hausser, 2011), and [3] The synchronized activation of axons is repeated several times in a relatively short period of time (100 ms) (Milojkovic et al., 2004; Suzuki et al., 2008; Polsky et al., 2009). Clustered and repetitive glutamatergic activity is necessary for buildup of glutamate levels in the extracellular space (Fig. 2C, red cloud) (Suzuki et al., 2008; Chalifoux and Carter, 2011; Oikonomou et al., 2012).
Fig. 4. Recruitment of neurons into neural ensembles.
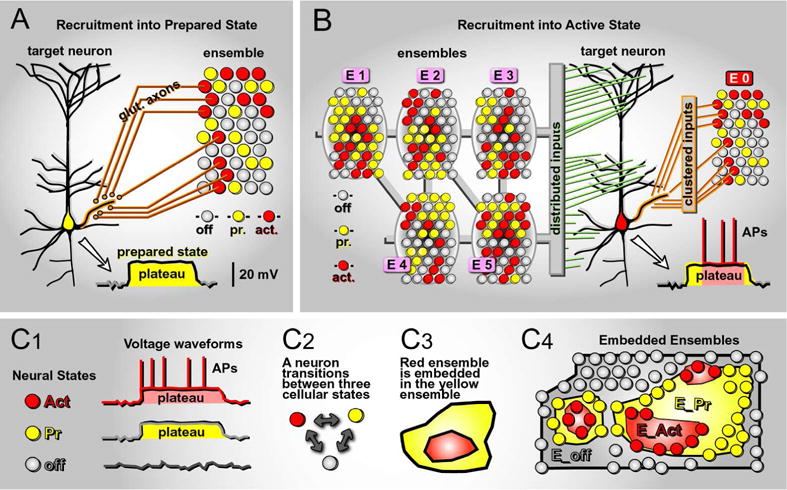
(A) A pyramidal neuron is receiving glutamatergic projections from a brain region comprised of neurons in three characteristic states of activity (OFF, Pr and Act). A fraction of spiking cells (red) sends their glutamatergic projections (glut. axons) onto the target pyramidal neuron, clustering on one particular basal branch. A computational task engaging the neural ensemble (red cell ensemble activity) is poised to recruit the target neuron into a Prepared state (somatic plateau depolarization, amplitude ≈ 20 mV, duration ≈ 300 ms), via the generation of a local dendritic plateau potential (basal dendrite - orange halo).
(B) A target pyramidal cell is receiving two types of glutamatergic inputs, clustered and distributed. “Clustered inputs” arrive from ensemble E0. Thus, ensemble E0 has the capacity to recruit our neuron into Prepared state, by triggering local dendritic plateau potential (same as in A). “Distributed inputs” arrive from various active brain regions (E1 – E5) and they scatter across the entire dendritic tree, including basal, oblique and apical tuft branches. Distributed inputs have the capacity to drive AP firing, but only if E0 had been successful in recruiting this cell into a Prepared state.
(C1) A characteristic somatic voltage waveform applies to each neuronal state, OFF, Prepared and Active. (C2) Each neuron transitions between 3 basic states, depending on the ongoing pattern of excitatory glutamatergic inputs. (C3) A group of Active cells (red contour) are recruited from a group of Prepared cells (yellow contour). (C4) Cartoon of a brain region shown at “cellular resolution”, each circle represents one neuron. The ensemble made of neurons in Active state (E_Act, red) is just a subset of the ensemble of neurons in Prepared state (E_Pr, yellow), which itself is a subset of neurons in the OFF state (E_off, gray).
PART 3: NEURAL ENSEMBLES
In this section, we adopt a notion that brains process and store information using neuronal ensembles. A neuronal ensemble is a dynamic structure composed of synchronously activated neurons engaged in the same task (Hebb, 1949; Eichenbaum, 1993; Engel and Singer, 2001). At one instant of time a neuron is a member of one ensemble, while in the next instant of time the same neuron participates meaningfully in the function of another neuronal ensemble (Desimone et al., 1984; Eichenbaum, 1993; Wilson and McNaughton, 1993; Engel and Singer, 2001; Legenstein and Maass, 2017). This “time-sharing” feature of the ensemble-organization principle assures a very high number of neuronal ensembles in the mammalian brain that can be assigned to a very high number of specific objects, including perceptual and mental objects – just as the pixel on your screen is only a tiny piece of an image and will be lit up for many other images as well.
Complex percepts, such as a black bear shown in Fig. 3A, are composed of sensory details (fragments) including, color, size, orientation, odor, emotional valence, etc. Each fragment of the sensory experience is processed by a specific cortical column or specific segment of a brain region specialized for that fragment of experience, and the combination of active and non-active regions thus represents a neural code for this class of objects (Fig. 3A). Both activated and silent brain regions contribute important clues about the nature (class) and/or identity of the object. Gathering information from as many regions as possible is a procedure that serves to reduce ambiguity. Since these attributes belong to different sensory modalities, and each modality is hosted by a different brain lobe (in two hemispheres simultaneously), it is inevitable that large neural ensembles (Fig. 3B, master neural ensembles) are composed of active neurons residing in many remote brain regions, including the thalamus, primary-sensory and association areas of the cerebral cortex, striatum and amygdala. Hippocampus, entorhinal cortex and midbrain are also activated, because the fundamental nature of sensory perception demands that current sensory percepts are compared with previously stored ones, as a measure of novelty (Lisman and Grace, 2005), while hypothalamus, amygdala and nucleus accumbens promptly assign emotional valence (Damasio, 1994). Experimental measurements show that the distribution of neurons involved in one functional neural ensemble is not restricted to the cerebral cortex, but it is likely to include subcortical gray matter (Fig. 3C, amygdala and hippocampus) (Brecht et al., 1998; Ziaei et al., 2013). Cortex-wide Ca2+ imaging in mice performing decision-making behavior revealed robust activation of neurons distributed across the majority of dorsal cortex (Allen et al., 2017). In summary, these activated groups of cells are widely distributed across different areas of the brain, each specialized in signaling a different attribute of the object or different element within the scene (Perrett et al., 1982; Mountcastle, 1997; Singer, 1999; Yu and Ferster, 2010).
Master Ensembles
The “master neural ensemble” is a functional construct (Fig. 3B, gray contour) that is formed when several member ensembles (E1 – E5) are activated simultaneously. Each member ensemble (sub-ensembles E1 – E5) combines some first order features (such as two co-occurring visual orientations) into a second order feature (such as checkered pattern). Detection of a checkered pattern on a given object is just one small step towards recognizing that object. This type of feature-binding is often performed locally in the primary sensory cortex (Muir et al., 2017), hence a sub-ensemble can, in general, be restricted to a given cortical region. In contrast, master ensembles are not encapsulated by one brain structure even if the brain structure is very large and multifaceted (e.g. cerebral cortex). This idea has repercussions on the actual experimental strategies. For example, studying the cellular determinants of face recognition by measuring electrophysiological responses from the cortical area specialized for face recognition (fusiform gyrus) might be an inadequate strategy, because the face recognition task requires an interplay between several brain structures including at least four visual cortices (V1–V4), fusiform gyrus, amygdala (Pujol et al., 2009) and thalamic nuclei. It would be difficult to identify the cellular mechanism of any given computational task, without physiological data from all these regions. For this reason, the current trend in experimental neuroscience is development of experimental recording methods for sampling voltage transients from many brain cells in many brain regions, simultaneously (Antic et al., 2016).
As a first approximation, the constellation of active brain regions is a code for one class of objects, or for one identifiable familiar object. However, the number of [a] object classes, [b] identifiable objects, [c] maps, [d] paths, [e] sequences and [f] strategies that need coding in everyday life, surpasses the combinatorial power of a few known cortical areas. The new view proposed in the present account states that within each activated specialized brain area, there is further refinement of the assembles of active neurons. Not all neurons in the activated specialized brain region (Fig. 3C, pink regions) are put into a Prepared state (Fig. 3C, yellow cells). Then, among the set of neurons in Prepared state, only a selected few cells will go one step further and generate APs, Active state (Fig. 3C, red cells). The question is what cellular process determines the switch of one cell from the OFF state to Prepared state, and what determines the switch from the Prepared state to the Active state. We propose that the switch from OFF to Prepared state is mediated by clustered glutamatergic inputs, while the generation of APs (Active state) is predominantly the consequence of distributed synaptic inputs (see below).
Time Window 200 – 500 ms
The majority of brain processes related to the feeling of awareness require that neural activity lasts for 200 - 500 ms (Wu et al., 2015). This window of time is perhaps a minimum amount of time needed to guarantee interactions among multiple brain regions. The 200 - 500 ms of sustained firing triggers the awareness of a stimulus either directly by producing significant glutamatergic output in target brain areas, or indirectly by allowing the feedforward stream from thalamus to interact appropriately with feedback streams from higher cortical areas (Cauller, 1995; Lamme and Roelfsema, 2000; Engel et al., 2001; Ro et al., 2003; Larkum, 2012). The 200 - 500 ms time window of sustained neuronal depolarization may be the consequence of reverberant activity closing the loop between past and present features of a moving object, or by closing the loop between long-term memory traces and the current sensory percept (reviewed in (Tononi and Koch, 2008)). Interestingly, the duration of synaptically-evoked somatic plateau depolarizations (Fig. 2A) is also in the range of 200 – 500 ms (Milojkovic et al., 2004; Oikonomou et al., 2012).
Global neural workspace
Psychological cognitive experiments suggest that a brain process exists (best termed consciousness), which enables the unification of otherwise separate neuronal functions. The unification of separate neuronal functions may be a serial process in which information travels from a node to a node (less likely) or occurring simultaneously in multiple nodes (more likely). It was previously suggested that distinct subsystems (brain regions), are competing for consciousness in the global workspace (Baars, 2002). This type of “competition” implies the existence of specific structures or specific networks performing exclusive integrating functions to keep track of the global workspace (Smallwood et al., 2012). If there were local structures exclusively responsible for multi-network integration, they would be expected to locally mirror activity from all member ensembles that they integrate. In line with these predictions, EEG and fMRI recordings identified several brain regions containing a significant number of converging network signals, in contrast to other regions in the same brain with much simpler function and activity patterns (John et al., 2001; Braga et al., 2013; Mittner, 2013). The neural underpinnings of this putative global brain architecture, in which specific structures or networks fulfill integrating functions associated with the global workspace, are currently unknown. The proposed theories (Dehaene et al., 2001; Engel and Singer, 2001; Llinas and Ribary, 2001; Baars, 2002; Tononi and Koch, 2008) are focused on identifying brain regions engaged in the process, but they do not offer cellular (synaptic and membrane) mechanisms utilized by individual neurons in this process. Here we propose the embedded ensemble encoding principle, which employs the ability of dendrites to trigger local glutamate-mediated regenerative membrane potentials (Fig. 2A, dend. plateau spike). In order to compete for access into the global workspace, the candidate brain structures (member ensemble) influence the responsiveness of cells in the higher order processing area, structures involved in multi-network integration (John et al., 2001; Braga et al., 2013). The goal of synaptic integration in this context is to trigger a dendritic plateau potential in the target brain area, which switches the state of the host neuron from OFF to Prepared. This is best achieved by clustered synaptic activity (Fig. 4A). A “cluster” is just a group of presynaptic axon terminals arriving on the same dendritic branch and carrying closely related information. Which presynaptic axons converge to one dendritic branch is determined by the “connectome”. The connectome is based on initial developmental wiring, then later on the life experiences, learning and memory. The connectome dictates that some cells are better connected with a particular functional ensemble than others. A functional ensemble is made of neurons carrying the same informational content in a given unit of time. The hardwiring is in essence equivalent to the idea of engrams. The engram can be considered similar to a memory trace, whereas formation of transient neural ensembles by synchronized action potential firing is similar to memory retrieval or ecphory. The term “ecphory” applies to a process in which an engram stored in the brain is retrieved and expressed behaviorally through interactions with strong retrieval cues, such as sensory input, ongoing behavior or voluntary goals (Josselyn et al., 2015). Experimental evidence supports the idea that mental representations, memory storage and memory retrieval all operate using neural ensembles. Multi-unit electrophysiological recordings performed in rodents showed that patterns of neuronal activity that occurred during the time of encoding are detected hours or days later, and when they do repeat, they influence the animal’s behavior. Modern studies based on neuron “tag-and-erase” methods have shown that silencing the so called “engram” neurons block the memory usage, and thus established that activation of “engram” neurons is necessary for successful retrieval of memories. Conversely, artificial stimulation of the “engram” neurons induces artificial memory recovery, providing strong evidence that activation of “engram” neurons is sufficient for retrieval (reviewed in (Josselyn et al., 2015). The “connectome-favorite” target cells receive large number of inputs, spatially segregated in their dendritic tree (clustered inputs, Fig. 4B). Only cells in Prepared state will be able to tune into synaptic activity constantly impinging across the entire dendritic tree (distributed inputs, Fig. 4B) and convert these distributed synaptic inputs into AP firing output in order to join the network of active neurons. AP firing patterns are then used for binding (Engel and Singer, 2001).
PART 4: EMBEDDED ENSEMBLE ENCODING
In this section, we combine: a) clustered glutamatergic inputs, b) dendritic plateau potentials, c) sustained depolarization of the cell body, d) distributed glutamatergic inputs, e) temporal coding and f) frequency coding, into one unified principle for transient recruitment of CNS neurons into dynamic neural ensembles. We dubbed this principle: embedded ensemble encoding, EEE theory.
Rate coding and temporal coding merge
Neural synchrony with a millisecond precision may be crucial for processing of information in mammalian brain, underlying many aspects of cognitive functions including the arousal, sensory perception, attentional selection and working memory. Current models suggest that synchrony can occur through reciprocal connectivity among groups of neurons (Bair, 1999; Salinas and Sejnowski, 2001; Buzsaki and Silva, 2012). The EEE theory modifies this by hypothesizing that, on a particular cycle of ensemble of neurons in the Active state (EAct), this connectivity will only be effective among current members of EAct. For the same level of firing, synchronous input is more effective on postsynaptic neurons than asynchronous input (Schneidman et al., 1998; London et al., 2002). Such synchronous firing might then not only signal cooperative binding during this period of an EAct, but would also activate sets of cells that are not involved in the current EAct to set up a new EAct for the subsequent period. Additional oscillatory activity associated with alpha activation will facilitate synchronous interactions by providing a restricted time frame within the longer period of the plateau depolarization (neuronal Prepared state) that underlies EAct. In our view, those oscillatory time intervals are superimposed on the glutamate-mediated dendritic plateau potentials (Fig. 2D, Prepared state) to enhance spike and burst synchronization. EEE theory is innovative in that it postulates two different ways to define cell assemblies, rather than the one usually assumed. The dendritic plateau activation in multiple cells leads to the ensemble of prepared cells (Fig. 4C4, Yellow). Spike generation is permissive in members of this ensemble only, which leads to the more transient production of multiple activity-synchronized embedded ensembles EAct at different times during the duration of the plateau (Fig. 4C4, Red). EEE theory proposes that larger “Master” ensembles (Fig. 3B) are made of neural sub-ensembles (member ensembles) simultaneously organized in two planes, space and time: [1] Sub-ensembles as physically distributed entities (Fig. 4C4); and [2] Sub-ensembles as transient, temporal entities – temporal coding embedded in rate coding (Fries et al., 2007; Ainsworth et al., 2012). These two related concepts are transformational because they pull together two concepts (rate vs. temporal coding) that are typically seen to be in opposition (Ahissar et al., 2000).
Bayesian predictive coding
Depolarized prepared state” provides bases for choosing one solution over another, which, in essence, is “Bayesian process”. In statistics, probability of some event is represented by its frequency. The more often some phenomenon occurs, the more probable it is. In a Bayesian process, probability is interpreted as reasonable expectation representing a state of knowledge, or as quantification of a personal belief. Knowledge is previous experience “recorded” by glutamatergic connections in the brain (e.g. LTP). Glutamatergic inputs trigger dendritic plateau potentials (Figs 2 & 4). Personal belief is comprised of knowledge and emotional state, both supported by the activity of hippocampus and limbic system, which project their glutamatergic inputs into the cerebral cortex, where we conceptualize dendritic plateau potentials in this article (Fig. 3). “Gathering information from as many regions as possible (Allen et al., 2017) is a procedure that serves to reduce ambiguity in computational tasks. The neuronal depolarized state (Prepared state) could be conceived as the Bayesian expectation that is then ready to be played into by input from either: hierarchically higher brain areas, or hierarchically lower areas, depending on a specific task.
Bayesian predictive coding theory suggests “how information about the environment, the individual’s needs, motivational states, and previous experience are represented” (Clifford et al., 2015). In Bayesian theory, information streams are theorized to interact and provide information integration via projections between hierarchically-lower and hierarchically-higher cortical areas (as well as from amygdala, thalamus and other areas (Friston, 2008)). Higher cortical areas provide predictions or internal models that are then used to interpret incoming sensory information. Ongoing Bayesian expectation information processing is nicely explained in the context of the problem of recognizing a coworker when you see them outside of work. At the work place, neurons that code for the list of coworkers are kept in a Prepared state each time a person enters our visual field. Outside the work place, this preparatory signal is missing, and a coworker may go unrecognized.
Another good example of the Bayesian expectation task is the need to identify the same object at almost any angle or perspective even if that object has never been seen before at that particular angle. In the multimodal association cortex, the neuronal depolarized state (Prepared state) could be conceived as the Bayesian expectation that is then ready to be played into by projections from the primary sensory areas. Presented with a multitude of choices and pressed to solve complex cognitive tasks, a healthy brain is in a permanent and dire need for: [a] context and [b] reasonable expectations. Chance favors the prepared mind/neuron. You won’t recognize something that you are entirely unprepared for.
Searchlight hypothesis
The “searchlight hypothesis” (Crick, 1984) shares many features with the present embedded ensemble encoding hypothesis (EEE). First, both theories are fundamentally grounded in the idea that neural ensembles are central for brain function. In the searchlight hypothesis, the searchlight appears to be focused on one important object, and it is controlled by reticular nuclei of thalamus. In the EEE theory, there is no focus on one object, but rather all (i) possible, (ii) similar and (iii) reasonable object vectors are silently turned ON at the same time, waiting for sensory input or memory retrieval signal to pick the best active ensemble embedded in a much larger ensemble of cells waiting in a depolarized prepared state. Second, in the searchlight hypothesis the reticular thalamic nucleus is working through von der Malsburg synapses – strong synapses formed by previous experiences. The EEE theory equally depends on excitatory connections formed by previous life experiences and learning. One major difference is that EEE theory specifies that connections which carry similar information or closely related information must impinge on the same dendritic branch. Clustered together in space and time, the EEE synapses exploit both synaptic and intrinsic dendritic excitabilities for amplifying afferent signals and converting excitatory postsynaptic potentials into dendritic plateau potentials, which, in turn, maintain the cell body in a sustained depolarized state for 200 – 500 ms (Milojkovic et al., 2004).
Closing remarks
In summary, the same general type of cell recruitment process is used across the entire telencephalon (cortex, striatum, accumbens, amygdala and hippocampus). In any given brain region at any given moment of time, nerve cells are found in one of the 3 basic states, OFF, Prepared and Active (Fig. 4C1). Each spiny neuron is capable of transitioning between 3 basic states, back and forth (Fig. 4C2). For the reasons explained in Fig. 2, the proper transition from the neuronal OFF to the Active state requires one obligatory step, a Prepared state. Since, the recruitment of neurons into the Active state is most easily accomplished from neurons in the Prepared state (Fig. 4B), the group of Active cells is going to be a subgroup of Prepared cells (Fig. 4C3–4). The ensemble of Active cells is embedded in the ensemble of Prepared cells. Active cell is the cell that is spiking, hence providing the substrate to two types of temporal coding: [1] Rate coding, which will allow binding with a subset of Prepared cells that reached AP firing threshold; and [2] Spike-time coding, which will allow binding with a subset of cells in the Active state (e.g. gamma oscillation).
Among several classes of dendritic spikes (Schiller et al., 2000; Golding et al., 2002; Brandalise et al., 2016), the glutamate-mediated plateaus (Milojkovic et al., 2004) and apical calcium plateaus (Larkum et al., 1999) produce the strongest and longest depolarization at the action potential initiation site and therefore have the most direct influence on cell firing via two mechanisms: [a] depolarization amplitude approaching the AP voltage threshold and [b] shortening of the neuronal membrane time constant. As a result, small synaptic inputs, previously ineffective, now gain the ability to drive the initiation of action potentials – “Chance favors the prepared cell.” Our central hypothesis is that the dendritic plateaus lead to a prepared neuronal state that favors spike generation, which results in the emergence of the embedded ensemble of prepared cells (Fig. 4, yellow color). Dendritic plateau potentials and the resulting plateau depolarizations of the cell body (Prepared state) produce notable changes in the neuronal electrical behavior (Fig. 2D), which are poised to spill over into the network dynamics, and are therefore too important to be left out. It would be interesting to examine in computational models, dynamic features of ongoing network activity that are gained when model pyramidal neurons, members of the large-scale network, are provided with the ability to produce dendritic plateau potentials (Antic et al., 2007; Plotkin et al., 2011; Gambino et al., 2014; Bittner et al., 2015). We hypothesize that “embedded ensemble encoding” (Fig. 4C4) may be the pivotal organizing principle in cortical networks of neurons.
Acknowledgments
We are grateful to Salvador Dura and other members of the Antic, Hines and Lytton laboratories for critical comments.
Support information:
This work was supported by NIH grants R01MH063503, U01MH109091, R01NS11613 and R01EB022903.
Footnotes
Conflict of Statement
The authors declare no conflicts of interest.
References
- Agmon-Snir H, Carr CE, Rinzel J. The role of dendrites in auditory coincidence detection. Nature. 1998;393:268–272. doi: 10.1038/30505. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature. 2000;406:302–306. doi: 10.1038/35018568. [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, Traub RD, Kopell NJ, Whittington MA. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron. 2012;75:572–583. doi: 10.1016/j.neuron.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Allen WE, Kauvar IV, Chen MZ, Richman EB, Yang SJ, Chan K, Gradinaru V, Deverman BE, Luo L, Deisseroth K. Global Representations of Goal-Directed Behavior in Distinct Cell Types of Mouse Neocortex. Neuron. 2017;94:891–907 e896. doi: 10.1016/j.neuron.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic SD, Empson RM, Knopfel T. Voltage imaging to understand connections and functions of neuronal circuits. J Neurophysiol. 2016;116:135–152. doi: 10.1152/jn.00226.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic SD, Acker CD, Zhou WL, Moore AR, Milojkovic BA. The Role of Dendrites in the Maintenance of the UP State. In: Timofeev I, editor. Mechanisms of spontaneous active states in the neocortex. Kerala, India: Research Signpost; 2007. pp. 45–72. [Google Scholar]
- Antic SD, Zhou WL, Moore AR, Short SM, Ikonomu KD. The decade of the dendritic NMDA spike. J Neurosci Res. 2010;88:2991–3001. doi: 10.1002/jnr.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariav G, Polsky A, Schiller J. Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons. J Neurosci. 2003;23:7750–7758. doi: 10.1523/JNEUROSCI.23-21-07750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn Sci. 2002;6:47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- Bair W. Spike timing in the mammalian visual system. Curr Opin Neurobiol. 1999;9:447–453. doi: 10.1016/S0959-4388(99)80067-1. [DOI] [PubMed] [Google Scholar]
- Bartram J, Kahn MC, Tuohy S, Paulsen O, Wilson T, Mann EO. Cortical Up states induce the selective weakening of subthreshold synaptic inputs. Nat Commun. 2017;8:665. doi: 10.1038/s41467-017-00748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci. 2015;18:1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono J, Clopath C. Modeling somatic and dendritic spike mediated plasticity at the single neuron and network level. Nat Commun. 2017;8:706. doi: 10.1038/s41467-017-00740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga RM, Sharp DJ, Leeson C, Wise RJ, Leech R. Echoes of the brain within default mode, association, and heteromodal cortices. J Neurosci. 2013;33:14031–14039. doi: 10.1523/JNEUROSCI.0570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Hausser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron. 2011;69:885–892. doi: 10.1016/j.neuron.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandalise F, Carta S, Helmchen F, Lisman J, Gerber U. Dendritic NMDA spikes are necessary for timing-dependent associative LTP in CA3 pyramidal cells. Nat Commun. 2016;7:13480. doi: 10.1038/ncomms13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol. 2002;543:49–70. doi: 10.1113/jphysiol.2002.018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Singer W, Engel AK. Correlation analysis of corticotectal interactions in the cat visual system. J Neurophysiol. 1998;79:2394–2407. doi: 10.1152/jn.1998.79.5.2394. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Silva FL. High frequency oscillations in the intact brain. Prog Neurobiol. 2012;98:241–249. doi: 10.1016/j.pneurobio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Yuste R. Input summation by cultured pyramidal neurons is linear and position-independent. Journal of Neuroscience. 1998;18:10–15. doi: 10.1523/JNEUROSCI.18-01-00010.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller L. Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav Brain Res. 1995;71:163–170. doi: 10.1016/0166-4328(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. Glutamate spillover promotes the generation of NMDA spikes. J Neurosci. 2011;31:16435–16446. doi: 10.1523/JNEUROSCI.2777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Rochefort NL, Sakmann B, Konnerth A. Reactivation of the same synapses during spontaneous up states and sensory stimuli. Cell Rep. 2013;4:31–39. doi: 10.1016/j.celrep.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Cichon J, Gan WB. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015;520:180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford CW, Mareschal I, Otsuka Y, Watson TL. A Bayesian approach to person perception. Conscious Cogn. 2015;36:406–413. doi: 10.1016/j.concog.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Compte A, Constantinidis C, Tegner J, Raghavachari S, Chafee MV, Goldman-Rakic PS, Wang XJ. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. Journal of Neurophysiology. 2003;90:3441–3454. doi: 10.1152/jn.00949.2002. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ Error: Emotion, Reason, and the Human Brain. New York: Putnam Publishing; 1994. [Google Scholar]
- Dayan P, Feller M, Feldman D. Networks, circuits and computation. Curr Opin Neurobiol. 2011;21:661–663. doi: 10.1016/j.conb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- DeBello WM, McBride TJ, Nichols GS, Pannoni KE, Sanculi D, Totten DJ. Input clustering and the microscale structure of local circuits. Front Neural Circuits. 2014;8:112. doi: 10.3389/fncir.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dura-Bernal S, Wennekers T, Denham SL. Top-down feedback in an HMAX-like cortical model of object perception based on hierarchical Bayesian networks and belief propagation. PLoS One. 2012;7:e48216. doi: 10.1371/journal.pone.0048216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature Neuroscience. 2000;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Thinking about brain cell assemblies. Science. 1993;261:993–994. doi: 10.1126/science.8351525. [DOI] [PubMed] [Google Scholar]
- Elston GN. Cortex, cognition and the cell: New insights into the pyramidal neuron and prefrontal function. Cerebral Cortex. 2003;13:1124–1138. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Friston K. Hierarchical models in the brain. PLoS Comput Biol. 2008;4:e1000211. doi: 10.1371/journal.pcbi.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino F, Pages S, Kehayas V, Baptista D, Tatti R, Carleton A, Holtmaat A. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature. 2014;515:116–119. doi: 10.1038/nature13664. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Migliore M, Magee JC. On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J Neurosci. 2004;24:11046–11056. doi: 10.1523/JNEUROSCI.2520-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Functional organization of the visual cortex. Progress in Brain Research. 1983a;58:209–218. doi: 10.1016/S0079-6123(08)60022-9. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Clustered intrinsic connections in cat visual cortex. Journal of Neuroscience. 1983b;3:1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gordon U, Polsky A, Schiller J. Plasticity compartments in basal dendrites of neocortical pyramidal neurons. J Neurosci. 2006;26:12717–12726. doi: 10.1523/JNEUROSCI.3502-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene MR, Oliva A. The briefest of glances: the time course of natural scene understanding. Psychol Sci. 2009;20:464–472. doi: 10.1111/j.1467-9280.2009.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Grundemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, Luthi A, Schnitzer MJ. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543:670–675. doi: 10.1038/nature21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Chen X, Konnerth A. Dendritic function in vivo. Trends Neurosci. 2015;38:45–54. doi: 10.1016/j.tins.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron. 2015;86:106–139. doi: 10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organisation of Behaviour: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Hill DN, Varga Z, Jia H, Sakmann B, Konnerth A. Multibranch activity in basal and tuft dendrites during firing of layer 5 cortical neurons in vivo. Proc Natl Acad Sci U S A. 2013;110:13618–13623. doi: 10.1073/pnas.1312599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. 1952 [classical article]. [Review] [16 refs] Bulletin of Mathematical Biology. 1990;52:25–71. doi: 10.1007/BF02459568. [DOI] [PubMed] [Google Scholar]
- Jadi M, Behabadi BF, Poleg-Polsky A, Schiller J, Mel BW. An augmented 2-layer model captures nonlinear analog spatial integration effects in pyramidal neuron dendrites. Conf Proc IEEE Eng Med Biol Soc. 2014 doi: 10.1109/JPROC.2014.2312671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Prichep LS, Kox W, Valdes-Sosa P, Bosch-Bayard J, Aubert E, Tom M, di Michele F, Gugino LD. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10:165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Kohler S, Frankland PW. Finding the engram. Nat Rev Neurosci. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- Kastellakis G, Cai DJ, Mednick SC, Silva AJ, Poirazi P. Synaptic clustering within dendrites: an emerging theory of memory formation. Prog Neurobiol. 2015;126:19–35. doi: 10.1016/j.pneurobio.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72:1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Koch C, Segev I. The role of single neurons in information processing. Nature Neuroscience. 2000;3:1171–1177. doi: 10.1038/81444. [DOI] [PubMed] [Google Scholar]
- Koch C, Poggio T, Torre V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc Natl Acad Sci U S A. 2004;101:15482–15487. doi: 10.1073/pnas.0406343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Larkman AU. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: III. Spine distributions. Journal of Comparative Neurology. 1991;306:332–343. doi: 10.1002/cne.903060209. [DOI] [PubMed] [Google Scholar]
- Larkum M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T. Synaptic clustering by dendritic signalling mechanisms. Curr Opin Neurobiol. 2008;18:321–331. doi: 10.1016/j.conb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science. 2009;325:756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature. 2012;490:397–401. doi: 10.1038/nature11451. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kreitzer AC, Singer AC, Schiff ND. Illuminating Neural Circuits: From Molecules to MRI. J Neurosci. 2017;37:10817–10825. doi: 10.1523/JNEUROSCI.2569-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legenstein R, Maass W. Branch-specific plasticity enables self-organization of nonlinear computation in single neurons. J Neurosci. 2017;31:10787–10802. doi: 10.1523/JNEUROSCI.5684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Fellous JM, Wang XJ. A Role For Nmda-Receptor Channels in Working Memory. Nature Neuroscience. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- Llinas R, Ribary U. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann N Y Acad Sci. 2001;929:166–175. [PubMed] [Google Scholar]
- London M, Schreibman A, Hausser M, Larkum ME, Segev I. The information efficacy of a synapse. Nature Neuroscience. 2002;5:332–340. doi: 10.1038/nn826. [DOI] [PubMed] [Google Scholar]
- Magee JC. Observations on clustered synaptic plasticity and highly structured input patterns. Neuron. 2011;72:887–888. doi: 10.1016/j.neuron.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72:1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso-Nanclares L, Antille N, Arsever S, Kahou GA, Berger TK, Bilgili A, Buncic N, Chalimourda A, Chindemi G, Courcol JD, Delalondre F, Delattre V, Druckmann S, Dumusc R, Dynes J, Eilemann S, Gal E, Gevaert ME, Ghobril JP, Gidon A, Graham JW, Gupta A, Haenel V, Hay E, Heinis T, Hernando JB, Hines M, Kanari L, Keller D, Kenyon J, Khazen G, Kim Y, King JG, Kisvarday Z, Kumbhar P, Lasserre S, Le Be JV, Magalhaes BR, Merchan-Perez A, Meystre J, Morrice BR, Muller J, Munoz-Cespedes A, Muralidhar S, Muthurasa K, Nachbaur D, Newton TH, Nolte M, Ovcharenko A, Palacios J, Pastor L, Perin R, Ranjan R, Riachi I, Rodriguez JR, Riquelme JL, Rossert C, Sfyrakis K, Shi Y, Shillcock JC, Silberberg G, Silva R, Tauheed F, Telefont M, Toledo-Rodriguez M, Trankler T, Van Geit W, Diaz JV, Walker R, Wang Y, Zaninetta SM, DeFelipe J, Hill SL, Segev I, Schurmann F. Reconstruction and Simulation of Neocortical Microcircuitry. Cell. 2015;163:456–492. doi: 10.1016/j.cell.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McBride TJ, DeBello WM. Input clustering in the normal and learned circuits of adult barn owls. Neurobiol Learn Mem. 2015;121:39–51. doi: 10.1016/j.nlm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Shu YS, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: Mechanisms of generation and effects on neuronal excitability. Cerebral Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- Mel BW. Synaptic integration in an excitable dendritic tree. Journal of Neurophysiology. 1993;70:1086–1101. doi: 10.1152/jn.1993.70.3.1086. [DOI] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Antic SD. A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J Neurosci. 2005a;25:3940–3951. doi: 10.1523/JNEUROSCI.5314-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojkovic BA, Zhou WL, Antic SD. Voltage and Calcium Transients in Basal Dendrites of the Rat Prefrontal Cortex. J Physiol. 2007;585:447–468. doi: 10.1113/jphysiol.2007.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Goldman-Rakic PS, Antic SD. Burst generation in rat pyramidal neurones by regenerative potentials elicited in a restricted part of the basilar dendritic tree. J Physiol. 2004;558:193–211. doi: 10.1113/jphysiol.2004.061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojkovic BA, Wuskell JP, Loew LM, Antic SD. Initiation of sodium spikelets in basal dendrites of neocortical pyramidal neurons. J Membr Biol. 2005b;208:155–169. doi: 10.1007/s00232-005-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittner M. Functional integration of large-scale brain networks. J Neurosci. 2013;33:18710–18711. doi: 10.1523/JNEUROSCI.4084-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Muir DR, Molina-Luna P, Roth MM, Helmchen F, Kampa BM. Specific excitatory connectivity for feature integration in mouse primary visual cortex. PLoS Comput Biol. 2017;13:e1005888. doi: 10.1371/journal.pcbi.1005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neymotin SA, Lee H, Park E, Fenton AA, Lytton WW. Emergence of physiological oscillation frequencies in a computer model of neocortex. Front Comput Neurosci. 2011;5:19. doi: 10.3389/fncom.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou KD, Short SM, Rich MT, Antic SD. Extrasynaptic glutamate receptor activation as cellular bases for dynamic range compression in pyramidal neurons. Front Physiol. 2012;3:334. doi: 10.3389/fphys.2012.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou KD, Singh MB, Sterjanaj EV, Antic SD. Spiny neurons of amygdala, striatum, and cortex use dendritic plateau potentials to detect network UP states. Front Cell Neurosci. 2014;8:292. doi: 10.3389/fncel.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Padival M, Quinette D, Rosenkranz JA. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38:1748–1762. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Shai AS, Reeve JE, Anderson HL, Paulsen O, Larkum ME. NMDA spikes enhance action potential generation during sensory input. Nat Neurosci. 2014;17:383–390. doi: 10.1038/nn.3646. [DOI] [PubMed] [Google Scholar]
- Palmer SE, Marre O, Berry MJ, 2nd, Bialek W. Predictive information in a sensory population. Proc Natl Acad Sci U S A. 2015;112:6908–6913. doi: 10.1073/pnas.1506855112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson ME, Fransen E. Long-lasting small-amplitude TRP-mediated dendritic depolarizations in CA1 pyramidal neurons are intrinsically stable and originate from distal tuft regions. Eur J Neurosci. 2012;36:2917–2925. doi: 10.1111/j.1460-9568.2012.08199.x. [DOI] [PubMed] [Google Scholar]
- Pinchas M, Baranes D. Dendritic branch intersections are structurally regulated targets for efficient axonal wiring and synaptic clustering. PLoS One. 2013;8:e82083. doi: 10.1371/journal.pone.0082083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotsis DA, Brincat SL, Miller EK. On memories, neural ensembles and mental flexibility. Neuroimage. 2017;157:297–313. doi: 10.1016/j.neuroimage.2017.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JL, Day M, Surmeier DJ. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat Neurosci. 2011;14:881–888. doi: 10.1038/nn.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Brannon T, Mel BW. Pyramidal neuron as two-layer neural network. Neuron. 2003;37:989–999. doi: 10.1016/s0896-6273(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Polsky A, Mel BW, Schiller J. Computational subunits in thin dendrites of pyramidal cells. Nat Neurosci. 2004;7:621–627. doi: 10.1038/nn1253. [DOI] [PubMed] [Google Scholar]
- Polsky A, Mel B, Schiller J. Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J Neurosci. 2009;29:11891–11903. doi: 10.1523/JNEUROSCI.5250-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Harrison BJ, Ortiz H, Deus J, Soriano-Mas C, Lopez-Sola M, Yucel M, Perich X, Cardoner N. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychol Med. 2009;39:1177–1187. doi: 10.1017/S003329170800500X. [DOI] [PubMed] [Google Scholar]
- Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968;31:884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models to experimental data. Physiological Reviews. 1992;72:S159–186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Ro T, Breitmeyer B, Burton P, Singhal NS, Lane D. Feedback contributions to visual awareness in human occipital cortex. Curr Biol. 2003;13:1038–1041. doi: 10.1016/s0960-9822(03)00337-3. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff DB, Zagha E, Yuzgec O, McCormick DA. Synaptic Mechanisms of Tight Spike Synchrony at Gamma Frequency in Cerebral Cortex. J Neurosci. 2015;35:10236–10251. doi: 10.1523/JNEUROSCI.0828-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Toleikyte G, Aitchison L, Roth A, Clark BA, Branco T, Hausser M. Active dendritic integration as a mechanism for robust and precise grid cell firing. Nat Neurosci. 2017;20:1114–1121. doi: 10.1038/nn.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman E, Freedman B, Segev I. Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Computation. 1998;10:1679–1703. doi: 10.1162/089976698300017089. [DOI] [PubMed] [Google Scholar]
- Seibt J, Richard CJ, Sigl-Glockner J, Takahashi N, Kaplan DI, Doron G, de Limoges D, Bocklisch C, Larkum ME. Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents. Nat Commun. 2017;8:684. doi: 10.1038/s41467-017-00735-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai AS, Koch C, Anastassiou CA. Spike-timing control by dendritic plateau potentials in the presence of synaptic barrages. Front Comput Neurosci. 2014;8:89. doi: 10.3389/fncom.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield ME, Dombeck DA. Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature. 2015;517:200–204. doi: 10.1038/nature13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Zeki S. The functional organization of area V2, I: specialization across stripes and layers. Vis Neurosci. 2002;19:187–210. doi: 10.1017/s0952523802191164. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Smith SL, Smith IT, Branco T, Hausser M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature. 2013;503:115–120. doi: 10.1038/nature12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky H. Computational neuroscience: beyond the local circuit. Curr Opin Neurobiol. 2014;25:xiii–xviii. doi: 10.1016/j.conb.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of Neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kodama S, Hoshino C, Izumi T, Miyakawa H. A plateau potential mediated by the activation of extrasynaptic NMDA receptors in rat hippocampal CA1 pyramidal neurons. Eur J Neurosci. 2008;28:521–534. doi: 10.1111/j.1460-9568.2008.06324.x. [DOI] [PubMed] [Google Scholar]
- Tao C, Zhang G, Xiong Y, Zhou Y. Functional dissection of synaptic circuits: in vivo patch-clamp recording in neuroscience. Front Neural Circuits. 2015;9:23. doi: 10.3389/fncir.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. Reentry and the problem of integrating multiple cortical areas: simulation of dynamic integration in the visual system. Cereb Cortex. 1992;2:310–335. doi: 10.1093/cercor/2.4.310. [DOI] [PubMed] [Google Scholar]
- Tran-Van-Minh A, Caze RD, Abrahamsson T, Cathala L, Gutkin BS, DiGregorio DA. Contribution of sublinear and supralinear dendritic integration to neuronal computations. Front Cell Neurosci. 2015;9:67. doi: 10.3389/fncel.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujfalussy BB, Makara JK, Branco T, Lengyel M. Dendritic nonlinearities are tuned for efficient spike-based computations in cortical circuits. Elife. 2017;4 doi: 10.7554/eLife.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits AL, Morrie RD, Tran-Van-Minh A, Bleckert A, Gainer CF, DiGregorio DA, Feller MB. A Role for Synaptic Input Distribution in a Dendritic Computation of Motion Direction in the Retina. Neuron. 2016;89:1317–1330. doi: 10.1016/j.neuron.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Rajan K, Abbott LF. Neural network dynamics. Annu Rev Neurosci. 2005;28:357–376. doi: 10.1146/annurev.neuro.28.061604.135637. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave sleep. J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Up and down states. Scholarpedia. 2008;3:1410. doi: 10.4249/scholarpedia.1410. revision #91903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. Journal of Neuroscience. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wu CT, Crouzet SM, Thorpe SJ, Fabre-Thorpe M. At 120 msec you can spot the animal but you don’t yet know it’s a dog. J Cogn Neurosci. 2015;27:141–149. doi: 10.1162/jocn_a_00701. [DOI] [PubMed] [Google Scholar]
- Yu J, Ferster D. Membrane potential synchrony in primary visual cortex during sensory stimulation. Neuron. 2010;68:1187–1201. doi: 10.1016/j.neuron.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cudmore RH, Lin DT, Linden DJ, Huganir RL. Visualization of NMDA receptor-dependent AMPA receptor synaptic plasticity in vivo. Nat Neurosci. 2015;18:402–407. doi: 10.1038/nn.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cordeiro Matos S, Jego S, Adamantidis A, Seguela P. Norepinephrine drives persistent activity in prefrontal cortex via synergistic alpha1 and alpha2 adrenoceptors. PLoS One. 2013;8:e66122. doi: 10.1371/journal.pone.0066122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei M, Peira N, Persson J. Brain systems underlying attentional control and emotional distraction during working memory encoding. Neuroimage. 2013;87C:276–286. doi: 10.1016/j.neuroimage.2013.10.048. [DOI] [PubMed] [Google Scholar]


