Abstract
Hepatitis C virus (HCV) is highly prevalent in incarcerated populations. The high cost of HCV therapy places a major burden on correctional system healthcare budgets, but the burden of untreated HCV is not known. We investigated the economic impact of HCV through comparison of length of stay (LOS), frequency of 30-day readmission, and costs of hospitalizations in inmates with and without HCV using a 2004–2014 administrative claims database. Inmates with HCV had longer LOS, higher frequency of 30-day readmission, and increased cost of hospitalizations. Costs were higher in inmates with HCV even without advanced liver disease and in inmates with HIV/HCV compared to HCV alone. We conclude that although HCV treatment may not avert all of the observed increases in hospitalization, modest reductions in hospital utilization with HCV cure could help offset treatment costs. Policy discussions on HCV treatment in corrections should be informed by the costs of untreated HCV infection.
Keywords: Inmate, Hepatitis C, Readmission, HIV, Hospitalization, Cost
Introduction
The approval of all oral HCV treatment regimens transformed HCV infection from a chronic disease to a readily curable condition [1–3]. HCV infection in correctional settings is highly prevalent (18–83%), and HCV-related liver disease is one of the leading causes of mortality [4–7]. Treating all people with chronic HCV infection is recommended, with a heightened priority placed on treatment of people with HIV/HCV co-infection and people with advanced liver disease [3]. Despite these recommendations, a national survey of prison administrators found that < 1% of inmates with HCV in the USA have received treatment [8]. Untreated, incarcerated people with HCV provide a viral reservoir for continued expansion of the HCV epidemic—an unaddressed public health crisis [9].
Expanded HCV treatment in corrections may provide cost savings over time, but up-front increases in prison budgets are necessary [10, 11]. Given the high prevalence of HCV in prisons and the high cost of therapy (US $24,000 to $80,000 per course), treating every HCV-infected inmate could threaten the solvency of correctional system pharmacy budgets. Medical care in corrections is frequent provided through a public-private partnership, with medical services contracted to private companies with annual capitated per-inmate coverage [12]. Massachusetts is a state that contracts the majority of medical services to private correctional healthcare companies, and there is concern that broadened HCV treatment would irreparably disrupt the budget. As an example, treating all HCV-positive inmates in Rhode Island (a state with similar HCV prevalence) would require $34 million, twice the overall correctional health budget and13 times the corrections pharmacy budget [13].
Budget projections may overestimate cost of HCV treatment by not including cost savings from HCV cure, including avoidance of complications of end-stage liver disease including hepatocellular carcinoma (HCC), extra-hepatic manifestations of HCV, and illnesses like diabetes and cardiovascular disease exacerbated by untreated HCV [14, 15]. While the costs of HCV therapy are immediate and clear, the costs of managing untreated HCV infection are poorly understood. Using an administrative claims database from the primary inpatient facility serving inmates in Massachusetts, we investigated differences in length of stay, frequency of 30-day readmission, and costs of hospitalizations between inmates with and without HCV.
Materials and Methods
Database
With approval from the Massachusetts Department of Public Health IRB, we accessed a de-identified database of all ≥ 18-year-old inmate admissions occurring between 2004 and 2014 at Lemuel Shattuck Hospital (LSH)—the preferred inpatient healthcare location for all incarcerated populations in MA. Variables included race, ethnicity, age, gender, month/year of admissions and discharges, length of stay (LOS), disposition after hospitalization, and all International Classification of Disease, 9th Edition (ICD-9 codes), associated with the initial admission classified as primary, secondary, or other. Elixhauser ICD-9-CM (ECM) index, a score calculated with points for the presence of 31 comorbidities frequently used as a risk-adjustment measure, was calculated for all admissions [16]. HCC is included within the comorbidity index (ICD-9 code 155.0), but given relationship to HCV, we analyzed it separately as well. The cost of hospitalization was estimated through DRG code in the database [17, 18]. We translated the DRG into a US dollar monetary variable based on the Health Care Cost and Utilization Project (HCUP) Cost to Charge Ratio Files for 2013 [19].
Comparison Groups
The primary comparison group was hospitalized inmates with and without HCV. Similar to previous studies [20–22], the presence of HCV was based on the existence of ICD-9 codes for HCV on initial admission: 070.41, 070.44, 070.51, 070.54, 070.70, and 070.71. The ICD-9 code for HCV could be present as primary, secondary, or other diagnosis.
We conducted two subgroup analyses to further investigate disease associations with increase hospital utilization. Interested in the potential costs of HCV in the absence of liver disease, subgroup A excluded all people from the database who had an ICD-9 code for advanced liver (defined by ECM category “Chronic Liver Disease”) [16]. Recognizing that HIV accelerates liver disease progression in people with HCV [23], subgroup B is a comparison of hospital utilization between HIV/HCV co-infection (classified as the ICD-9 code 042 in addition to a HCV ICD-9 code) and HCV mono-infection.
Outcomes of Interest
Length of stay: Days hospitalized during index hospitalization (first hospitalization during the 2004–2014 time period).
30-day readmission: This outcome was chosen because (A) it is a metric used to assess quality of health care following hospitalization for several illneses including cirrhosis [24, 25] and (B) since our database only had hospitalizations when the patient was incarcerated, limiting the outcome to 30-day readmission decreased the possibility of missing readmissions of inmates who were released post-hospitalization and hospitalized as a non-inmate.
Cost of hospitalization episode: Sum cost of the index hospitalization and any readmissions that happen within 30 days of discharge from the initial admission.
Statistical Analysis
We described continuous variables using the mean and standard deviation or median and interquartile range and tested differences between groups using a t test or the Wilcoxon rank-sum test as appropriate. Variables were checked for skewness, and if skewed, a dichotomous variable around the median was used.
We constructed a multivariable logistic regression models of the odds of 30-day readmission following index admission using a step-wise forward elimination process. The final model included variables with variance inflation factor of less than 6.0 and an overall p value of less than 0.25 in the step-wise regression.
In crude cost comparisons, mean cost was used for our analysis because it allows scaling individual costs to population [26]. In line with recommendations from health economists and literature, the negative binomial model was used to examine relationship between exposure of HCV and outcome of cost of hospitalization episode [26–28]. The output of the negative binomial regression is an incidence ratio, interpreted as an X% increase in cost. Any covariate with a p value of less than 0.05 was included in the multivariable model. Inmates who died during their initial hospitalization were included in the descriptive analysis and the financial analysis, but excluded from the readmission analysis because they could not be readmitted.
Software
All analyses were performed with SAS software 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
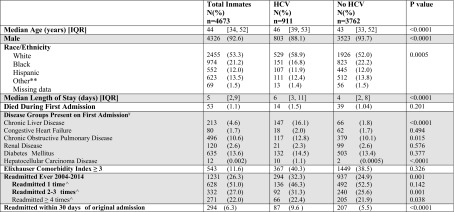
The data set included 4673 index admissions. The average number of total admissions each year was 457 (370–683, standard deviation 102). Fifty-three patients (1.1%) died during index admissions. There were 354 readmissions within 30 days in 294 patients. About one-fifth (19.5%) of the cohort had HCV, and 2.5% had both HIV and HCV. Patients with HCV were older, more likely to be White, and more likely to have chronic liver disease, and had longer LOS (6 vs. 4 days, p < 0.0001; Table 1). Almost twice as many patients with HCV were readmitted within 30 days compared to inmates without HCV (9.6 vs. 5.5%, p < 0.0001). Of the 87 people with HCV who were readmitted within 30 days, 26% had HCV which was listed as the primary/secondary reason for readmission, 16% had liver disease, and 1% had HCC. In the 207 people without HCV who were readmitted within 30 days, the primary reason for admission was liver disease in 5 people (2%), and no one was readmitted for HCC.
Table 1.
Demographic and hospitalization-related characteristics of inmates from initial admission to Lemuel Shattuck Hospital 2004–2014
HCV hepatitis C virus, IQR interquartile range
**other race includes Asian, Pacific Islanders, Native American
ѰICD-9 code classification of disease groups based on the categories designated using the Elixhauser Comorbidity Index
&ECM co-morbidity index includes primary, secondary, and other diagnoses
^Denominator for percentage is people ever readmitted
In multivariable modeling, HCV infection remained associated with a 73% increase in the odds of 30-day readmission when compared to patients without HCV infection (adjusted odds ratio (AOR) 1.73, 95% CI [1.31, 2.29]; Table 2). In subgroup A (no liver disease), HCV was still associated with a 72% increased risk of 30-day readmission (AOR 1.72, 95% CI [1.29, 2.29], p < 0.001, data not shown). In subgroup B (only inmates with HCV), HIV/HCV co-infection was not associated with increased risk of 30-day readmission (AOR 1.15, 95% CI [0.60, 2.19]), p = 0.67, data not shown).
Table 2.
Demographic and disease-related factors related to outcomes of (1) 30-day readmission and (2) cost of hospitalization episode
| Logistic regression: 30-day readmission (n = 4620&) | Negative binomial regression: cost of hospitalization episode (n = 4673) | ||||
|---|---|---|---|---|---|
| Crude odds ratio | Adjusted odds ratio | Crude incidence ratio | Adjusted incidence ratio | ||
| HCV | No | REF | REF | REF | REF |
| Yes | 1.84 (1.42, 2.40) | 1.73 (1.31, 2.29) | 1.18 (1.14, 1.23) | 1.06 (1.02, 1.10) | |
| Sex | Female | REF | REF | REF | -- |
| Male | 0.95 (0.61, 1.47) | -- | 0.98 (0.93, 1.04) | -- | |
| LOS | ≤ 4 | REF | REF | REF | REF |
| > 4 | 1.45 (1.14, 1.84) | 1.36 (1.07, 1.73) | 1.25 (1.21, 1.29) | 1.19 (1.16, 1.22) | |
| Age | < 30 years | REF | REF | REF | REF |
| 30–39 | 0.69 (0.45, 1.06) | 0.66 (0.43, 1.02) | 0.96 (0.91, 1.00) | 0.97 (0.93, 1.01) | |
| 40–49 | 0.95 (0.65, 1.39) | 0.82 (0.56, 1.21) | 1.03 (0.98, 1.08) | 1.01 (0.97, 1.05) | |
| 50–59 | 1.48 (1.01, 2.17) | 1.19 (0.81, 1.77) | 1.07 (1.02, 1.13) | 1.02 (0.98, 1.07) | |
| 60–69 | 1.29 (0.79, 2.10) | 1.07 (0.65, 1.77) | 1.15 (1.08, 1.23) | 1.14 (1.08, 1.21) | |
| 70 + | 2.91 (1.64, 5.18) | 2.66 (1.48, 4.78) | 1.09 (0.99, 1.20) | 0.99 (0.91, 1.08) | |
| Race | White | REF | REF | REF | REF |
| Non-White** | 0.68 (0.53, 0.86) | 0.77 (0.60, 0.99) | 1.03 (1.00, 1.07) | 0.99 (0.97, 1.02) | |
| Liver diseaseѰ | No | REF | REF | REF | REF |
| Yes | 2.53 (1.53, 4.17) | 2.05 (1.21, 3.48) | 1.25 (1.15, 1.37) | 1.06 (0.98, 1.15) | |
| HIV/AIDSѰ | No | REF | -- | REF | REF |
| Yes | 1.12 (0.64, 1.96) | -- | 1.30 (1.21, 1.40) | 1.20 (1.12, 1.28) | |
| Modified ECM∞ | None | REF | REF | REF | REF |
| 1 or 2 | 1.24 (0.98, 1.57) | 1.33 (1.04, 1.71) | 0.89 (0.86, 0.92) | 0.88 (0.86, 0.91) | |
| 3 or 4 | 2.06 (0.26, 16.54) | 1.76 (0.21, 14.50) | 0.78 (0.55, 1.09) | 0.77 (0.58, 1.04) | |
| 30-day readmission | No | Not applicable | REF | REF | |
| Yes | 2.40 (2.28, 2.54) | 2.35 (2.22, 2.48) | |||
&53 people died during first admission, removed from analysis
**Non-white ethnicity includes African American, Hispanic, Asian, Pacific Islanders, Native American, and other races not defined as White
∞Modified Elixhauser Comorbidities exclude advanced liver disease and HIV/AIDS from the sum variable; both are included individually in the model
“--” indicates that a predictor was excluded from the adjusted model due to p value of 0.25 in the crude model
The mean cost of hospitalization episode was 18% higher in patients with HCV than without ($10,969 vs. $9264). Costs were 17% higher in people with HCV even in the absence of advanced liver disease ($10,794 vs. $9240), and costs for HIV/HCV hospitalization episodes were more than those for HCV mono-infection episodes ($12,634 vs. $10,721). HCV was associated with 6% increased cost per hospitalization episode (incidence rate ratio = 1.06; 95% CI (1.02, 1.10)). Increased LOS, increased age, and non-White ethnicity were also associated with increased cost.
Discussion
Increasing access to curative HCV treatment in corrections is necessary to quell the national epidemic and improve public health. We demonstrate two important findings that should inform correctional health policy. First, untreated HCV infection is associated with increased healthcare utilization in inmates. There is a substantial cost to deferring HCV treatment even in patients with early disease, due to progression to cirrhosis, complications of decompensated liver disease, and other diseases accelerated by chronic HCV infection [15, 29]. Our findings add to the current literature by supporting that incarcerated populations with earlier stage HCV, often thought to have a “silent infection,” have considerable healthcare needs with associated health care costs [30].
Second, the current high costs of HCV treatment make expanded treatment financially difficult, even with proposed healthcare cost savings. From our data, in the average year, there were 457 admissions at the hospital, and about 20% (~91) of admissions were in inmates with HCV. As HCV was associated with an additional $1705 per hospitalization episode, the additional cost of untreated HCV in the average year (91 × $1705) is approximately $155,000. This is essentially the cost of three courses of HCV treatment at current prices. HCV treatment costs are forecasted to drop with increased market competition, but the costs are unlikely to drop enough to fit into current corrections’ budgets. There needs to be an innovative approach to securing appropriations for HCV treatment, potentially through negotiating discounted costs with pharmaceutical companies, or developing criteria for prioritization of inmates for HCV treatment. In states like Massachusetts where Medicaid covers treatment for HCV in the community, programs that link inmates with short periods of incarceration to HCV treatment after release may also be a prudent investment.
Administrative billing codes are frequently used in HCV-hospital utilization analyses [20]; however, reliance on billing codes is a limitation of this study. The presence of HCV by ICD-9 code may represent serologic evidence of HCV without active HCV, which can happen through spontaneous clearance of the virus in about 20% of people [31]. The absence of an HCV ICD-9 code may reflect HCV-negative status, undiagnosed infection or unreported infection. There is no ICD-9 code for liver fibrosis, preventing our ability to describe a potential gradient of impact between HCV without liver damage, HCV with fibrosis, and HCV with cirrhosis.
Analysis of data in 2013 US dollars may slightly increase the costs; however, overall, our analysis likely underestimates the costs of healthcare related to HCV infection in the prison population. One fourth of inmates were readmitted 4 or more times, and we did not include the costs of any admissions that occurred outside of 30 days in the cost analysis. If inmates are critically ill while in prison, they could be sent to a tertiary care facility for evaluation and stabilization prior to transfer to the Lemuel Shattuck Hospital or transfer back to the facility; the costs of their care at other hospitals are not captured with this analysis. In our study, we found that hospital utilization in the HCV group (50% increased length of stay, 70% increase in risk of readmission) was increased more than were measurements of cost (6% increase in multivariable analysis). The incongruity between hospital utilization and cost has been reported in other studies [32] and is likely related to limitations of using DRGs to represent cost. DRGs are highly dependent on physician documentation, susceptible to coding and classification errors, and better used to categorize patients into cost groups than estimated costs per patient [33, 34]. We based our costs analysis with the robust literature using DRGs to estimate costs of care for community patients with HCV [20–22, 35], but hospital utilization may be a better estimate of the increased financial burden related to HCV infection.
Conclusion
Appreciation of increased healthcare resource utilization among HCV-infected people in the corrections setting is critical to making sound policy and informing adequate medical care. Addressing HCV in correctional settings will require political will to prioritize ending the HCV epidemic by additional investment in correctional health.
Acknowledgements
Thank you to the staff of the Lemuel Shattuck Hospital for their support of this study.
Funding
This work was supported by NIH CTSA [UL1TR001064], NIH Training Grant [T32 AI055412-10], CFAR Grant Lifespan/Tufts/Brown Center for AIDS Research (P30 AI042853), Bristol-Myers Squibb Fellow’s Virology Grant, CHERISH (Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV) and NIDA (R25 DA037190-01).
References
- 1.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312(6):631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 2.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AASLD and IDSA, HCV Guidance: recommendations for Testing, Managing and Treating Hepatitis C. www.hcvguidelines.org. Accessed 2 Jan 2018.
- 4.Harzke AJ, Baillargeon JG, Kelley MF, Diamond PM, Goodman KJ, Paar DP. HCV-related mortality among male prison inmates in Texas, 1994-2003. Ann Epidemiol. 2009;19(8):582–589. doi: 10.1016/j.annepidem.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larney S, Mahowald MK, Scharff N, Flanigan TP, Beckwith CG, Zaller ND. Epidemiology of hepatitis C virus in Pennsylvania state prisons, 2004-2012: limitations of 1945-1965 birth cohort screening in correctional settings. Am J Public Health. 2014;104(6):e69–e74. doi: 10.2105/AJPH.2014.301943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varan AK, Mercer DW, Stein MS, Spaulding AC. Hepatitis C seroprevalence among prison inmates since 2001: still high but declining. Public Health Rep. 2014;129(2):187–195. doi: 10.1177/003335491412900213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenbachler BT, Smith BD, Seña AC, et al. Hepatitis C Virus Testing and Linkage to Care in North Carolina and South Carolina Jails, 2012-2014. Public Health Rep. 2016;131(Suppl 2):98–104. doi: 10.1177/00333549161310S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckman AL, Bilinski A, Boyko R, Camp GM, Wall AT, Lim JK, Wang EA, Bruce RD, Gonsalves GS. New hepatitis C drugs are very costly and unavailable to many state prisoners. Health Aff (Millwood) 2016;35(10):1893–1901. doi: 10.1377/hlthaff.2016.0296. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding AC, Anderson EJ, Khan MA, Taborda-Vidarte CA, Phillips JA. HIV and HCV in U.S. prisons and jails: the correctional facility as a bellwether over time for the community’s infections. AIDS Rev. 2017;19(3):134–147. doi: 10.24875/AIDSRev.M17000006. [DOI] [PubMed] [Google Scholar]
- 10.He T, Li K, Roberts MS, et al. Prevention of hepatitis C by screening and treatment in U.S. prisons. Ann Intern Med. 2016;164(2):84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48(5):1387–1395. doi: 10.1002/hep.22509. [DOI] [PubMed] [Google Scholar]
- 12.Galik L, Gilroy L. Public-private partnerships in correctional health care. Washington, DC: Reason Foundation; 2014.
- 13.Nguyen JT, Rich JD, Brockmann BW, Vohr F, Spaulding A, Montague BT. A budget impact analysis of newly available hepatitis C therapeutics and the financial burden on a state correctional system. J Urban Health. 2015;92(4):635–649. doi: 10.1007/s11524-015-9953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepanova M, Younossi ZM. Economic burden of hepatitis C infection. Clin Liver Dis. 2017;21(3):579–594. doi: 10.1016/j.cld.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599–1608. doi: 10.1053/j.gastro.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services. Medicare Provider Utilization and Payment Data: inpatient. 2015. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient.html. Accessed September 28, 2015.
- 18.Heerey A, McGowan B, Ryan M, Barry M. Microcosting versus DRGs in the provision of cost estimates for use in pharmacoeconomic evaluation. Expert Rev Pharmacoecon Outcomes Res. 2002;2(1):29–33. doi: 10.1586/14737167.2.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Health Care Research and Quality R, MD. HCUP Cost-to-Charge Ratio Files (CCR). Rockville: Health Care Cost and Utilization Project (HCUP); 2006-2009.
- 20.Galbraith JW, Donnelly JP, Franco R, Overton T, Rodgers JB, Wang HE. National estimates of healthcare utilization by individuals with hepatitis C virus infection in the United States. Clin Infect Dis. 2014;59(6):755–764. doi: 10.1093/cid/ciu427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younossi ZM, Otgonsuren M, Henry L, Arsalla Z, Stepnaova M, Mishra A, Venkatesan C, Hunt S. Inpatient resource utilization, disease severity, mortality and insurance coverage for patients hospitalized for hepatitis C virus in the United States. J Viral Hepat. 2015;22(2):137–145. doi: 10.1111/jvh.12262. [DOI] [PubMed] [Google Scholar]
- 22.Xu F, Tong X, Leidner AJ. Hospitalizations and costs associated with hepatitis C and advanced liver disease continue to increase. Health Aff (Millwood) 2014;33(10):1728–1735. doi: 10.1377/hlthaff.2014.0096. [DOI] [PubMed] [Google Scholar]
- 23.López-Diéguez M, Montes ML, Pascual-Pareja J, Quereda C, von Wichmann M, Berenguer J, Tural C, Hernando A, González-García J, Serrano L, Arribas JR, GESIDA 37/03-FIPSE 36465/03-NEAT IG5 Study Group The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS. 2011;25(Journal Article):899–904. doi: 10.1097/QAD.0b013e3283454174. [DOI] [PubMed] [Google Scholar]
- 24.Services CfMM. 2015 measure information about the 30-day all-cause hospital readmission measure, calculated fort he value-based payment modifier program. Baltimore: Services CFNM; 2015. p. 1–26.
- 25.Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64(2):569–581. doi: 10.1002/hep.28558. [DOI] [PubMed] [Google Scholar]
- 26.Drummond M SM, Torrance GW, O’Brien B, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
- 27.Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916. doi: 10.1002/hec.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC, Ghali WA, Tu JV. A comparison of several regression models for analysing cost of CABG surgery. Stat Med. 2003;22(17):2799–2815. doi: 10.1002/sim.1442. [DOI] [PubMed] [Google Scholar]
- 29.Negro F, Forton D, Craxi A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149(6):1345–1360. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Sarbah SA, Younossi ZM. Hepatitis C: an update on the silent epidemic. J Clin Gastroenterol. 2000;30(2):125–143. doi: 10.1097/00004836-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Smith DJ, Jordan AE, Frank M, Hagan H. Spontaneous viral clearance of hepatitis C virus (HCV) infection among people who inject drugs (PWID) and HIV-positive men who have sex with men (HIV+ MSM): a systematic review and meta-analysis. BMC Infect Dis. 2016;16:471. doi: 10.1186/s12879-016-1807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreassen AES, Jacobsen CM, de Blasio B, White R, Kristiansen IS, Elstrøm P. The impact of methicillin-resistant S. aureus on length of stay, readmissions and costs: a register based case-control study of patients hospitalized in Norway. Antimicrob Resist Infect Control. 2017;6:74. doi: 10.1186/s13756-017-0232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel AD, Kavaler F. The debate over diagnosis related groups. J Community Health. 1985;10(2):81–92. doi: 10.1007/BF01326513. [DOI] [PubMed] [Google Scholar]
- 34.Sophy KG. Diagnosis related groups and the price of cost containment. J Contemp Health Law Policy. 1986;2:305–326. [PubMed] [Google Scholar]
- 35.Kieran JA, Norris S, O’Leary A, et al. Hepatitis C in the era of direct-acting antivirals: real-world costs of untreated chronic hepatitis C; a cross-sectional study. BMC Infect Dis. 2015;15:471. doi: 10.1186/s12879-015-1208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]