Abstract
Purpose
The current TNM (Tumor Node Metastasis) staging system is inadequate at identifying high-risk colorectal cancer (CRC) patients. Using a systematic and comprehensive-biomarker discovery and validation approach, we aimed to identify a miRNA-recurrence classifier (MRC) that can improve upon the current TNM-staging as well as superior to currently offered molecular assays.
Experimental Design
Three independent genome-wide miRNA-expression profiling datasets were used for biomarker discovery (N=158) and in-silico validation (N=109 and N=40) to identify a miRNA signature for predicting tumor recurrence in CRC patients. Subsequently, this signature was analytically trained and validated in retrospectively collected independent patient cohorts of fresh frozen (N=127, cohort 1) and FFPE (N=165, cohort 2 and N=139, cohort 3) specimens.
Results
We identified an 8-miRNA signature that significantly predicted recurrence free interval (RFI) in the discovery (p=0.002) and two independent publicly available datasets (p=0.00006 and p=0.002). The RT-PCR based validation in independent clinical cohorts revealed that MRC-derived high-risk patients succumb to significantly poor RFI in stage II and III CRC patients [cohort 1: HR: 3.44 (1.56–7.45), P=0.001, cohort 2: HR: 6.15 (3.33–11.35), P=0.001 and cohort 3: HR: 4.23 (2.26–7.92), P=0.0003]. In multivariate analyses, MRC emerged as an independent predictor of tumor recurrence, and achieved superior predictive accuracy than the currently available molecular assays. The RT-PCR based MRC risk score = (−0.1218×miR−744) + (−3.7142×miR-429) + (−2.2051×miR-362) + (3.0564×miR-200b) + (2.4997×miR-191) + (−0.0065×miR-30c2) + (2.2224×miR-30b) + (−1.1162×miR-33a).
Conclusions
This novel miRNA-recurrence classifier works superior to currently used clinicopathological features, as well as NCCN criteria, and works independent of adjuvant chemotherapy status in identifying high-risk stage II and III CRC patients. This can be readily deployed in clinical practice with FFPE specimens for decision making pending further model testing and validation.
Keywords: Colorectal cancer, recurrence, miRNA, adjuvant therapy, prognosis
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related mortality worldwide, with an estimated 49,190 deaths recorded in the United States alone in 2016(1). Survival in CRC patients is primarily associated with the tumor stage at diagnosis; as 5-year relative survival rates range from 65% for all stages and approximately 93.2% for stage I, 82.5% for stage II, 59.5% for stage III, and 8.1% for stage IV (2).
Post-surgery, 30% of stage II and 50–60% of stage III CRC patients develop a recurrence within 5-years (3). Although there is a general agreement that adjuvant chemotherapy in stage III CRC patients improves patient survival, (4–6) the use of such treatments in stage II cancers remains debatable due to lack of risk stratification for identifying true high-risk patients (7,8). Current NCCN (National Comprehensive Cancer Network) guidelines recommend adjuvant chemotherapy for patients with high-risk stage II patients, where the risk is primarily defined by the clinicopathological features such as tumor size, number of lymph nodes investigated, degree of differentiation, tumor perforation, bowel obstruction and lymphovascular invasion (7,9). However, several studies have highlighted the inadequacy at these pathological features in identifying such high-risk patients, providing a potential explanation for the lack of clinical benefit from adjuvant therapy in these patients (10–12). Furthermore, a significant proportion of stage III patients suffer from adverse effects of adjuvant chemotherapy (2). Collectively, these data highlight the imperative need to develop molecular markers for identifying true high-risk population of stage II and III CRC patients to facilitate optimal treatment modalities.
With regards to availability of other potential prognostic biomarkers in CRC, the association of BRAF and KRAS mutations, CIMP (CpG island methylator phenotype) and MSI (microsatellite instability) status have been studied extensively. It has been shown that MSI patients demonstrate an inherently better survival, and do not benefit from 5-fluorouracil (5FU)-based adjuvant chemotherapy in stage II patients (13–18). A more recent effort in this context, involving a gene expression-based consensus molecular subtyping (CMS), has identified that CRC patients with CMS4 subtype associate with poor prognosis (19). Although gene expression based biomarkers may be promising, technical concerns involving specimen preservation and mRNA integrity, particularly in formalin-fixed paraffin embedded (FFPE) specimens limits their clinical translation. In contrast, due to their short length, noncoding RNAs such as miRNAs, are emerging as important biomarker candidates by virtue of their ability to resist RNAase-mediated degradation, and their intact expression in a variety of bodily fluids as well as FFPE tissues.
Previously, we discovered that miR-200 family is an important driver for CMS4 subtype in CRC patients (20). Building upon this evidence, herein, we have performed an unbiased, systematic and comprehensive genome-wide discovery to identify a novel and robust miRNA-based classifier that can predict tumor recurrence in stage II and III CRC patients. By analyzing multiple clinical cohorts totally 736 stage II and III CRC patients, we demonstrate that this miRNA recurrence classifier (MRC) has superior predictive power than clinicopathological risk determinants, currently available commercial assays, and its robust performance even in FFPE tissues, making it attractive for relatively immediate clinical translation.
Materials and Methods
Patients Cohorts
This study included multiple clinical cohorts with a total of 736 patients. These cohorts included patients from the publicly available dataset from the TCGA (N=158 and 107), the GSE29623 dataset (N=40), as well two clinical validation cohorts of 431 stage II and III CRC patients who underwent surgery without neoadjuvant chemotherapy. Clinicopathological parameters of the clinical validation cohorts are provided in Table 1. The first cohort (cohort 1) comprised of fresh frozen tissues from 127 patients who were enrolled at the National Cancer Center Hospital (NCCH), Tokyo, Japan from 2004–2006; and consisted of 28 recurrences with a median follow-up of 67 months. The second cohort included formalin fixed paraffin embedded (FFPE) tissues from 304 patients enrolled at the Tokyo Medical and Dental University Hospital (TMDU), Tokyo, Japan between 2007–2011; and consisted of 82 recurrences with a median follow-up of 47 months. Based upon the year of enrollment, this cohort was subdivided into a training (cohort 2) and a validation (cohort 3) cohort, respectively. Random splitting of this cohort into training and validation also resulted in similar outcomes (Data not shown). Our study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the subjects and the respective institutional review boards approved the study. A reporting recommendations for tumor marker prognostic studies (REMARK) (21) compliance checklist is provided in Supplementary Table 1.
Table 1.
Patient characteristics
| In-silico discovery and validation | Clinical training and Validation | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | TCGA Hi-Seq (Discovery, N=158) |
TCGA GA (Validation 1, N=109) |
GSE29623 (Validation 2, N=40) |
Cohort 1 (Fresh frozen, N=127) |
Cohort 2 (FFPE training, N=165) |
Cohort 3 (FFPE validation, N=139) |
| Age | ||||||
| Median | 68 | 69 | 69 | NA | 69 | 69 |
| Gender | ||||||
| Male | 88 | 51 | 22 | 73 | 90 | 86 |
| Female | 70 | 58 | 18 | 54 | 75 | 53 |
| Localization | ||||||
| left | NA | NA | NA | 107 | 112 | 94 |
| right | NA | NA | NA | 20 | 53 | 45 |
| Grade | ||||||
| well/moderate | NA | NA | 32 | 122 | 152 | 126 |
| poor | NA | NA | 8 | 5 | 13 | 13 |
| Stage | ||||||
| 2 | 88 | 65 | 22 | 53 | 92 | 70 |
| 3 | 70 | 44 | 18 | 74 | 73 | 69 |
| Relapse | ||||||
| Yes | 34 | 16 | 7 | 28 | 42 | 40 |
| No | 124 | 93 | 33 | 99 | 123 | 99 |
| Lymphatic Invasion | ||||||
| Yes | NA | NA | NA | 81 | 91 | 72 |
| No | NA | NA | NA | 46 | 74 | 67 |
| Venous Invasion | ||||||
| Yes | NA | NA | NA | 45 | 147 | 122 |
| No | NA | NA | NA | 82 | 18 | 17 |
| Lymph Nodes Investigated | ||||||
| >/=12 | NA | NA | NA | 110 | 133 | 118 |
| </=12 | NA | NA | NA | 17 | 32 | 21 |
| Adjuvant Therapy | ||||||
| Yes | 56 | 60 | 23 | 56 | 60 | 52 |
| No | 71 | 105 | 17 | 71 | 105 | 87 |
| MSI | ||||||
| Yes | 59 | 32 | NA | 5 | 15 | 10 |
| No | 99 | 76 | NA | 111 | 147 | 125 |
| NA | 40 | 11 | 3 | 4 | ||
| CEA | ||||||
| </=5 | NA | NA | NA | 84 | 96 | 86 |
| >/=5 | NA | NA | NA | 43 | 69 | 53 |
| Non-CMS4 | 100 | 80 | NA | NA | NA | NA |
| CMS4 | 50 | 24 | NA | NA | NA | NA |
| NA | 8 | 5 | 40 | 127 | 165 | 139 |
MSI, microsatellite instability; CEA, carcinoembryonic antigen; FFPE, formalin fixed paraffin embedded
Identification of the miRNA signature from genome-wide small RNA sequencing data
Two public data sets (3 cohorts) were analyzed in the discovery phase, CRC miRNA sequencing data from TCGA(22) and GSE29623(23) from Gene Expression Omnibus (GEO). The TCGA CRC data set includes 265 stage II and III patients with corresponding miRNA sequencing data, derived from two different platforms, Illumina Hi-Seq (TCGA-HiSeq, n=158) and Genome Analyzer (TCGA-GA, n=109). More specifically, level-3 miRNA expression data were downloaded from Firehose Broad GDAC portal (http://gdac.broadinstitute.org/, accessed on Nov 1, 2015). The miRNA expression levels, measured by reads per million miRNA mapped (RPM), were first log2-transformed. 680 miRNAs in common between two platforms were kept for the following analysis. Differential miRNA expression analysis was subsequently performed between patients with and without recurrence in 3 years using Wilcoxon signed-rank test. For in silico validation of identified miRNAs, we analyzed one additional independent cohort (GSE29623). The GSE29623 set includes expression levels of 664 miRNAs for 65 tumor tissue samples, based on NIH Taqman Human MicroRNA Array v.2 microarray platform, of which 40 samples were from stage II & III patients. The miRNA expression profiles were normalized using the robust multi-array average (RMA) algorithm in R (23). We downloaded preprocessed data from GEO using Bioconductor package ‘GEOquery’. Using multivariate Cox regression analysis, we calculated risk scores and assessed the prognosis performance of the miRNA signature based survival analysis, using the median value of the predicted risk scores in each dataset as the cut-off.
Nucleic acid isolation and miRNA expression analysis
Total RNA from the fresh frozen tissues was isolated using RNeasy Mini Kit (QIAGEN), and both RNA and DNA from the FFPE cohort were isolated using Allprep FFPE kit (QIAGEN). The miRNA expression analysis was performed using QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA). All miRNA Taqman probes were purchased from Thermo Fischer Scientific (Waltham, MA). The qRT-PCR assays were conducted using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) using SensiFAST™ Probe Lo-ROX Kit (Bioline, USA). The relative expression of miRNAs were determined by 2-Δct method using snRNA U6 as a normalizer, as described previously (24) and we observed no difference in snRNA U6 between recurrent and non-recurrent patients.
Microsatellite instability analysis
The microsatellite instability (MSI) analysis was performed using the five mononucleotide repeat microsatellite markers (BAT-25, BAT-26, NR-21, NR-24 and NR-27) in a pentaplex PCR system, as described previously (25–27).
Statistical analysis
Statistical analyses were performed using IBM SPSS version 23, GraphPad Prism version 6.0 and R 3.2.4. Statistical differences between miRNAs and various clinicopathological factors were determined by the χ2 test. Benjamini-Hochberg’s method was used to correct for multiple hypothesis testing wherever applicable. All statistical tests were two-sided and a p-value of less than 0.05 was considered significant. Recurrence free interval (RFI) was defined from the day of surgery to the recurrence or the end of follow-up, and was analyzed by log-rank test. We performed receiver-operating-characteristic (ROC) curve analysis to evaluate the predictive power of MRC. All 8-miRNA expression values derived from the RT-PCR were used to build MRC using Cox’s proportional hazard regression. The risk scores derived from the 8-gene MRC Cox model was used to plot the area under the curves (AUC). The risk-scores were calculated using the formula derived from the cox-model as following: The RT-PCR based MRC risk score = (−0.1218×miR-744) + (−3.7142×miR-429) + (−2.2051×miR-362) + (3.0564×miR-200b) + (2.4997×miR-191) + (−0.0065×miR-30c2) + (2.2224×miR-30b) + (−1.1162×miR-33a). To plot the Kaplan Meier (KM) curves, we dichotomized the patients into low or high-risk, based on X-tile derived cut-off values (X-tile software 3.6.1, Yale University School of Medicine, USA). Additionally, we performed univariate and multivariate Cox proportional hazard regression models using clinicopathological variables and MRC to calculate estimate hazard ratios (HRs). Only the significant variables in the univariate model were used to perform the multivariate analysis.
Results
Discovery and validation of an eight-gene miRNA classifier for predicting recurrence in stage II and III colorectal cancer patients
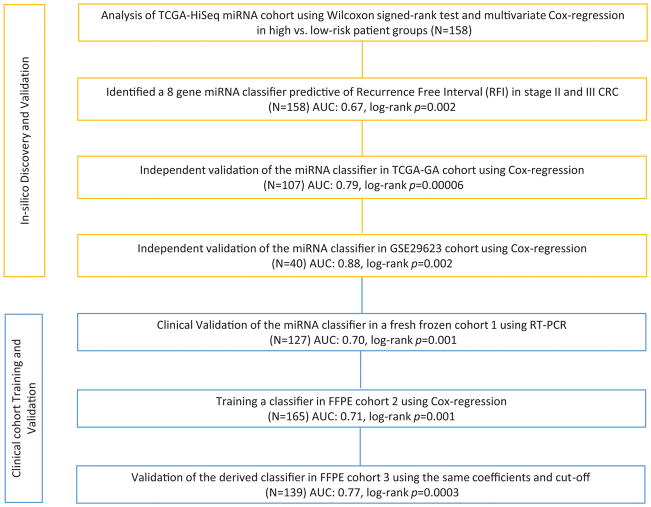
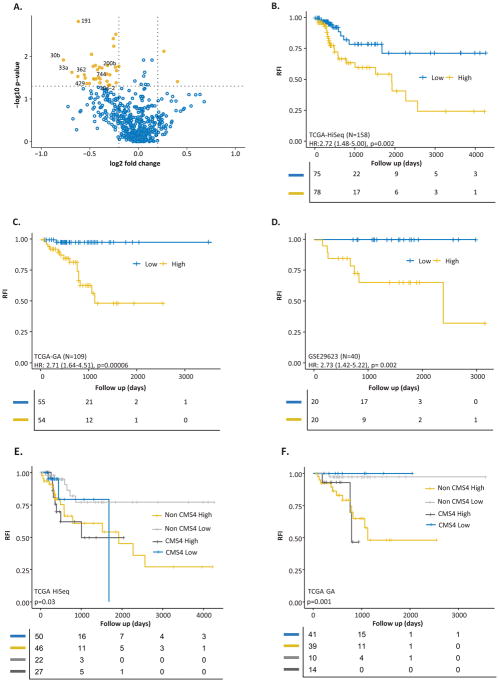
Based upon the study design illustrated in Figure 1, we performed a genome-wide, unbiased, biomarker discovery to identify a miRNA signature that allowed stratification of low and high-risk stage II and III CRC patients. Since TCGA dataset consisted of miRNA sequencing profiles from two different platforms, we used one of these for biomarker discovery (TCGA-HiSeq, N=158) and the other for validation (TCGA-GA, N=109) purposes. In the TCGA-HiSeq discovery cohort, we compared miRNA expression profiles between high and low-risk groups who had at least a minimum of 3 year follow-up, and identified 25 targets with an absolute log2 fold change difference of 0.2, a p-value less than 0.05 (Wilcoxon signed-rank test), and an average expression level of greater than 3 transcripts per million. Based on multivariate cox regression analysis using atotal 25 miRNAs, eight candidates with top statistical significance (p-value <0.2) were further selected which includes hsa-mir-191, hsa-mir-200b, hsa-mir-30b, hsa-mir-30c2, hsa-mir-33a, hsa-mir-362, hsa-mir-429 and hsa-mir-744 (Figure 2A, Supplementary Table 3).
Figure 1.
Study Design
Figure 2.
A miRNA classifier Volcano plot and KM curves predicting recurrence-free interval in the TCGA discovery, validation and GSE validation cohorts. A) Volcano plot showing the significant and differentially regulated miRNAs selected in the TCGA discovery cohort. Selected miRNAs are depicted in the figure. The Kaplan Meier survival plots for recurrence-free interval stratified by MRC scores in the: B) TCGA discovery cohort (N=158), C) TCGA validation cohort (N=109), and D) the GSE29623 validation cohort (N=40). E) and F) The Kaplan Meier plots illustrating that both CMS4 and non-CMS4 patients with high miRNA risk scores exhibited shorter recurrence-free interval in the TCGA discovery and validation cohorts, respectively.
Further validation of the MRC using Kaplan Meier and log-rank analysis significantly predicted RFI in all three cohorts: the TCGA-HiSeq cohort (Figure 2B, HR=2.72; 95% CI 1.48–5.00; p=0.002), the TCGA-GA cohort (Figure 2C, HR=2.71; 95% CI 1.64–4.51; p=0.00006), and the GSE29623 cohort (Figure 2D, HR=2.73; 95% CI 1.42–5.22; p=0.002). The AUC values for predicting the tumor recurrence in both validation cohorts are 0.79 (95% CI 0.67–0.89) and 0.88 (95% CI 0.78–0.99), respectively, highlighting the validity of the miRNA classifier.
miRNA classifier predicted cancer recurrence independent of gene expression-based CMS status of CRC patients
We were also curious to investigate whether our MRC can predict recurrence irrespective of the CMS subtype in CRC patients. The CMS labels of TCGA cohort were obtained from the CRC Subtyping Consortium (CRCSC)’s repository (19). The long-rank analysis demonstrated that regardless of the gene expression subtype, our MRC was able to significantly predict RFI in both TCGA cohorts (Figure 2E and F). Especially, for prediction of recurrence, our MRC outperforms CMS classifier significantly in both TCGA cohorts (p=0.00975 and 0.0000187 respectively, DeLong’s test) Supplementary Figure 3.
Validation of the miRNA classifier in fresh frozen tissues from stage II and III CRC patients
The Supplementary Figure 1 illustrates the CONSORT diagram for all clinical validation cohorts.
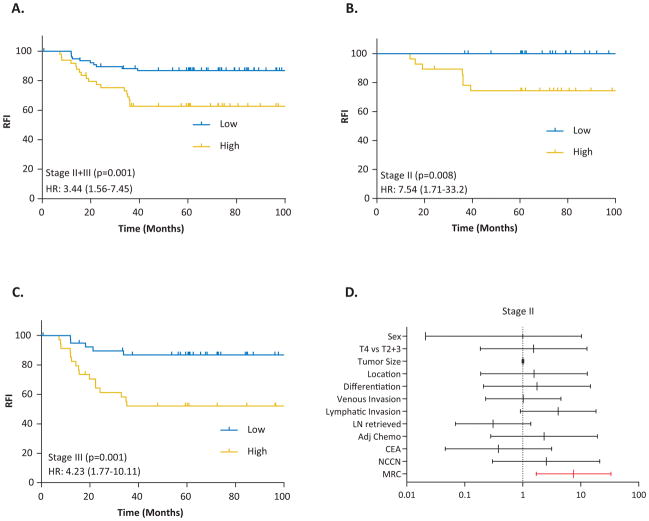
To determine whether our MRC derived from the in-silico datasets was robust, we first evaluated its performance in cohort-1, comprising of 127 fresh frozen tissues from stage II and III CRC patients. We measured expression level of all 8 miRNAs in CRC tissues and used Cox’s proportional hazard models to build a prognostic classifier. As depicted in Figure 3A, the five-year RFI significantly dropped from 87% to 63% in MRC-derived low vs. high-risk patients (p<0.001) with a Hazard Ratio (HR) of 3.44 (1.56–7.45). The HR in stage II patients was 7.54 (1.71–33.2; Figure 3B), while in stage III it was 4.23 (1.77–10.11; Figure 3C). Furthermore, the MRC achieved an AUC of 0.70 in both stages, with a superior recurrence prediction in stage II (AUC: 0.89) vs. stage III (AUC: 0.72) CRC patients, which is clinically quite exciting.
Figure 3.
Stage-wise survival curves predicting recurrence-free interval in fresh-frozen specimens in clinical cohort-1. The Kaplan Meier survival plots for recurrence-free interval stratified by MRC scores in: A) combined stage II and III CRC patients, B) stage II patients, C) stage III CRC patients, and D) Hazard Ratios of the miRNA classifier, NCCN risk classification and other clinicopathological variables presented for stage II CRC patients.
In the univariate analysis, among the three significant variables, MRC emerged as the strongest predictor of recurrence vs. tumor stage and lymphatic invasion (Table 2). However, in the multivariate analysis MRC remained as the only significant predictor of recurrence (HR, 2.54; 95% CI 1.29–4.99). From a clinical viewpoint, we were enthused to observe that our MRC-based risk stratification was significant in predicting recurrence in stage II CRC patients, while the NCCN criteria didn’t work (Figure 3D).
Table 2.
Univariate and multivariate analysis of MRC and clinicopathological factors in all three clinical cohorts
| Cohort 1 | Cohort 2 | Cohort 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
|
| ||||||||||||
| P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | |
| Age (<65 vs >65) | NA | NA | NA | NA | 0.40 | 1.32 (0.69–2.51) | 0.35 | 0.74 (0.39–1.4) | ||||
| Gender (M vs. F) | 0.06 | 2.32 (0.99–5.45) | 0.61 | 1.17 (0.64–2.16) | 0.53 | 0.82 (0.44–1.53) | ||||||
| Location | 0.19 | 2.62 (0.62–11.06) | 0.64 | 0.85 (0.43–1.69) | 0.11 | 0.49 (0.23–1.06) | ||||||
| Differentiation | 0.89 | 0.87 (0.12–6.4) | 0.80 | 0.86 (0.26–2.77) | 0.87 | 1.09 (0.39–3.07) | ||||||
| T4 vs. T2&3 | 0.06 | 2.10 (0.97–4.53) | 0.08 | 1.74 (0.94–3.25) | 0.19 | 1.61 (0.79–3.3) | ||||||
| Tumor Size | 0.84 | 1 (0.98–1.02) | 0.72 | 1 (0.99–1.02) | 0.46 | 1 (0.99–1.01) | ||||||
| Venous Invasion | 0.19 | 1.78 (0.76–4.18) | 0.37 | 1.71 (0.53–5.53) | 0.16 | 2.79 (0.67–11.56) | ||||||
| Lymphatic Invasion | 0.01 | 2.58 (1.22–5.46) | 0.14 | 1.82 (0.82–4.07) | 0.68 | 1.14 (0.62–2.1) | 0.01 | 2.43 (1.24–4.79) | 0.12 | 1.83 (0.84–3.99) | ||
| Stage (III vs II) | 0.03 | 2.56 (1.09–6.02) | 0.23 | 1.75 (0.7–4.39) | 0.02 | 2.16 (1.16–4.03) | 0.03 | 2.01 (1.07–3.74) | 0.06 | 1.85 (0.97–3.51) | 0.44 | 1.33 (0.64–2.78) |
| CEA (Low vs High) | 0.90 | 0.95 (0.43–2.1) | 0.10 | 1.67 (0.91–3.06) | 0.60 | 1.19 (0.63–2.23) | ||||||
| Lymph Node Number | 0.18 | 0.54 (0.22–1.33) | 0.64 | 1.22 (0.54–2.74) | 0.13 | 0.55 (0.25–1.2) | ||||||
| Adjuvant Chemotherapy | 0.35 | 1.43 (0.68–2.99) | 0.61 | 0.85 (0.45–1.6) | 0.22 | 1.48 (0.79–2.75) | ||||||
| MSI | 0.81 | 0.78 (0.10–5.79) | 0.16 | 0.04 (0–3.65) | 0.53 | 0.63 (0.15–2.62) | ||||||
| miRNA classifier | 0.001 | 3.44 (1.56–7.45) | 0.01 | 2.54 (1.29–4.99) | 0.0001 | 6.15 (3.33–11.35) | 0.00004 | 5.11 (2.26–11.53) | 0.0003 | 4.23 (2.26–7.92) | 0.00001 | 3.94 (1.64–9.43) |
M, male; F, female; MSI, microsatellite instability; CEA, carcinoembryonic antigen; HR, hazards ratio; CI, confidence interval
Training and Validation of the miRNA classifier in independent FFPE cohorts to evaluate its translational potential
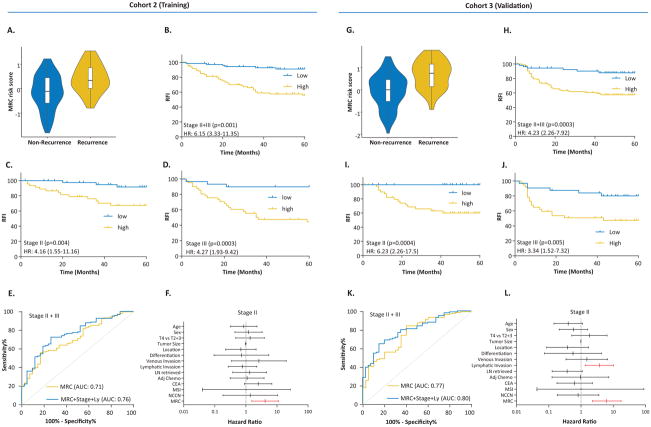
To evaluate the translational potential of our MRC in identifying high-risk patients, we deliberately examined its performance in FFPE tissues, which are routinely available in the clinical settings. To this end, we divided our large FFPE cohort into a training (cohort 2) and a validation set (cohort 3). Using Cox Proportional Hazards model, we initially trained a classifier on the 8-miRNA signature, and subsequently applied the coefficients derived from this model to the validation cohort.
The risk scores for each patient in the training cohort were as follows: MRC risk score=(−0.1218×miR-744) + (−3.7142×miR-429) + (−2.2051×miR-362) + (3.0564×miR-200b) + (2.4997×miR-191) + (−0.0065×miR-30c2) + (2.2224×miR-30b) + (−1.1162×miR-33a). Based upon the Cox-model derived risk scores (Figure 4A and G), patients from both the training and validation cohorts were stratified into MRC-low and high-risk groups, using a cutoff threshold of −0.04. When we assessed the distribution of risk scores and recurrence status, we observed that the high-risk patients had a significantly shorter RFI vs. low-risk patients in both cohorts with a HR of 6.15 (3.33–11.35) and 4.23 (2.26–7.92), respectively. Likewise, the 5 year recurrence free probability in high-risk stage II and III patients was 56% and 57%, while it was 91% and 88% in low-risk patients in the training and validation cohorts, respectively (Figure 4B and H). To verify the stage-wise tumor recurrence risk, we performed the log-rank analysis separately for each stage. In line with the results from cohort 1, in addition to excellent risk stratification for both stages, HRs for stage II patients were significantly higher in both cohorts (Figure 4C, D and I, J).
Figure 4.
Training and validation of the miRNA recurrence classifier in the FFPE specimens in clinical cohorts 2 and 3. A and G) Depict MRC risk score violin plots from Cox regression model of the 8-miRNA signature in the training and validation cohorts, respectively. B, C, D and H, I, J) Stage-wise Kaplan Meier plots for the recurrence-free interval in the training (N=165) and validation (N=139) cohorts, stratified based on the MRC risk scores and E and K) Receiver Operating Characteristic (ROC) curves achieved with MRC risk-scores as well as its combination with the tumor stage and lymphatic invasion in the training and validation cohorts, respectively. F and L) Hazard Ratios of the miRNA classifier, NCCN risk classification and other clinicopathological variables presented for stage II CRC from both cohorts.
We next assessed the accuracy of our miRNA classifier in recurrence prediction by performing ROC analysis. As illustrated in Figures 4E and K, our MRC achieved an AUC of 0.71 (0.63–0.80) and 0.77 (0.68–0.85) in predicting recurrence in stage II and III patients in both cohorts, respectively. Univariate analysis revealed that together with MRC, tumor stage and lymphatic invasion were significantly associated in predicting recurrence in stage II and III CRC patients. However, in multivariate analysis, MRC emerged as the only significant predictor of tumor recurrence in both cohorts (Table 2). In view of these findings, we combined MRC risk scores with tumor stage and lymphatic invasion, which further improved the AUC for recurrence prediction in both stages of CRC patients to 0.76 (0.68–0.84) and 0.80 (0.72–0.87) in the training and validation cohorts, respectively (Figure 4E and F). As was the case in cohort 1, compared to NCCN criteria and other clinicopathological variables that failed, our MRC was successful in identifying true, high-risk, stage II CRC patients with excellent accuracy; and was superior to predictive accuracies of currently available Coloprint and Oncotype Dx assays, especially in FFPE specimens (Figure 4F and L). We have performed Spearman’s rho correlation between MRC and clinicopathological risk-factors for all the clinical cohorts and as it is evident, the MRC is highly correlated with recurrence (Supplementary Table 2).
The MRC predicts tumor recurrence independent of adjuvant chemotherapy status in CRC patients
A large subset of patients in our clinical validation cohorts were treated with adjuvant chemotherapy, which could potentially affect tumor recurrence prediction. To assess any such potential confounding effects, we analyzed the associations between MRC-derived risk subgroups and tumor recurrence separately in stage II and III patients who did and did not receive 5FU-based chemotherapy. In untreated patients, our MRC was still significantly associated with poor RFI in stage II and III CRC patients (Supplementary Figure 2, panels A, B, C and D). In contrast, in patients who received 5FU, while we did not see any significant associations in stage II patients (probably due to small sample size), we noted that our MRC was robust in identifying high-risk stage III patients (Supplementary Figure 2, panels E, F, G and H), highlighting its recurrence prediction potential regardless of the adjuvant chemotherapy status in CRC patients.
MRC low risk microsatellite stable patients benefited from 5FU adjuvant therapy alone, while the high risk group did not
In our FFPE cohort (Both cohort 2 and 3) only fluoropyrimidine was administered for adjuvant chemotherapy. To estimate whether MRC could predict the benefit of fluoropyrimidine adjuvant chemotherapy in stage III patients, we investigated the association between MRC risk and RFI among patients who did and did not receive fluoropyrimidine adjuvant therapy. While there is no significant difference when MSI patients are included in the analysis (Supplementary Figure 4A, B, C, E, F and G), the analysis in microsatellite stable patients revealed that treatment with fluoropyrimidine adjuvant therapy was associated with a significant gain in five years recurrence free probability (5 year RFI 87% with chemotherapy vs. 67% with no chemotherapy, HR: 3.57 (0.96–13.25)) in the stage III MRC low risk patient population (Supplementary Figure 4H). On the other hand there is no significant difference between patients who did and did not receive fluoropyrimidine adjuvant therapy in the stage III MRC high risk patient population (Supplementary Figure 4D).
Discussion
In our quest to develop a robust CRC prognostic signature, we have successfully developed an 8-miRNA recurrence classifier (MRC) that achieved excellent predictive values in tumor recurrence, both in stage II and III CRC patients, which were validated in two independent clinical cohorts. Furthermore, our miRNA classifier remained as the strongest prognostic indicator regardless of the adjuvant chemotherapy status, when compared to the other clinicopathological risk factors. To further highlight the clinical significance of our findings, while the NCCN criteria failed to identify high-risk stage II CRC patients, our MRC significantly stratified patients from all clinical cohorts into high and low-risk subgroups rather robustly.
Previously, a 6-gene miRNA-based classifier was reported to predict tumor recurrence in stage II CRC patients (28); however, in this study, our miRNA classifier performed significantly better, and illustrated its ability to predict recurrence in not just stage II, but stage III CRC patients as well. A recent gene expression-based consensus molecular subtyping (CMS) (19) identified a CMS4 mesenchymal subtype of CRC patients, which associated with poor prognosis. However, an eventual clinical translation of such a gene expression panel-based approach is challenging, primarily due to two reasons: a) the number of genes involved in CMS4 subtyping is quite large, and b) any gene expression based assay will require high quality, fresh frozen, RNA-preserved tissues – which isn’t always practical in the routine clinical practice. Based upon our recent findings that miR-200 family plays a central role in orchestrating the CMS4 subtype (20), and in view of the relative stability of miRNAs in a variety of biological fluids and FFPE tissues, these short noncoding RNAs present as attractive targets for biomarker development in CRC patients.
Although we didn’t perform a direct comparison, the recurrence prediction values for our MRC were superior to gene expression-based signatures offered by the ColoPrint and OncotypeDx (29) assays. It would also be interesting to validate our markers or stratify based on CDX2 as well as recently published immune scores in future (30,31). An ideal prognostic classifier for CRC risk prediction should be robust, reproducible and most importantly, be potentially feasible in FFPE materials- which would eliminate the need to plan and invest methodologies to collect and preserve fresh frozen tumor specimens. Our miRNA classifier successfully overcomes these barriers as evidenced from its superior performance and independent validation in large cohort of FFPE specimens. Availability of ideal prognostic and predictive biomarkers is essential for achieving the clinical goals in refining the therapeutic decisions, and thereby improving the survival and quality of life of CRC patients. Although, in this study, we didn’t have access to blood specimens, but we feel encouraged that given the stability and relative abundance of miRNAs in circulation, it is very likely that our miRNA-signature may eventually be translated into a blood-based, predictive and surveillance assay.
A recent study published by Cantini et al., (32) reported miRNAs differentially expressed across gene-expression based colorectal cancer subtypes. They have showed that miRNA 200b, 33a, 362 and 429 which are present in our MRC are associated with poor colorectal cancer CMS4 subtype. Furthermore, we and others have shown previously that mir200 and 429 are associated with EMT and stemness (20,33,34). The role of miRNA 362, 33a, 30c2, 30b, 744 and 191 in cancer progression, EMT and chemotherapy resistance have been reported earlier (35–41). This further exemplifies the important of miRNAs we found with an unbiased and systematic approach to be associated with poor prognosis in CRC.
With regards to potential limitations, our current study is retrospective in nature, and our results must be validated in future, prospective, multi-center clinical trials.
In conclusion, we provide a novel evidence that our miRNA-based recurrence classifier can effectively stratify stage II and III CRC patients into high and low-risk groups based upon clinical outcomes; thereby offering a significantly improved prognostic biomarker potential compared to the currently used clinicopathological risk factors. Notably, our study has several strengths related to the study design and analytical methods. The miRNA classifier was validated in independent in-silico datasets, as well as two independent population-based clinical cohorts. Since we developed a ‘risk prediction model’ using our 8 miRNA signature, this scores can be readily applied to independent, future prospective cohorts. Although our assay also demonstrated effectiveness in FFPE tissues, we noted that the expression of three of the miRNAs were discrepant (not significant) in our validation cohort compared to the TCGA dataset used in the initial discovery. This effect may be due to the following reasons: 1) the biological differences between fresh frozen and FFPE tissues. 2) a fairly common issue for the existence of ‘co-linearity’ in biomarker studies, i.e., some of these miRNAs may have correlated expression levels, which might confound each other in the linear regression model; hence the observed differences in model coefficients between the two cohorts. Nonetheless, pending further optimization and validation in future studies, such a miRNA classifier potentially offers tremendous clinical value in directing personalized treatment regimens and clinical management of patients with stage II and III colorectal cancer.
Supplementary Material
CONSORT diagram for patients and specimens from both fresh-frozen and FFPE clinical validation cohorts. A) Fresh-frozen cohort (cohort-1), B) FFPE cohort (cohorts 2 and 3).
The MRC significantly predicted recurrence-free interval regardless of the adjuvant chemotherapy treatment. The Kaplan Meier plots from: A, B, C and D) untreated patients; E, F, G and H) patients treated with 5-FU; A, B, E and F) from the fresh-frozen specimens in cohort-1; and C, D, G and H) from the FFPE specimens in cohorts 2 and 3.
Recurrence prediction comparison between CMS and MRC in the A) TCGA discovery and B) Validation cohorts
MRC significantly predicted benefit of adjuvant chemotherapy in microsatellite stable stage III colorectal cancers
KM plots depicted in panel A, B, C and D belong to MRC high risk category, while the KM plots in panel E, F, G and H belong to MRC low risk category. KM plots in panel A and E represents the analysis performed on combined stage II and III CRC patients, while panel B and F represents stage II analysis and panel C and G represents stage III patient’s analysis separately. KM plots depicted in panel D and H represents the analysis performed on microsatellite stable stage III CRC patients
REMARK checklist
Spearman’s rho correlation coefficients of MRC vs clinicopathological risk-factors for all the clinical cohorts
Data of miRNAs which are analyzed in the TCGA discovery cohort with associated statistical parameters
Acknowledgments
Grant support
This work was supported by the CA72851, CA181572, CA184792, CA187956 and CA202797 grants from the National Cancer Institute, NIH; RP140784 from the Cancer Prevention Research Institute of Texas; grants from the Baylor Foundation and Baylor Scott & White Research Institute, Dallas, TX, USA awarded to Ajay Goel, and a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. CityU 21101115), as well as a grant from The Science Technology and Innovation Committee of Shenzhen (JCYJ20170307091256048) awarded to Xin Wang.
Footnotes
Disclosure of Potential Conflicts of Interest
The author shave declared no conflict of interest
Author contributions
RK and FG are involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript. TM, TI, HU, NT, YY and SK are involved in critical revision of the manuscript for important intellectual content and material support. AG and XW are involved in study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, material support and study supervision
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 3.Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93(9):1115–22. doi: 10.1002/bjs.5349. [DOI] [PubMed] [Google Scholar]
- 4.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 5.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–71. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322(6):352–8. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–74. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ABB, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology Recommendations on Adjuvant Chemotherapy for Stage II Colon Cancer. Journal of Clinical Oncology. 2004;22(16):3408–19. doi: 10.1200/jco.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29(25):3381–8. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang SH, Efron JE, Berho ME, Wexner SD. Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. J Am Coll Surg. 2014;219(5):1056–69. doi: 10.1016/j.jamcollsurg.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Quasar Collaborative G. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 13.Ogino S, Cantor M, Kawasaki T, Brahmandam M, Kirkner GJ, Weisenberger DJ, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55(7):1000–6. doi: 10.1136/gut.2005.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 15.Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012;18(6):1506–12. doi: 10.1158/1078-0432.CCR-11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinicrope FA, Rego RL, Halling KC, Foster N, Sargent DJ, La Plant B, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131(3):729–37. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 17.de Cuba EM, Snaebjornsson P, Heideman DA, van Grieken NC, Bosch LJ, Fijneman RJ, et al. Prognostic value of BRAF and KRAS mutation status in stage II and III microsatellite instable colon cancers. Int J Cancer. 2016;138(5):1139–45. doi: 10.1002/ijc.29855. [DOI] [PubMed] [Google Scholar]
- 18.Zong L, Abe M, Ji J, Zhu WG, Yu D. Tracking the Correlation Between CpG Island Methylator Phenotype and Other Molecular Features and Clinicopathological Features in Human Colorectal Cancers: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2016;7:e151. doi: 10.1038/ctg.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fessler E, Jansen M, De Sousa EMF, Zhao L, Prasetyanti PR, Rodermond H, et al. A multidimensional network approach reveals microRNAs as determinants of the mesenchymal colorectal cancer subtype. Oncogene. 2016;35(46):6026–37. doi: 10.1038/onc.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97(16):1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DT, Hernandez JM, Shibata D, McCarthy SM, Humphries LA, Clark W, et al. Complementary strand microRNAs mediate acquisition of metastatic potential in colonic adenocarcinoma. J Gastrointest Surg. 2012;16(5):905–12. doi: 10.1007/s11605-011-1815-0. discussion 12–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14(5):844–52. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buhard O, Cattaneo F, Wong YF, Yim SF, Friedman E, Flejou JF, et al. Multipopulation analysis of polymorphisms in five mononucleotide repeats used to determine the microsatellite instability status of human tumors. J Clin Oncol. 2006;24(2):241–51. doi: 10.1200/JCO.2005.02.7227. [DOI] [PubMed] [Google Scholar]
- 26.Goel A, Nagasaka T, Hamelin R, Boland CR. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One. 2010;5(2):e9393. doi: 10.1371/journal.pone.0009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123(6):1804–11. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14(13):1295–306. doi: 10.1016/S1470-2045(13)70491-1. [DOI] [PubMed] [Google Scholar]
- 29.Park YY, Lee SS, Lim JY, Kim SC, Kim SB, Sohn BH, et al. Comparison of prognostic genomic predictors in colorectal cancer. PLoS One. 2013;8(4):e60778. doi: 10.1371/journal.pone.0060778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, et al. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med. 2016;374(3):211–22. doi: 10.1056/NEJMoa1506597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantini L, Isella C, Petti C, Picco G, Chiola S, Ficarra E, et al. MicroRNA-mRNA interactions underlying colorectal cancer molecular subtypes. Nat Commun. 2015;6:8878. doi: 10.1038/ncomms9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11(9):670–7. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–95. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 35.Nagpal N, Ahmad HM, Chameettachal S, Sundar D, Ghosh S, Kulshreshtha R. HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFbeta-signaling in hypoxic microenvironment. Sci Rep. 2015;5:9650. doi: 10.1038/srep09650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagpal N, Ahmad HM, Molparia B, Kulshreshtha R. MicroRNA-191, an estrogen-responsive microRNA, functions as an oncogenic regulator in human breast cancer. Carcinogenesis. 2013;34(8):1889–99. doi: 10.1093/carcin/bgt107. [DOI] [PubMed] [Google Scholar]
- 37.Kang H, Kim C, Lee H, Rho JG, Seo JW, Nam JW, et al. Downregulation of microRNA-362-3p and microRNA-329 promotes tumor progression in human breast cancer. Cell Death Differ. 2016;23(3):484–95. doi: 10.1038/cdd.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Sun T, Hu J, Zhang R, Rao Y, Wang S, et al. miR-33a promotes glioma-initiating cell self-renewal via PKA and NOTCH pathways. J Clin Invest. 2014;124(10):4489–502. doi: 10.1172/JCI75284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyamae M, Komatsu S, Ichikawa D, Kawaguchi T, Hirajima S, Okajima W, et al. Plasma microRNA profiles: identification of miR-744 as a novel diagnostic and prognostic biomarker in pancreatic cancer. Br J Cancer. 2015;113(10):1467–76. doi: 10.1038/bjc.2015.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tormo E, Adam-Artigues A, Ballester S, Pineda B, Zazo S, Gonzalez-Alonso P, et al. The role of miR-26a and miR-30b in HER2+ breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Sci Rep. 2017;7:41309. doi: 10.1038/srep41309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K, et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun. 2013;4:1393. doi: 10.1038/ncomms2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT diagram for patients and specimens from both fresh-frozen and FFPE clinical validation cohorts. A) Fresh-frozen cohort (cohort-1), B) FFPE cohort (cohorts 2 and 3).
The MRC significantly predicted recurrence-free interval regardless of the adjuvant chemotherapy treatment. The Kaplan Meier plots from: A, B, C and D) untreated patients; E, F, G and H) patients treated with 5-FU; A, B, E and F) from the fresh-frozen specimens in cohort-1; and C, D, G and H) from the FFPE specimens in cohorts 2 and 3.
Recurrence prediction comparison between CMS and MRC in the A) TCGA discovery and B) Validation cohorts
MRC significantly predicted benefit of adjuvant chemotherapy in microsatellite stable stage III colorectal cancers
KM plots depicted in panel A, B, C and D belong to MRC high risk category, while the KM plots in panel E, F, G and H belong to MRC low risk category. KM plots in panel A and E represents the analysis performed on combined stage II and III CRC patients, while panel B and F represents stage II analysis and panel C and G represents stage III patient’s analysis separately. KM plots depicted in panel D and H represents the analysis performed on microsatellite stable stage III CRC patients
REMARK checklist
Spearman’s rho correlation coefficients of MRC vs clinicopathological risk-factors for all the clinical cohorts
Data of miRNAs which are analyzed in the TCGA discovery cohort with associated statistical parameters