Abstract
Probiotics that promote the gut microbiota have been reported to reduce stress responses, and improve memory and mood. Whether and how antibiotics that eliminate or inhibit pathogenic and commensal gut bacteria also affect central nervous system functions in humans is so far unknown. In a double-blinded randomized study, 16 healthy volunteers (27.00 ± 1.60 years; 9 males) received either rifaximin (600 mg/day) (a poorly absorbable antibiotic) or placebo for 7 days. Before and after the drug intervention, brain activities during rest and during a social stressor inducing feelings of exclusion (Cyberball game) were measured using magnetoencephalography. Social exclusion significantly affected (p < 0.001) mood and increased exclusion perception. Magnetoencephalography showed brain regions with higher activations during exclusion as compared to inclusion, in different frequency bands. Seven days of rifaximin increased prefrontal and right cingulate alpha power during resting state. Low beta power showed an interaction of intervention (rifaximin, placebo) × condition (inclusion, exclusion) during the Cyberball game in the bilateral prefrontal and left anterior cingulate cortex. Only in the rifaximin group, a decrease (p = 0.004) in power was seen comparing exclusion to inclusion; the reduced beta-1 power was negatively correlated with a change in the subjective exclusion perception score. Social stress affecting brain functioning in a specific manner is modulated by rifaximin. Contrary to our hypothesis that antibiotics have advert effects on mood, the antibiotic exhibited stress-reducing effects similar to reported effects of probiotics (supported by NeuroGUT, a EU 7th Framework Programme ITN no. 607652; ClinicalTrials.gov identifier number NCT02793193).
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0627-2) contains supplementary material, which is available to authorized users.
Keywords: Gut–brain axis, Antibiotic, Stress, Cyberball, MEG
Introduction
The gut–brain axis is essential for communication between the enteric nervous system and central nervous system (CNS) functions. The commensal gut microbiota, therefore, may play an important role on this axis through neural, immune, and endocrine pathways. Previous studies have found altered gut microbiota (GM) composition not only to affect enteric nervous system functions [1] but also to change CNS functions in animals and humans [2–5]. Among the most frequently investigated CNS functions are social functions, learning and memory functions, and stress responsiveness in animals, yet few data are available in humans [6–9].
Although many tools are available to manipulate the microbiota in animals (germ-free or specifically colonized animals, antibiotic elimination of the microbiota, fecal microbiota transfer between animals and from humans to animals, etc.) [10–13], few allow similar approaches in humans. As we have recently summarized [14], probiotics have been used to affect CNS functions in animals and humans, with a variety of different bacterial strains but predominantly Lactobacillus and Bifidobacterium.
Although some of these were available as nutrient supplements even before their therapeutic benefit became evident, others have been developed specifically for the purpose to act as therapeutic agents in intestinal (e.g., irritable bowel syndrome, IBS; inflammatory bowel diseases) and extraintestinal disorders (atopy, diabetes). More recently, disturbed CNS functions, e.g., of neurological (autism spectrum disorder) or psychiatric nature (mood disorders), have come into focus [15–17], and first studies have evaluated the effects of probiotics on related CNS functions in healthy volunteers, whereas studies in respective patient populations are still scarce [18].
The mechanisms by which probiotics affect central functions are incompletely understood, but include—among others—direct effects on the commensal gut microbiota (via increased diversity and/or competitive colonization), enhancement of their metabolic functions, or stimulation of the enteric neural or immune system, all of which could directly or indirectly stimulate the gut–brain axis and elicit such central effects.
Despite its wide use in everyday medicine, antibiotics have rarely been investigated for their effects on central functions, presumably for two reasons: their use in healthy subjects is limited by their ability to induce antibiotic resistance that may be detrimental in case antibiotics are needed for treatment of acute bacterial infections; and patients that are in need for antibiotics are suffering from acute infections, and any central effect seen may as well be a consequence of the acute disease rather than antibiotics-induced CNS consequences of manipulating the gut microbiota. Although antibiotics-induced peripheral consequences, e.g., diarrhea and irritable bowel syndrome-type symptoms, are well established, long-term CNS consequences of antibiotic use have not been investigated so far.
Rifaximin is a locally (intestinally) acting broadband antibiotic with poor bioavailability (< 1% systemic absorption), thus with minimal risk for provoking antibiotic resistance [19]. This specific property allows its use both in healthy volunteers as well as in patients without severe infection, e.g., in traveler’s diarrhea [20]. A few trials have shown clinical efficacy in IBS [21, 22] and in small intestinal bacterial overgrowth [23, 24], but here the mechanism of action is less unclear.
Most animal work of “psychobiotics” relates to their effects on standardized stress paradigms, specifically social stressors such as open-field [15], maternal separation [25], and defensive burying test [15, 26]. In search for a stress paradigm that would allow the testing of social stress with neuroimaging methods in humans, we have identified the “Cyberball game” (CBG), which is a virtual game that is often used to study social stress by exclusion/rejection [27]. Different from other human stress tasks, such as the Trier Social Stress test [28], the CBG can be employed easily in a brain scanner, thus allowing direct evaluation of associated neurobiological processes, to compensate for the lack of direct physiological stress markers in human research, as compared to animal studies.
During this CBG, participants play a computer-simulated ball and feel distressed during the period when the other players barely throw a ball to him/her. Some studies also showed physiological changes such as raised cortisol level, higher skin conductance, and increased facial temperature [29–34]. A systematic review of neuroimaging studies summarized the relevant brain activities and showed its reliability to induce social stress in healthy volunteers [35]. Accordingly, regions of the insula, anterior cingulate cortex (ACC), and temporal and prefrontal cortex (PFC) were activated to social exclusion [36–38]. Neural oscillations are thought to play a key role in processing neural information, and different types of oscillatory activities are being studied for their functions. Exclusion-induced changes in neural oscillations such as alpha and theta frequency bands have also been reported in these areas [39–41].
In order to investigate the neural oscillations during social stress and their modulation by rifaximin, we conducted an exploratory experiment in healthy volunteers using magnetoencephalography (MEG), as a functional neuroimaging technique with fine spatial resolution and high temporal [42]. Because of a putative antibiotic effect of rifaximin on the commensal microbiota, we hypothesized that rifaximin would, in contrast to known probiotic effects on CNS functions [14], increase the stress response following social exclusion.
Materials and Method
Participants
Sixteen volunteers participated in the study. All participants met our inclusion criteria: 1) nonsmoker for at least 3 months, 2) with a body mass index of 18 to 30, 3) without any chronic allergies, 4) willing to discontinue the consumption of probiotic- and prebiotic-containing foods or potentially immune-enhancing dietary supplements, 5) receiving no immune-suppressing intervention and not having any immunosuppressive illness within the last year, 6) receiving no antibiotic therapy within the last 2 months, 7) having no psychiatric or gastrointestinal disorder, and 8) having no nonremovable metal parts in the body. Informed consent was obtained from all participants prior to joining the study. The protocol has been approved by the Ethics Board of the University of Tübingen Medical School (No. 503/2015BO1, approved on 26 August 2015) and registered at ClinicalTrials.gov (identifier number NCT02793193).
Study Design
Our pilot study was a randomized, double-blinded, and parallel-group design, in which the participants visited our laboratory twice for measurements: at baseline and 1 day after the end of drug intake. The intervention and the control group took the antibiotic rifaximin (3 × 200 mg/day) or placebo pills, respectively, for 7 days; drugs and placebos were provided by the university hospital pharmacy. The randomization scheme was unblinded after completion of the experiment and the data evaluation.
During the intervention period, participants were instructed to avoid the consumption of food containing probiotics/prebiotics, or potentially immune-enhancing dietary supplements. This was supported by providing them with a list of “prohibited” foods (Appendix 1).
Questionnaires
To survey participants’ health status during each of the 2 visits, the 36-item short-form health survey (SF-36) was used [43]. The SF-36 includes 8 concepts: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions.
After each of the inclusion and exclusion blocks (see below), participants needed to complete 2 questionnaires to assess their acute level of distress. We employed the self-report measures of the Need Threat Scale (NTS), Mood questionnaire (MQ), and the subjective “exclusion perception” (SEP) on a scale rating between 1 and 5 (Appendix 2); all these scales are validated standard for the CBG [27, 44].
Cyberball Game
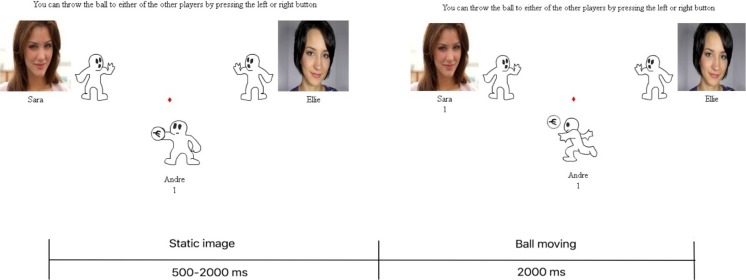
In the CBG, the participants were asked to play a ball-tossing game with 2 other virtual players programmed by the experimenter. They were made to believe that the 2 players were real and were playing the game. To control for gender effects, male participants played with 2 female players, and female participants played with 2 male players. The volunteering participants could throw the ball to either of the other 2 players on the left or right, by pressing the button on the response box (Fig. 1).
Fig. 1.
Schematic outline of a trial in the Cyberball game
The CBG consisted of 4 blocks: inclusion–exclusion–inclusion–exclusion conditions, in fixed order. In each inclusion blocks, there were 108 trials, and each of the players has equal chance to receive the ball. The 1/3 of total 108 trials in the inclusion block when the virtual players threw the ball to each other and not to the participant were called “not my turn” events. To equalize the numbers of analyzed trials when the virtual players threw the ball to each other and not to the participant, despite the 3 trials when participants received the ball for maintaining their attention, the remaining 36 “rejection” events were used for comparison with the 36 “not my turn” events in the inclusion block. The trial began with the ball being presented in the cartoon for 500–2000 ms randomly to imitate a real-life situation. Then, the ball was moving for 2000 ms before reaching the target player (Fig. 1). After each of the inclusion and exclusion blocks, participants completed the NTS, MQ, and SEP.
Magnetoencephalography Recording
Brain magnetic fields were measured with a 275-channel whole-head magnetoencephalograph. Participants were studied in supine position. During each visit, 5-min resting state was recorded prior to while playing the CBG. During resting-state measurements, participants were instructed to move as little as possible and to be awake while keeping their eyes closed. During the CBG, task instructions were projected onto a screen in front of the participants. MEG signals were sampled at a rate of 585.94 Hz with an anti-aliasing filter set to 292.97 Hz.
In order to overlay the brain activity derived from MEG on anatomical scans, high-resolution (1 mm, isotropic) T1-weighted structural MR images were acquired using an MPRAGE sequence with a 3-T MR scanner (University Hospital Tübingen, Germany) for each participant.
Data Analysis
Data Analysis: Questionnaires
To test the intervention-related changes in participants’ health status scored by SF-36, changes from before to after the 7-day intervention were computed by subtracting the baseline assessment from postintervention values. To control the intervention-related changes on the NTS, the MQ, and the SEP during the CBG, changes after each intervention were computed for each condition.
Data were analyzed using SPSS 21 (IBM, Armonk, NY, USA). Changes in SF-36 were entered into a 2–independent-sample Mann–Whitney U test of intervention (rifaximin vs placebo), as this data was not normally distributed. The changes in NTS, MQ, and SEP were entered in a 2 × 2 ANOVA of intervention as a between-subject factor (rifaximin vs placebo) × condition as a within-subject factor (exclusion vs inclusion). If significant main effects or interactions were observed, pairwise comparisons were used to assess each time point with a Bonferroni-adjusted threshold (α = 0.025).
Data Analysis: MEG Data
Preprocessing
Analysis of the MEG data was carried out using MATLAB (MathWorks, Natick, MA, USA) and the open-source toolbox Fieldtrip [45]. The resting-state dataset were cut into time windows of 2 s. Data in this time window were filtered using a 4-Hz high-pass frequency filter. Nonphysiological jumps in the MEG signal and trials with jump and muscle artifacts were excluded by an automatic rejection algorithm. Any trial in each channel whose variance exceeded 10−25 T2 was excluded from further analysis. The continuously recorded dataset of the CBG was segmented with respect to stimulus onset (when 1 player threw the ball) and baseline adjusted using a [−1000: 2000] ms trial interval. Trials in which the other players threw the ball toward each other during the inclusion blocks were defined as “inclusion” condition, and those during exclusion blocks were defined as “exclusion” conditions.
Time–Frequency Analysis
The time–frequency analysis used the multitaper windowed fast Fourier transform “MTMFFT” implemented in Fieldtrip. The “multitaper method” is based on Slepian sequences as tapers. The frequency of interest ranged from 4 to 30 Hz with step of 2 Hz and the smoothing window is +/− 3 Hz.
Source Analysis
Using the time–frequency determined by the analysis described above, oscillatory sources of theta, alpha, beta-1, beta-2, and beta-3 bands (6, 11, 16, 21, and 26 Hz) were localized using beamformer techniques. The Dynamical Imaging of Coherent Sources method was applied [46]. In order to estimate the individual source activity, each participant’s brain recorded as T1-MR image was divided in a regular three-dimensional grid with a 1-cm resolution and a spatial inverse filter was computed from both conditions and both visits, as common filter. This common filter was applied to each condition and each visit separately in order to obtain the respective source power. The MEG data was coregistrated with the individual structural MR images.
Source Statistics
For testing the effects of stress induced by the CBG, the source power in each frequency band from all the participants at the baseline visit was entered in a paired-samples t test comparing exclusion with inclusion condition. For analyzing the effect of rifaximin, we performed source-level statistics for the data obtained from the resting-state condition and the CBG, respectively. For resting state, changes in the source power in each frequency band were computed by subtracting the baseline from the postintervention. The changes of the source power were entered into an independent t test with intervention (rifaximin vs placebo) as between factor. For the CBG, changes in the source power were computed by subtracting the baseline from the postintervention in each condition in each frequency band. Changes of source power were entered in a 2-way ANOVA of interventions (rifaximin vs placebo) × conditions (exclusion vs inclusion). To localize significant activations, the cluster-based permutation method for multiple comparisons (corrected) was used with a significance level of alpha of 0.05.
Correlation Between Behavioral and MEG Data
To correlate the change in neural activity with change in the subjective reports by rifaximin, correlations were done for the resting-state task and the CBG, respectively. For the resting-state task, averaged source power was calculated for clusters that differed significantly between both visits, and was correlated with changes in health status. For the CBG, for each condition and each intervention, source power in the clusters that differed significantly between both visits was averaged. The averaged source power was correlated with changes in the scores of NTS, MQ, and SEP in each condition and each group, using Pearson correlations. These correlations were considered significant at a corrected threshold of p < 0.05.
Results
Sixteen healthy participants met the inclusion criteria of the study and completed the experiment (9 males, age 27.00 ± 1.60 years age; BMI 22.21 ± 0.48). Eight participants completed the intervention with rifaximin (6 males, age 26.50 ± 1.05; BMI 22.48 ± 0.58) and 8 with placebo (3 males, mean age 27.50 ± 3.12; BMI 21.94 ± 0.81).
Stress Effect by the Cyberball Game
There was a significant difference between inclusion and exclusion in the global score of the NTS (t15 = 5.06, p < 0.001). In the exclusion condition, participants reported higher scores of the global NTS score. The score of the MQ is significantly lower in the exclusion condition compared to inclusion condition (t15 = − 5.40, p < 0.001). Also, in the SEP, participants revealed a significantly higher score of exclusion perception (t15 = 13.64, p < 0.001).
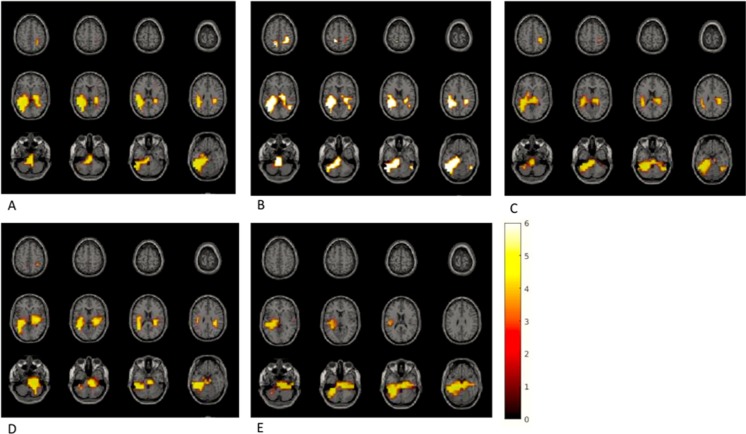
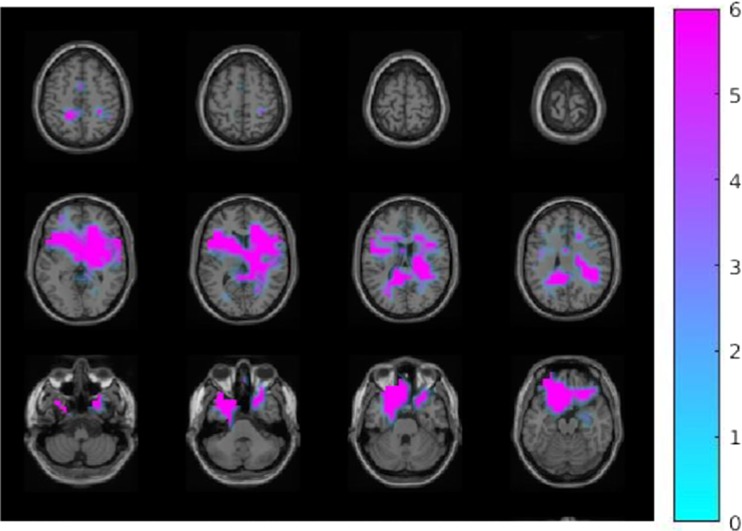
Source statistics of MEG power in each frequency band showed brain regions that had significantly higher activations during the exclusion compared to the inclusion condition (Fig. 2).
Fig. 2.
Activation in different frequency bands during exclusion versus inclusion condition. (A) Theta frequency band (6 Hz): the left fusiform gyrus, the right inferior and superior parietal lobule, the right thalamus, and the left middle occipital gyrus (p = 0.008). (B) Alpha frequency band (11 Hz): the bilateral posterior cingulate gyrus, the bilateral inferior and superior parietal lobule, the left hippocampus and parahippocampal gyrus, and the left fusiform gyrus (p = 0.002). (C) Beta-1 frequency band (16 Hz): the bilateral inferior and middle temporal lobule, the bilateral fusiform gyrus, the bilateral hippocampus, and the left inferior and middle occipital cortex (p = 0.01). (D) Beta-2 frequency band (21 Hz): the right cingulate gyrus, the bilateral fusiform gyrus, the bilateral hippocampus, the left occipital cortex, and the right parahippocampal gyrus (p = 0.008). (E) Beta-3 frequency band (26 Hz): the left posterior cingulate gyrus; the bilateral fusiform gyrus; the bilateral hippocampus and parahippocampal gyrus; the bilateral inferior, middle, and superior temporal lobule; and the bilateral thalamus (p = 0.01)
Intervention Effect by Rifaximin
Physical and Psychological Health Status
Comparing the scores of the questionnaires between the rifaximin and the placebo group at baseline, no group differences were found for any score of the SF-36 item survey. After intervention, only the difference in “emotional well-being,” a subitem of the SF-36 item survey, was significant between groups (U = 11, p = 0.02). There was a significantly higher increase of “emotional well-being” in the rifaximin group (median, 11.13) than in the placebo group (median, 5.88).
Effects of Rifaximin on Resting-State MEG
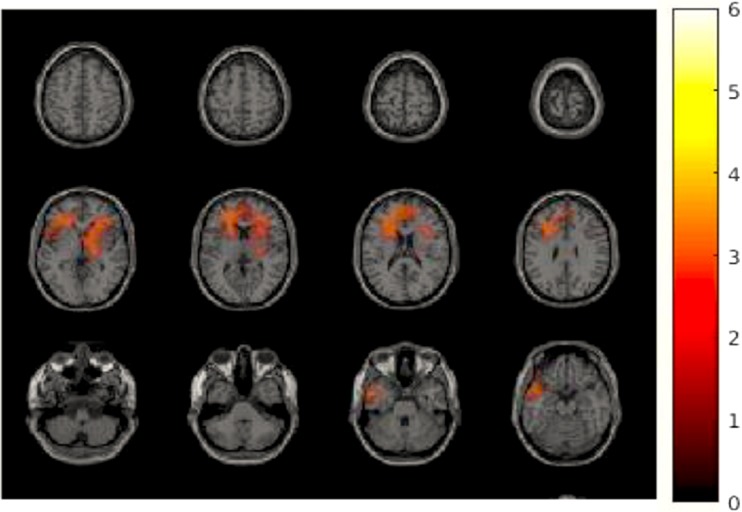
We tested the effects of the intervention on the source power in each frequency band during resting state. An independent t test showed a significant cluster of increased power in the alpha band (11 Hz) in the right superior and inferior frontal cortex and the bilateral middle cingulate gyrus extending to the left insula in the rifaximin group as compared to the placebo group (Fig. 3, p < 0.05).
Fig. 3.
Difference of changed brain activity during resting state comparing rifaximin versus placebo. A cluster including the left inferior and middle frontal cortex, the bilateral superior frontal cortex, the right middle cingulate gyrus, and the left insula showed significantly more increased power in alpha band (11 Hz), p < 0.05
Resting State and Health Status
To investigate whether the increase of neural activity during resting state was correlated to the participants’ health state, we correlated the averaged power changes in the significantly activated cluster with the changes in SF-36 scores. However, no correlation was found, neither across both groups nor within each group.
Rifaximin Effect on Neural Response to the Cyberball Game
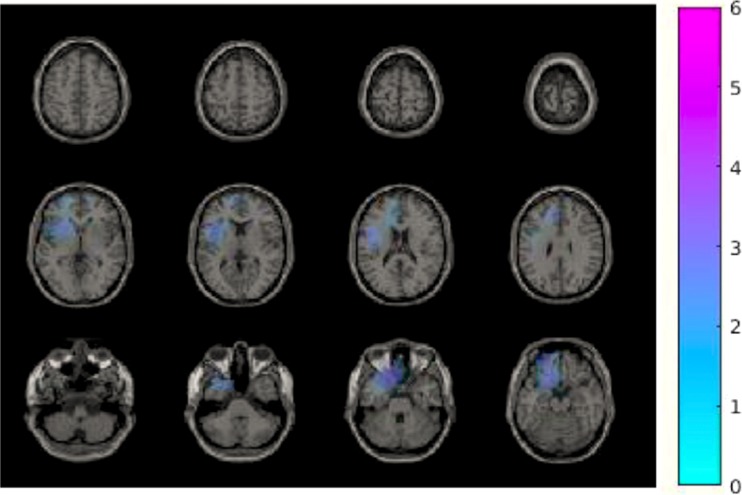
To test the effects of interventions and conditions on the neural response to the CBG, a 2-way ANOVA test was carried out. No significant main effect of intervention or condition was found. Only in beta-1 band (16 Hz), a significant interaction of interventions and conditions was found in the left inferior and superior and the bilateral middle frontal cortex and the left anterior cingulate gyrus (Fig. 4; p < 0.05). As a post hoc analysis, we compared brain activity changes between two conditions (exclusion vs inclusions) for each intervention group separately. Only in the rifaximin group, a decrease in beta-1 power, in the bilateral inferior, superior, and middle frontal cortex; the bilateral anterior, middle, and posterior cingulate gyrus; and the bilateral parietal and postcentral cortex, could be demonstrated for exclusion as compared with inclusion (Fig. 5; p = 0.002). A summary of frequency bands and neuroanatomical areas found to be related with social stress, rifaximin intervention, and their interaction is provided (Table 1).
Fig. 4.
Difference of neural activity change in 16 Hz during the Cyberball game by interaction of interventions and conditions effects. A cluster including the left inferior and superior and the bilateral middle frontal cortex, and the left anterior cingulate gyrus, showed a reduced neural activity change in beta-1 band (16 Hz) comparing exclusion versus inclusion and rifaximin versus placebo, p < 0.05
Fig. 5.
Difference of neural activity change in 16 Hz during the Cyberball game comparing exclusion versus inclusion conditions in the rifaximin group. Regions of the bilateral inferior, superior, and middle frontal cortex; the bilateral anterior, middle, and posterior cingulate gyrus; and the bilateral parietal and postcentral cortex showed a reduced neural activity change in beta-1 band (16 Hz) comparing exclusion versus inclusion conditions, p < 0.002
Table 1.
Summarized frequency bands and neuroanatomical areas found to be associated with social stress, the application of rifaximin, and interaction of both
| Comparison | Frequency band | Brain region | Hemisphere | p value |
|---|---|---|---|---|
| Stress effect: exclusion versus inclusion | Theta | Fusiform gyrus | Left | 0.008 |
| Inferior parietal lobule | Right | |||
| Superior parietal lobule | Right | |||
| Thalamus | Right | |||
| Middle occipital gyrus | Left | |||
| Alpha | Posterior cingulate gyrus | Bilateral | 0.002 | |
| Inferior parietal lobule | Bilateral | |||
| Superior parietal lobule | Bilateral | |||
| Hippocampus | Left | |||
| Parahippocampal gyrus | Left | |||
| Fusiform gyrus | Left | |||
| Beta-1 | Inferior temporal cortex | Bilateral | 0.01 | |
| Middle temporal cortex | Bilateral | |||
| Fusiform cortex | Bilateral | |||
| Hippocampus | Bilateral | |||
| Inferior occipital cortex | Left | |||
| Beta-2 | Cingulate gyrus | Right | 0.008 | |
| Fusiform cortex | Bilateral | |||
| Hippocampus | Bilateral | |||
| Parahippocampal gyrus | Right | |||
| Occipital cortex | Left | |||
| Beta-3 | Posterior cingulate gyrus | Left | 0.01 | |
| Fusiform gyrus | Bilateral | |||
| Hippocampus | Bilateral | |||
| Parahippocampal gyrus | Bilateral | |||
| Interior temporal lobule | Bilateral | |||
| Middle temporal lobule | Bilateral | |||
| Superior temporal lobule | Bilateral | |||
| Thalamus | Bilateral | |||
| Rifaximin effect on resting state: | Alpha | Inferior frontal gyrus | Left | 0.05 |
| Middle frontal gyrus | Left | |||
| Superior frontal gyrus | Bilateral | |||
| Middle cingulate gyrus | Right | |||
| Insula | Left | |||
| Rifaximin effect * stress effect on CBG: | Beta-1 | Inferior frontal gyrus | Left | 0.05 |
| Superior frontal gyrus | Left | |||
| Middle frontal gyrus | Bilateral | |||
| Anterior cingulate gyrus | Left |
Neural Activity and Subjective Report of the Social Exclusion
A correlation between neural activity change and subjective report changes of social exclusion was tested only in case of a significant difference of neural activity changes between intervention groups. Therefore, we only tested correlations between neural activity changes in the cluster with changes of the subjective report of NTS, MQ, and SEP for each condition during the CBG, respectively. Only in the rifaximin group, a significant correlation was obtained between SEP score changes and beta-1 power changes (r = 0.86, p = 0.006).
Discussion
Using MEG, we observed an effect of stress, induced by the CBG, on the neural oscillations in different frequency bands and at distributed brain areas. Daily intervention of 600 mg rifaximin for 1 week significantly increased the frontal and cingulate alpha power during resting state and decreased prefrontal and cingulate low beta power during social exclusion compared with inclusion. These neural changes were only observed in the group that consumed rifaximin, indicating that 1-week intervention of rifaximin did have effects on CNS functions in healthy volunteers. The observed neural effects of rifaximin are suggested to be mediated by its effects on the gut microbiota.
Stress Effect
Our study is the first one using MEG that has found that social exclusion produced larger neural oscillatory activities in various frequency bands in certain brain areas. The fusiform facial area was more activated in all the frequency bands from theta, alpha, to beta waves. This finding is consistent with previous studies, suggesting an enhanced processing of the other players’ faces and learning of the social value of their faces according to the unpleasant/negative social exclusive event. Activations of the parietal area were found in the low-frequency band—theta and alpha waves, and temporal activations in beta bands. In a recent scoping review of neuroimaging data of CBG, the summarized key neural processing of social exclusion pointed out that parietal activity was involved in an early stage process including perception and attention [35]. Hereby, we may suggest that theta and alpha oscillations play a crucial role in perception and attention of the exclusion event. Temporal activations in beta bands may reflect emotional arousal and event appraisal processes [36, 47]. Interestingly, in addition to the cingulate gyrus which has been reported by many previous studies, we have also obtained significant activations of hippocampus and parahippocampal gyrus in alpha and beta bands, indicating stronger memory consolidation and retrial during the social exclusion.
Rifaximin Effect
Rifaximin has been approved for the use in humans to treat acute and chronic gastrointestinal infections and disorders. However, few studies have been done to investigate its role in the gut–brain axis, whereas probiotics have been more commonly addressed for its positive effects on both gut and brain functions [14, 48–50]. Several cognition functions, including working memory in patients with liver cirrhosis, have been reported to improve with rifaximin [51, 52]. In the study by Ahluwalia et al. [51], an 8-week intake of rifaximin enhanced working memory and inhibitory control with altered brain activities in patients with liver cirrhosis. The authors suggested the central effects of rifaximin are mediated by the gut–liver–brain axis by modulating gut bacteria, serum bilirubin, and systemic endotoxemia.
From our results, one could conclude that rifaximin is beneficial in improving mental health by relieving stress, both in resting conditions and in stressful situations. Finding that volunteers taking rifaximin became less nervous, calmer, and happier after the intervention (as is reflected by the respective SF-36 items), an improvement of emotional well-being was achieved.
Currently, we do not know whether the effects of rifaximin on the neuronal activity are direct or mediated indirectly via an improvement of bodily well-being. Nevertheless, based on a large number of studies showing frontal alpha as neuronal signature of participants entering a more relaxed state—e.g., as an effect of music therapy leading to reduced levels of anxiety [53, 54]—the larger frontal and cingulate power for the rifaximin group during resting state is a convincing index for states of stronger relaxation.
This improvement may be associated with altered brain activities during the resting state after the rifaximin intervention. The larger frontal and cingulate alpha power for the rifaximin group during resting state may be related to increased relaxation—this has often been observed when participants were more relaxed, e.g., as effect of music therapy leading to reduced anxiety levels [53, 54]. Together, participants’ reported improvement in emotional well-being and altered resting brain activity may indicate an effect of rifaximin on relieving stress in general.
During socially stressful situations as induced by the exclusion condition in the CBG, a decreased frontal and cingulate low beta power was found for the rifaximin group. Also, beta-1 band power reduction was negatively correlated to the change of SEP after rifaximin intervention, showing that as the participants perceived more exclusion, there was higher reduction of the beta-1 power in the PFC and ACC. Frontal beta power has been reported to occur in mental fatigue and appears negatively related to mental stress level [55]. In a study investigating the central effects of the phosphatidylserine supplement, which can decrease perceived stress and improve mood, a reduced frontal beta-1 power by phosphatidylserine after an induction of stress has been reported [56]. Similarly, a moderate massage also decreased the beta activity in the brain as well as participants’ stress level [57, 58]. As activations in the PFC and ACC have been reported to be involved in emotion regulation during social exclusion [35, 47], the reduced beta-1 power band in these areas may be related to higher demands for emotion regulation of the perceived exclusion as well as to reduce mental stress.
Additional lines of evidence can be provided by two fMRI studies investigating alteration of neural processes of emotional stimuli by probiotics intervention. One study using fermented milk products with probiotic reported a shift of brain activity from arousal-based resting-state network to a regulatory network and reduced neural activities in affective and viscerosensory cortices to emotional stimuli after 4 weeks of intervention [59]. A recent study in IBS patients showed Bifidobaterium longum NCC3001 also reduced neural responses in amygdala and frontolimbic regions to fearful stimuli [18]. Changed activations in amygdala and frontolimbic have indicated modulated hypothalamic–pituitary axis activity and emotion regulation due to the stimuli [60, 61]. According to these convergent lines of evidence, the reduced power in the frontal and cingulate regions in beta power found in our study may indicate less mental fatigue and a higher level of ongoing mental regulation during the stressful event. In summary, rifaximin may have effects of improving relaxation and reducing anxiety levels and stress responses by modulating central processing of emotion.
Rifaximin is known because of its benefits in modulating the gastrointestinal functions and treating IBS. Furthermore, rifaximin has been found in some studies to promote beneficial bacteria in the gut such as Bifidobacteria and Lactobacilli [62–64]. Rifaximin-induced changes of CNS functions might be mediated by rifaximin-induced altered gut GM composition or diversity that lead to changes of metabolites such as short-chain fatty acids and tryptophan, which in turn can influence the CNS [15, 65]. Similar to probiotics, rifaximin influences immune system mucosal inflammation by reducing the level of certain interleukins and tumor necrosis factor α [64]. Thereby, the improved immune function could affect the endocrine and nervous systems [66]. Probiotics have been regarded as having positive effects on gastrointestinal and increasingly also on central functions by modulating the gut microbiota through the gut–brain axis. However, our findings indicate that rifaximin may act on the CNS by modulating the GM in a similar way as probiotics.
The present study had some limitations that need discussion. First, as a pilot study, we did not collect stool samples for microbiome analysis, which could give more insight into the mechanism of action of rifaximin, e.g., whether rifaximin alters the commensal gut microbiota itself, alters the metabolic activity of the microbiota, or acts via the immune system, that in turn may be responsible for the CNS effects. Second, the stress response induced by the CBG was testified by subjective measures of distress, but neither physiological nor hormonal stress responses were measured. Third, no power calculation was performed because of the exploratory character of the trial, which was supposed to generate a hypothesis that was planned to be tested in a future study, but the serendipity of the finding motivated us to report it separately. The present study provides evidence that it is worthwhile to compare the effects of rifaximin and probiotics as well as their combination on central nervous system functions. Further research should in particular study the effect of rifaximin comprehensively by correlating the microbiological, physiological, psychological, and neural responses due to stress and intervention effects.
Conclusion
Using MEG, we were able to identify a neural signature of social stress and its modulation due to rifaximin. Oscillatory neuromagnetic activity in different frequency bands and brain areas reflected aspects of neural processes during social exclusion. One week of rifaximin intervention influenced the prefrontal and cingulate alpha oscillation in the resting state and the prefrontal and cingulate low beta oscillation as a response to social stress. Further studies investigating this effect in a larger population are expected to confirm these findings and might highlight even more subtle effects on brain activities and social well-being. Including peripheral physiological parameters in the study and testing patients with functional gastrointestinal disorders appear to be promising to elucidate the pathways and mechanisms on how GM affects brain functions.
Electronic Supplementary Material
(PDF 998 kb)
Acknowledgments
The research leading to these results has received funding from the People Programme of the European Union’s Seventh Framework Programme under Research Executive Agency Grant agreement no. 607652 (NeuroGUT).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Appendix 1. Probiotic-/Prebiotic-Containing Food not to Eat During Intervention
Probiotic–rich-containing food:
Yogurt containing probiotics (e.g., Dannon Activia, Yakult, or any other brands you know)
Goat’s milk
Soy milk
Kefir
Sauerkraut
Pickles
Kimchi
Umeboshi plums
Tempeh
Dark chocolate
Microalgae
Natto
Poi (kind of mashing cooked taro plant)
Miso soup
Kombucha tea
Prebiotic–rich-containing food:
Raw chicory root
Raw Jerusalem artichoke
Raw dandelion greens
Raw garlic
Raw leek
Raw onion
Appendix 2: Assessment of Need Threat Scale, Mood Questionnaire, and Exclusion Perception
All items need to be rated on a scale from 1 (“not at all”) to 5 (“very much”). (R) = reversed scored
Need
Belonging:
1. I felt disconnected with one or more players.
2. I felt rejected by other players.
3. I felt like an outsider.
4. I felt belonged to the group. (R)
5. The other players interacted with me a lot. (R)
Self-esteem:
6. I felt good about myself. (R)
7. My self-esteem was high. (R)
8. I felt I was liked. (R)
9. I felt insecure.
10. I felt satisfied. (R)
Meaningful existence:
11. I felt invisible.
12. I felt meaningless.
13. I felt nonexistent.
14. I felt important. (R)
15. I felt useful. (R)
Control:
16. I felt powerful. (R)
17. I felt I had control over the course of the game. (R)
18. I felt I had the ability to significantly alter events. (R)
19. I felt I was unable to influence the actions of others.
20. I felt the other players decided everything.
Mood
During the game I felt:
1. Good (R)
2. Bad
3. Happy (R)
4. Sad
5. Pleasant (R)
6. Angry
7. Friendly (R)
8. Unfriendly
Exclusion perception
1. I was ignored.
2. I was excluded.
Author Contributions
PE is responsible for the integrity of the work—the inception of the study and publication of the work. HW contributed to the design of the study, data collection and analysis, drafting of the manuscript, and critical revisions of the manuscript. CB and PE contributed to the design of the study, data analysis, and critical revisions of the manuscript. All authors approved the final version of the manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Contributor Information
Huiying Wang, Phone: +49-7071-2987717, Email: wanghuiying36@hotmail.com.
Christoph Braun, Phone: +49-707-2987705, Email: christoph.braun@uni-tuebingen.de.
Paul Enck, Email: paul.enck@uni-tuebingen.de.
References
- 1.Hyland NP, Cryan JF. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016;417(2):182–187. doi: 10.1016/j.ydbio.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 3.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 4.Cryan JF, Dinan TG. More than a gut feeling: the microbiota regulates neurodevelopment and behavior. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40(1):241–2. doi: 10.1038/npp.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox LM, Weiner HL. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2018;15(1):135–45. doi: 10.1007/s13311-017-0598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor C, Thiennimitr P, Chattipakorn N, Chattipakorn SC. Diet, gut microbiota and cognition. Metab Brain Dis. 2017;32(1):1–17. doi: 10.1007/s11011-016-9917-8. [DOI] [PubMed] [Google Scholar]
- 7.Gareau MG. Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol. 2014;817:357–371. doi: 10.1007/978-1-4939-0897-4_16. [DOI] [PubMed] [Google Scholar]
- 8.O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014;277:885–901. doi: 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Molecular Psychiatry. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. [DOI] [PMC free article] [PubMed]
- 11.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–264, e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Hu X, Liang S, Li W, Wu X, Wang L, et al. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef Microbes. 2015;6(5):707–717. doi: 10.3920/BM2014.0177. [DOI] [PubMed] [Google Scholar]
- 13.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66(4):806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans—a systematic review. J Neurogastroenterol Motil. 2016. [DOI] [PMC free article] [PubMed]
- 15.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantak PA, Bobrow DN, Nyby JG. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG) Behav Pharmacol. 2014;25(1):71–79. doi: 10.1097/FBP.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 17.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017;153(2):448–459 e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 19.DuPont HL. Biologic properties and clinical uses of rifaximin. Expert Opin Pharmacother. 2011;12(2):293–302. doi: 10.1517/14656566.2011.546347. [DOI] [PubMed] [Google Scholar]
- 20.DuPont HL, Jiang ZD, Okhuysen PC, Ericsson CD, de la Cabada FJ, Ke S, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142(10):805–812. doi: 10.7326/0003-4819-142-10-200505170-00005. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel M, Morales W, Chua K, Barlow G, Weitsman S, Kim G, et al. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci. 2011;56(7):2067–2072. doi: 10.1007/s10620-011-1728-5. [DOI] [PubMed] [Google Scholar]
- 22.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145(8):557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 23.Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45(5):604–616. doi: 10.1111/apt.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarpellini E, Gabrielli M, Lauritano CE, Lupascu A, Merra G, Cammarota G, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25(7):781–786. doi: 10.1111/j.1365-2036.2007.03259.x. [DOI] [PubMed] [Google Scholar]
- 25.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience. 2010;170(4):1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26(11):1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 27.Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38(1):174–180. doi: 10.3758/BF03192765. [DOI] [PubMed] [Google Scholar]
- 28.Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev. 2014;38:94–124. doi: 10.1016/j.neubiorev.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 29.McQuaid RJ, McInnis OA, Matheson K, Anisman H. Distress of ostracism: oxytocin receptor gene polymorphism confers sensitivity to social exclusion. Soc Cogn Affect Neurosci. 2015;10(8):1153–1159. doi: 10.1093/scan/nsu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beekman JB, Stock ML, Marcus T. Need to Belong, Not Rejection Sensitivity, Moderates Cortisol Response, Self-Reported Stress, and Negative Affect Following Social Exclusion. J Soc Psychol. 2016;156(2):131–138. doi: 10.1080/00224545.2015.1071767. [DOI] [PubMed] [Google Scholar]
- 31.Blackhart GC, Eckel LA, Tice DM. Salivary cortisol in response to acute social rejection and acceptance by peers. Biol Psychol. 2007;75(3):267–276. doi: 10.1016/j.biopsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52(4):318–327. doi: 10.1016/S0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- 33.Kelly M, McDonald S, Rushby J. All alone with sweaty palms—physiological arousal and ostracism. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2012;83(3):309–314. doi: 10.1016/j.ijpsycho.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Paolini D, Alparone FR, Cardone D, van Beest I, Merla A. “The face of ostracism”: The impact of the social categorization on the thermal facial responses of the target and the observer. Acta Psychologica. 2016;163:65–73. doi: 10.1016/j.actpsy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Braun C, Enck P. How the brain reacts to social stress (exclusion)—A scoping review. Neurosci Biobehav Rev. 2017;80:80–88. doi: 10.1016/j.neubiorev.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Bolling DZ, Pelphrey KA, Vander Wyk BC. Unlike adults, children and adolescents show predominantly increased neural activation to social exclusion by members of the opposite gender. Soc Neurosci. 2016;11(5):475–486. doi: 10.1080/17470919.2015.1117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, et al. Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(9):2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cristofori I, Harquel S, Isnard J, Mauguiere F, Sirigu A. Monetary reward suppresses anterior insula activity during social pain. Soc Cogn Affect Neurosci. 2015;10(12):1668–1676. doi: 10.1093/scan/nsv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamoto T, Nittono H, Ura M. Cognitive, Affective, and Motivational Changes during Ostracism: An ERP, EMG, and EEG Study Using a Computerized Cyberball Task. Neuroscience Journal. 2013;2013:304674. doi: 10.1155/2013/304674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Noordt SJ, White LO, Wu J, Mayes LC, Crowley MJ. Social exclusion modulates event-related frontal theta and tracks ostracism distress in children. Neuroimage. 2015;118:248–255. doi: 10.1016/j.neuroimage.2015.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen D. Magnetoencephalography: detection of the brain's electrical activity with a superconducting magnetometer. Science (New York, NY). 1972;175(4022):664–666. doi: 10.1126/science.175.4022.664. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72(1):134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001;98(2):694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Dev Sci. 2011;14(6):1431–1444. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romijn AR, Rucklidge JJ. Systematic review of evidence to support the theory of psychobiotics. Nutr Rev. 2015;73(10):675–693. doi: 10.1093/nutrit/nuv025. [DOI] [PubMed] [Google Scholar]
- 49.Mazurak N, Broelz E, Storr M, Enck P. Probiotic Therapy of the Irritable Bowel Syndrome: Why Is the Evidence Still Poor and What Can Be Done About It? J Neurogastroenterol Motil. 2015;21(4):471–485. doi: 10.5056/jnm15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 51.Ahluwalia V, Wade JB, Heuman DM, Hammeke TA, Sanyal AJ, Sterling RK, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with Rifaximin in Cirrhosis: implications for the gut-liver-brain axis. Metab Brain Dis. 2014;29(4):1017–1025. doi: 10.1007/s11011-014-9507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13(3):125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fachner J, Gold C, Erkkila J. Music therapy modulates fronto-temporal activity in rest-EEG in depressed clients. Brain Topogr. 2013;26(2):338–354. doi: 10.1007/s10548-012-0254-x. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka M, Ishii A, Watanabe Y. Neural effects of mental fatigue caused by continuous attention load: a magnetoencephalography study. Brain Res. 2014;1561:60–66. doi: 10.1016/j.brainres.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Baumeister J, Barthel T, Geiss KR, Weiss M. Influence of phosphatidylserine on cognitive performance and cortical activity after induced stress. Nutr Neurosci. 2008;11(3):103–110. doi: 10.1179/147683008X301478. [DOI] [PubMed] [Google Scholar]
- 57.Diego MA, Field T, Sanders C, Hernandez-Reif M. Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate. Int J Neurosci. 2004;114(1):31–44. doi: 10.1080/00207450490249446. [DOI] [PubMed] [Google Scholar]
- 58.Field T, Ironson G, Scafidi F, Nawrocki T, Goncalves A, Burman I, et al. Massage therapy reduces anxiety and enhances EEG pattern of alertness and math computations. Int J Neurosci. 1996;86(3–4):197–205. doi: 10.3109/00207459608986710. [DOI] [PubMed] [Google Scholar]
- 59.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Eack SM, Wojtalik JA, Barb SM, Newhill CE, Keshavan MS, Phillips ML. Fronto-Limbic Brain Dysfunction during the Regulation of Emotion in Schizophrenia. PLoS One. 2016;11(3):e0149297. doi: 10.1371/journal.pone.0149297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65(12):2556–2565. doi: 10.1093/jac/dkq345. [DOI] [PubMed] [Google Scholar]
- 63.Ponziani FR, Scaldaferri F, Petito V, Paroni Sterbini F, Pecere S, Lopetuso LR, et al. The Role of Antibiotics in Gut Microbiota Modulation: The Eubiotic Effects of Rifaximin. Dig Dis. 2016;34(3):269–278. doi: 10.1159/000443361. [DOI] [PubMed] [Google Scholar]
- 64.Xu D, Gao J, Gillilland M, 3rd, Wu X, Song I, Kao JY, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146(2):484–496 e4. doi: 10.1053/j.gastro.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14(3):143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 998 kb)