Abstract
Glial cell types were classified less than 100 years ago by del Rio-Hortega. For instance, he correctly surmised that microglia in pathologic central nervous system (CNS) were “voracious monsters” that helped clean the tissue. Although these historical predictions were remarkably accurate, innovative technologies have revealed novel molecular, cellular, and dynamic physiologic aspects of CNS glia. In this review, we integrate recent findings regarding the roles of glia and glial interactions in healthy and injured spinal cord. The three major glial cell types are considered in healthy CNS and after spinal cord injury (SCI). Astrocytes, which in the healthy CNS regulate neurotransmitter and neurovascular dynamics, respond to SCI by becoming reactive and forming a glial scar that limits pathology and plasticity. Microglia, which in the healthy CNS scan for infection/damage, respond to SCI by promoting axon growth and remyelination—but also with hyperactivation and cytotoxic effects. Oligodendrocytes and their precursors, which in healthy tissue speed axon conduction and support axonal function, respond to SCI by differentiating and producing myelin, but are susceptible to death. Thus, post-SCI responses of each glial cell can simultaneously stimulate and stifle repair. Interestingly, potential therapies could also target interactions between these cells. Astrocyte–microglia cross-talk creates a feed-forward loop, so shifting the response of either cell could amplify repair. Astrocytes, microglia, and oligodendrocytes/precursors also influence post-SCI cell survival, differentiation, and remyelination, as well as axon sparing. Therefore, optimizing post-SCI responses of glial cells—and interactions between these CNS cells—could benefit neuroprotection, axon plasticity, and functional recovery.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0630-7) contains supplementary material, which is available to authorized users.
Key Words: Spinal cord injury, Glia, Astrocyte, Microglia, Oligodendrocyte precursor cell, Neuroinflammation
Introduction
Glial cells—non-neuronal cells that populate the nervous system—have critical roles in health and disease. Recounting the initial discovery and classification of these cells offers a fascinating historical perspective [1]. The presence of non-neuronal substance in the central nervous system (CNS) was first postulated in the mid-nineteenth century, when Virchow [2] predicted the presence of CNS connective tissue. Although his drawings did not definitively show non-neuronal cells, Virchow observed that the connective tissue of the brain differed from that of other organs. He called this unique tissue of the CNS “Nervenkitt,” or neuroglia. In turn, Golgi (1885–1886; see Somjen [1]) used his precise silver staining techniques to identify CNS cells that lacked axon projections and appeared non-neuronal. Glial cells were classified gradually over the following 40 years: Ramon y Cajal identified fibrous and protoplasmic cells as astrocytes; later, Ramon y Cajal’s protégé del Rio-Hortega revealed the presence of interfascicular glia (oligodendrocytes) and microglia. Indeed, some of del Rio-Hortega’s remarkable drawings from the 1920s and 1930s depicted microglia in healthy tissue (described as “bodyguards [that] extend their tentacles in every direction”) and in tissue with encephalitis (described as “voracious monsters” and “valuable assistants in cleaning the tissue”) [1, 3].
Now, a century later, we continue to ask: What are glia, and what are their functions in health and disease?
In this review, we will first discuss how glia and other non-neuronal cells function in the healthy CNS. Next, we will consider the beneficial and detrimental roles of glial cells, and their interactions, after spinal cord injury (SCI). Finally, we will discuss how glial cell responses and interactions can be manipulated to improve nervous system repair. Overall, this review will first highlight key active roles of non-neuronal cells in maintaining CNS homeostasis; it will then emphasize the importance—and therapeutic potential—of optimizing glial responses and interactions after SCI.
Glia and Vascular Cells: Heterogeneous Cells that Maintain Homeostasis in the Healthy CNS
Glial cells are non-neuronal cell types that reside in the nervous system to support and enable effective nervous system function. The focus of this review is the CNS glial cells, which include astrocytes, oligodendrocytes and their progenitors, and microglia. Beyond the scope of this review are other related cell types: nonglial CNS-resident cells include vascular cells such as endothelial cells and pericytes [4, 5], peripheral nervous system (PNS) glia include Schwann cells and satellite cells (reviewed by [6, 7]). In this section, we will focus on the roles of glial cells in the healthy spinal cord.
Although non-neuronal cell types in the neural parenchyma have classically been grouped under the umbrella term “glia,” divergent developmental origin, programming, and mature expression patterns highlight the fact that these cell types are quite distinct from one another [8]. During development, astrocytes and oligodendrocytes both derive from ectoderm (neural tube), but emerge from divergent dorsal-ventral regions and progenitor cells [9, 10]. In contrast, microglia populate the CNS from primitive macrophage progenitors in the yolk sac [11–13]. These distinct developmental cell origins manifest as differences in the adult as well: recent studies using modern techniques (e.g., cell sorting, RNA sequencing) have established that disparate glial cell types have widespread differences in protein/gene expression signatures [14–16]. Interestingly, even within a cell type, there exist differences in phenotype by brain region. For instance, based on microarray profiles, cerebellar microglia are quite different from cortical microglia, whereas cortical and striatal microglia are relatively similar [17]. Thus, in studying CNS health and pathology, researchers should consider the heterogeneity of cells—both between cell types and between CNS regions.
Astrocytes: Ubiquitous Cells that Enable Effective CNS Signaling and Function
Astrocytes constitute ~ 20–40% of all cells in the mammalian CNS [18]. These cells have a “bushy” morphology, and individual astrocytes maintain distinct territories from one another—individually labeled astrocytes tile among each other in three-dimensional space and display minimal overlap [19, 20]. Subtypes of astrocytes have been identified in the white matter (protoplasmic) and the gray matter (fibrous) [21]. Astrocyte processes are complex, and they interact with nearly all cell types and structures throughout the brain. Given their ubiquitous distribution and extensive network of processes, these cells are ideally suited for CNS-wide support of homeostatic mechanisms [20]. Indeed, astrocytes control neuron activity and health through neurometabolic coupling (dynamically linking blood flow and energetics to local neuron needs; [22, 23]), and by removing excess neurotransmitters, potassium, and glutamate from the extracellular space [24–26]. Astrocytes also control delivery and removal (“paravascular clearance”) of solutes in the cerebrospinal fluid; this requires astrocyte expression of the water-permeable channel aquaporin-4, linking astrocytic water transport to solute movement [27]. Impaired glymphatic clearance of solutes (including amyloid-β) contributes to age-related cognitive decline and possibly other neuropathologies [28]. In addition, astrocytes have endfeet that communicate with pericytes in capillary walls to control cerebrovascular tone [29]. Astrocytes also modulate circuit-level transitions between brain states, such as between sleep-wake cycles [30]. Although astrocytes are not electrically active, they do display calcium waves that act in a delayed and prolonged timescale compared to neuronal action potentials [31–33]. Further, calcium waves in one astrocyte can be propagated to nearby astrocytes via gap junctions [34–36]. Astrocytic calcium waves are observed in awake mice during locomotion and arousal, likely via endogenous release of norepinephrine [37, 38]. The cellular and physiologic importance of these calcium waves remain uncertain, and highlight that we still have an incomplete understanding of how astrocytes broadly influence CNS function and physiology [31]. Overall, it is becoming clear that astrocytes regulate CNS function from the molecular-microenvironment level (e.g., neurotransmitter turnover) to the physiologic level (e.g., brain states).
Microglia: Resident CNS Immune Cells that Refine Synaptic Connections
Microglia make up 5–10% of all CNS cells. Microglia are the main immune cell type resident to CNS parenchyma; however, other CNS macrophage types associate with CNS structures, including meningeal, perivascular, and choroid plexus macrophages [39]. Microglial survival throughout life requires activation of the receptor CSF1R by one of its two ligands, CSF-1 or IL-34 [40–42]. Effective steady-state microglial activity is ensured by constant communication through specific receptor systems. For instance, interactions between CD200R (receptor expressed on microglia) and CD200 (cell surface ligand expressed on neurons) are required to maintain microglia in an inactivated state [43, 44]. Microglia in the healthy CNS have a ramified morphology, and their processes dynamically scan parenchyma for signs of infection or damage [45]. Under healthy conditions, microglia possess minimal antigen-presenting machinery compared to professional antigen-presenting cells (e.g., dendritic cells—which may also reside in healthy CNS [46]) [44]. Activated microglia upregulate a suite of proteins involved in immune activation, such as the antigen-presenting molecule MHC II (see below for more detail).
Although microglia are renowned for their immunocompetence, these cells are also versatile orchestrators of nervous system development and homeostatic control [39]. Microglial processes intimately interact with synapses. Microglia use the complement system—classically defined as an immune defense mechanism—to remove synapses and refine circuits [47–49], and to modulate synaptic activity [50]. Further, microglia regulate and coordinate neuron activity in the brain of mice, under healthy conditions and after cerebral ischemia [51]. Microglia are also involved in phagocytosing cellular [52, 53] and myelin components [54] as part of healthy CNS maintenance. In addition, microglia have key roles in regulating development and responses of other cells. For instance, microglia produce trophic factors that support neuron survival [55] and axon growth [56] during development. Microglia also aid oligodendrocyte precursor survival and myelination [57, 58]. Finally, microglia direct endothelial cells to increase brain vascular complexity [59]. Thus, microglia display an impressive array of functions that regulate CNS development and homeostatic balance.
Oligodendrocytes: Myelinating Glia that Optimize Axon Integrity, Structure, and Conduction
Oligodendrocytes are glia that myelinate axons. Myelin is a specialized membrane that extends from glia to enwrap axon segments, which accelerates axon conduction and supports axonal function. Oligodendrocytes extend multiple processes to myelinate several segments simultaneously [60, 61]. Myelin accelerates axon signal transduction by promoting saltatory conduction—which is fast action potential propagation enabled by lower membrane capacitance in the presence of myelin (on axons > 1 μm), between nodes of Ranvier (short, myelin-free axon segments with high ion channel density) [62, 63]. Interestingly, myelin sheath length is intrinsically programmed in oligodendrocytes by region: spinal cord oligodendrocytes produce longer myelin sheaths than cortical oligodendrocytes [64]. Individual axons may modify the myelination process via neuregulin-1 type III [65, 66] and Fyn kinase [67] signaling.
Although oligodendrocytes are known for their ability to myelinate, these cells have other key active roles in the CNS (see McTigue and Tripathi [68]). Oligodendrocytes preferentially myelinate larger-caliber axons; in turn, the myelination process feeds back to further increase axon diameter [69]. Myelination and oligodendrocytes are also required to initiate and maintain sodium channel clustering in the nodes, which is essential for saltatory conduction [63, 70]. In addition, oligodendrocyte and myelin contact on axons ensures effective transport of cytoskeletal components [71]. Recent data reveal the existence of oligodendrocyte–axon metabolic coupling: oligodendrocytes produce and use lactate, but can also supply lactate to axons [72, 73]; similarly, oligodendrocyte glucose uptake is linked to neuron activity and supports action potential propagation [74–76]. Finally, axons and their cell bodies require oligodendrocytes for trophic support: removing key CNS myelin proteins elicits axon degeneration [77], and oligodendrocyte-derived growth factors support survival of several neuron populations [68, 78, 79] (although note that these trophic support studies were completed in vitro; future work should establish whether oligodendrocytes support neuron survival in vivo). The wide-ranging roles of oligodendrocytes in the healthy CNS suggest that they likely influence physiologic function. Indeed, ablating oligodendrocytes caused secondary axon damage in the CNS, which was associated with early-onset and long-lasting neuropathic pain symptoms (cold and mechanical pain), as well as motor deficits [80]. Thus, oligodendrocytes and axons have an intimate, active relationship that enables steady-state axon integrity, structure, and conduction—and ultimately influences animal physiology and behavior.
Oligodendrocytes are derived from oligodendrocyte precursor cells (OPCs). OPCs (or “NG2+ cells”) are progenitor cells that migrate and differentiate from neuroepithelial layers during development (for details, see van Tilborg et al. and Bergles and Richardson [10, 81]. In the adult CNS, OPCs are the main proliferative cell type and constitute ~ 5% of all cells [82, 83]. OPCs have a lattice-like distribution throughout the healthy CNS parenchyma; these cells constantly divide and are removed to maintain cell density. OPCs exhibit exquisite homeostatic control of cell density: loss of a single OPC—by differentiation, ablation, or apoptosis—causes a nearby OPC to rapidly divide to replace the lost OPC [84]. In this manner, tiled OPCs may be optimally distributed to differentiate or (re-)myelinate in response to local requirements [81]. Although NG2+ OPCs predominantly generate oligodendrocytes [85, 86], they can differentiate into other cell types including neurons [87, 88].
Glial Cell Responses to Spinal Cord Injury
SCI causes peri-lesional glial cells to robustly alter their phenotypes and activities. Whereas spinal astrocytes typically regulate neurotransmitter availability and blood flow, post-SCI reactive astrocytes form a glial scar that limits both lesion expansion and axon regeneration. Whereas microglia in healthy spinal cord scan for pathogens and control synapse density, post-SCI microglia can promote plasticity, yet also become hyper-activated for prolonged periods and worsen damage. Whereas OPCs and oligodendrocytes in healthy spinal cord maintain effective axon conduction through myelination and trophic support, post-SCI OPCs and oligodendrocytes attempt to proliferate and remyelinate damaged axons.
Thus, SCI elicits both reparative and unproductive responses from each glial cell type. These responses shift over time (Fig. 1) and are defined by spatial location (Fig. 2) relative to the lesion. In this section, we detail glial cell responses to SCI, and how these characteristic responses help or hinder spinal cord repair.
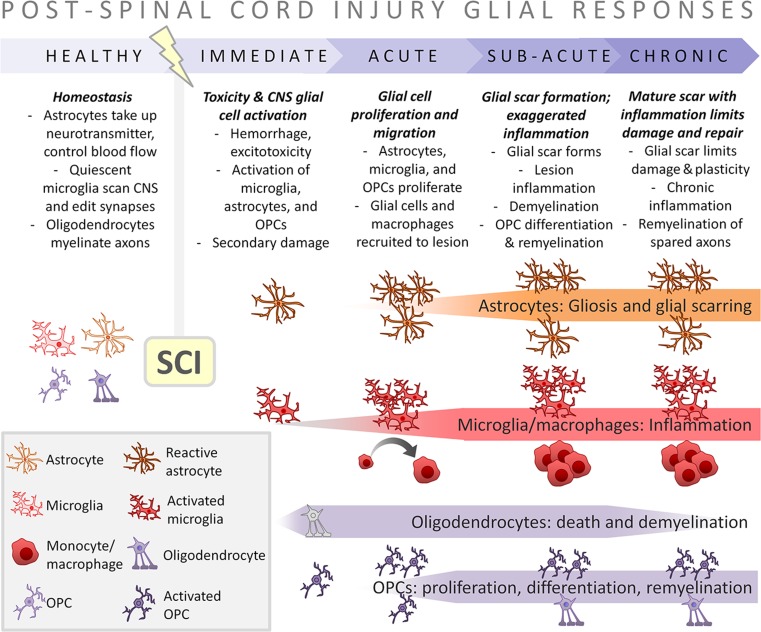
Fig. 1.
Temporal dynamics of SCI-elicited glial cell activation. In healthy CNS tissue, astrocytes, microglia, and oligodendrocytes/OPCs have key roles in maintaining homeostasis. SCI causes primary trauma, eliciting hemorrhage, and spreading cell death. Immediately after SCI, astrocytes, microglia, and OPCs become activated: they proliferate, secrete cytokines, and contribute to secondary damage. At acute times post-injury, hematogenous immune cells including macrophages are recruited to the lesion site. In addition, astrocytes, microglia, and other glial cells migrate toward the lesion site and the glial scar forms. At sub-acute to chronic times post-SCI, the glial scar matures. The glial scar prevents axon plasticity, but also inflammation-elicited lesion expansion. Oligodendrocytes in perilesion zones are susceptible to cytotoxicity, but their death is partially compensated for by OPC proliferation, differentiation, and remyelination. Differentiation and remyelination by OPCs can proceed into chronic post-SCI times
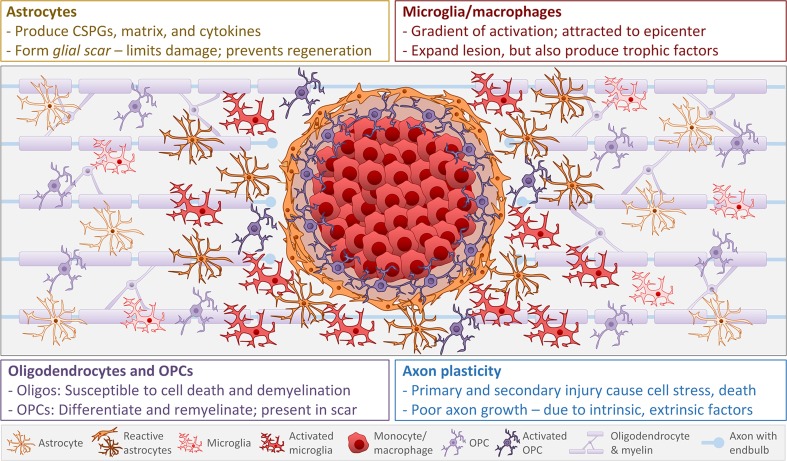
Fig. 2.
Spatial dynamics of SCI-elicited glial cell activation. At sub-acute to chronic times post-SCI, glial cell responses have stabilized to limit further damage and axon plasticity. Glial cells form a gradient of activation, with peak activation at or near the epicenter. Astrocytes (gold) become reactive, proliferate, and contribute to the glial scar. The glial scar limits axon plasticity, but also restricts secondary damage. Microglia and macrophages (red) become hyperactivated for prolonged periods, and hematogenous macrophages are recruited to the lesion epicenter. Microglia and macrophages exacerbate secondary damage, but microglia/macrophage-derived factors can also promote axon growth and remyelination. Oligodendrocytes and oligodendrocyte precursor cells (OPCs) (purple) are susceptible to cytotoxicity. OPCs can differentiate and remyelinate, but they also produce growth inhibitory factors and contribute to the glial scar. Axons (light blue) die back from the injury site due to interactions with microglia/macrophages and other cells; potential plasticity is prevented by proteoglycans and cells present in the glial scar (as well as a poor intrinsic growth response). Spared axons near the lesion site can be demyelinated; these may or may not be remyelinated by chronic times. Schematic shows relative density of glial cells (and not other cells) to simplify presentation. Cells with deeper shading and bold borders represent activated/reactive cells near the lesion. The close proximity of several activated glial cell types highlights the importance of understanding post-SCI glial cell reactions and interactions
Astrocytes React to SCI by Forming a Glial Scar, Which Limits Both Secondary Damage and Axon Regeneration
Neuropathology causes astrocytes to take on a stereotypic suite of molecular, morphologic, and functional changes that together constitute “astrogliosis” (formed by “reactive” astrocytes) [89]. Astrogliosis after SCI exists along a gradient of intensity defined by the injury severity, time postinjury, and relative spinal location of astrocytes to the lesion epicenter [90, 91]. The most minor signs of astrogliosis include modest molecular and morphologic changes. Reactive astrocytes begin to upregulate key astrocyte intermediate filaments, including glial fibrillary acidic protein (GFAP) (whose upregulation is a hallmark of gliosis), and various secreted factors (e.g., proteoglycans, cytokines, etc.) [92]. In addition, these low-intensity reactive astrocytes show cellular and nuclear hypertrophy; however, this slight stimulation induces minimal astrocyte proliferation and does not cause individual astrocyte domains to overlap with one another [93]. Reactive astrocytes exposed to moderate-to-severe local stimuli show more robust upregulation of GFAP, exaggerated hypertrophy, and upregulated secretion of cytokines (e.g., TGF-β, IL-1β, IL-6) and other factors (e.g., COX-2, iNOS, S100β) [90]. Further, these astrocytes proliferate and begin to impinge on each other’s parenchymal domains [91, 94]. The most robust astrogliosis is observed in the area surrounding the frank lesion: SCI-elicited blood–brain barrier breakdown and inflammation cause reactive astrocytes to proliferate, migrate, and align to contribute to a glial scar that surrounds the lesion (see Cregg et al. [95]). This glial scar forms between 5 and 14 days post-SCI in rodents [96], and remains intact throughout chronic SCI stages in rodents [96] and humans [97]. Perilesional reactive astrocytes—along with other cells such as fibroblasts and OPCs—form a mesh-like entanglement that creates a physical barrier, and they secrete various molecules that shape later inflammatory and repair processes [95] (Fig. 3).
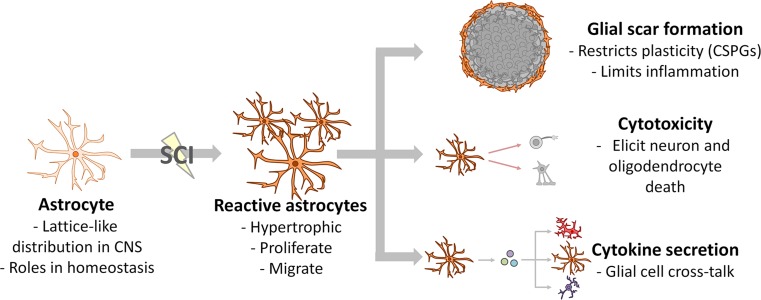
Fig. 3.
Astrocyte responses after SCI. Astrocytes respond soon after SCI by becoming reactive, proliferating, and migrating toward the lesion. Surviving astrocytes closest to the lesion reorient to form a dense meshwork. This meshwork of cells, combined with secreted extracellular matrix molecules such as chondroitin sulfate proteoglycans, form the glial scar. The glial scar limits axon plasticity, but also restricts the spread of cytotoxic inflammation. Reactive astrocytes can produce cytotoxic factors, and secrete cytokines to communicate with other glial cells
Astrocytes: Beneficial Roles in SCI Repair
Reactive astrocytes near the lesion have roles that are both beneficial and detrimental to SCI repair. First, beneficial effects will be considered. As in other tissues, fibrosis during pathology was likely evolutionarily adaptive due to its tissue-preserving (and potentially life-saving) benefits [98]. Typically, the SCI lesion epicenter contains abundant inflammatory cells, such as macrophages and microglia, which produce secreted factors that can exacerbate tissue damage (“secondary damage”). Within acute times post-SCI, the lesion epicenter is devoid of parenchyma or surviving endogenous CNS cells—many CNS cells at the lesion site will have died through necrosis or apoptosis. The epicenter gradually becomes surrounded and contained by the glial scar preventing the spread of necrotic and apoptotic cell death.
The glial scar’s role in confining inflammation to the lesion epicenter has been studied extensively by Sofroniew’s group. Early studies showed that genetically ablating astrocytes had severe consequences for postinjury pathology and recovery [99–101]. Ablating astrocytes using a ganciclovir-thymidine kinase strategy greatly worsens pathology: a simple stab wound SCI in mice lacking astrocytes robustly worsened inflammation, lesion expansion, and motor dysfunction [100]. The ability of astrocytes to limit inflammatory spread requires signal transducers and activators of transcription (STAT)-3 signaling. STAT3 is a transcription factor that drives expression of several injury-induced cytokines and trophic factors, including IL-6, IL-10, TGF-α, EGF, LIF, and CNTF [102]. Astrocytes in uninjured spinal cord that lacked STAT3 had typical morphology and density; however, in mice that received L1/L2 moderate crush injury, conditional deletion of STAT3 in astrocytes prevented typical scar formation and exacerbated inflammation, lesion expansion, and motor dysfunction [94, 96]. Disrupting scar formation by ablating astrocytes or by removing astrocyte STAT3 also prevented growth factor-elicited axon regeneration [103, 104]. Limiting astrogliosis or removing astrocytes can be detrimental in other models of CNS pathology, including ischemia and experimental autoimmune encephalomyelitis [105, 106]. It is notable, however, that specific aspects of SCI-elicited astrogliosis worsen inflammation [107, 108]. Indeed, a recent compelling study highlighted the complex nature of post-SCI astrocyte dynamics: mice lacking the complement factor C5a receptor showed initial locomotor improvements at 7 days post-SCI, but had worsened recovery at chronic times versus wild-type mice; early improvements in deficient mice were associated with reduced astrocyte proliferation and epicenter inflammation [109]. This suggests that future preclinical and therapeutic strategies should consider the complex temporal dynamics of glial scar formation. Overall, these studies suggest that scar-forming astrocytes can help restrict the spread of toxic aspects of inflammation, thereby preventing lesion expansion and further loss of function.
Astrocytes: Detrimental Roles in SCI Repair
Astrocytes also have detrimental roles after SCI. The physical and molecular properties of the scar limit the spread of toxic inflammation, but they also prevent axon regrowth. Densely packed astrocytes present a physical barrier to regenerating axons ([96]; however, simply removing the scar may not be useful—see above and [104]). SCI-elicited breakdown of scaffolding in the extracellular matrix (ECM) likely softens tissue and contributes to regeneration failure [110, 111]. In addition, pioneering research in the 1980s and 1990s by Silver’s group established that scar-localized astrocytes generate a long-lasting molecular barrier to axon regeneration. Early in vitro studies suggested that mature astrocytes form an entangled scaffold that prevents axon extension and that sulfated proteoglycans are inhibitory to neurite outgrowth [112–114]. Soon after, chondroitin sulfate proteoglycans (CSPGs) and extracellular proteins called tenascins in the scar were identified as local inhibitors of axon growth [115]. Indeed, inhibiting CSPGs using antibodies improved neurite growth on glial scars in vivo [116]. CSPGs are deposited into the ECM within 24 h of SCI, and remain around the epicenter for months postinjury [117].
An especially effective strategy for attenuating CSPG inhibitory activity occurs by removing glycosaminoglycan (GAG) side chains (see [110, 118]). CSPGs are composed of a protein core with attached GAG side chains; GAGs can be removed using the bacterial enzyme chondroitinase ABC (ChABC). Bradbury et al. [119] found that ChABC treatment after C4 dorsal column crush lesion improved corticospinal axon regeneration, functional axon reconnection (using electrophysiology), and recovery of sensorimotor function (tape removal and walking tests). This initial work led to a flood of research highlighting the widespread effects of ChABC as a treatment for SCI (current number of PubMed results for “chondroitinase ABC spinal cord injury” = 185). Subsequent studies have shown that ChABC promotes plasticity of various axon systems, including primary afferents [120, 121] and descending axons derived from brainstem nuclei [122, 123]. ChABC also affects the response of non-neuronal cells: ChABC reduces lesion size and causes epicenter macrophages to take on a less damaging, anti-inflammatory phenotype [124], and ChABC relieves CSPG-dependent inhibition of OPC recruitment and morphological differentiation [90, 125]. In addition, ChABC has been used recently in effective combinatorial strategies. Combining ChABC with peripheral nerve graft and growth factors or other treatments increases post-SCI axon plasticity and recovery in various rodent SCI models, including after chronic SCI [103, 126–129]. Although challenges related to ChABC safety and delivery have delayed clinical trials in humans [130], these studies using ChABC underscore the potential of modulating the glial scar for effective stand-alone or combinatorial SCI therapies.
Additional research has revealed CSPG-specific receptors that mediate axon growth inhibition [131]. Disrupting signaling by either receptor protein tyrosine phosphatase (PTP)-σ [132, 133] or leukocyte common antigen-related phosphatase [134, 135] improved the post-SCI regenerative capacity of CNS axons; however, corticospinal axon regeneration beyond the scar with these treatments was limited suggesting persistent inhibitory signaling via unidentified CSPG receptors, additional extrinsic factors (e.g., myelin-associated inhibitors), and/or an insufficient neuron-intrinsic growth response. CSPGs also indirectly activate an EGFR-dependent pathway in neurons to inhibit axon growth [136, 137]. Dampening intracellular signaling pathways within reactive astrocytes also shows promise: increasing the microRNA miR-21, which likely limits activation of several intracellular signaling pathways, reduced astrogliosis and improved axon plasticity [138].
Thus, reactive astrocytes in the glial scar have beneficial roles—they help confine toxic elements to the epicenter and limit secondary damage—and detrimental roles—they produce molecules and matrix that prevent axon plasticity and limit post-SCI repair. Unmodified post-SCI astrocyte responses can be deleterious to OPC and neuron survival, and may further activate microglia (discussed below). Another intriguing and under-studied possibility is that activated peri-lesional astrocytes become “preoccupied” with pathology and neglect their typical homeostatic roles, thereby incidentally exacerbating cell damage and death. Ultimately, effective treatments related to astrogliosis will not completely ablate cells or the scar indiscriminately; rather, therapies will involve a more nuanced approach that modifies the glial environment at an optimal postinjury time to promote axon regrowth and remyelination, while maintaining a barrier against lesion expansion.
Activated Microglia (and Macrophages) Around the Lesion Secrete Factors that Promote Plasticity and Toxicity
Given that microglia are the main CNS-resident immune cell type, it follows that they are strongly activated by spinal cord trauma. Indeed, microglia activation state is tightly linked to the absence or presence of immunomodulatory factors in the CNS microenvironment. Trauma (or other pathology) causes build-up of excess extracellular damage-associated molecular patterns (DAMPs). DAMPS include proteins (e.g., hemoglobin and its products), purine metabolites (e.g., ATP), RNA, and DNA that are either typically found inside the cell (so once released extracellularly, they signal that damage has occurred) or are factors that are actively secreted to initiate inflammation [139–143]. These DAMPs bind to pattern recognition receptors, such as toll-like receptors, which can strongly activate microglia [7, 144]. In addition, SCI elicits massive upregulation of other secreted factors, such as cytokines, that bind cell surface receptors to further activate microglia. Post-SCI immune receptor activation causes microglia to engage signaling pathways that upregulate transcription factors, immunomodulatory receptors, and secreted factors, which constitute a robust pro-inflammatory response that amplifies inflammation and exacerbates pathology (secondary damage) [145]. As with astrocytes, microglia show graded activation phenotypes after SCI: inactivated microglia are ramified, with many long and fine processes; “primed” microglia do not secrete appreciable amounts of pro-inflammatory factors but show morphological evidence of activation and are molecularly sensitized by prior stimulation for a robust inflammatory response; moderately activated microglia show hypertrophy with shorter processes and induced inflammatory factor expression; and strongly activated microglia take on an amoeboid (spherical) morphology in parallel with a robust molecular inflammatory response. It is likely that perilesional microglia migrate and proliferate in the lesion epicenter to form strongly activated microglia; however, these hyperactivated microglia are nearly indistinguishable from hematogenous macrophages, which are likely more prevalent in the lesion epicenter. Indeed, hematogenous macrophages—which have no access to the healthy adult CNS—invade the lesion site after SCI, attracted by chemotactic factors and by the breakdown of the blood–brain barrier (BBB) [146]. These recruited macrophages (and microglia) make up a significant proportion of cells in the lesion epicenter within 7 days post-SCI [147], and remain within the lesion site into chronic postinjury times in rodents and humans. Like microglia, macrophages display plastic responses that depend on factors present in their local milieu. (Other immune cells infiltrate the epicenter as well, but are beyond the scope of this review; see Anwar et al. [148].)
Macrophage responses to CNS injury can be better understood by first considering the immune response in the periphery (non-CNS tissue). How does a healthy peripheral macrophage response proceed? After injury in the periphery (e.g., injury to skin or peripheral nerve), recruited macrophages take on a transient pro-inflammatory phenotype that involves release of cytotoxic molecules that sterilize the wound. Although these cytotoxic factors also cause bystander damage of tissue, this pro-inflammatory portion of the response helps remove pathogens and is temporary [149, 150]. After 1 week, the response shifts toward a more anti-inflammatory proliferative response—this involves the release of anti-inflammatory molecules that help remodel, repair, and regenerate the peripheral tissue. During the final remodeling phase (within weeks), inflammation is resolved as excess immune cells are removed via apoptosis or the circulation (see Gaudet et al., Gensel and Zhang, and Novak and Koh [7, 151, 152]).
Unfortunately, the remarkable effectiveness of the peripheral immune response is not recapitulated in the adult mammalian CNS. The immune response to injury in non-CNS tissue successfully sterilizes, remodels, and repairs the wound within weeks [7, 153]. In contrast, inflammation begins soon after SCI, and a disproportionate pro-inflammatory response persists into chronic phases [154–158] without appreciably enhancing tissue repair or functional recovery. Given that microglia/macrophages display phenotypic plasticity in time based on local conditions [159], it appears that the chronic SCI environment promotes and maintains exaggerated pro-inflammatory microglia/macrophage polarization. In this section, we describe these differences, and consider the beneficial and detrimental aspects of post-SCI microglial/macrophage responses (Fig. 4).
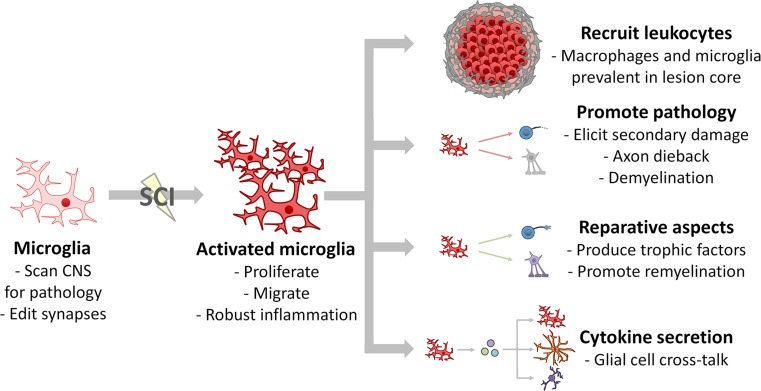
Fig. 4.
Microglial responses after SCI. Microglia respond to SCI in graded manner; they proliferate, produce cytokines, and orchestrate a massive inflammatory response. Microglia help break down the blood–brain barrier, which enables leukocyte infiltration of the lesion epicenter. Aspects of the microglial response to injury are maladaptive—they cause cytotoxicity, axon dieback, and demyelination—while other aspects are reparative—microglia can elicit axon growth and remyelination. Post-SCI microglia also produce myriad cytokines and trophic factors to influence nearby CNS glial cells
Microglia and Macrophages: Beneficial Roles in SCI Repair
The microglial and macrophage response to SCI has beneficial aspects [160]. Activated microglia and macrophages produce factors that encourage axon growth. Intraspinal injection of the inflammatory stimulus zymosan, which activates macrophages and microglia, destroyed axons around the injection site [161]. Interestingly, transplanted GFP+ neurons showed increased axon growth toward the area of macrophage activation [161], suggesting these activated macrophages have concomitant growth-promoting and neurotoxic properties. When dystrophic adult axons contact activated microglia or macrophages, retraction is induced (axon dieback; [162, 163]). This suggests that microglia/macrophages promote axon growth from a distance, but can suppress axon growth or maintenance upon contact.
Our recent data using a novel co-culture system underscore that macrophages can support axon growth and neuron survival. Adult dorsal root ganglion neurons survived and extended neurites on macrophages (in the absence of laminin or any other substrate coating the wells) [164]. Pro-inflammatory macrophages—which model the cell type found in the SCI epicenter—were less growth-supportive and more cytotoxic, whereas anti-inflammatory macrophages enhanced neurite outgrowth. This highlights that shifting post-SCI polarization of microglia and macrophages could help support axon plasticity after SCI.
Beneficial aspects of inflammation can be harnessed by activating specific microglia/macrophage receptors and intracellular signaling cascades, or by promoting release of reparative molecules. Activating the immune receptor toll-like receptor 2 (which is expressed on microglia/macrophages as well as other resident CNS cells) increased post-SCI inflammation, yet also reduced secondary damage and axon dieback [161, 165]. Spinal cord-infiltrating macrophages may also secrete protective factors, such as the anti-inflammatory cytokine interleukin-10 [166], and boosting release of protective molecules would be advantageous. In addition, MMP-2, which is upregulated during wound remodeling at 7–14 days post-SCI, is beneficial for SCI recovery: MMP-2-deficient mice showed exacerbated lesion expansion, scar formation, vascular instability, and locomotor deficits [167, 168].
Microglia and Macrophages: Detrimental Roles in SCI Repair
Unlike the PNS macrophage response, the CNS microglial/macrophage response is ineffective at repair or resolution. Early studies by Popovich and colleagues highlighted a role for macrophages in spinal cord repair. Microglia and macrophages were found in the lesion epicenter at 3–7 days post-SCI [169]. Depleting macrophages in rats using acute clodronate treatment improved tissue pathology (with minor locomotor improvements) [170], and activating microglia/macrophages (and other glial cells) in the healthy spinal cord using zymosan created an inflammatory lesion [171]. Thus, microglial/macrophage activation appeared necessary to create typical post-SCI tissue pathology and sufficient to cause lesions in uninjured spinal cord.
More recently, it has become clear that the post-SCI phenotype of microglia/macrophages (not just their presence or absence) helps define extent of pathology. Unlike macrophages in the periphery, microglia/macrophages in the CNS do not switch to a reparative phenotype that leads to resolution of inflammation. At 7 days post-SCI, lesion-localized microglia/macrophages displayed a balance between pro- and anti-inflammatory phenotypes; however, by 28 days post-SCI (a chronic time in rodents), microglia/macrophages expressed predominantly pro-inflammatory markers and persisted in the lesion [159]. When in vitro-transformed anti-inflammatory macrophages were injected into the SCI environment, they rapidly switched to a pro-inflammatory phenotype [159]. Thus, the environment of the injured CNS signals to cognate microglia/macrophages to take on a persistent, and ultimately damaging, pro-inflammatory phenotype [159, 172].
Emerging technologies are increasingly effective at delineating post-SCI microglia and macrophage responses. Using mice expressing enhanced green fluorescent protein (EGFP) on a lysozyme M promoter (EGFP expressed in macrophages, but not microglia; however, note that lysozyme M is also expressed by neutrophils [173]), Greenhalgh and David [174] found that microglia were responsible for early (1–3 days) post-SCI phagocytosis, whereas macrophages were the major phagocytic cell from 7 to 42 days post-SCI. Interestingly, microglia were better at clearing intracellular phagocytosed debris; macrophages contained persistent phagocytic debris and were more susceptible to apoptotic and necrotic cell death. Another distinction is that macrophages, but not microglia, showed SCI-elicited upregulation of the anti-inflammatory enzyme arginase-1 [175]. In a mouse model of multiple sclerosis (called experimental autoimmune encephamyelitis), preventing infiltration of hematogenous macrophages (by using parabiosis donors lacking the chemokine receptor Ccr2) substantially ameliorated disease progression and severity [176]. This suggests that infiltrating macrophages in particular could worsen the course of neurologic diseases, and that microglia and macrophages can have distinct responses during pathology [177]. Recent research has identified other microglia-specific markers, including Sall1, Fcrls, and P2ry12 [41, 178]. Future studies should use these and other tools to compare post-SCI responses of microglia versus macrophages.
Improving the resolution or reparative capacity of post-SCI microglia/macrophages can benefit spinal cord repair. Intraperitoneal treatment with the pro-resolution mediator Maresin 1 shifted intraspinal microglial/macrophage responses toward an anti-inflammatory phenotype; this improved phagocytosis, neuroprotection, and locomotor recovery [179]. Reducing early SCI-induced expression of specific matrix metalloproteinases (MMP-9 or MMP-12), which are enzymes that degrade extracellular proteins and remodel the environment, better preserved BBB integrity and improved neuroprotection, locomotor recovery, and bladder function [146, 180–182]. Shifting the microglia and macrophage SCI response toward an anti-inflammatory phenotype can also be protective. Genetic deletion of the pro-inflammatory cytokine TNF increased post-SCI anti-inflammatory markers arginase-1 and CD206, and led to improved locomotor recovery in an open field [172]. Similarly, inhibiting the NOX enzyme—which increases release of reactive oxygen species—reduced post-SCI oxidative stress, pro-inflammatory microglia/macrophage polarization, miroglial/macrophage density, and locomotor deficits [183, 184].
Another promising neuroprotective approach is to modulate key upstream regulators of CNS macrophage polarization. To that end, our group studied a critical pro-inflammatory microRNA, miR-155. microRNAs act by binding to complementary oligonucleotide regions in the 3′ untranslated region of specific mRNAs to cause their downregulation or degradation; miR-155 targets several anti-inflammatory mRNAs, including SHIP-1, C/ebpb, and others, and contributes to inflammatory disorders [185–187]. Indeed, our group found using microarrays that miR-155 is required for a strong induction of pro-inflammatory macrophage polarization [188]. miR-155 also drives pro-inflammatory polarization of microglia [189, 190]. When co-cultured with dorsal root ganglion neurons, miR-155 knockout macrophages better supported axon growth and neuron survival [164]. These results corroborated with our in vivo findings: miR-155 knockout mice with SCI showed improved regeneration of CNS axons, neuroprotection, and locomotor recovery [164]. Thus, a more reparative inflammatory response may be created by targeting microRNAs or other key microglia/macrophage signaling hubs.
Therefore, post-SCI activated microglia/macrophages produce cytotoxic factors that cause secondary damage; a prolonged and exaggerated pro-inflammatory response hinders tissue repair and resolution of the immune response. Revealing novel therapies that shift the microglial/macrophage response to preferentially stimulate beneficial immune effects could help repair the injured spinal cord.
Oligodendrocytes and OPCs After SCI Are Susceptible to Apoptosis, but Also Have the Ability to Repopulate, Remyelinate, and Communicate
SCI-induced oligodendrocyte death in rat occurs within 15 min and oligodendrocytes continue to die for at least 3 weeks [191–194]. After contusion SCI in rat, there was a 50% decrease in OPC and a 93% decrease in oligodendrocyte numbers in the lesion epicenter [195]. Oligodendrocytes succumb to apoptotic or necrotic death via several post-SCI processes [68]: ischemia and oxidative damage, with oligodendrocytes being particularly sensitive [196, 197]; glutamate- or ATP-elicited excitotoxicity, which can elicit in oligodendrocytes toxic accumulation of exorbitant calcium [198–200]; pathologic lipid signaling, which can activate cell death pathways [201]; and an extreme inflammatory environment, which includes cytokines that can kill oligodendrocytes directly or indirectly (see [191]).
Within hours of SCI, degenerating myelin and loss of myelin proteins has been observed [202], and demyelination occurs within 2 weeks post-SCI in rodents and cats [203, 204]. Similarly, humans with chronic SCI present with variable amounts of demyelination in the lesion penumbra [205]. Therefore, demyelination of spared axons in the lesion border could restrict axon conduction and contribute to post-SCI functional deficits.
OPCs in the adult mammal have the ability to proliferate, differentiate, and remyelinate [78, 206]. Indeed, groundbreaking studies by Richard and Mary Bunge showed that remyelination occurs after demyelinating lesion ([207, 208]; see Bunge and Bunge [209]), and that a population of small glia proliferate and form remyelinating cells [210]. These remyelinating progenitors proliferate in response to demyelination, but not in a purely inflammatory lesion [211]. Recent data suggest that CNS remyelination by oligodendrocytes and Schwann cells is a dynamic and protracted process: newly generated myelin after SCI initially forms thinner and shorter sheaths, but these expand and extend over time to form myelin similar to control myelin [212–215].
SCI causes OPCs to become activated, which includes shortening of the cell cycle, altered cell morphology (stubby, thicker branches, and cell hypertrophy), and accumulation of the cells into a dense cellular meshwork surrounding the lesion site [216]. McTigue’s group and others have revealed that post-SCI OPCs proliferate and offer modest intrinsic replenishment of endogenous oligodendrocytes (see [191]). NG2+ OPCs showed significant proliferation in and near the lesion at 1, 2, and 4 (but not 10) weeks post-SCI [195]. Interestingly, despite proliferation, the absolute number of NG2+ cells did not increase in parallel, suggesting that proliferating OPCs replenished the population but was likely balanced by cell death [195]; although note that other cells such as pericytes and macrophages can express NG2 [217]. In the perilesional area, OPCs proliferated particularly in the first week post-SCI and differentiated into mature oligodendrocytes [218]. Others have shown that SCI-elicited OPC increases were sustained for at least 42 days [219]. Although most new oligodendrocytes are generated after SCI from OPCs, some are derived from ependymal cells [220, 221].
As discussed above, oligodendrocytes and OPCs after SCI are often considered to respond to other cells as passive bystanders—rather than active players—but accumulating evidence suggests a more active role for these cells in beneficial and detrimental processes after SCI (Fig. 5).
Fig. 5.
OPC and oligodendrocyte responses after SCI. Oligodendrocytes are sensitive to perturbation and can die or demyelinate after SCI. Surviving oligodendrocytes have some inherent ability to remyelinate. OPCs respond to SCI by becoming activated, proliferating, and migrating to replace lost cells. OPCs can differentiate into oligodendrocytes, and then remyelinate denuded axons. In addition, OPCs contribute to the glial scar, where they can inhibit axon growth (e.g., through exposure of NG2 proteoglycan) and may also enable some plasticity. OPCs can also communicate to other glial cells to influence post-SCI processes
Oligodendrocytes and OPCs: Beneficial Roles in SCI Repair
Several manipulations can improve post-SCI oligodendrocyte differentiation and remyelination (for comprehensive review, see Plemel et al. [78]). Major approaches include cell transplantation and promoting endogenous repair. Remyelination can be improved by transplanting various cells types; cell transplants that encourage oligodendrocyte differentiation and remyelination can enhance spinal cord repair and neurologic recovery [222]. Transplant of human embryonic stem cell-derived OPCs improved remyelination and locomotor recovery. Indeed, OPCs transplanted into rats at 7 days (but not 10 months) post-SCI integrated and migrated in tissue, and differentiated into oligodendrocytes to promote remyelination [223]. Similarly, transplant of mouse neural precursor cells (supported by concomitant growth and immunosuppressive factor treatment) into the injured rat spinal cord supported remyelination and functional recovery when transplanted at 2 but not 8 weeks post-SCI [224]. Transplanted cells could encourage recovery through direct myelination by transplanted cells, but also by producing factors that modulate other key physiologic processes (e.g., endogenous myelination, gliosis, inflammation, etc.). Issues related to cell transplantation (e.g., immunosuppression or unchecked cell division [78]) could be circumvented by identifying and using efficacious transplant-derived molecular constituents. In a model of multiple sclerosis, the beneficial effects of mesenchymal stem cell treatment were recapitulated by delivering a single key secreted factor, hepatocyte growth factor [225]. Similar strategies could be used to discover effective transplant-derived molecules for improving post-SCI repair.
Several treatment strategies could be used to promote endogenous repair. Boosting OPC recruitment and remyelination directly can be achieved by increasing spinal levels of OPC mitogens. Transplant of fibroblasts engineered to express the growth factors neurotrophin-3 or brain-derived neurotrophic factor into the 2-day post-SCI rat lesion supported oligodendrocyte differentiation, myelination, and axon penetration in the graft (assessed at 10 weeks post-SCI) [226]. Delivering the growth factor neuregulin-1 alone or in combination with fibroblast growth factor in mice improved post-SCI OPC proliferation, oligodendrogenesis, and neurologic recovery [227, 228].
Remyelination after SCI can be improved through myelination by Schwann cells, which typically myelinate cells in the PNS. After SCI, the majority of Schwann cells in the CNS are likely derived from OPCs [212, 229, 230]. Recent data support a role for neuregulin-1 in improving myelination by Schwann cells: neuregulin-1 deletion was associated with deficient post-SCI remyelination by CNS-derived Schwann-like cells, as well as worsened axon conduction and locomotor recovery [229]. In addition, relieving inhibition of oligodendrogenesis and myelination can benefit post-SCI outcomes [231]. In demyelinating models, injection of myelin impairs remyelination [232], and myelin clearance is inefficient after SCI—SCI myelin debris persists for at least 3 years in humans [233]. Thus, rendering OPCs insensitive to extracellular myelin [234] or improving phagocytic capacity of immune cells [235] could expedite oligodendrocyte differentiation and remyelination.
Oligodendrocytes and OPCs: Detrimental Roles in SCI Repair
OPCs respond to SCI by producing factors that can hinder spinal cord repair [236]. NG2+ cells can secrete growth-inhibitory CSPGs neurocan and versican [237]. In addition, the NG2 proteoglycan, which is a CSPG expressed on the membranes of these progenitor cells, has complex roles in axon growth after SCI. Cell surface NG2 may help entrap axons near the lesion; axons around the lesion formed stable synaptic-like contacts on NG2+ cells and NG2 knockout SCI mice had further axon dieback, suggesting that NG2 may help prevent axon dieback but also limits axon extension due to NG2’s “stickiness” [238]. In parallel, cell surface NG2 may help promote axon regrowth [238, 239]. Regenerating axons are more prevalent around NG2+ cells [240, 241], and NG2 knockout mice show reduced plasticity of serotonergic fibers [242].
In addition to being a cell-surface protein, NG2 can be cleaved by MMPs and liberated into the ECM [243]; this soluble NG2 can inhibit axon outgrowth. NG2 expression is increased in the glial scar [244], and NG2 as a substrate inhibits neurite outgrowth in vitro [245]. Treatment with an NG2 function-blocking antibody improved post-SCI axon regeneration, conduction, and neurologic recovery [246, 247]. Thus, cell-surface NG2 may have some permissive properties, but overall NG2 in the adult postinjury spinal cord appears to limit growth and entrap extending axons. Interestingly, NG2 and other CSPGs increased by SCI inhibit process outgrowth from oligodendrocytes [125, 248], suggesting that SCI-elicited CSPG deposition also impairs remyelination [249]. In addition, OPCs likely contribute to inflammatory reactions that can affect SCI outcomes ([236]; see below).
Post-SCI Glial Cell Interactions
In this section, the interactions between CNS glial cells will be considered. Although it is convenient to compartmentalize post-SCI processes by individual cell type, it is critical to consider and to study post-SCI cell–cell interactions. Cross-talk between cell types after SCI could lead to detrimental or beneficial feed-forward loops that amplify over time and could substantially alter the course of pathology. For instance, microglia and astrocytes cooperate to shape the neuroinflammatory response and influence oligodendrocyte dynamics. In turn, oligodendrocytes and OPCs can modify neuroinflammation. Here, we highlight how interactions between glial cell types helps define post-SCI neuroinflammation.
A Dynamic Duo: Astrocytes and Microglia Collaborate to Shape Neuroinflammation
Both astrocytes and microglia contribute to postinjury inflammation. Microglial inflammatory responses are so intense that they can mask astrocyte influences (e.g., even 5% microglia contamination of astrocyte cultures has massive effect on inflammatory outcomes) [250]; however, there is also evidence that astrocytes influence the phenotype of microglia. For instance, astrocytes can modulate microglial responses to inflammatory stimuli. The bacterial cell wall component lipopolysaccharide (LPS) upregulates pro-inflammatory factors in microglia but not astrocytes; however, microglia cultured with astrocytes show even stronger increases in pro-inflammatory factors than microglia alone [251], implying the existence of an astrocyte-microglia feedback loop.
Several astrocyte-derived inflammatory mediators have been identified. Astrocyte-derived galectin-9 drives microglial secretion of the pro-inflammatory cytokine TNF [252]. In addition, reactive astrocytes with activated NFκB-dependent transcription produce the complement factor C3; C3 acts on microglial C3aR to induce phagocytosis during acute neuroinflammation, but inhibits phagocytosis during chronic neuroinflammation [253]. Treatment with a C3aR antagonist improved phagocytosis and reduced microglial activation in a mouse model of Alzheimer’s disease [253]; similarly, removing C3 enhanced axon growth and neuron survival after SCI [254]. (Complement also has roles in the healthy CNS, including amyloid-β plaque removal [255] and synapse remodeling [256], highlighting the complexity and sensitivity of this system.) Growth-associated protein 43 (GAP-43, normally associated with actin cytoskeletal dynamics in intrinsic axon growth programs) is expressed in reactive astrocytes and GAP-43-expressing astrocyte-conditioned media-activated microglia to be less toxic and more growth-promoting [257]. Deficiency of aquaporin-4, a water-selective membrane transit protein, caused stimulated astrocytes to robustly upregulate pro-inflammatory cytokines IL-1β and TNF-α and to hyperactivate co-cultured microglia [258]. Astrocytes can also decrease microglial adhesion [259], inflammatory gene expression [260] and co-stimulatory molecule expression [261].
Activated microglia, in turn, strongly influence astrocyte responses. How do microglia regulate the phenotype of astrocytes? A recent study from the Barres group used cytokine arrays to identify key microglial factors that induce reactive astrocytes. Microglia-derived IL-1α, TNF, and C1q were sufficient to elicit detrimental hallmarks of astrogliosis: reduced ability to support neuron and oligodendrocyte survival, synaptogenesis, and phagocytosis, and increased cytotoxicity [262]. Several microglia-derived pro-inflammatory cytokines—including IL-1β, IL-1α, and IL-18—are activated by inflammasomes, intracellular conglomerates of proteins that cleave/activate cytokines and are expressed specifically in microglia but not astrocytes [139, 263–265]. Exaggerated microglial expression of the pro-inflammatory cytokine TNF-α can disrupt a delicate astrocyte homeostatic mechanism: TNF-α caused upregulation of astrocyte prostaglandin-E2, which ultimately elicited excess astrocyte release of glutamate [266]. In turn, excess extracellular glutamate can cause excitotoxicity [267]. Conditioned media from IL-6-treated microglia activated inflammatory signaling in astrocytes and facilitated their proliferation [268]. Microglia that produce the anti-inflammatory cytokine IL-10 act on astrocyte IL-10R1 receptors; in turn, these astrocytes produce the cytokine TGF-β that dampens microglial activation [269].
Overall, activated microglia and astrocytes exist in close proximity and share overlapping inflammatory functions, so it is difficult to delineate specific roles for each cell type. It is important that future studies use innovative cell culture, transgenic, and drug delivery strategies to better understand the individual and combined contributions of microglia and astrocytes. In addition, future research could systematically test the influence of graded activation states of the two cell types and the relationship between in vitro and in vivo phenotypes. Thus, microglia/macrophages and astrocytes communicate with each other, and likely collaborate in a pernicious post-SCI feedback loop to amplify inflammation.
Astrocytes and Microglia Regulate Post-SCI Oligodendrocyte/OPC Survival, Proliferation, and Differentiation
After SCI, reactive astrocytes limit differentiation and myelination by OPCs. In vitro, CSPGs prevented OPC process outgrowth and differentiation into mature oligodendrocytes [270]. Adding ChABC improved post-SCI OPC migration and differentiation [271]. Interestingly, CSPGs also limit survival and migration of experimentally engrafted neural precursor cells [128]. In addition, post-SCI reactive astrocytes upregulate bone morphogenetic proteins (BMPs), which caused OPCs to differentiate into astrocytes instead of oligodendrocytes; BMPs reduced remyelination and recovery of function [272]. Another study found that tenascin-C-elicited quiescent astrocytes inhibited myelination, whereas ciliary neurotrophic factor stimulated astrocytes to promote myelination [273]. The prolonged prevalence of CSPGs and tenascin-C in the injured spinal cord [110] suggests that astrocytes likely limit OPC differentiation and remyelination, and that these processes could be improved with appropriate therapeutics. Recent studies show that astrocytes activated by microglia produce soluble factors that kill oligodendrocytes and neurons [262].
Microglia modulate post-SCI survival/death of oligodendrocytes, and survival, differentiation, and myelination by OPCs. After SCI, activated microglia promote oligodendrocyte apoptosis, even long distances from lesion epicenter; oligodendrocyte death peaks around 8 days post-SCI and likely contributes to demyelination of spared axons [274]. Several microglia-derived factors have been implicated in oligodendrocyte death: activated microglia increase TNF-α, IL-1β, nitric oxide and reactive oxygen species, and extracellular glutamate, all of which can contribute (directly or indirectly) to oligodendroglial death [198, 275–279]. Upregulation of complement factors during inflammation can tag myelin for microglia-mediated phagocytosis [280–282]. Similarly, microglia can contribute to OPC remyelination failure and toxicity [283, 284]. Focal LPS-elicited activation of microglia-driven inflammation caused loss of oligodendrocytes, with subsequent replenishment by OPC division [285]. Indeed, while activated microglia exacerbate oligodendrocyte/OPC death, microglial factors have seemingly paradoxical effects by promoting OPC proliferation, differentiation, and remyelination [286]. Activated microglia produce a suite of factors that promote OPC migration and remyelination [235, 279]. In a cuprizone model of demyelination/remyelination, treatment with the anti-inflammatory drug minocycline reduced remyelination and this correlated with suppressed ciliary neurotrophic factor [287]. Interestingly, reducing TNF-α decreases OPC proliferation and remyelination via the receptor TNFR2 [288, 289], suggesting a dual role for the cytokine. A subset of CD11c+ microglia produce insulin-like growth factor 1, which supports myelination in the developing CNS [290]. Another study showed that pro-inflammatory microglia/macrophages support OPC proliferation, whereas anti-inflammatory microglia/macrophages—which are sparse after SCI—promote oligodendrocyte differentiation and remyelination via activin-A [291]. In addition, demyelinating models have highlighted the importance of microglial/macrophage-mediated phagocytosis of myelin debris. Suppressing the phagocytic response impairs remyelination [292, 293]. Given the complex roles of microglia in OPC/oligodendrocyte cell dynamics, it is perhaps unsurprising that the size of demyelinated lesions correlates both with intensity of inflammation and accumulation of OPCs [294].
Oligodendrocytes and OPCs Modify the Post-SCI Inflammatory Milieu
OPCs can affect the response of other glial cells. Rodriguez et al. [295] identified a detrimental role of cell-intrinsic OPC responses in SCI repair. Wnt-β-catenin signaling is important in OPC-oligodendrocyte development. Interestingly, selectively deleting β-catenin from OPCs reduced OPC proliferation, but also reduced microglial/macrophage activation, astrogliosis, and axon growth inhibition [295, 296]. Thus, β-catenin-mediated support of OPC proliferation is counter-balanced by detrimental influences on neuroinflammatory dynamics.
There is evidence that oligodendrocytes express immune receptors and cytokines, which could modulate activation of adjacent glial cells [279, 297]. Human oligodendrocytes infected with bacteria upregulated the cytokines IL-6, IL-8, and CCL-2 [298]. Similarly, IFN-γ, which is produced by neutrophils and macrophages after SCI [299], caused oligodendrocytes to upregulate several chemokines [300]. OPCs and oligodendrocytes can express the pro-inflammatory cytokine IL-1β [301, 302], which worsens post-SCI pathology [303]. After cerebral hypoperfusion stress, OPCs upregulated MMP-9, thereby eliciting BBB breakdown and neutrophil infiltration; in this manner, early pathological OPC activation caused white matter injury [304]. In a rat model of fetal growth restriction-induced brain damage, transcriptomic and gene network analyses showed that postnatal day 4 oligodendrocytes upregulated inflammatory pathways; these included RNAs related to TNF, IL-6-JAK-STAT3, and complement signaling [305]. Future studies should use similar network analyses after SCI to better understand intrinsic responses of oligodendrocytes and OPCs, and whether these cells produce anti-inflammatory (as well as pro-inflammatory) mediators.
Overall, much remains unknown about how CNS cells interact to influence post-SCI tissue dynamics. Microglia (and macrophages) mount a particularly robust inflammatory response, and participate in cross-talk with astrocytes to shape the neuroinflammatory response. These cells both affect oligodendrocyte and OPC cell responses, but there is also some evidence that oligodendrocytes and OPCs can alter astrocyte and microglial phenotype.
Future Directions: Manipulating Glial Responses to Improve Nervous System Repair
As described above, rodent models have been used extensively for development of potentially translatable SCI therapies. This large body of work shows that several glial-related processes could be targeted to improve spinal cord repair, including reactive astrogliosis and glial scar formation, microglia/macrophage-driven inflammation, and oligodendrocyte-OPC survival and remyelination. Innovative transgenic technologies can help elucidate mechanisms underlying post-SCI changes in astrocytes [104], microglia/macrophages [306], and oligodendrocytes [295]. To further clarify the relationship between glial cells, it is critical to study how these cells respond and interact after SCI, considering both temporal dynamics (Fig. 1) and the spatial relationship between cells (Fig. 2).
One of the most promising treatments in rodent models of SCI involves degrading glial scar CSPGs using ChABC. ChABC administration after rodent SCI can improve axon plasticity [307], inflammation [124], and remyelination [131], as well as recovery of locomotor [119, 122], respiratory [308], and bladder function [309, 310]. Future studies should design and optimize delivery of a ChABC analogue in humans.
In humans, the magnitude of acute SCI-induced inflammatory cytokines in cerebrospinal fluid correlates with later sensorimotor deficit [311], suggesting that neuroinflammatory modulators could help improve post-SCI neuroprotection. Several candidate therapies are in clinical trial [312]. Minocycline, a BBB-permeable antibiotic, inhibits microglial and glial cell activation and related neuroinflammation after rodent SCI [313–315], and has shown some safety and modest efficacy in a phase II placebo-controlled randomized trial [316] (though more subjects and statistical power would be desired [317]). It is important to note that minocycline has broad anti-inflammatory activities (it is not microglia-specific), that some patients treated with minocycline experience deleterious side effects, and that promising preclinical data with minocycline did not translate well in clinical trials for at least one CNS disease, amyotrophic lateral sclerosis [318–320]. Therapeutic hypothermia is protective after cardiac arrest [313] and reduces systemic inflammation [321]. In improving poststroke outcomes, therapeutic hypothermia confers neuroprotection via several coordinated mechanisms: hypothermia reduces CNS metabolism, decreases reactive nitro-oxidative and glutamate toxicity, reduces inflammation and cell death, and dampens edema [322]. The effectiveness of therapeutic hypothermia after SCI has been studied in rodent models; therapeutic hypothermia will also be tested in an upcoming phase II/III trial, which will assess the safety and efficacy of treating within 6 h post-SCI [312]. Other potential neuroprotective agents that are already in use clinically for other indications have shown promise in rodent models of SCI, but have not yet been tested in human trials; these include erythopoietin and nonsteroidal anti-inflammatory drugs (NSAIDS; e.g., ibuprofen) [323]. In translating promising preclinical SCI findings to the clinic, several hurdles must be overcome [324]: challenges include effective study design/blinding, patient recruitment, therapeutic post-SCI time windows, heterogeneity of injuries (e.g., severity, lesion level), and variable degree/type of concomitant polytrauma. Thus, deciphering particularly effective preclinical treatments and relevant/responsive patient populations is critical to enable best use of research time and funds. Regardless, future preclinical studies will continue to identify promising post-SCI immunomodulatory candidates; effective treatments targeting microglia/macrophages will likely boost anti-inflammatory responses, dampen inflammation, and/or capitalize on specific beneficial aspects of the pro-inflammatory response.
Cell or nerve transplants after SCI can also help improve recovery [222]. Cells used for transplant could promote repair by remyelinating axons, populating the lesion cavity, providing a substrate, and/or secreting reparative factors. Potentially effective cells for transplant include Schwann or other myelinating cells, stem/progenitor cells, neural and glial precursor cells, and bone marrow stromal cells. Cell transplant as a therapy has several challenges: these challenges relate to replicating findings; to lack of efficacy/testing in chronic SCI; and to encouraging regenerating axons to leave grafts and re-enter host CNS. Further, there can be challenges related to the specific stem cell used in clinical trial. A recent study used immunodeficient SCI mice that received human stem cells that did not effectively improve human SCI [325]. The mice also did not show significant improvement, despite the fact that other similar human stem cell lines had improved post-SCI recovery; this underscores the importance of selecting, maintaining, and testing effective cell lines prior to clinical trial. In addition, collecting and implanting certain nonautologous cells raises concerns that are ethical (e.g., stem cell collection and use) and safety-related (immune rejection or suppression; unrestrained proliferation of stem cells). Once an effective cell transplant is identified, ethical and safety issues could be avoided by revealing and using the effective mechanical or molecular constituents.
One mechanical strategy for improving spinal cord repair involves implanting biomaterials that modulate glial responses and support axon growth. For instance, hydrogel polymers can support cell migration, trophic factor delivery, and cell transplant [326–328]; they can also be altered to improve cell differentiation and neuroprotection. Several other biomaterials promote post-SCI repair, including agarose, collagen, and hyaluronan/methylcellulose [312]. In addition, self-assembling nanofibers, which form at body temperature a structure similar to in situ ECM, improved post-SCI astrogliosis, neuroprotection, and axon plasticity [329].
Another strategy for understanding how to promote complete repair of the spinal cord is to use comparative models. Zebrafish, frogs, and salamanders provide useful models of effective spinal cord and/or tissue regeneration [330, 331]. Comparative models of repair can also be studied in mammals: neural repair and regeneration occur more effectively in the immature mammalian CNS [332, 333] and in the adult mammalian PNS [7].
Given the complexity and challenge of improving post-SCI reparative responses, it is possible that spinal cord repair will be maximized through combinatorial approaches. Indeed, promising preclinical SCI studies have used several strategies in parallel. After complete transection SCI, a combination of transplanted Schwann cells, olfactory ensheathing cells, and ChABC injection improved axon regeneration and locomotor scores [127]. ChABC combined with exercise [334] or peripheral nerve graft [335] promoted recovery in chronically injured rats. Intraspinal implant of human induced pluripotent stem cells, combined with fibrin matrix and a trophic factor cocktail, elicited neuronal differentiation and long-distance axon growth [336]. Integrating therapeutic stimulation or brain-computer interfaces with biological therapies could also prove effective [337, 338]. For instance, flexible nanowires have been developed that are biocompatible and stretchable for integration in the spinal cord; these enabled optoelectronic interrogation of spinal cord circuits in chronically implanted, free-moving mice [339]. These engineered technologies will be further improved (e.g., to measure single-neuron action potentials), and could be included in a combinatorial SCI therapy. In addition, better understanding cell–cell interactions using in vitro (co-culture, media transfer) and in vivo strategies would further aid development of combinatorial strategies [164, 262]. Although combining treatments presents challenges (e.g., number of groups, treatment compatibility/safety, optimal timing) [340], revealing new effective treatments and thoughtfully combining complementary strategies could lead to robust improvements and novel therapies.
Conclusions
Glial cell biology has progressed remarkably since these cells were first classified in the 1920s. We now know much more about del Rio-Hortega’s microglia that presented as “voracious monsters”—for instance, that they are activated by specific extracellular cues, that activation involves engaging intracellular signaling cascades, and that these signaling cascades effect morphological and functional changes in the cell. Further, it is clear that SCI causes a set of stereotypic changes to glial cells that both hinder and help repair. The glial scar formed largely by astrocytes limits axon plasticity, but also restricts spread of toxic lesion-derived components. Inflammation directed by microglia and macrophages elicits secondary damage, but also drives modest axon plasticity and remyelination. Oligodendrocytes and OPCs may succumb to bystander damage, but also achieve differentiation and remyelination. Given that the response of each of these cells has beneficial aspects, completely abolishing one of these cells or responses is unlikely to be effective. Instead, modifying the post-SCI response of glial cells to promote protective and reparative phenotypes could ameliorate spinal cord pathology. Further, targeting the interactions between these cells could offer synergistic benefits: shifting post-SCI astrocyte–microglia cross-talk could improve the physical and molecular reparative properties of the extracellular milieu; similarly, enhancing astrocyte, microglial, and oligodendroglial responses could boost remyelination and axon sparing. Therefore, modifying SCI-elicited glial cell responses and their interactions presents a promising avenue for therapeutic development. Future studies should identify novel targets and strategies that improve post-SCI reparative responses of glial cells.
Electronic supplementary material
(PDF 1224 kb)
Acknowledgements
The authors are thankful for support over the years from excellent advisers, including Drs. Randy Nelson, Steven Maier, Phillip Popovich, Matt Ramer, and Linda Watkins. The authors appreciate citation management by Heather D’Angelo. Funding was provided by the Craig H. Neilsen Foundation (A.D.G with Steven F. Maier) and the Wings for Life Foundation (A.D.G. and Linda R. Watkins).
Required Author Forms Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1(1):2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- 2.Letterer E. Virchow's contribution to modern pathology; on the 100th anniversary of cellular pathology, August 20, 1858. Hippokrates. 1958;29(16):505–11. [PubMed] [Google Scholar]
- 3.del Rio-Hortega P. Art and artifice in the science of histology. 1933. Histopathology. 1993;22(6):515–25. doi: 10.1111/j.1365-2559.1993.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 4.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333(6039):238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 5.Oudega M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012;349(1):269–88. doi: 10.1007/s00441-012-1440-6. [DOI] [PubMed] [Google Scholar]
- 6.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7(7):a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masgrau R, Guaza C, Ransohoff RM, Galea E. Should we stop saying 'Glia' and 'Neuroinflammation'? Trends Mol Med. 2017;23(6):486–500. doi: 10.1016/j.molmed.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol. 2014;7(1):a020362. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tilborg E, de Theije CGM, van Hal M, Wagenaar N, de Vries LS, Benders MJ, et al. Origin and dynamics of oligodendrocytes in the developing brain: implications for perinatal white matter injury. Glia. 2018;66(2):221–38. doi: 10.1002/glia.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 13.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16(12):1896–905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–47. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19(3):504–16. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62(9):1377–91. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 19.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22(1):183–92. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18(7):942–52. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimelberg HK. Functions of mature mammalian astrocytes: a current view. Neuroscientist. 2010;16(1):79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- 22.Nortley R, Attwell D. Control of brain energy supply by astrocytes. Curr Opin Neurobiol. 2017;47:80–5. doi: 10.1016/j.conb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Rose CR, Chatton JY. Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience. 2016;323:121–34. doi: 10.1016/j.neuroscience.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 25.Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, et al. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci. 2015;35(13):5187–201. doi: 10.1523/JNEUROSCI.4255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 2013;4:102. doi: 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–61. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016;19(12):1619–27. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A. 2016;113(19):E2675–84. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poskanzer KE, Molofsky AV. Dynamism of an astrocyte in vivo: perspectives on identity and function. Annu Rev Physiol. 2017. [DOI] [PMC free article] [PubMed]
- 32.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19(2):182–9. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 33.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–3. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 34.Fujii Y, Maekawa S, Morita M. Astrocyte calcium waves propagate proximally by gap junction and distally by extracellular diffusion of ATP released from volume-regulated anion channels. Sci Rep. 2017;7(1):13115. doi: 10.1038/s41598-017-13243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2(4):E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enkvist MO, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J Neurochem. 1992;59(2):519–26. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- 37.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82(6):1263–70. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shigetomi E, Patel S, Khakh BS. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol. 2016;26(4):300–12. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2017. [DOI] [PubMed]
- 40.Blevins G, Fedoroff S. Microglia in colony-stimulating factor 1-deficient op/op mice. J Neurosci Res. 1995;40(4):535–44. doi: 10.1002/jnr.490400412. [DOI] [PubMed] [Google Scholar]
- 41.Buttgereit A, Lelios I, Yu X, Vrohlings M, Krakoski NR, Gautier EL, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17(12):1397–406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13(8):753–60. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290(5497):1768–71. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 44.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 45.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 46.D'Agostino PM, Gottfried-Blackmore A, Anandasabapathy N, Bulloch K. Brain dendritic cells: biology and pathology. Acta Neuropathol. 2012;124(5):599–614. doi: 10.1007/s00401-012-1018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 48.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82(1):195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 51.Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15(4):209–16. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- 54.Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19(8):995–8. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–51. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 56.Pont-Lezica L, Beumer W, Colasse S, Drexhage H, Versnel M, Bessis A. Microglia shape corpus callosum axon tract fasciculation: functional impact of prenatal inflammation. Eur J Neurosci. 2014;39(10):1551–7. doi: 10.1111/ejn.12508. [DOI] [PubMed] [Google Scholar]
- 57.Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, et al. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017;134(3):441–58. doi: 10.1007/s00401-017-1747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pang Y, Fan LW, Tien LT, Dai X, Zheng B, Cai Z, et al. Differential roles of astrocyte and microglia in supporting oligodendrocyte development and myelination in vitro. Brain Behav. 2013;3(5):503–14. doi: 10.1002/brb3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 61.Bunge MB, Bunge RP, Pappas GD. Electron microscopic demonstration of connections between glia and myelin sheaths in the developing mammalian central nervous system. J Cell Biol. 1962;12:448–53. doi: 10.1083/jcb.12.2.448. [DOI] [PubMed] [Google Scholar]
- 62.Huxley AF, Stampfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol. 1949;108(3):315–39. doi: 10.1113/jphysiol.1949.sp004335. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, et al. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386(6626):724–8. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- 64.Bechler ME, Byrne L, Ffrench-Constant C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr Biol. 2015;25(18):2411–6. doi: 10.1016/j.cub.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59(4):581–95. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56(3):284–93. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- 67.Czopka T, Ffrench-Constant C, Lyons DA. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell. 2013;25(6):599–609. doi: 10.1016/j.devcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107(1):1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA. Oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci. 1996;16(16):5095–105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rasband MN, Peles E. The Nodes of Ranvier: molecular assembly and maintenance. Cold Spring Harb Perspect Biol. 2015;8(3):a020495. doi: 10.1101/cshperspect.a020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirkpatrick LL, Witt AS, Payne HR, Shine HD, Brady ST. Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons. J Neurosci. 2001;21(7):2288–97. doi: 10.1523/JNEUROSCI.21-07-02288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–21. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–8. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, et al. Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep. 2018;22(9):2383–94. doi: 10.1016/j.celrep.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 75.Philips T, Rothstein JD. Oligodendroglia: metabolic supporters of neurons. J Clin Invest. 2017;127(9):3271–80. doi: 10.1172/JCI90610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91(1):119–32. doi: 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366–74. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 78.Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23(12):4967–74. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gritsch S, Lu J, Thilemann S, Wortge S, Mobius W, Bruttger J, et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat Commun. 2014;5:5472. doi: 10.1038/ncomms6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8(2):a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20(17):6404–12. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115(2):535–51. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- 84.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668–76. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Q, Whittemore SR, Devries WH, Zhao X, Kuypers NJ, Qiu M. Dorsally-derived oligodendrocytes in the spinal cord contribute to axonal myelination during development and remyelination following focal demyelination. Glia. 2011;59(11):1612–21. doi: 10.1002/glia.21203. [DOI] [PubMed] [Google Scholar]
- 87.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11(12):1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robins SC, Trudel E, Rotondi O, Liu X, Djogo T, Kryzskaya D, et al. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One. 2013;8(10):e78236. doi: 10.1371/journal.pone.0078236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014;7(2):a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46(2):251–64. doi: 10.1007/s12035-012-8287-4. [DOI] [PubMed] [Google Scholar]
- 91.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 93.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103(46):17513–8. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870–86. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, et al. Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol. 2007;7:17. doi: 10.1186/1471-2377-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lech M, Anders HJ. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989–97. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/S0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 100.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129(Pt 10):2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 102.Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296(5573):1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 103.Alluin O, Delivet-Mongrain H, Gauthier MK, Fehlings MG, Rossignol S, Karimi-Abdolrezaee S. Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS One. 2014;9(10):e111072. doi: 10.1371/journal.pone.0111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28(3):468–81. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 106.Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29(37):11511–22. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202(1):145–56. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, et al. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108(21):8867–72. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brennan FH, Gordon R, Lao HW, Biggins PJ, Taylor SM, Franklin RJ, et al. The complement receptor C5aR controls acute inflammation and astrogliosis following spinal cord injury. J Neurosci. 2015;35(16):6517–31. doi: 10.1523/JNEUROSCI.5218-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gaudet AD, Popovich PG. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol. 2014;258:24–34. doi: 10.1016/j.expneurol.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moeendarbary E, Weber IP, Sheridan GK, Koser DE, Soleman S, Haenzi B, et al. The soft mechanical signature of glial scars in the central nervous system. Nat Commun. 2017;8:14787. doi: 10.1038/ncomms14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10(11):3594–603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith GM, Silver J. Transplantation of immature and mature astrocytes and their effect on scar formation in the lesioned central nervous system. Prog Brain Res. 1988;78:353–61. doi: 10.1016/S0079-6123(08)60304-0. [DOI] [PubMed] [Google Scholar]
- 114.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109(1):111–30. doi: 10.1016/S0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 115.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11(11):3398–411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136(1):32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 117.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182(2):399–411. doi: 10.1016/S0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 118.Dyck SM, Karimi-Abdolrezaee S. Chondroitin sulfate proteoglycans: key modulators in the developing and pathologic central nervous system. Exp Neurol. 2015;269:169–87. doi: 10.1016/j.expneurol.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 119.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 120.Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, et al. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28(46):11998–2009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27(9):2176–85. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107(8):3340–5. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vavrek R, Pearse DD, Fouad K. Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J Neurotrauma. 2007;24(10):1667–73. doi: 10.1089/neu.2007.0290. [DOI] [PubMed] [Google Scholar]
- 124.Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34(14):4822–36. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Doring A, Sloka S, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72(3):419–32. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- 126.DePaul MA, Lin CY, Silver J, Lee YS. Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Sci Rep. 2017;7(1):9018. doi: 10.1038/s41598-017-09432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25(5):1169–78. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30(5):1657–76. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu D, Klaw MC, Connors T, Kholodilov N, Burke RE, Tom VJ. Expressing constitutively active rheb in adult neurons after a complete spinal cord injury enhances axonal regeneration beyond a chondroitinase-treated glial scar. J Neurosci. 2015;35(31):11068–80. doi: 10.1523/JNEUROSCI.0719-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 2014;13(12):1241–56. doi: 10.1016/S1474-4422(14)70144-9. [DOI] [PubMed] [Google Scholar]
- 131.Karimi-Abdolrezaee S, Schut D, Wang J, Fehlings MG. Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PLoS One. 2012;7(5):e37589. doi: 10.1371/journal.pone.0037589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58(4):423–33. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- 133.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592–6. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31(40):14051–66. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518(7539):404–8. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Erschbamer M, Pernold K, Olson L. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J Neurosci. 2007;27(24):6428–35. doi: 10.1523/JNEUROSCI.1037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310(5745):106–10. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 138.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, et al. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32(50):17935–47. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fonken LK, Frank MG, Kitt MM, D'Angelo HM, Norden DM, Weber MD, et al. The Alarmin HMGB1 mediates age-induced neuroinflammatory priming. J Neurosci. 2016;36(30):7946–56. doi: 10.1523/JNEUROSCI.1161-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kigerl KA, Lai W, Wallace LM, Yang H, Popovich PG. High mobility group box-1 (HMGB1) is increased in injured mouse spinal cord and can elicit neurotoxic inflammation. Brain Behav Immun. 2017. [DOI] [PMC free article] [PubMed]
- 141.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 142.Gladwin MT, Ofori-Acquah SF. Erythroid DAMPs drive inflammation in SCD. Blood. 2014;123(24):3689–90. doi: 10.1182/blood-2014-03-563874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang YK, Liu JT, Peng ZW, Fan H, Yao AH, Cheng P, et al. Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. J Neuroinflammation. 2013;10:112. doi: 10.1186/1742-2094-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noble LJ, Wrathall JR. Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol. 1989;103(1):34–40. doi: 10.1016/0014-4886(89)90182-9. [DOI] [PubMed] [Google Scholar]
- 146.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–35. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res. 2008;86(9):1944–58. doi: 10.1002/jnr.21659. [DOI] [PubMed] [Google Scholar]
- 148.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci. 2011;31(35):12533–42. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, Lornet G, et al. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation. 2012;9:176. doi: 10.1186/1742-2094-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619;2015:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 152.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183(5):1352–63. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gaudet AD, Leung M, Poirier F, Kadoya T, Horie H, Ramer MS. A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol. 2009;220(2):320–7. doi: 10.1016/j.expneurol.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 154.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133(Pt 2):433–47. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pajoohesh-Ganji A, Byrnes KR. Novel neuroinflammatory targets in the chronically injured spinal cord. Neurotherapeutics. 2011;8(2):195–205. doi: 10.1007/s13311-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pruss H, Kopp MA, Brommer B, Gatzemeier N, Laginha I, Dirnagl U, et al. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain Pathol. 2011;21(6):652–60. doi: 10.1111/j.1750-3639.2011.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gaudet AD, Sweet DR, Polinski NK, Guan Z, Popovich PG. Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair. Mol Cell Neurosci. 2015;64:84–94. doi: 10.1016/j.mcn.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494(4):578–94. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury—past, present and future. Exp Neurol. 2014;258:91–104. doi: 10.1016/j.expneurol.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 161.Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29(12):3956–68. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28(38):9330–41. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McPhail LT, Stirling DP, Tetzlaff W, Kwiecien JM, Ramer MS. The contribution of activated phagocytes and myelin degeneration to axonal retraction/dieback following spinal cord injury. Eur J Neurosci. 2004;20(8):1984–94. doi: 10.1111/j.1460-9568.2004.03662.x. [DOI] [PubMed] [Google Scholar]
- 164.Gaudet AD, Mandrekar-Colucci S, Hall JC, Sweet DR, Schmitt PJ, Xu X, et al. miR-155 deletion in mice overcomes neuron-intrinsic and neuron-extrinsic barriers to spinal cord repair. J Neurosci. 2016;36(32):8516–32. doi: 10.1523/JNEUROSCI.0735-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain. 2014;137(Pt 3):707–23. doi: 10.1093/brain/awt341. [DOI] [PubMed] [Google Scholar]
- 166.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26(39):9841–50. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trivedi A, Zhang H, Ekeledo A, Lee S, Werb Z, Plant GW, et al. Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord. Exp Neurol. 2016;284(Pt A):50–62. [DOI] [PMC free article] [PubMed]
- 169.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443–64. doi: 10.1002/(SICI)1096-9861(19970120)377:3<443::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 170.Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158(2):351–65. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 171.Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol. 2002;61(7):623–33. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 172.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83(5):1098–116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 173.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–77. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 174.Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci. 2014;34(18):6316–22. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Greenhalgh AD, Passos Dos Santos R, Zarruk JG, Salmon CK, Kroner A, David S. Arginase-1 is expressed exclusively by infiltrating myeloid cells in CNS injury and disease. Brain Behav Immun. 2016;56:61–7. [DOI] [PubMed]
- 176.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–9. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 177.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339(6116):156–61. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Francos-Quijorna I, Santos-Nogueira E, Gronert K, Sullivan AB, Kopp MA, Brommer B, et al. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci. 2017;37(48):11731–43. doi: 10.1523/JNEUROSCI.1395-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Levine JM, Cohen ND, Fandel TM, Levine GJ, Mankin J, Griffin JF, et al. Early blockade of matrix metalloproteinases in spinal-cord-injured dogs results in a long-term increase in bladder compliance. J Neurotrauma. 2017;34(18):2656–67. doi: 10.1089/neu.2017.5001. [DOI] [PubMed] [Google Scholar]
- 181.Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, et al. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23(31):10107–15. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM, et al. Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. J Neurosci. 2011;31(44):15894–903. doi: 10.1523/JNEUROSCI.3943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khayrullina G, Bermudez S, Byrnes KR. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J Neuroinflammation. 2015;12:172. doi: 10.1186/s12974-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 2016;39(12):862–79. doi: 10.1016/j.tins.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fonken LK, Gaudet AD, Gaier KR, Nelson RJ, Popovich PG. MicroRNA-155 deletion reduces anxiety- and depressive-like behaviors in mice. Psychoneuroendocrinology. 2016;63:362–9. doi: 10.1016/j.psyneuen.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 186.Gaudet AD, Fonken LK, Gushchina LV, Aubrecht TG, Maurya SK, Periasamy M, et al. miR-155 deletion in female mice prevents diet-induced obesity. Sci Rep. 2016;6:22862. [DOI] [PMC free article] [PubMed]
- 187.Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist. 2017:1073858417721150. [DOI] [PMC free article] [PubMed]
- 188.Jablonski KA, Gaudet AD, Amici SA, Popovich PG, Guerau-de-Arellano M. Control of the inflammatory macrophage transcriptional signature by miR-155. PLoS One. 2016;11(7):e0159724. doi: 10.1371/journal.pone.0159724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol. 2015;77(1):75–99. doi: 10.1002/ana.24304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135(1):73–88. doi: 10.1111/j.1365-2567.2011.03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8(2):262–73. doi: 10.1007/s13311-011-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3(1):73–6. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 193.Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001;168(2):273–82. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- 194.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17(14):5395–406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17(2):83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 197.Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67(3):1014–22. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- 198.Domercq M, Sanchez-Gomez MV, Sherwin C, Etxebarria E, Fern R, Matute C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol. 2007;178(10):6549–56. doi: 10.4049/jimmunol.178.10.6549. [DOI] [PubMed] [Google Scholar]
- 199.Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27(35):9525–33. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–7. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 201.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–39. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 202.Banik NL, Powers JM, Hogan EL. The effects of spinal cord trauma on myelin. J Neuropathol Exp Neurol. 1980;39(3):232–44. doi: 10.1097/00005072-198005000-00002. [DOI] [PubMed] [Google Scholar]
- 203.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2(4):299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 204.Gledhill RF, Harrison BM, McDonald WI. Demyelination and remyelination after acute spinal cord compression. Exp Neurol. 1973;38(3):472–87. doi: 10.1016/0014-4886(73)90169-6. [DOI] [PubMed] [Google Scholar]
- 205.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192(2):384–93. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 206.Kondiles BR, Horner PJ. Myelin plasticity, neural activity, and traumatic neural injury. Dev Neurobiol. 2018;78(2):108–22. doi: 10.1002/dneu.22540. [DOI] [PubMed] [Google Scholar]
- 207.Bunge MB, Bunge RP, Ris H. Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord. J Biophys Biochem Cytol. 1961;10:67–94. doi: 10.1083/jcb.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bunge RP, Bunge MB. Rish. Electron microscopic study of demyelination in an experimentally induced lesion in adult cat spinal cord. J Biophys Biochem Cytol. 1960;7:685–96. doi: 10.1083/jcb.7.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 210.Carroll WM, Jennings AR, Mastaglia FL. The origin of remyelinating oligodendrocytes in antiserum-mediated demyelinative optic neuropathy. Brain. 1990;113(Pt 4):953–73. doi: 10.1093/brain/113.4.953. [DOI] [PubMed] [Google Scholar]
- 211.Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28(4–5):365–81. doi: 10.1023/A:1007069815302. [DOI] [PubMed] [Google Scholar]
- 212.Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JA, Manesh SB, et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci. 2017;37(36):8635–54. doi: 10.1523/JNEUROSCI.2409-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hesp ZC, Goldstein EZ, Miranda CJ, Kaspar BK, McTigue DM. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J Neurosci. 2015;35(3):1274–90. doi: 10.1523/JNEUROSCI.2568-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Powers BE, Lasiene J, Plemel JR, Shupe L, Perlmutter SI, Tetzlaff W, et al. Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J Neurosci. 2012;32(15):5120–5. doi: 10.1523/JNEUROSCI.0002-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, et al. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci U S A. 2013;110(10):4075–80. doi: 10.1073/pnas.1210293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59(6):869–81. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- 217.Levine J. The reactions and role of NG2 glia in spinal cord injury. Brain Res. 2016;1638(Pt B):199–208. [DOI] [PMC free article] [PubMed]
- 218.Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55(7):698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- 219.Rabchevsky AG, Sullivan PG, Scheff SW. Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia. 2007;55(8):831–43. doi: 10.1002/glia.20508. [DOI] [PubMed] [Google Scholar]
- 220.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470–82. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 221.Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31(6–7):469–80. doi: 10.1023/A:1025739630398. [DOI] [PubMed] [Google Scholar]
- 222.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–82. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377–89. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15(6):862–70. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18(14):5354–65. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57(3):270–85. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Whittaker MT, Zai LJ, Lee HJ, Pajoohesh-Ganji A, Wu J, Sharp A, et al. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia. 2012;60(2):281–94. doi: 10.1002/glia.21262. [DOI] [PubMed] [Google Scholar]
- 229.Bartus K, Galino J, James ND, Hernandez-Miranda LR, Dawes JM, Fricker FR, et al. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain. 2016;139(Pt 5):1394–416. doi: 10.1093/brain/aww039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578–90. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Plemel JR, Manesh SB, Sparling JS, Tetzlaff W. Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia. 2013;61(9):1471–87. doi: 10.1002/glia.22535. [DOI] [PubMed] [Google Scholar]
- 232.Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26(1):328–32. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, et al. Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain. 2005;128(Pt 2):356–64. doi: 10.1093/brain/awh355. [DOI] [PubMed] [Google Scholar]
- 234.Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, Ffrench-Constant C, et al. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain. 2009;132(Pt 2):465–81. doi: 10.1093/brain/awn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rawji KS, Kappen J, Tang W, et al. Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in ageing mice visualized by ex vivo live multiphoton Imaging. J Neurosci. 2018. [DOI] [PMC free article] [PubMed]
- 236.Hackett AR, Lee JK. Understanding the NG2 glial scar after spinal cord injury. Front Neurol. 2016;7:199. doi: 10.3389/fneur.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22(6):2225–36. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, et al. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci. 2014;34(49):16369–84. doi: 10.1523/JNEUROSCI.1309-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26(14):3829–39. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23(28):9276–88. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol. 2006;65(4):406–20. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- 242.de Castro R, Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192(2):299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 243.Asher RA, Morgenstern DA, Properzi F, Nishiyama A, Levine JM, Fawcett JW. Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci. 2005;29(1):82–96. doi: 10.1016/j.mcn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 244.Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22(7):2792–803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14(12):7616–28. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Petrosyan HA, Hunanyan AS, Alessi V, Schnell L, Levine J, Arvanian VL. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J Neurosci. 2013;33(9):4032–43. doi: 10.1523/JNEUROSCI.4702-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26(18):4729–39. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPsigma. Exp Neurol. 2013;247:113–21. doi: 10.1016/j.expneurol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 249.Harlow DE, Macklin WB. Inhibitors of myelination: ECM changes. CSPGs and PTPs. Exp Neurol. 2014;251:39–46. doi: 10.1016/j.expneurol.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Barbierato M, Facci L, Argentini C, Marinelli C, Skaper SD, Giusti P. Astrocyte-microglia cooperation in the expression of a pro-inflammatory phenotype. CNS Neurol Disord Drug Targets. 2013;12(5):608–18. doi: 10.2174/18715273113129990064. [DOI] [PubMed] [Google Scholar]
- 252.Steelman AJ, Li J. Astrocyte galectin-9 potentiates microglial TNF secretion. J Neuroinflammation. 2014;11:144. doi: 10.1186/s12974-014-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lian H, Litvinchuk A, Chiang AC, Aithmitti N, Jankowsky JL, Zheng H. Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer's disease. J Neurosci. 2016;36(2):577–89. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Peterson SL, Nguyen HX, Mendez OA, Anderson AJ. Complement protein C3 suppresses axon growth and promotes neuron loss. Sci Rep. 2017;7(1):12904. doi: 10.1038/s41598-017-11410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28(25):6333–41. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in development and disease. Adv Immunol. 2017;135:53–79. doi: 10.1016/bs.ai.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 257.Hung CC, Lin CH, Chang H, Wang CY, Lin SH, Hsu PC, et al. Astrocytic GAP43 Induced by the TLR4/NF-kappaB/STAT3 axis attenuates astrogliosis-mediated microglial activation and neurotoxicity. J Neurosci. 2016;36(6):2027–43. doi: 10.1523/JNEUROSCI.3457-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sun H, Liang R, Yang B, Zhou Y, Liu M, Fang F, et al. Aquaporin-4 mediates communication between astrocyte and microglia: implications of neuroinflammation in experimental Parkinson's disease. Neuroscience. 2016;317:65–75. doi: 10.1016/j.neuroscience.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 259.Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the alpha6beta1 integrin. J Neurosci. 2002;22(5):1562–72. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Aloisi F, Penna G, Cerase J, Menendez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J Immunol. 1997;159(4):1604–12. [PubMed] [Google Scholar]
- 261.Acevedo G, Padala NK, Ni L, Jonakait GM. Astrocytes inhibit microglial surface expression of dendritic cell-related co-stimulatory molecules through a contact-mediated process. J Neurochem. 2013;125(4):575–87. doi: 10.1111/jnc.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF. The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: a role for the NLRP3 inflammasome. Brain Behav Immun. 2016;55:215–24. doi: 10.1016/j.bbi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, et al. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One. 2015;10(6):e0130624. doi: 10.1371/journal.pone.0130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2016;113(24):E3441–50. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4(7):702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 267.Lepore AC, O'Donnell J, Kim AS, Yang EJ, Tuteja A, Haidet-Phillips A, et al. Reduction in expression of the astrocyte glutamate transporter, GLT1, worsens functional and histological outcomes following traumatic spinal cord injury. Glia. 2011;59(12):1996–2005. doi: 10.1002/glia.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Molet J, Mauborgne A, Diallo M, Armand V, Geny D, Villanueva L, et al. Microglial Janus kinase/signal transduction and activator of transcription 3 pathway activity directly impacts astrocyte and spinal neuron characteristics. J Neurochem. 2016;136(1):133–47. doi: 10.1111/jnc.13375. [DOI] [PubMed] [Google Scholar]
- 269.Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62(6):881–95. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119(1):176–88. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- 271.Siebert JR, Stelzner DJ, Osterhout DJ. Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp Neurol. 2011;231(1):19–29. doi: 10.1016/j.expneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 272.Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31(16):6053–8. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Nash B, Thomson CE, Linington C, Arthur AT, McClure JD, McBride MW, et al. Functional duality of astrocytes in myelination. J Neurosci. 2011;31(37):13028–38. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50(5):798–808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 275.Kigerl KA, Ankeny DP, Garg SK, Wei P, Guan Z, Lai W, et al. System x(c)(−) regulates microglia and macrophage glutamate excitotoxicity in vivo. Exp Neurol. 2012;233(1):333–41. doi: 10.1016/j.expneurol.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102(28):9936–41. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Merrill JE. Effects of interleukin-1 and tumor necrosis factor-alpha on astrocytes, microglia, oligodendrocytes, and glial precursors in vitro. Dev Neurosci. 1991;13(3):130–7. doi: 10.1159/000112150. [DOI] [PubMed] [Google Scholar]
- 278.Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151(4):2132–41. [PubMed] [Google Scholar]
- 279.Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014;141(3):302–13. doi: 10.1111/imm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kopper TJ, Gensel JC. Myelin as an inflammatory mediator: myelin interactions with complement, macrophages, and microglia in spinal cord injury. J Neurosci Res. 2017. [DOI] [PMC free article] [PubMed]
- 281.Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168(1):458–65. doi: 10.4049/jimmunol.168.1.458. [DOI] [PubMed] [Google Scholar]
- 282.Zajicek JP, Wing M, Scolding NJ, Compston DA. Interactions between oligodendrocytes and microglia. A major role for complement and tumour necrosis factor in oligodendrocyte adherence and killing. Brain. 1992;115(Pt 6):1611–31. doi: 10.1093/brain/115.6.1611-a. [DOI] [PubMed] [Google Scholar]
- 283.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166(2):464–75. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 284.Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, et al. Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res. 2010;88(8):1632–44. doi: 10.1002/jnr.22335. [DOI] [PubMed] [Google Scholar]
- 285.Goldstein EZ, Church JS, Pukos N, Gottipati MK, Popovich PG, McTigue DM. Intraspinal TLR4 activation promotes iron storage but does not protect neurons or oligodendrocytes from progressive iron-mediated damage. Exp Neurol. 2017;298(Pt A):42–56. [DOI] [PMC free article] [PubMed]
- 286.Plemel JR, Liu WQ, Yong VW. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16(9):617–34. doi: 10.1038/nrd.2017.115. [DOI] [PubMed] [Google Scholar]
- 287.Tanaka T, Murakami K, Bando Y, Yoshida S. Minocycline reduces remyelination by suppressing ciliary neurotrophic factor expression after cuprizone-induced demyelination. J Neurochem. 2013;127(2):259–70. doi: 10.1111/jnc.12289. [DOI] [PubMed] [Google Scholar]
- 288.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4(11):1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 289.Madsen PM, Motti D, Karmally S, Szymkowski DE, Lambertsen KL, Bethea JR, et al. Oligodendroglial TNFR2 mediates membrane TNF-dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J Neurosci. 2016;36(18):5128–43. doi: 10.1523/JNEUROSCI.0211-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. Embo j. 2017;36(22):3292–308. doi: 10.15252/embj.201696056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–8. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35(3):204–12. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- 293.Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10(1):96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125(Pt 2):338–49. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 295.Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci. 2014;34(31):10285–97. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Duncan GJ, Assinck P, Hilton BJ. Canonical Wnt signalling in PDGFRalpha-expressing cells is a critical regulator of astrogliosis and axon regeneration following CNS injury. J Neurosci. 2014;34(49):16163–5. doi: 10.1523/JNEUROSCI.4052-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Cannella B, Raine CS. Multiple sclerosis: cytokine receptors on oligodendrocytes predict innate regulation. Ann Neurol. 2004;55(1):46–57. doi: 10.1002/ana.10764. [DOI] [PubMed] [Google Scholar]
- 298.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J Neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Guerrero AR, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WE, et al. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD, et al. Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci. 2007;27(8):2013–24. doi: 10.1523/JNEUROSCI.4689-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Boghozian R, McKenzie BA, Saito LB, Mehta N, Branton WG, Lu J, et al. Suppressed oligodendrocyte steroidogenesis in multiple sclerosis: Implications for regulation of neuroinflammation. Glia. 2017;65(10):1590–606. doi: 10.1002/glia.23179. [DOI] [PubMed] [Google Scholar]
- 302.Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20(3):489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- 303.Sato A, Ohtaki H, Tsumuraya T, Song D, Ohara K, Asano M, et al. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J Neuroinflammation. 2012;9:65. doi: 10.1186/1742-2094-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123(2):782–6. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rideau Batista Novais A, Pham H, Van de Looij Y, Bernal M, Mairesse J, Zana-Taieb E, et al. Transcriptomic regulations in oligodendroglial and microglial cells related to brain damage following fetal growth restriction. Glia. 2016;64(12):2306–20. doi: 10.1002/glia.23079. [DOI] [PubMed] [Google Scholar]
- 306.Wieghofer P, Prinz M. Genetic manipulation of microglia during brain development and disease. Biochim Biophys Acta. 2016;1862(3):299–309. doi: 10.1016/j.bbadis.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 307.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26(42):10856–67. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011;475(7355):196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord. 2009;47(10):727–32. doi: 10.1038/sc.2009.10. [DOI] [PubMed] [Google Scholar]
- 310.Lee YS, Lin CY, Jiang HH, Depaul M, Lin VW, Silver J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33(26):10591–606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kwon BK, Stammers AM, Belanger LM, Bernardo A, Chan D, Bishop CM, et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma. 2010;27(4):669–82. doi: 10.1089/neu.2009.1080. [DOI] [PubMed] [Google Scholar]
- 312.Ahuja CS, Fehlings M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 2016;5(7):914–24. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. [DOI] [PubMed]
- 314.Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24(9):2182–90. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126(Pt 7):1628–37. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 316.Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012;135(Pt 4):1224–36. doi: 10.1093/brain/aws072. [DOI] [PubMed] [Google Scholar]
- 317.Kramer JL, Curt A. When is the time right for a Phase III clinical study in spinal cord injury (P = 0.05)? Brain. 2012;135(Pt 11):e220. doi: 10.1093/brain/aws217. [DOI] [PubMed] [Google Scholar]
- 318.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6(12):1045–53. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 319.Moller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, et al. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia. 2016;64(10):1788–94. doi: 10.1002/glia.23007. [DOI] [PubMed] [Google Scholar]
- 320.Fanning WL, Gump DW, Sofferman RA. Side effects of minocycline: a double-blind study. Antimicrob Agents Chemother. 1977;11(4):712–7. doi: 10.1128/AAC.11.4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kwon BK, Mann C, Sohn HM, Hilibrand AS, Phillips FM, Wang JC, et al. Hypothermia for spinal cord injury. Spine J. 2008;8(6):859–74. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 322.Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12(3):275–84. doi: 10.1016/S1474-4422(13)70013-9. [DOI] [PubMed] [Google Scholar]
- 323.Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 2011;28(8):1545–88. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kwon BK, Sekhon LH, Fehlings MG. Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine (Phila Pa 1976). 2010;35(21 Suppl):S263–70. doi: 10.1097/BRS.0b013e3181f3286d. [DOI] [PubMed] [Google Scholar]
- 325.Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA, Cummings BJ. Preclinical efficacy failure of human CNS-derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Reports. 2017;8(2):249–63. doi: 10.1016/j.stemcr.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–9. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 327.Itosaka H, Kuroda S, Shichinohe H, Yasuda H, Yano S, Kamei S, et al. Fibrin matrix provides a suitable scaffold for bone marrow stromal cells transplanted into injured spinal cord: a novel material for CNS tissue engineering. Neuropathology. 2009;29(3):248–57. doi: 10.1111/j.1440-1789.2008.00971.x. [DOI] [PubMed] [Google Scholar]
- 328.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28(14):3814–23. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Diaz Quiroz JF, Echeverri K. Spinal cord regeneration: where fish, frogs and salamanders lead the way, can we follow? Biochem J. 2013;451(3):353–64. doi: 10.1042/BJ20121807. [DOI] [PubMed] [Google Scholar]
- 331.Mokalled MH, Patra C, Dickson AL, Endo T, Stainier DY, Poss KD. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science. 2016;354(6312):630–4. doi: 10.1126/science.aaf2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hilton BJ, Bradke F. Can injured adult CNS axons regenerate by recapitulating development? Development. 2017;144(19):3417–29. doi: 10.1242/dev.148312. [DOI] [PubMed] [Google Scholar]
- 333.Nicholls J, Saunders N. Regeneration of immature mammalian spinal cord after injury. Trends Neurosci. 1996;19(6):229–34. doi: 10.1016/0166-2236(96)10021-7. [DOI] [PubMed] [Google Scholar]
- 334.Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31(25):9332–44. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, et al. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29(47):14881–90. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83(4):789–96. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ievins A, Moritz CT. Therapeutic stimulation for restoration of function after spinal cord injury. Physiology (Bethesda). 2017;32(5):391–8. doi: 10.1152/physiol.00010.2017. [DOI] [PubMed] [Google Scholar]
- 338.Moritz CT, Ruther P, Goering S, Stett A, Ball T, Burgard W, et al. New perspectives on neuroengineering and neurotechnologies: NSF-DFG Workshop Report. IEEE Trans Biomed Eng. 2016;63(7):1354–67. doi: 10.1109/TBME.2016.2543662. [DOI] [PubMed] [Google Scholar]
- 339.Lu C, Park S, Richner TJ, Derry A, Brown I, Hou C, et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Sci Adv. 2017;3(3):e1600955. doi: 10.1126/sciadv.1600955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Oudega M, Bradbury EJ, Ramer MS. Combination therapies. Handb Clin Neurol. 2012;109:617–36. doi: 10.1016/B978-0-444-52137-8.00038-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)