Abstract
Purpose
Early and consistent evaluation of cardiac morphology and function throughout an atrophic stimulus is critically important for the design and optimization of interventions. This randomized, controlled trial was designed to: 1) characterize the time course of unloading-induced morpho-functional remodeling, and 2) to examine the effects of exercise with and without low dose testosterone supplementation on cardiac biomarker, structural, and functional parameters during unloading.
Methods
Twenty six subjects completed 70 days of head down tilt bed rest (BR): 9 were randomized to exercise training (Ex), 8 to EX and low dose testosterone (ExT), and 9 remained sedentary (CONT). Exercise consisted of high intensity, continuous, and resistance exercise. Cardiac morphology (left ventricular mass; LVM) and mechanics (longitudinal, radial, and circumferential strain and twist), cardiovascular biomarkers, and cardiorespiratory fitness (VO2peak) were assessed before, during, and following BR.
Results
Sedentary BR resulted in a progressive decline in LVM, longitudinal, radial, and circumferential strain in CONT, while Ex and ExT mitigated decreases in LVM and function. Twist was increased throughout bed rest in sedentary BR, while after an initial increase at BR7, there were no further changes in twist in Ex and ExT. HDL cholesterol was significantly decreased in all groups compared to pre-bed rest (p<0.007). There were no significant changes in other cardiovascular biomarkers. Change in twist was significantly related to change in VO2max (R=0.68, p<0.01).
Conclusion
An integrated approach with evaluation of cardiac morphology, mechanics, VO2peak, and biomarkers provides extensive phenotyping of cardiovascular atrophic remodeling. Exercise training and exercise training with low dose testosterone supplementation abrogates atrophic remodeling.
Keywords: NASA 70 day bed rest study, cardiac mechanics, left ventricular mass, spaceflight
INTRODUCTION
The heart responds with remarkable morpho-functional plasticity and adaptability in response to various stimuli (1). In recent years, although the extent and functional consequences of pathological (2) and physiological hypertrophy (3) have been studied intensively, little is known regarding the impact, magnitude, and time course of atrophic remodeling. Emerging evidence indicates that unveiling these mechanisms may be of central importance for numerous populations exposed to mechanical unloading (e.g. physical inactivity, prolonged bed rest (4), weightlessness during space travel (5), or augmented catabolic state (e.g. cancer) (6). Importantly, early and consistent evaluation of cardiac morphology and function throughout an atrophic stimulus is critically important for the design and optimization of interventions.
Exercise training is one intervention that has been shown to abrogate declines in LV mass and function during unloading (7), nevertheless, the format and intensity of exercise required to induce optimal cardiac improvements has not been investigated. Notably, the maintenance of LV mass and function is a key determinant of cardiorespiratory fitness (VO2peak) (8), a strong predictor of both all-cause and cardiovascular disease mortality (9).
Testosterone therapy is another intervention prescribed to patients exposed to atrophic stimuli (10) that has been shown to significantly increase muscle strength and bone mineral density (11), even at lower doses (12). However, the effects of androgens on the cardiovascular system remain incompletely understood (13), and the effects of exercise training with concurrent low dose testosterone supplementation on atrophic cardiovascular remodeling has not been investigated in humans.
This randomized, controlled trial was designed to 1) comprehensively characterize the time course of unloading-induced morpho-functional remodeling, and 2) examine the effects of high intensity exercise training and low dose testosterone with concurrent high intensity exercise training on cardiac structural and functional parameters.
METHODS
Subjects, Setting, and Procedures
Twenty-six subjects completed 70 days of 6° head down tilt bed rest (BR). Full details of the study methods are reported in companion paper (Cromwell et al.). In brief, subjects were recruited and screened in two phases according to standard National Aeronautics and Space Administration (NASA) Flight Analog Project procedures. Subjects with a history of musculoskeletal injury that could affect exercise performance or expose the subject to an increased risk of injury were excluded from the study. Subjects were confined to BR for the entire 70 days, including personal hygiene, although they were allowed to raise up on one elbow for meals only. Diets were strictly controlled (55% carbohydrate, 30% fat, and 15% protein).
To obtain matched groups, subjects were stratified by baseline characteristics (Table 1) and then randomly assigned to three study groups: 9 were randomized to exercise training (Ex), 8 to EX and testosterone (ExT), and 9 remained sedentary (CONT). All study procedures were reviewed and approved by NASA and University of Texas Medical Branch institutional review boards. All subjects signed a written consent prior to the initiation of any study-related procedures.
Table 1.
Participant characteristics
| CONT (n=9) | Ex (n=9) | ExT (n=8) | |
|---|---|---|---|
| Age (years) | 36 ± 6 | 34 ± 6 | 33 ± 6 |
| Height (cm) | 176 ± 8 | 179 ± 4 | 182 ± 8 |
| Weight (kg) | 79 ± 9 | 76 ± 4 | 77 ± 14 |
| Body surface area (m2) | 1.9 ± 0.2 | 1.9 ± 0.1 | 1.9 ± 0.1 |
| VO2peak (ml/kg/min) | 36.1 ± 6.4 | 42.3 ± 6.3 | 42.5 ± 5.7 |
| Left ventricular mass (g) | 142 ± 16 | 144 ± 12 | 152 ± 11 |
Data are means ± SD or n.
CONT, control group; Ex, exercise group; ExT, exercise and testosterone group.
Exercise Training
The countermeasure consisted of high-intensity aerobic training combined with resistive strength exercise as described previously (14). Subjects exercised 6 d/wk. Supine aerobic exercise was performed using the Standalone Zero Gravity Locomotion Simulator (sZLS) vertical treadmill and a supine cycle ergometer, and resistance exercise was performed on the Horizontal Exercise Fixture. High-intensity interval aerobic exercise and continuous aerobic exercise were performed on alternating days. Resistance exercise was performed 3d/wk on the same day as the continuous aerobic exercise, separated by 4–6 h. The aerobic exercise intensities were prescribed as a percentage of VO2peak achieved during the pre–bed rest upright peak cycle test. Exercise intensity was assessed one time per week throughout bed rest during a continuous, 2-min interval, or 4-min interval aerobic exercise session as described previously (15). Continuous exercise intensity was targeted at 80% of VO2peak. For the interval days, one of three interval protocols were performed: 1) 6 × 2-min stages at target intensities of 70%, 80%, 90%, 100%, 90%, and 80% of VO2peak with a 2-min rest; 2) 8 × 30-s at maximal effort with a 15-s active rest; 3) 4 × 4 min at a target intensity of 85% VO2peak with a 3-min active rest. Each interval was performed once per week. Heart rate was monitored continuously during training sessions. Resistance exercise sessions consisted of three sets of 6, 8, or 12 repetitions (alternating each resistance day) on each of four lifts (supine squat, supine leg press, supine heel raise, and prone lying leg curl).
Testosterone Treatment
The countermeasure consisted of a low-dose, intermittent testosterone regimen throughout the BR period. Placebo (Ex and CONT; saline) or testosterone enanthate injections (ExT; 100 mg/wk, intramuscular) were administered in 2-week intervals during BR (injections occurred on BR−1, BR7, BR28, BR35, BR56, and BR63).
Cardiorespiratory Fitness
Cardiorespiratory fitness was assessed during peak cycle ergometry tests (Lode Excalibur Sport; Lode B.V., Groningen, the Netherlands) performed before (BR−3), during (BR4, 25, 46, 68) and following (BR+0) BR. Peak tests were performed in the upright position before and following BR, and were performed supine during BR. The protocol consisted of a 3-min warm-up at 50 W, followed by 25-W increases each minute. Tests were terminated at volitional fatigue. HR and rhythm were monitored continuously (Q-Stress ECG monitor; Quinton Instruments, Seattle, WA). Metabolic gas exchange was measured continuously during exercise and averaged over 30-second intervals (ParvoMedics TrueOne® 2400, Sandy, UT). Peak VO2 was defined as the highest VO2 value for a given 30-second interval within the last 60 seconds of exercise.
Cardiac Structure and Function
Participants underwent two-dimensional transthoracic and pulsed-Doppler imaging by use of a commercial ultrasound system (iE33, Phillips Healthcare) at least 12 h after the most recent training session before (BR−2), during (BR7, 21, 31, 70), and following (BR+0, +3) BR. Images were obtained by experienced, registered sonographers in the long axis, short axis and apical 4 chamber views according to the American Society of Echocardiography guidelines.(16) Cardiac structural measurements were made in accord with current guidelines and LV mass (LVM) was obtained through 3D quantification. LV volumes were calculated using the biplane Simpson method. The LV length was measured in the apical 4-chamber view and was defined as the end-diastolic length from the mitral valve hinge point plane to the most distal endocardium at the LV apex.(17) Pulsed Doppler recordings were employed to assess diastolic filling; in particular, early (E) and atrial (A) peak mitral inflow velocities were measured and the ratio of early to late diastolic filling velocity (E:A) was calculated. Tissue Doppler data were used to assess E’. Images were analyzed off-line by a single experienced technician blinded to group allocation. A minimum of three consecutive cardiac cycles were measured and averaged. Resting heart rates were obtained on the final loop of each study, and blood pressures were obtained daily at 0600.
LV Strain, Twist, and Untwisting
Radial displacements, circumferential and radial strain as well as strain rate data were averaged between the basal and apical slices. The apical window was utilized for longitudinal strain assessment. In both orientations frame rates were maximized (>60 frames per second). For the purpose of LV rotation assessment, short-axis imaging standardization within and across subjects was maximized using the following criteria. The basal level was defined as the highest basal imaging plane at which uniform full thickness myocardium was observed surrounding the mitral valve at end-systole. As the location of apical imaging acquisition has been shown to confer significant variability in the measurement of apical rotation, we carefully standardized apical imaging. Specifically, from multiple apical acquisitions distal to the papillary muscles, the apical level was chosen as the imaging plane with no visible papillary muscles that most closely approximated an end-diastolic ratio of LV cavity diameter to total LV diameter of 0.5 (17).
Using the original two-dimensional images, a single experienced technician performed offline measurements of the longitudinal, radial, and circumferential planes using a dedicated software package (Q-Lab, Phillips Healthcare). This system tracks acoustic markers within the myocardium, frame-by-frame, over the entire cardiac cycle. The spatial displacement of an acoustic marker indicates local tissue movement. A tracking setting was selected with a width between endocardium and epicardium to include as much myocardium as possible. Sonographer specific coefficient of variation data ranged from 4.9 to 7.1% for strain and twist. A minimum of three consecutive cardiac cycles were measured and averaged. All data were exported to a spreadsheet program (Excel, Microsoft Corporation, Redmond, Washington). Cubic spline interpolation (Matlab, Mathworks, USA) was used to determine data points at 2% increments of systolic duration throughout the cardiac cycle (18). To adjust all parameters for inter-subject differences in heart rate and imaging frame rate, event timing was calculated as a percentage of systole, with the onset of QRS being 0% and aortic valve closure equivalent to 100%. For each participant systole occurred from 0 to 100%, whereas diastole was from 100% onward. Peak systolic LV twist was calculated as the maximum instantaneous difference between peak systolic apical and basal rotation. The timing of peak systolic LV twist was determined as a percentage of systolic duration. Peak early diastolic untwisting rate was defined as the peak untwisting velocity occurring in early diastole. The LV twist is reported as absolute and as LV length-corrected values.
Cardiac Biomarkers and Plasma Volume
Venous blood samples were obtained from fasting participants and immediately processed and stored at −80°C. For each blood sample, 5 mL of whole blood was drawn from an antecubital vein and collected in serum-gel tubes before (BR−3), during (BR28), and following (BR+0, +5) HDBR. Ten cardiovascular biomarkers were measured: cholesterol, low and high density lipoproteins (LDL, HDL), triglyceride, C-reactive protein (CRP), phospholipids, superoxide dismustase (SOD), hematocrit (HCT), hemoglobin (HB), and total antioxidant capacity. Biochemical analyses were performed by standard commercial techniques, as described previously (19).
Plasma volume was assessed four times (BR−2, BR7, BR31, BR+0) using the carbon monoxide rebreathing technique as previously reported (20). Briefly, the subjects breathed 100% oxygen for 2 min through a closed breathing circuit fitted with a two-way nonrebreathing valve (Model 2700; Hans Rudolph, Kansas City, MO). Carbon monoxide (60 ml) was then added to the system and was rebreathed for 10 min. Plasma volume was then calculated.
Statistical Analysis
All statistical analyses were performed using Stata, IC software (v 141, StataCorp LP, College Station, TX) setting 2-tailed alpha to reject the null hypothesis at 0.05. All of our outcomes are continuously scaled and appropriately analyzed with parametric statistical techniques; all statistical assumptions were tested prior to interpreting results. Statistical assumptions were tested prior to hypothesis testing by careful residual evaluations. Subjects’ repeated measures outcomes were submitted to a mixed-effects linear regression analysis with a-priori contrasts comparing baseline (pre-bedrest) to each of the subsequent six observations made during and post-bedrest. Random y-intercept terms were included to accommodate for the repeated-measures experimental design, and Bonferroni adjustments to p-values were used to adjust for the inflated Type I error risk associated with multiple comparisons. The data from all of our outcomes met the distributional requirements for the techniques employed without requiring data transformations or non-linear modeling, however we did eliminate occasional observations when the standardized residual exceeded ± 3.0. This resulted in no more than one of 63 possible observations being eliminated from any given analysis. Data are expressed as using either ± standard deviation or with 95% confidence intervals (CI).
RESULTS
Cardiorespiratory Fitness
Compared to pre-bed rest, VO2peak was significantly decreased in all groups at BR4 (p<0.007): CONT (BR−2: 2.9 ± 0.6 L/min vs. BR4: 2.5 ± 0.4 L/min), Ex (BR−2: 3.2 ± 0.6 L/min vs. BR4: 2.7 ± 0.5 L/min), ExT (BR−2: 3.2 ± 0.6 L/min vs. BR4: 2.8 ± 0.3 L/min). Following the initial decline at BR4, there were no significant differences from pre-bed rest at any other time point for Ex and ExT groups; VO2peak was significantly decreased at all other time points in the CONT group (p<0.007).
Cardiac Structure, Resting Heart Rate, and Blood Pressure
Time course of change in cardiac structural measurements are detailed in Table 2. Image quality was sufficient to obtain 3D LVM in 25 subjects. Among the 25 subjects with a possible total of 175 images, image quality was sufficient in 94%. LV atrophy developed in CONT subjects. Specifically, there was a significant decrease in LV end-diastolic volume (LVEDV), internal diameter wall thickness (LVIDd), and LVM commencing at BR7 (p<0.007). No change in structural measurements occurred throughout bed rest in Ex or ExT groups. There was no change in systolic or diastolic blood pressures in any group. Resting heart rate was preserved in Ex (BR−2: 56 ± 8 bpm; BR70: 61 ± 9 bpm; BR+0: 67 ± 8 bpm) and ExT (BR−2: 58 ± 9 bpm; BR70: 59 ± 7 bpm; BR+0: 61 ± 10 bpm); however resting heart rate was significantly increased in CONT subjects at time points BR70 and BR+0 (BR−2: 62 ± 9 bpm; BR70: 75 ± 12 bpm; BR+0: 93 ± 9 bpm; p<0.007).
Table 2.
LV Structural Parameters
| Time Point | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BR−2 | BR7 | BR21 | BR31 | BR70 | BR+0 | BR+3 | |
| LVEDV, ml | |||||||
| CONT | 137.8 (122.8–152.8) | 122.2 * (107.2–137.2) | 112.6 * (97.2–128.1) | 111.8 * (96.8–126.8) | 114.5 * (99.5–129.5) | 114.6 * (98.9–130.3) | 128.8 (113.7–144.0) |
| Ex | 142.5 (133.2–151.8) | 136.1 (126.8–145.5) | 140.0 (130.7–149.3) | 141.7 (132.4–151.1) | 136.2 (126.9–145.5) | 130.2 (120.6–139.8) | 144.2 (134.9–153.6) |
| ExT | 143.3 (133.2–153.4) | 141.7 (131.6–151.8) | 139.6 (129.2–150.0) | 141.4 (131.3–151.5) | 140.9 (130.8–151.0) | 142.0 (131.7–152.4) | 142.7 (132.6–152.8) |
| LVESV, ml | |||||||
| CONT | 46.5 (40.4–52.6) | 40.5 * (34.4–46.6) | 38.0 * (31.6–44.4) | 38.0 * (31.8–44.1) | 39.2 * (33.1–45.3) | 38.8 * (32.3–45.4) | 44.3 (38.0–50.5) |
| Ex | 53.5 (48.7–58.4) | 46.4 * (41.6–51.3) | 46.9 * (42.1–51.7) | 48.1 (43.3–52.9) | 44.6 * (39.8–49.4) | 48.1 (43.1–53.1) | 51.3 (46.4–56.1) |
| ExT | 49.2 (44.6–53.8) | 50.8 (46.2–55.5) | 47.0 (42.2–51.8) | 50.9 (46.3–55.5) | 48.9 (44.2–53.5) | 51.9 (47.1–56.8) | 52.8 (48.2–57.4) |
| LVIDd, cm | |||||||
| CONT | 5.22 (4.92–5.51) | 5.09 (4.79–5.38) | 4.92 * (4.63–5.22) | 4.84 * (4.55–5.14) | 4.84 * (4.55–5.14) | 4.77 * (4.48–5.07) | 5.02 * (4.72–5.31) |
| Ex | 5.21 (5.05–5.37) | 5.16 (5.00–5.32) | 5.08 (4.91–5.24) | 5.15 (4.99–5.31) | 5.11 (4.95–5.27) | 5.14 (4.98–5.30) | 5.10 (4.94–5.26) |
| ExT | 5.10 (4.81–5.39) | 5.08 (4.79–5.37) | 5.06 (4.76–5.35) | 5.02 (4.72–5.31) | 5.17 (4.88–5.46) | 5.16 (4.87–5.45) | 5.31 (5.01–5.60) |
| LV PW, cm | |||||||
| CONT | 0.81 (0.73–0.90) | 0.77 * (0.68–0.85) | 0.75 * (0.67–0.84) | 0.72 * (0.64–0.81) | 0.73 * (0.64–0.81) | 0.72 * (0.64–0.81) | 0.72 * (0.64–0.80) |
| Ex | 0.79 (0.71–0.86) | 0.80 (0.73–0.87) | 0.79 (0.71–0.86) | 0.81 (0.74–0.88) | 0.80 (0.73–0.87) | 0.81 (0.74–0.88) | 0.81 (0.74–0.88) |
| ExT | 0.71 (0.62–0.80) | 0.71 (0.62–0.80) | 0.69 (0.60–0.78) | 0.70 (0.61–0.79) | 0.72 (0.63–0.81) | 0.72 (0.63–0.81) | 0.73 (0.64–0.82) |
| LV IVSW, cm | |||||||
| CONT | 0.75 (0.65–0.84) | 0.76 (0.66–0.85) | 0.75 (0.66–0.84) | 0.71 (0.62–0.80) | 0.70 * (0.60–0.79) | 0.68 * (0.59–0.77) | 0.67 * (0.58–0.76) |
| Ex | 0.73 (0.67–0.79) | 0.73 (0.67–0.79) | 0.74 (0.68–0.80) | 0.75 (0.69–0.81) | 0.75 (0.69–0.81) | 0.75 (0.69–0.81) | 0.76 (0.70–0.82) |
| ExT | 0.68 (0.59–0.76) | 0.68 (0.60–0.76) | 0.69 (0.60–0.77) | 0.68 (0.60–0.76) | 0.68 (0.59–0.76) | 0.70 (0.61–0.78) | 0.69 (0.61–0.78) |
| LV mass, g | |||||||
| CONT | 142 (129–154) | 126 * (114–138) | 122 * (109–134) | 119 * (107–131) | 123 * (111–135) | 117 * (104–130) | 133 * (120–145) |
| Ex | 144 (136–152) | 146 (138–154) | 140 (132–148) | 144 (136–151) | 145 (137–152) | 140 (132–148) | 148 (140–156) |
| ExT | 152 (144–161) | 151 (142–160) | 153 (144–162) | 155 (146–163) | 153 (144–162) | 146 (137–155) | 150 (141–159) |
| LVM/EDV | |||||||
| CONT | 1.03 (0.97–1.09) | 1.04 (0.98–1.09) | 1.08 (1.02–1.10) | 1.07 (1.01–1.10) | 1.08 (1.02–1.12) | 1.02 (0.97–1.09) | 1.04 (0.98–1.09) |
| Ex | 1.07 (1.00–1.14) | 1.07 (1.00–1.14) | 1.09 (1.02–1.15) | 1.09 (1.02–1.16) | 1.10 (1.02–1.16) | 1.04 (0.97–1.11) | 1.06 (0.98–1.13) |
| ExT | 1.03 (0.97–1.09) | 1.04 (0.98–1.11) | 1.02 (0.96–1.06) | 1.02 (0.96–1.08) | 1.07 (1.01–1.12) | 1.08 (1.02 –1.15) | 1.02 (0.98–1.09) |
| LV length, cm | |||||||
| CONT | 8.09 (7.78–8.41) | 8.01 (7.70–8.32) | 7.97 (7.66–8.29) | 7.92 (7.61–8.23) | 7.78 * (7.46–8.09) | 7.73 * (7.41–8.04) | 7.88 * (7.56–8.19) |
| Ex | 8.37 (8.11–8.63) | 8.27 (8.01–8.53) | 8.28 (8.02–8.54) | 8.30 (8.04–8.55) | 8.27 (8.02–8.53) | 8.35 (8.09–8.60) | 8.33 (8.08–8.59) |
| ExT | 8.21 (7.93–8.48) | 8.12 (7.84–8.39) | 8.17 (7.90–8.45) | 8.13 (7.85–8.40) | 8.13 (7.86–8.41) | 8.10 (7.83–8.38) | 8.20 (7.93–8.48) |
BR, bed rest day; LV, left ventricular; EDV, end diastolic volume; ESV, end systolic volume; IDd, internal diameter diastole; PW, posterior wall; IVSW, intraventricular septal wall. Values are mean and 95% CI.
p<0.007 vs. BR−2.
Cardiac Function: Conventional and Tissue Doppler Parameters
Time course of change in measures of cardiac systolic and diastolic parameters are detailed in Table 3. There was no change in LV ejection fraction in any group. Diastolic function assessed by E/A and E’ was preserved in Ex and ExT groups, but was significantly decreased in the CONT group (p<0.007) beginning at BR7.
Table 3.
Conventional Function Parameters
| Time Point | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BR−2 | BR7 | BR21 | BR31 | BR70 | BR+0 | BR+3 | |
| LVSV, ml | |||||||
| CONT | 91.3 (81.4–101.2) | 81.7 * (71.9–91.6) | 74.6 * (64.3–84.9) | 73.9 * (64.0) | 75.3 * (65.5–85.2) | 75.9 * (65.3–86.5) | 84.6 * (74.6–94.7) |
| Ex | 88.9 (82.0–95.9) | 89.7 (82.7–96.7) | 93.1 (86.1–100.1) | 96.0 (89.0–103.0) | 91.6 (84.6–98.6) | 81.9 (74.7–89.1) | 95.2 (88.2–102.2) |
| ExT | 94.1 (83.4–104.8) | 90.9 (80.2–101.6) | 92.4 (81.4–103.3) | 94.9 (84.2–105.6) | 94.9 (84.1–105.6) | 87.9 (77.0–98.9) | 96.9 (86.2–107.6) |
| LVEF, % | |||||||
| CONT | 66.4 (64.3–68.6) | 66.9 (64.8–69.0) | 66.2 (63.8–68.6) | 65.9 (63.8–68.1) | 65.9 (63.8–68.0) | 65.7 (63.1–68.3) | 66.1 (63.8–68.3) |
| Ex | 62.4 (59.5–65.2) | 65.9 (63.1–68.7) | 66.6 (63.8–69.4) | 66.1 (63.3–69.0) | 67.3 (64.5–70.1) | 63.5 (60.5–66.4) | 64.2 (61.4–67.0) |
| ExT | 65.6 (61.6–69.5) | 64.0 (60.0–67.9) | 66.1 (62.0–70.1) | 63.9 (59.9–67.8) | 64.9 (61.0–68.9) | 63.0 (58.9–67.0) | 62.6 (58.7–66.6) |
| LV Sa (cm/s) | |||||||
| CONT | 10.4 (9.1–11.7) | 10.8 (9.5–12.0) | 11.1 (9.8–12.4) | 10.2 (8.9–11.5) | 11.0 (9.8–12.3) | 12.9 * (11.7–14.2) | 10.5 (9.3–11.8) |
| Ex | 10.3 (9.0–11.6) | 11.6 (10.3–12.8) | 11.3 (10.0–12.6) | 11.3 (10.0–12.6) | 12.4 (11.1–13.7) | 12.6 (11.3–13.8) | 10.7 (9.4–12.0) |
| ExT | 10.7 (9.1–12.2) | 11.2 (9.6–12.8) | 10.8 (9.3–12.3) | 10.8 (9.2–12.3) | 11.0 (9.4–12.5) | 10.0 (8.5–11.5) | 10.0 (8.4–11.5) |
| Transmitral E/A | |||||||
| CONT | 2.1 (1.8–2.4) | 1.7 * (1.4–2.0) | 1.4 * (1.1–1.7) | 1.4 * (1.1–1.7) | 1.4 * (1.1–1.6) | 0.9 * (0.6–1.2) | 1.7 * (1.4–2.0) |
| Ex | 2.0 (1.6–2.4) | 2.0 (1.6–2.4) | 1.9 (1.5–2.3) | 1.9 (1.5–2.3) | 1.6 (1.2–2.0) | 1.6 (1.1–1.9) | 2.0 (1.6–2.4) |
| ExT | 1.9 (1.5–2.4) | 1.9 (1.4–2.4) | 1.8 (1.3–2.3) | 2.2 (1.8–2.7) | 1.8 (1.4–2.3) | 1.6 (1.2–2.1) | 2.1 (1.6–2.6) |
| Mitral Deceleration (ms) | |||||||
| CONT | 207.6 (175.7–239.4) | 208.7 (176.8–240.6) | 212.8 (180.9–244.7) | 207.2 (175.4–239.1) | 229.8 (196.7–262.8) | 160.6 * (128.7–192.5) | 216.3 (184.4–248.2) |
| Ex | 220.4 (194.2–246.6) | 206.4 (180.2–232.6) | 208.6 (182.4–234.8) | 201.3 (175.1–227.5) | 198.3 (172.1–224.5) | 209.8 (183.6–236.0) | 220.5 (194.3–246.7) |
| ExT | 208.5 (179.3–237.8) | 215.1 (185.9–244.4) | 191.6 (162.3–220.8) | 244.1 (214.9–273.4) | 203.2 (173.9–232.4) | 202.0 (172.7–231.2) | 234.9 (205.6–264.1) |
| LV Ea (cm/s) | |||||||
| CONT | 14.7 (13.1–16.3) | 12.0 * (10.4–13.6) | 12.4 * (10.8–14.0) | 11.5 * (9.9–13.1) | 11.5 * (9.9–13.1) | 11.3 * (9.7–12.9) | 13.2 (11.6–14.8) |
| Ex | 15.3 (13.9–16.8) | 14.7 (13.3–16.2) | 13.4 (12.0–14.9) | 15.7 (14.2–17.1) | 14.1 (12.7–15.6) | 13.0 (11.5–14.4) | 15.1 (13.7–16.6) |
| ExT | 16.4 (14.5–18.3) | 15.0 (13.1–16.8) | 14.9 (13.0–16.8) | 15.3 (13.5–17.2) | 14.6 (12.8–16.5) | 13.8 (11.9–15.7) | 14.7 (12.8–16.6) |
BR, bed rest day; LV, left ventricular; SV, stroke volume; EF, ejection fraction; Sa, lateral wall systolic peak tissue velocity; Ea, lateral wall early diastolic peak tissue velocity; RV, right ventricular. Values are mean and 95% CI.
p<0.007 vs. BR−2.
Cardiac Function: LV Rotation, LV Twist, and Untwisting
LV strain, twist and untwist data are summarized in Table 4. Image quality was sufficient to obtain LV strain and twist in 25 subjects. Among the 25 subjects with a possible total of 175 images, image quality was sufficient to obtain longitudinal strain, radial strain, circumferential strain, and twist in 94%, 93%, 92% and 92%, respectively. Longitudinal and circumferential strain were unaffected in the three groups throughout bed rest. Compared to pre-bed rest, there was a significant decrease in radial strain at BR70 and BR+0 in the CONT group; no differences were observed in the exercise groups. At BR7, there was a significant increase in peak systolic apical rotation but no change in basal rotation, which translated into a significant increase in peak systolic LV twist in all groups. There were no significant differences from pre-bed rest at any other time point for Ex and ExT groups. Apical rotation and LV twist were significantly increased at all other time points in the CONT group compared to pre-bed rest (p<0.007). Peak early diastolic untwisting was increased significantly in the CONT group at BR7, 21, 31, 70 and +0. The time to peak apical and basal rotation and LV twist did not change significantly throughout bed rest in any group. Finally, with all groups combined there was a significant correlation between change in peak systolic LV twist and change in VO2peak (r2 = 0.61, p < 0.001).
Table 4.
Strain, Twist and Untwist Parameters
| Time Point | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| BR−2 | BR7 | BR21 | BR31 | BR70 | BR+0 | BR+3 | |
| Longitudinal Strain (%) | |||||||
| CONT | −17.5 (−19.3 −15.8) | −16.2 (−18.0 −14.5) | −15.8 (−17.6 −14.1) | −16.2 (−17.9 −14.4) | −15.5 (−17.3 −13.7) | −15.8 (−17.6 −14.0) | −16.9 (−18.6 −15.1) |
| Ex | −18.2 (−20.4 −15.9) | −17.8 (−20.0 −15.5) | −18.6 (−20.9 −16.4) | −18.0 (−20.3 −15.8) | −16.9 (−19.1 −14.7) | −17.1 (−19.3 −14.9) | −18.6 (−20.8 −16.4) |
| ExT | −19.5 (−21.7 −17.3) | −17.0 (−19.2 −14.8) | −18.1 (−20.3 −15.9) | −17.0 (−19.2 −14.8) | −16.0 (−18.1 −13.8) | −16.4 (−17.5 −13.2) | −17.1 (−19.3 −14.9) |
| Circumferential Strain (%) | |||||||
| CONT | −22.2 (−24.6 −19.8) | −22.0 (−24.3 −19.6) | −21.8 (−24.1 −19.4) | −21.8 (−24.2 −19.4) | −19.6 (−22.0 −17.2) | −20.1 (−22.5 −17.8) | −21.8 (−24.2 −19.4) |
| Ex | −20.0 (−23.4 −16.5) | −21.5 (−24.9 −18.0) | −22.8 (−26.3 −19.4) | −21.6 (−25.0 −18.1) | −23.9 (−27.3 −20.4) | −23.6 (−27.0 −20.1) | −23.1 (−26.6 −19.7) |
| ExT | −20.9 (−22.7 −19.1) | −20.8 (−22.5 −19.0) | −22.3 (−24.0 −20.5) | −21.7 (−23.5 −20.0) | −20.3 (−22.1 −18.6) | −20.8 (−22.5 −19.0) | −20.2 (−22.0 −18.5) |
| Radial Strain (%) | |||||||
| CONT | 39.3 (32.9– 45.7) | 35.5 (29.1–41.9) | 32.6 (26.2–39.0) | 33.2 (26.8–39.5) | 31.6 * (25.3–38.0) | 29.7 * (23.3–36.1) | 35.0 (28.6–41.3) |
| Ex | 39.8 (35.3–44.3) | 38.1 (33.6–42.7) | 40.6 (36.0–45.1) | 35.1 (28.5–37.6) | 37.8 (33.2–42.3) | 36.6 (32.1–41.2) | 38.0 (33.5–42.5) |
| ExT | 37.8 (33.8–41.7) | 40.6 (36.6–44.5) | 37.3 (33.3–41.2) | 38.1 (34.2–42.0) | 37.2 (33.2–41.1) | 39.4 (35.5–43.3) | 37.8 (33.9–41.7) |
| Basal Rotation (°) | |||||||
| CONT | −8.3 (−10.3 −6.3) | −7.8 (−9.8 −5.8) | −7.9 (−9.9 −5.9) | −8.5 (−10.5 −6.5) | −9.5 (−11.5 −7.5) | −9.0 (−11.0 −7.0) | −8.8 (−10.8 −6.9) |
| Ex | −6.1 (−7.3 −5.0) | −6.1 (−7.3 −4.9) | −7.1 (−8.2 −6.0) | −6.7 (−7.8 −5.6) | −6.2 (−7.3 −5.1) | −6.3 (−7.4 −5.1) | −5.6 (−6.7 −4.5) |
| ExT | −6.9 (−8.2 −5.6) | −7.3 (−8.6 −6.0) | −8.1 (−9.4 −6.8) | −7.9 (−9.2 −6.6) | −8.2 (−9.4 −6.9) | −8.6 (−9.9 −7.3) | −7.4 (−8.7 −6.1) |
| Apical Rotation (°) | |||||||
| CONT | 8.8 (6.1–11.4) | 13.6 * (10.9–16.2) | 12.8 * (10.2–15.5) | 15.8 * (13.1–18.4) | 15.0 * (12.3–17.6) | 15.1 * (12.5–17.7) | 12.1* (9.4–14.7) |
| Ex | 8.5 (6.2–10.9) | 12.9 * (10.6–15.3) | 11.3 (8.9–13.7) | 11.0 (8.6–13.4) | 10.4 (8.0–12.7) | 10.5 (8.2–12.9) | 10.7 (8.3–13.0) |
| ExT | 11.3 (8.6–14.0) | 14.5 * (11.8–17.2) | 12.6 (9.9–15.3) | 12.5 (9.8–15.2) | 14.3 (11.6–17.0) | 10.6 (7.9–13.3) | 9.9 (7.2–12.6) |
| Peak Twist (°) | |||||||
| CONT | 16.6 (13.5–19.7) | 21.5 * (18.4–24.6) | 20.1 * (17.0–23.2) | 23.9 * (20.8–27.0) | 24.1 * (21.0–27.2) | 23.7 * (20.7–26.8) | 20.6* (17.5–23.7) |
| Ex | 14.2 (12.0–16.4) | 18.9 * (16.6–21.1) | 17.1 (14.9–19.4) | 16.8 (14.6–19.0) | 15.8 (13.5–18.0) | 16.7 (14.4–18.9) | 14.2 (12.0–16.5) |
| ExT | 17.0 (14.2–19.8) | 21.8 * (19.0–24.6) | 19.7 (16.9–22.5) | 20.1 (17.3–22.9) | 21.0 (18.2–23.8) | 17.9 (15.1–20.7) | 16.2 (13.4–19.0) |
| Normalized Twist (°/cm) | |||||||
| CONT | 2.1 (1.7–2.5) | 2.7 * (2.3–3.1) | 2.6 (2.1–2.9) | 3.0 * (2.7–3.4) | 3.1 * (2.7–3.5) | 3.1 * (2.7–3.5) | 2.6* (2.2–3.0) |
| Ex | 1.7 (1.5–1.9) | 2.3 * (2.0–2.5) | 2.1 (1.8–2.3) | 2.0 (1.8–2.3) | 1.9 (1.7–2.1) | 2.0 (1.7–2.2) | 1.7 (1.5–2.0) |
| ExT | 2.1 (1.7–2.4) | 2.7 * (2.3–3.0) | 2.4 (2.1–2.8) | 2.5 (2.1–2.8) | 2.6 (2.2–2.9) | 2.2 (1.9–2.6) | 2.0 (1.6–2.3) |
| Peak Untwist (°/s) | |||||||
| CONT | −182.1 (−232.7 −131.4) | −244.3 * (−295.0 −193.7) | −281.4 * (−332.1 −230.8) | −237.0 * (−287.6 −186.4) | −255.5 * (−306.1 −204.9) | −248.3 * (−298.9 −197.7) | −202.4* (−256.5 −148.3) |
| Ex | −218.5 (−269.2 −167.8) | −223.3 (−274.0 −172.6) | −243.7 (−294.4 −193.0) | −250.4 (−301.1 −199.7) | −259.8 (−310.5 −209.1) | −226.0 (−276.8 −175.3) | −228.8 (−279.5 −178.1) |
| ExT | −237.3 (−290.1 −184.5) | −295.9 (−348.7 −243.0) | −257.2 (−310.1 −204.4) | −264.2 (−317.1 −211.4) | −235.9 (−288.8 −183.1) | −331.5 (−384.3 −278.6) | −249.2 (−302.0 −196.3) |
| Time to peak basal rotation (% systole) | |||||||
| CONT | 94.9 (87.1–102.8) | 92.6 (85.3–100.0) | 87.1 (79.7–94.4) | 86.5 (79.1–93.8) | 87.1 (79.8–94.5) | 90.1 (82.7–97.5) | 89.8 (82.5–97.2) |
| Ex | 94.6 (86.5–102.8) | 97.5 (94.4–110.7) | 98.2 (90.1–106.4) | 93.5 (85.3–101.6) | 91.2 (83.1–99.4) | 85.5 (77.4–93.7) | 88.7 (80.5–96.9) |
| ExT | 90.0 (81.4–98.5) | 91.6 (83.0–100.1) | 95.7 (87.2–104.2) | 91.1 (82.6–99.6) | 94.6 (86.1–103.1) | 86.7 (78.2–95.3) | 93.0 (90.5–107.5) |
| Time to peak apical rotation (% systole) | |||||||
| CONT | 89.8 (82.8–96.8) | 87.9 (80.9–94.9) | 88.8 (81.8–95.9) | 86.1 (79.1–93.1) | 90.2 (83.2–97.2) | 90.4 (83.4–97.5) | 84.0 (77.0–91.1) |
| Ex | 92.0 (88.8–103.1) | 91.5 (84.4–98.6) | 91.2 (84.0–98.3) | 93.3 (86.2–100.5) | 91.0 (83.5–98.6) | 93.4 (85.9–101.0) | 95.8 (93.7–97.9) |
| ExT | 89.7 (80.2–95.3) | 92.4 (90.3–104.5) | 94.5 (87.4–101.6) | 88.9 (81.9–96.0) | 91.3 (84.2–98.3) | 92.0 (84.9–99.1) | 89.1 (82.0–96.1) |
| Time to peak twist rotation (% systole) | |||||||
| CONT | 89.3 (83.6–95.1) | 90.5 (84.8–96.3) | 92.1 (86.4–97.9) | 85.2 (79.5–91.0) | 90.3 (84.6–96.1) | 92.4 (86.6–98.1) | 86.1 (80.4–91.9) |
| Ex | 93.0 (85.5–100.5) | 97.9 (90.4–105.4) | 92.6 (85.1–100.1) | 93.2 (85.7–100.7) | 92.1 (84.6–99.6) | 93.1 (85.6–100.6) | 96.6 (89.1–104.1) |
| ExT | 88.0 (82.0–93.9) | 95.6 (89.6–101.5) | 93.1 (87.2–99.0) | 88.9 (83.0–94.8) | 92.5 (86.5–98.4) | 86.7 (80.8–92.7) | 93.1 (87.2–99.1) |
BR, bed rest day; LV, left ventricular. Values are mean and 95% CI.
p<0.007 vs. BR−2.
Cardiac Biomarkers and Plasma Volume
Results from the blood chemistry panel are presented in Table 5. HDL cholesterol was significantly decreased in all groups compared to pre-bed rest (p<0.007). CRP was significantly increased in the CONT group at BR+5 compared to pre-bed rest; no changes were observed in the exercise groups. There were no significant changes in other cardiovascular biomarkers. Compared to pre-bed rest, plasma volume was significantly decreased in all groups at BR7 (p<0.01): CONT (BR−2: 3.0 ± 0.4 L, BR7: 2.5 ± 0.4 L), Ex (BR−2: 3.2 ± 0.5 L, BR7: 2.7 ± 0.5 L), ExT (BR−2: 3.0 ± 0.4 L, BR7: 2.7 ± 0.4 L). There were no significant differences from pre-bed rest at BR31in any group; plasma volume was significantly decreased in CONT and Ex subjects at BR+0 (CONT: 2.7 ± 0.5 L; Ex: 2.8 ± 0.4 L), but not in ExT (2.7 ± 0.6 L).
Table 5.
Blood biomarkers
| Time Point | ||||
|---|---|---|---|---|
|
| ||||
| BR−3 | BR28 | BR+0 | BR+5 | |
| Cholesterol (mg/dL) | ||||
| CONT | 193 (169–218) | 182 (157–206) | 184 (159–208) | 173 (148–197) |
| Ex | 175 (157–193) | 175 (157–193) | 172 (154–190) | 167 (149–185) |
| ExT | 164 (148–179) | 162 (146–178) | 154 (138–170) | 150 (134–166) |
| LDL (mg/dL) | ||||
| CONT | 119 (100–139) | 112 (93–131) | 115 (95–134) | 111 (91–130) |
| Ex | 110 (94–127) | 111 (95–128) | 109 (93–126) | 105 (88–121) |
| ExT | 98 (83–113) | 98 (83–113) | 94 (79–109) | 91 (75–106) |
| HDL (mg/dL) | ||||
| CONT | 45 (40–50) | 39* (34–44) | 38* (33–43) | 39* (34–44) |
| Ex | 46 (43–49) | 41* (38–44) | 41* (38–44) | 40* (37–43) |
| ExT | 48 (42–53) | 45* (39–50) | 41* (35–46) | 42* (36–47) |
| Triglyceride (mg/dL) | ||||
| CONT | 146 (104–189) | 153 (110–195) | 153 (110–195) | 116 (73–158) |
| Ex | 92 (73–112) | 111 (92–131) | 109 (90–128) | 108 (89–128) |
| ExT | 88 (60–116) | 98 (70–127) | 98 (70–126) | 91 (62–119) |
| CRP (mg/L) | ||||
| CONT | 0.98 (0.13–1.83) | 0.63 (0.22–1.48) | 0.89 (0.03–1.74) | 3.98* (3.13–4.83) |
| Ex | 0.93 (0.25–1.61) | 0.72 (0.04–1.41) | 1.50 (0.78–2.23) | 1.24 (0.51–1.96) |
| ExT | 0.68 (0.12–1.23) | 0.70 (0.16–1.24) | 0.62 (0.08–1.16) | 0.85 (0.30–1.40) |
| Phospholipids (mg/g) | ||||
| CONT | 2.01 (1.85–2.16) | 2.01 (1.85–2.16) | 1.98 (1.83–2.14) | 2.16 (2.00–2.31) |
| Ex | 2.17 (1.97–2.37) | 2.09 (1.89–2.29) | 2.12 (1.91–2.32) | 2.10 (1.90–2.30) |
| ExT | 2.13 (1.89–2.37) | 2.24 (2.00–2.48) | 2.19 (1.95–2.44) | 2.22 (1.98–2.46) |
| SOD (U/g) | ||||
| CONT | 1475 (1361–1588) | 1519 (1405–1632) | 1590 (1476–1703) | 1531 (1418–1645) |
| Ex | 1513 (1385–1642) | 1566 (1437–1694) | 1715 (1580–1851) | 1626 (1497–1754) |
| ExT | 1574 (1424–1724) | 1730 (1580–1880) | 1641 (1491–1791) | 1634 (1484–1784) |
| Total Antioxidant Capacity (mmol/L) | ||||
| CONT | 1.69 (1.58–1.80) | 1.67 (1.56–1.78) | 1.66 (1.55–1.77) | 1.62 (1.51–1.72) |
| Ex | 1.65 (1.58–1.72) | 1.66 (1.59–1.73) | 1.65 (1.57–1.72) | 1.62 (1.55–1.70) |
| ExT | 1.67 (1.55–1.79) | 1.67 (1.55–1.79) | 1.64 (1.52–1.75) | 1.63 (1.51–1.75) |
| HCT (%) | ||||
| CONT | 45 (40–50) | 39 (34–44) | 38 (33–43) | 39 (34–44) |
| Ex | 46 (43–49) | 41 (38–44) | 41 (38–44) | 40 (37–43) |
| ExT | 48 (42–53) | 45 (39–50) | 41 (35–46) | 42 (36–47) |
| HB (g/dL) | ||||
| CONT | 15.4 (14.7–16.1) | 16.3 (15.5–17.0) | 15.7 (15.0–16.5) | 14.5* (13.7–15.2) |
| Ex | 14.7 (14.1–15.3) | 15.3 (14.7–15.9) | 15.2 (14.6–15.8) | 14.4 (13.8–15.0) |
| ExT | 15.2 (14.4–15.9) | 15.9 (15.2–16.7) | 15.5 (14.7–16.2) | 14.7 (14.0–15.5) |
BR, bed rest day; LV, left ventricular; LDL, low density lipoprotein; HDL, high density lipoprotein; CRP, C-reactive protein; SOD, superoxide dismustase; HCT, hematocrit; HB, hemoglobin. Values are mean and 95% CI.
p<0.007 vs. BR−2.
DISCUSSION
We present novel, longitudinal data describing the time course of atrophic cardiovascular remodeling and the cardioprotective effects of exercise training and low dose testosterone supplementation. The principal new findings from this study were that: 1) alterations in cardiac morphology and function begin early (within 7 days of disuse); 2) augmentation in twist is an early indicator of cardiac adaptation to unloading; 3) the cardiovascular alterations that occur with sedentary bed rest were prevented by an integrated exercise program consisting of high intensity aerobic exercise and strength training; and 4) this integrated exercise training with concurrent low dose testosterone supplementation also mitigates atrophic cardiovascular remodeling. These findings suggest that an integrated approach with evaluation of cardiac morphology, LV mechanics, VO2peak, and biomarkers may be important complementary tools to characterize the overall cardiovascular impact of unloading. In turn, early identification of these cardiovascular alterations would help guide frequency of monitoring and optimize timing and type of interventions, including exercise.
Time Course of Cardiovascular Remodeling during Unloading
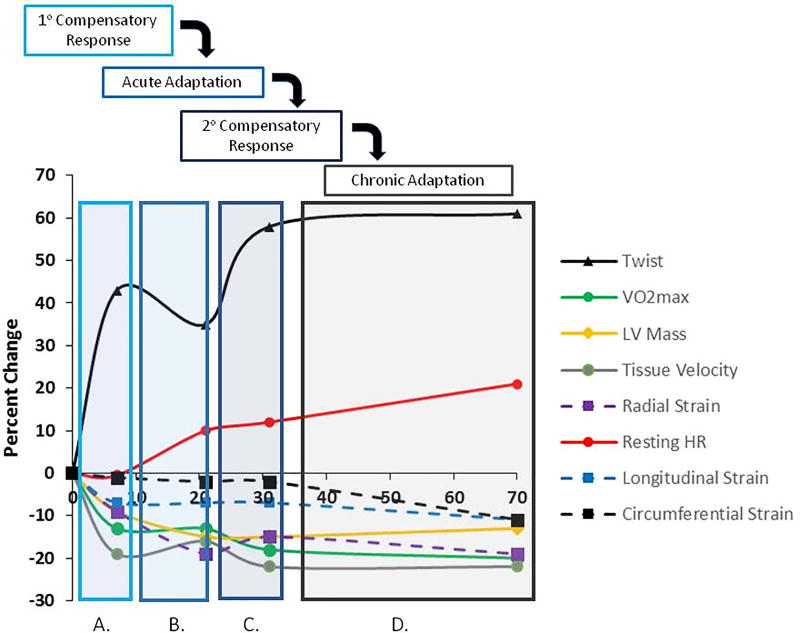
This is the first study to conduct temporal measures to comprehensively characterize the time course of both functional and morphological cardiovascular alterations; prior studies examining the cardiovascular impact of unloading consisted of pre and post measures. We found that significant declines in cardiac morphology, alterations in diastolic function and LV twist, and VO2peak occurred within the first 7 days of BR and persisted throughout disuse, significant increases in resting heart rate were observed towards the end of 70 days of unloading, and surprisingly few changes in cardiovascular biomarkers were observed. An outline of the integrated time course of remodeling is provided in Figure 1.
Figure 1. Time course of cardiovascular atrophic remodeling in control subjects.
Remodeling occurred in 4 phases: (A) primary compensatory response; (B) acute adaptation; (C) secondary compensatory responses; (D) chronic adaptation. In the primary compensatory phase there was a decline in tissue velocity and VO2peak and an increase in twist relative to pre BR. During the acute adaptation phase there was a decline in LV mass and radial strain, maintenance of tissue velocity and VO2peak, and decline in twist relative to primary compensatory phase. In the secondary compensatory response phase there was a further decline in tissue velocity and VO2peak and an increase in twist and resting HR relative to the acute adaptation phase. In the final chronic adaptation phase, there were declines in longitudinal and circumferential strain, augmentation in resting HR, and maintenance of increased twist and decreased VO2peak, LV mass, and tissue velocity. BR, bed rest; LV, left ventricular; HR, heart rate.
Cardiac Morphology
The changes in cardiac structure during short- and long-term bed rest have been under investigation for several years. Our present findings support earlier data by Perhonen et al. (4) that showed that the overall heart size (including both volume and mass) decreased after 2 wk of horizontal bed rest, and appeared to plateau after 5 wk of bed rest. Indeed, we found decreases in LVM and EDV at BR7 and BR21 compared with pre-BR, with little change thereafter. The initial changes in cardiac structure are thought to be the results of decreases in volume; within 24 h of a head-ward fluid shift, there is a salt and water diuresis, with a reduction in central venous pressure and stroke volume below supine values (5). Our present findings support earlier data suggesting that after stabilization of fluid changes, changes in cardiac myocyte cell size occurring after periods of disuse longer than 70 days could contribute to decreases in LVM (5). Nevertheless, echocardiographic estimates of LVM are dependent on LV volumes; cardiac magnetic resonance imaging is currently considered the reference technique for LV morphology measurements (21).
Cardiac Mechanics
Global measures of LV function, such as LVEF, are typically unchanged following unloading. LVEF, however, is load-, rate-, and contractility-dependent, and acute declines in myocardial function can be masked by initial compensations in LV mechanics that maintain cardiac output (22). Thus, LVEF change is a late marker, only becoming evident after significant alterations in cardiac performance have already occurred. Speckle tracking assessment of systolic function with strain is more sensitive for detecting altered myocardial performance beyond LVEF (23). In the clinical setting, reduced strain and strain rate revealed impaired myocardial function prior to LVEF decline (24) in cancer patients treated with anthracycline-containing therapy. Interestingly, we found no change in longitudinal or circumferential strain following 70 days of bed rest, suggesting alterations in these planes may also occur later in the remodeling sequelae.
We found that that LV apical rotation was altered, and not LV basal rotation, which is consistent with other myocardial deformation studies in athletes (25) and with the notion that the magnitude of apical counterclockwise rotation is the primary determinant of overall peak systolic LV twist. Initial increases in twist (within 7 days) may be due to alterations in loading. Indeed, a decrease in LV loading results in a compensatory increase in LV twist (26), as does increased vascular loading (27). Similar to previous studies reporting an acute loss of plasma volume (20), we found by BR7 plasma volume was significantly decreased in all groups suggesting increases in twist observed at BR7 may be due to decreased preload (26). Importantly however, chronic changes in LV twist in CONT may be due to specific alterations in the subendocardial layer. Normally, the subendocardial and subepicardial layers oppose each other during contraction due to their oblique fiber angle orientations (28). Greater force is generated in the epicardial layer as a result of the longer distance from the center of the ventricle (28). Previous research in healthy aging has demonstrated that subendocardial shortening is reduced, resulting in less opposition to epicardial contraction, ultimately leading to greater LV twist at rest (28). Combining the assessment of comprehensive LV mechanical function with indicators of molecular remodeling would further advance the current understanding of physiological adaptation of LV mechanics.
Cross-sectional reports have identified differences in LV twist when comparing cyclists and sedentary controls (29); specifically, individuals with high peak oxygen consumption had significantly lower apical rotation and twist. Here, we found a similar relationship between peak twist and aerobic capacity at the other end of the spectrum: a low peak oxygen consumption was associated with a high LV twist. Importantly, the change in aerobic capacity was associated with the change in LV twist, suggesting an important interplay between cardiac mechanics and exercise tolerance. It is possible that a high resting twist results in little ‘twist reserve’ with exercise (28), which significantly impairs the ability of the heart to augment preload, and thus increase cardiac output. We did not assess cardiac function during stress; future studies should assess the role of twist reserve on aerobic capacity and exercise tolerance.
Resting Heart Rate
Resting heart rate is a simple measure which can provide information regarding relative effects of autonomic tone in a given state (30). We found a progressive increase in resting heart rate throughout disuse; by BR70 resting heart rate was 21% (13 beats/min) higher. These findings highlight the potential time course of autonomic dysregulation, with changes occurring within 2 months of a stimulus onset. Importantly, epidemiological studies show that resting heart rate is an independent predictor of CVD and all-cause mortality in individuals with and without diagnosed CVD disease (30), suggesting that interventions are crucial early on.
Cardiac Biomarkers
Identification of biomarkers that predict risk of CVD could provide insight into the biology of the disease and could aid in developing targeted prevention strategies during the preclinical phase of CVD, when intervention may be more likely to alter disease progression. Previous studies have reported evidence of a shift towards a less healthy whole body metabolic profile during extreme reductions in activity (19); accordingly, we characterized the time course of change in several established cardiovascular biomarkers that are mechanistically relevant to CVD pathogenesis (31). We found no other alterations in cardiac biomarkers. In the current investigation, the diet was tightly controlled and consisted of a 55% carbohydrate (4.97 ± 0.30 g·kg−1), 30% fat (1.21 ± 0.07 g·kg−1), and 15% protein (1.36 ± 0.08 g·kg−1) macronutrient profile. These findings highlight the important role of diet in preventing metabolic alterations and CVD pathogenesis.
Cardioprotective Effects of Exercise Training
A number of investigators have used exercise training to prevent cardiovascular deconditioning associated with bed rest. For example, Watenpaugh and colleagues (32) employed supine treadmill running during simultaneous lower body negative pressure to establish a similar cardiovascular stress to exercise training in the 1 G upright position and found upright exercise capacity was preserved. Similarly, Hastings et al. (33) reported that exercise training maintained cardiac size, function, and aerobic capacity. The present report is consistent with and extends these previous studies by demonstrating that despite early fluid-shift-induced alterations in LV function and a decrease in cardiorespiratory fitness, a comprehensive training program incorporating submaximal intensity base training, higher-intensity intervals, and regular resistance training completely mitigates atrophic functional and morphological cardiovascular remodeling.
Implications of Exercise Training with Low Dose Testosterone Supplementation
Testosterone, given its role in bone and muscle maintenance, is often considered as a potential countermeasure for unloading-induced atrophy. Testosterone is known to promote muscle protein synthesis, (34) and several studies have shown that chronic administration of testosterone increases lean body and muscle mass and improves muscle strength (35). The heart is also a target organ for steroids; there are receptors with a high affinity for testosterone in cardiomyocytes (36), suggesting testosterone supplementation may also impact cardiac function. Here, we found similar cardiovascular responses between Ex and ExT groups, supporting two salient findings: (1) the addition of low dose testosterone supplementation does not further enhance an already cardioprotective stimulus (i.e., exercise), and (2) low dose testosterone does not significantly alter cardiac function. These findings suggest that although there may be no supplementary cardiovascular benefit to low dose testosterone, low dose androgen administration could be safely implemented as a muscle or bone countermeasure .
Implications
Physical inactivity is rapidly becoming a major global concern and is the fourth leading cause of death worldwide (37). Evidence indicates that physical inactivity increases the relative risk of coronary artery disease, stroke, and hypertension, by 45%, 60%, and 30%, respectively (38). Moreover, the health care costs of sedentary individuals are close to one-third higher than those of individuals who are physically active, representing at least 100 billion US dollars in excess healthcare costs (39). Exercise, in contrast, is a non-pharmacological, pleiotropic intervention that has the unique capacity to modulate, without toxicity, a plethora of cardiovascular-specific molecular and cell-signaling pathways implicated in inactivity-induced alterations. Indeed, we found that exercise intervention mitigated a 27%, 22%, 20%, and 14% decline in LV twist, VO2peak, LV mass, and resting HR, respectively. Together, these data indicate that exercise training and maintenance of physical fitness have important impacts on the prevalence and progression of cardiovascular diseases in humans.
Limitations and Future Directions
Several limitations and areas for future work are noteworthy. First, we performed a serial assessment of resting cardiac function and thus cannot comment on how our observations relate to myocardial function during exercise. To completely determine the physiological importance of atrophic remodeling, there is a need to move beyond the study of LV function at rest. Evaluation of the myocardial response to hemodynamic/exercise challenge may ultimately represent a method for characterizing exercise performance and also aid in characterizing cardiovascular remodeling. Second, VO2peak reflects the integrative capacity of the cardiovascular and musculoskeletal system to transport and utilize oxygen (O2) for ATP synthesis (40). Here, we focused on the cardiac specific aspects contributing to aerobic capacity; the contributions of musculoskeletal atrophy to decrements in functional performance were examined in a companion paper. Third, we evaluated relatively young, healthy subjects. This limits the generalizability of our findings to disease states and older populations.
Conclusions
The present study demonstrated that broad phenotyping integrating cardiac morphology, LV mechanics, VO2peak, and biomarkers could guide timing and optimization of interventions that may delay or prevent the onset of atrophic remodeling resulting from spaceflight, bed rest, augmented catabolic state, reduction in physical activity, or aging. To this end, our findings suggest that the cardiovascular alterations that occur with sedentary bed rest can be mitigated both by an integrated exercise program consisting of high intensity aerobic exercise and strength training and exercise training with concurrent low dose testosterone supplementation.
Acknowledgments
This study was supported by the Human Research Program of the National Aeronautics and Space Administration (NASA), and the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health. We thank members of the NASA Exercise Physiology and Countermeasures Laboratory for countless hours of subject training, the Flight Analogs Project for planning and implementation support, as well as the subjects who enthusiastically participated in the study. JMS is supported by grants from AKTIV Against Cancer, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Conflicts of Interest
Authors have no conflicts of interest. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–80. doi: 10.1056/NEJMra072139. Epub 2008/03/28. [DOI] [PubMed] [Google Scholar]
- 2.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. Epub 2013/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Gerche A, Heidbuchel H. Can intensive exercise harm the heart? You can get too much of a good thing. Circulation. 2014;130(12):992–1002. doi: 10.1161/CIRCULATIONAHA.114.008141. Epub 2014/09/17. [DOI] [PubMed] [Google Scholar]
- 4.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest :"cardiovascular deconditioning" or hypovolemia? Circulation. 2001;103(14):1851–7. doi: 10.1161/01.cir.103.14.1851. Epub 2001/04/11. [DOI] [PubMed] [Google Scholar]
- 5.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol. 2001;91(2):645–53. doi: 10.1152/jappl.2001.91.2.645. Epub 2001/07/18. [DOI] [PubMed] [Google Scholar]
- 6.Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 2011;71(5):1710–20. doi: 10.1158/0008-5472.CAN-10-3145. Epub 2010/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol (1985) 2010;108(5):1177–86. doi: 10.1152/japplphysiol.01408.2009. Epub 2010/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Gerche A, Burns AT, Taylor AJ, Macisaac AI, Heidbuchel H, Prior DL. Maximal oxygen consumption is best predicted by measures of cardiac size rather than function in healthy adults. Eur J Appl Physiol. 2012;112(6):2139–47. doi: 10.1007/s00421-011-2184-9. Epub 2011/10/04. [DOI] [PubMed] [Google Scholar]
- 9.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. Epub 2002/03/15. doi: 10.1056/NEJMoa011858 346/11/793 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. Epub 2008/01/03. [DOI] [PubMed] [Google Scholar]
- 11.Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA, Braith RW, Beck DT, Martin JS, Morrow M, Roessner S, Beggs LA, McCoy SC, Cannady DF, 2nd, Shuster JJ. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab. 2014;306(4):E433–42. doi: 10.1152/ajpendo.00592.2013. Epub 2013/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffield-Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon-Jones D, Durham WJ, Grady JJ, Urban RJ. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011;96(11):E1831–7. doi: 10.1210/jc.2011-1262. Epub 2011/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22(3):193–202. doi: 10.1097/MED.0000000000000161. Epub 2015/04/19. [DOI] [PubMed] [Google Scholar]
- 14.Ploutz-Snyder LL, Downs M, Ryder J, Hackney K, Scott J, Buxton R, Goetchius E, Crowell B. Integrated resistance and aerobic exercise protects fitness during bed rest. Med Sci Sports Exerc. 2014;46(2):358–68. doi: 10.1249/MSS.0b013e3182a62f85. Epub 2014/01/21. [DOI] [PubMed] [Google Scholar]
- 15.Scott JM, Hackney K, Downs M, Guined J, Ploutz-Snyder R, Fiedler J, Cunningham D, Ploutz-Snyder L. The metabolic cost of an integrated exercise program performed during 14 days of bed rest. Aviat Space Environ Med. 2014;85(6):612–7. doi: 10.3357/asem.3772.2014. Epub 2014/06/13. [DOI] [PubMed] [Google Scholar]
- 16.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Circulation. 2003;108(9):1146–62. doi: 10.1161/01.CIR.0000073597.57414.A9. Epub 2003/09/04. [DOI] [PubMed] [Google Scholar]
- 17.Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010;3(10):1001–9. doi: 10.1016/j.jcmg.2010.08.003. Epub 2010/10/16. [DOI] [PubMed] [Google Scholar]
- 18.Esch BT, Scott JM, Warburton DE, Thompson R, Taylor D, Baron JC, Paterson I, Haykowsky MJ. Left ventricular torsion and untwisting during exercise in heart transplant recipients. J Physiol. 2009;587(Pt 10):2375–86. doi: 10.1113/jphysiol.2009.170100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Heer M, Wang Z, Huntoon CL, Zwart SR. Long-duration space flight and bed rest effects on testosterone and other steroids. J Clin Endocrinol Metab. 2012;97(1):270–8. doi: 10.1210/jc.2011-2233. Epub 2011/11/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arzeno NM, Stenger MB, Lee SM, Ploutz-Snyder R, Platts SH. Sex differences in blood pressure control during 6 degrees head-down tilt bed rest. Am J Physiol Heart Circ Physiol. 2013;304(8):H1114–23. doi: 10.1152/ajpheart.00391.2012. Epub 2013/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Schmidt F, Galuschky C, Schummers G, Lang RM, Nesser HJ. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114(7):654–61. doi: 10.1161/CIRCULATIONAHA.106.626143. [DOI] [PubMed] [Google Scholar]
- 22.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–49. doi: 10.1161/CIRCULATIONAHA.104.500546. Epub 2005/06/02. doi: 111/21/2837 [pii] 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 23.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Assessment of left ventricular systolic function using echocardiography in patients with preserved ejection fraction and elevated diastolic pressures. Am J Cardiol. 2008;101(12):1766–71. doi: 10.1016/j.amjcard.2008.02.070. Epub 2008/06/14. doi: S0002-9149(08)00375-5 [pii] 10.1016/j.amjcard.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 24.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158(2):294–301. doi: 10.1016/j.ahj.2009.05.031. Epub 2009/07/22. doi: S0002-8703(09)00441-4 [pii] 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Nottin S, Doucende G, Schuster-Beck I, Dauzat M, Obert P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart. J Physiol. 2008;586(Pt 19):4721–33. doi: 10.1113/jphysiol.2008.156323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stohr EJ, Gonzalez-Alonso J, Pearson J, Low DA, Ali L, Barker H, Shave R. Dehydration reduces left ventricular filling at rest and during exercise independent of twist mechanics. J Appl Physiol (1985) 2011;111(3):891–7. doi: 10.1152/japplphysiol.00528.2011. Epub 2011/06/28. [DOI] [PubMed] [Google Scholar]
- 27.Weiner RB, Weyman AE, Khan AM, Reingold JS, Chen-Tournoux AA, Scherrer-Crosbie M, Picard MH, Wang TJ, Baggish AL. Preload dependency of left ventricular torsion: the impact of normal saline infusion. Circ Cardiovasc Imaging. 2010;3(6):672–8. doi: 10.1161/CIRCIMAGING.109.932921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns AT, La Gerche A, MacIsaac AI, Prior DL. Augmentation of left ventricular torsion with exercise is attenuated with age. J Am Soc Echocardiogr. 2008;21(4):315–20. doi: 10.1016/j.echo.2007.08.013. Epub 2007/10/02. [DOI] [PubMed] [Google Scholar]
- 29.Stohr EJ, McDonnell B, Thompson J, Stone K, Bull T, Houston R, Cockcroft J, Shave R. Left ventricular mechanics in humans with high aerobic fitness: adaptation independent of structural remodelling, arterial haemodynamics and heart rate. J Physiol. 2012;590(Pt 9):2107–19. doi: 10.1113/jphysiol.2012.227850. Epub 2012/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159(4):612–9. e3. doi: 10.1016/j.ahj.2009.12.029. Epub 2010/04/07. [DOI] [PubMed] [Google Scholar]
- 31.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–9. doi: 10.1056/NEJMoa055373. Epub 2006/12/22. [DOI] [PubMed] [Google Scholar]
- 32.Watenpaugh DE, O'Leary DD, Schneider SM, Lee SM, Macias BR, Tanaka K, Hughson RL, Hargens AR. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. J Appl Physiol (1985) 2007;103(6):1964–72. doi: 10.1152/japplphysiol.00132.2007. Epub 2007/10/20. [DOI] [PubMed] [Google Scholar]
- 33.Hastings JL, Krainski F, Snell PG, Pacini EL, Jain M, Bhella PS, Shibata S, Fu Q, Palmer MD, Levine BD. Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol (1985) 2012;112(10):1735–43. doi: 10.1152/japplphysiol.00019.2012. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol (1985) 1989;66(1):498–503. doi: 10.1152/jappl.1989.66.1.498. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 35.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335(1):1–7. doi: 10.1056/NEJM199607043350101. Epub 1996/07/04. [DOI] [PubMed] [Google Scholar]
- 36.Kinson GA, Layberry RA, Hebert B. Influences of anabolic androgens on cardiac growth and metabolism in the rat. Can J Physiol Pharmacol. 1991;69(11):1698–704. doi: 10.1139/y91-252. Epub 1991/11/01. [DOI] [PubMed] [Google Scholar]
- 37.Kraus WE, Bittner V, Appel L, Blair SN, Church T, Despres JP, Franklin BA, Miller TD, Pate RR, Taylor-Piliae RE, Vafiadis DK, Whitsel L. The national physical activity plan: a call to action from the American Heart Association: a science advisory from the American Heart Association. Circulation. 2015;131(21):1932–40. doi: 10.1161/CIR.0000000000000203. Epub 2015/04/29. [DOI] [PubMed] [Google Scholar]
- 38.Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics. 2007;28(2):146–57. doi: 10.1152/physiolgenomics.00174.2006. Epub 2006/10/13. [DOI] [PubMed] [Google Scholar]
- 39.Pratt M, Macera CA, Wang G. Higher direct medical costs associated with physical inactivity. Phys Sportsmed. 2000;28(10):63–70. doi: 10.3810/psm.2000.10.1237. Epub 2000/10/01. [DOI] [PubMed] [Google Scholar]
- 40.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. The Lancet Oncology. 2009;10(6):598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]