Abstract
Objective
To describe the frequency of co-occurring newly acquired cognitive impairment, disability in activities of daily living (ADLs), and depression among survivors of a critical illness and to evaluate predictors of being Post-Intensive Care Syndrome (PICS)-free (i.e., no PICS problems).
Design
Prospective cohort study
Setting
Medical and surgical ICUs from 5 U.S. centers
Patients
Patients with respiratory failure or shock, excluding those with preexisting cognitive impairment or disability in ADLs.
Interventions
None
Measurements and Main Results
At 3 and 12 months after hospital discharge we assessed patients for cognitive impairment, disability, and depression. We categorized patients into eight groups reflecting combinations of cognitive, disability, and mental health problems. Using multivariable logistic regression, we modeled the association between age, education, frailty, durations of mechanical ventilation, delirium, and severe sepsis with the odds of being PICS-free.
We analyzed 406 patients with a median age of 61 years and an APACHE II of 23. At 3 and 12 months, one or more PICS problems were present in 64% and 56%, respectively. Nevertheless, co-occurring PICS problems (i.e., in two or more domains) were present in 25% at 3 months and 21% at 12 months. PICS problems in all three domains were present in only 6% at 3 months and 4% at 12 months. More years of education was associated with greater odds of being PICS-free (P<0.001 at 3 and 12 months). More severe frailty was associated with lower odds of being PICS-free (P=0.005 at 3 months and P=0.048 at 12 months).
Conclusions
In this multicenter cohort study, one or more PICS problems were present in the majority of survivors, but co-occurring problems were present in only 1 out of 4. Education was protective from PICS and frailty predictive of the development of PICS. Future studies are needed to understand better the heterogeneous subtypes of PICS and to identify modifiable risk factors.
Keywords: Critical Illness, Cognitive Dysfunction, Activities of Daily Living, Depression, Survivors, Post Intensive Care Syndrome
INTRODUCTION
New or worsened cognitive impairment, disabilities in activities of daily living (ADLs), and mental health impairment arising after critical illness and persisting beyond acute care hospitalization is referred to as Post-Intensive Care Syndrome (PICS) (1, 2). Despite growing awareness of PICS, effective interventions remain elusive (3–5). This may relate, in part, to an incomplete understanding of the potential subtypes of PICS and of the associated factors that may predispose patients to, or protect them from, the development of PICS.
Cohort studies of survivors of the Acute Respiratory Distress Syndrome (ARDS) and sepsis report problems in cognition, disability, and/or mental health (6–11). Nevertheless, the co-occurrence of these problems (i.e., how often one, two, or all three are present) in individual patients remains unclear. Moreover, despite the high prevalence of PICS reported in prior studies, some patients survive critical illness without problems. Little is known about factors that may predict survival from critical illness without PICS problems.
To address these gaps in knowledge, we measured the co-occurrence of cognitive impairment, disability in activities of daily living, and depression among survivors of critical illness who did not have cognitive impairment or disability in ADLs prior to the index illness. We also evaluated potential predictors of being PICS-free (i.e., without clinically significant problems in any of the three PICS domains). We hypothesized that different subtypes of PICS would be present. We also hypothesized that factors present before and during critical illness would be associated with being PICS-free.
MATERIALS AND METHODS
We tested these hypotheses in a prospective cohort study nested within the identical BRAIN-ICU (NCT00392795) and MIND-ICU (NCT00400062) studies. We included participants who survived the index hospitalization and completed long-term follow-up (12). These original data have been presented in abstract form (13).
Setting and Study Participants
The study protocol and the eligibility criteria have been published and are presented in the supplemental digital content (12). We included adult patients age 18 years or older treated for respiratory failure or shock in the medical and surgical ICU from 5 U.S. centers (see supplemental digital content). The primary aim of the parent study was to prospectively evaluate the effects of acute critical illness on cognitive function, disability, and mental health outcomes in survivors of critical illness. We therefore excluded those at high-risk for preexisting cognitive impairment (i.e., severe dementia, suspected anoxic brain injury, neurodegenerative disease, or recent cardiac surgery), and, to facilitate prospective data collection about the present episode of critical illness, those with a previous episode of critical illness in the last 30 days and those with >72 hours of organ dysfunction prior to enrollment in the study. To facilitate follow-up, we also excluded those who were moribund, those with blindness, deafness, inability to speak English, active substance abuse, psychotic disorders, homelessness, or who lived >200 miles from an enrolling center. Further, because we sought to describe the co-occurrence of PICS problems, defined as the presence of new problems in two or more PICS domains, we also excluded those with less overt cognitive impairment (i.e., an Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) score of ≥3.6) (14), and preexisting disability (i.e., score of ≥1 on the Katz ADL) (15). Because no objective measure for preexisting depression or its severity was available in the original studies, we did not exclude those with depression from our primary analyses. Patients or their proxies provided informed consent. The institutional review boards at each center approved the study protocol.
Determining the Co-occurrence of Post-Intensive Care Syndrome Problems
At 3 and 12 months after hospital discharge, blinded study personnel performed in-person assessment for cognitive impairment, disability in activities of daily living, and mental health problems using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (19), the Katz ADL (15), and the Beck Depression Inventory Second Edition (BDI-II) (16), respectively (see supplemental digital content for detailed descriptions of these instruments). We chose depression as a representative measure of mental health problems because we wanted to study newly acquired problems, based on previous work in showing that depression is five times more common than post-traumatic stress disorder (PTSD) in survivors of critical illness (17), and because other mental health symptoms such as anxiety and PTSD frequently co-occur with depression (10).
We defined PICS problems using accepted limits to determine the presence of clinically significant cognitive impairment, disability in ADLs, and depression. We defined cognitive impairment as an RBANS score of 78 or less), disability as a Katz ADL score ≥1, and depression as a BDI-II score >13.
Predictors of Being Post-Intensive Care Syndrome-Free
We selected a priori potential predictors for being PICS-free at follow-up. We included age, years of education, Canadian Study of Health and Aging Clinical Frailty Scale score (18), and durations of severe sepsis, delirium (19), and mechanical ventilation (see supplemental digital content for complete descriptions).
Missing Data
We used predictive mean matching multiple imputation at the time of regression modeling to account for incomplete predictor and outcome data among patients who participated in follow-up testing at each time point (20).
Statistical Analysis
We used the cutoffs defined above and descriptive statistics to determine the co-occurrence of PICS problems. We categorized patients into eight groups ranging from having no problems to problems in all three PICS domains: (1) no problems, (2) cognitive impairment only, (3) disability in ADLs only, (4) depression only, (5) cognitive impairment and disability in ADLs, (6) cognitive impairment and depression, (7) disability in ADLs and depression, and (8) cognitive impairment, disability in ADLs, and depression. Data are reported as median and interquartile ranges (IQR).
We used multivariable logistic regression to determine the association and the odds of being PICS-free at 3 and 12 months, adjusting for covariates. We conducted two sensitivity analyses: 1) excluding patients with proxy reported history of depression and 2) substitution of Agency for Healthcare Research Quality (AHRQ) Index of Socioeconomic Status for years of education (21).
Associations with continuous covariates were allowed to be nonlinear using restricted cubic splines. Nonlinear terms were forced to be linear if the P-value of the global test for nonlinearity was >0.20. We used R (version 3.1.2) for all analyses. P-values <0.05 were considered significant.
RESULTS
Characteristics of the patients
Between January 2007 and December 2010, we enrolled 1047 patients (Figure 1). During the hospitalization, 7 patients withdrew consent and requested their data be destroyed. Of the remaining 1040 patients, 214 died and 45 withdrew from further participation during hospitalization. Of the 781 hospital survivors, we excluded 250 who had preexisting cognitive impairment and/or preexisting disability in ADLs, leaving 531 patients eligible for this long-term follow-up study. We assessed 384/465 (83%) of survivors at 3 months and 334/419 (80%) of survivors at 12 months.
Figure 1. Enrollment and Follow-up.
Overall, 406 unique patients, who were a median age of 61 (Interquartile Range [IQR]: 51–70) years old with high severity of illness (median APACHE II score of 23 [IQR: 16–29]) at admission, and the majority of whom were not frail (median Clinical Frailty Scale score of 3 [IQR: 2–4]) contributed data to these analyses (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients
| Characteristic | N = 406 |
|---|---|
| Age (years) | 61 (51–70) |
| Male Sex, n (%) | 256 (63%) |
| Education (years) | 12 (12–14) |
| Katz ADL scorea | 0 (0-0) |
| IQCODE scoreb | 3 (3-3) |
| Clinical Frailty Scale Score, n (%) | |
| 1 (Very Fit) | 21 (5%) |
| 2 (Well) | 87 (21%) |
| 3 (Well, with Treated Comorbidities) | 164 (40%) |
| 4 (Apparently Vulnerable) | 86 (21%) |
| 5 (Mildly Frail) | 28 (7%) |
| 6 (Moderately Frail) | 17 (4%) |
| 7 (Severely Frail) | 3 (1%) |
| Charlson Comorbidity Index Scorec | 2 (1–3) |
| APACHE II Score at admissiond | 23 (16–29) |
| Mean Daily SOFA Scoree | 7 (5–8) |
| Diagnoses at Admission, n (%) | |
| Sepsis, ARDS due to infection or septic shock | 118 (29%) |
| Acute Respiratory Failuref | 42 (10%) |
| Cardiogenic shock, CHF, myocardial infarction, or arrhythmia | 79 (19%) |
| Upper airway obstructiong | 40 (10%) |
| Gastric or colonic surgery | 26 (6%) |
| Neurologic disease or seizure | 5 (1%) |
| Other surgical procedureh | 58 (14%) |
| Other diagnosesi | 38 (9%) |
| Mechanical ventilation | |
| Patients, n (%) | 360 (89%) |
| Duration of mechanical ventilation among those who were ever mechanically ventilated, days | 3 (1–7) |
| Severe Sepsis | |
| Patients, n (%) | 259 (64%) |
| Duration of severe sepsis among those who were ever septic, days | 4 (2–8) |
| Delirium | |
| Patients, n (%) | 289 (71%) |
| Duration of delirium among those who were ever delirious, days | 3 (2–7) |
| Coma | |
| Patients, n (%) | 221 (54%) |
| Duration of coma among those who were ever comatose, days | 2 (1–5) |
Data are median (interquartile range) unless otherwise indicated. APACHE II, Acute Physiology And Chronic Health Evaluation, version II; ARDS, Acute Respiratory Distress Syndrome; CHF, Congestive Heart Failure; IQCODE, Short Informant Questionnaire on Cognitive Decline in the Elderly, assessment of pre-illness cognition; Katz ADL, Assessment of basic activities of daily living; SOFA, Sequential Organ Failure Assessment
Katz ADL scores range from 0 to 12, where higher scores indicate more severe disability in activities of daily living. A score of 0 indicates no disability.
IQCODE scores range from 1 to 5, with a score of 3 indicating no change in cognition over the past 10 years. Scores lower than 3 indicate improvement, whereas, scores greater than 3 indicate decline.
Charlson comorbidity scores range from 0 to 33, with higher scores indicating a greater burden of chronic illness
APACHEII scores range from 0 to 71, with higher scores indicating more severe critical illness.
SOFA scores range from 0 to 24, with higher scores indicating more severe organ dysfunction.
Acute respiratory failure includes ARDS, acute exacerbations of chronic obstructive pulmonary disease or asthma, pulmonary edema, pulmonary embolism, and pulmonary fibrosis.
Upper airway obstruction also includes patients intubated for airway protection
Other surgical procedures includes vascular, urologic, orthopedic, obstetric/gynecologic, hepatobiliary, otolaryngologic, and liver transplant surgery.
Other diagnoses include acute renal failure, acid/base disturbance, endocrinologic, hemorrhagic shock, gastrointestinal bleeding, coagulopathy, cirrhosis, and acute liver failure.
Co-occurrence of Post-Intensive Care Syndrome Problems
Among patients who participated in 3-month follow-up, we assessed 337 for cognitive impairment (88%), 383 for disability (99%), and 363 patients for depression (95%). Of patients assessed, 128 (38%) had cognitive impairment, 100 (26%) had disability and 121 (33%) had depression. At 12 months, we assessed 292 patients for cognitive impairment (87%), 332 for disability (99%), and 313 for depression in (94%). Of these, 97 (33%) had cognitive impairment, 69 (21%) had disability, and 97 (31%) had depression. The median scores on the each of the follow-up assessments are reported in table S1 of the supplemental digital content.
There were 330 patients (86% of survivors) and 285 (85% of survivors) who completed all three assessments at 3 and 12 months, respectively. A single PICS problem was present in 130 (39%) at 3 months and 101 (35%) at 12 months (Figure 2). Two problems were present in 62 patients (19%) at 3 months and 47 patients (16%) at 12 months. Only 19 patients (6%) and 12 patients (4%), had problems in all three domains at 3 and 12 months, respectively. Among those patients with any PICS problems, the proportion with one, two, or three problems was 62%, 29% and 9%, respectively at 3 months, and 63%, 29%, and 9%, respectively at 12 months. Approximately 4 out of every 10 patients were PICS-free (119/330 [36%] at 3 months and 125/285 [44%] at 12 months) (Figure 2). Though the proportion of patients who were PICS-free during follow-up increased from 3 months to 12 months, the total number of patients without any problems was similar (Figure 2).
Figure 2. Co-Occurring Post-Intensive Care Syndrome Problems at 3- and 12-Month Follow-Up.
This diagram illustrates the co-occurrence of PICS problems at 3 and 12 months. The proportion of patients with PICS problems in each domain at 3 months is presented in the left panel and at 12 months in the right panel. Cognitive impairment is represented by the red circle. Disability in activities of daily living by the yellow circle. Depression by the blue circle. The overlap between the circles represents the co-occurrence of 2 or 3 problems. Overall, 6 out of 10 patients had PICS. The most common pattern at both 3 and 12 months was problems in a single domain and was present in 4 out of 10 patients. Co-occurring problems (i.e., in 2 or 3 domains) were present in 2 out of 10 patients.
In a sensitivity analysis that excluded patients with a proxy report of pre-existing depression, the proportion of patients who had one or more PICS problems decreased by 5% at 3 months and 12 months (Figure S1 in supplemental digital content). This decrease was due, in large part, to fewer patients having PICS-related to depression either as a single problem or co-occurring with disability or cognitive impairment.
Among the 211 patients with at least one PICS problem at 3 months, 44 (21%) no longer had any PICS problems at 12 months, 115 (55%) still had at least one PICS problem, 19 (9%) died, and 33 (16%) patients withdrew or were lost to follow-up. Of the 119 patients who had no PICS problems at 3 months, 76 (64%) remained without PICS problems at 12 months, 19 (16%) developed at least one PICS problem, 8 (7%) died, and 16 (13%) patients withdrew or were lost to follow-up.
Predictors Being PICS-Free at Follow-up
Survivors who were PICS-free tended to be younger, more educated, less frail and had fewer comorbidities than those with PICS (Tables S2 and S3 in supplemental digital content). Although severity of illness scores at ICU admission were similar between those who developed PICS and those who were PICS-free, the proportion of patients who were mechanically ventilated, were septic, delirious, or comatose during their ICU stay was lower among those who were PICS-free. Moreover, the duration of each of these conditions was shorter in those who were PICS-free (Tables S2 and S3 in supplemental digital content).
After adjusting for covariates, more years of education independently predicted greater odds of being PICS-free at 3 and 12 months (P<0.001 at both time points) (Table 2 and Figure 3A and 3B). Conversely, higher Clinical Frailty Scale scores at ICU admission independently predicted lower odds of being PICS-free (P=0.005 at 3 months and P=0.048 at 12 months) (Table 2 and Figure 3C and 3D). Age, duration of delirium, duration of severe sepsis, and duration of mechanical ventilation were not associated with being PICS-free (Table 2).
Table 2.
Association between Baseline and Clinical Factors and the Odds of Being PICS-Free at Follow-up.
| Baseline or Clinical Factor | Comparison (75th vs 25th percentile) |
Odds Ratio (95% CI) at 3 months |
P | Odds Ratio (95% CI) at 12 months |
P |
|---|---|---|---|---|---|
| Years of education | 14 vs 12 years | 1.6 (1.3–2.0) | <0.001 | 1.6 (1.3–2.0) | <0.001 |
| Clinical Frailty Scale score | 4 vs 2 | 0.5 (0.3–0.8) | 0.005 | 0.6 (0.3–1.0) | 0.048 |
| Duration of Severe Sepsis | 6 vs 0 days | 0.7 (0.4–1.1) | 0.09 | 0.6 (0.3–1.3) | 0.42 |
| Age | 70 vs 51 years | 1.2 (0.9–1.7) | 0.30 | 1.1 (0.7–1.5) | 0.09 |
| Duration of Delirium | 5 vs 0 days | 0.8 (0.5–1.2) | 0.35 | 0.5 (0.2–1.1) | 0.20 |
| Duration of Mechanical Ventilation | 5 vs 1 day | 1.0 (0.8–1.3) | 0.97 | 1.2 (0.6–2.5) | 0.80 |
Each odds ratio represents the odds being symptom-free at follow-up in a comparison of patients who have values of the exposure of interest at 75th percentile with patients who have values at the 25th percentile. Because the P-values consider all beta coefficients together, in cases where the 95% confidence interval includes 1, but the P-value is <0.05, the P-value is correct. Interpretive example, in a comparison of two patients alike in all other ways (that is, all covariates adjusted to their respective median or mode value) the patient with 14 years of education would have, on average, 60% greater odds of being PICS-free compared to a patient with 12 years of education.
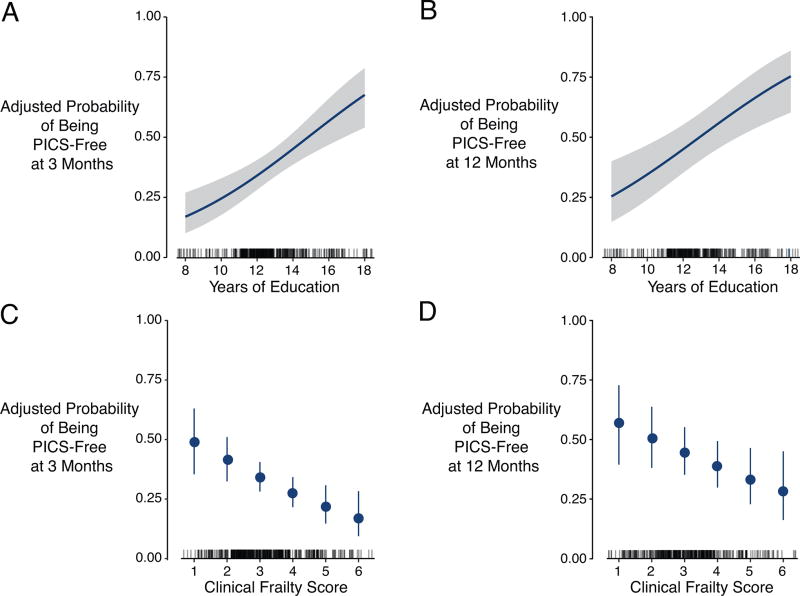
Figure 3. Associations between Years of Education and Clinical Frailty Scale Score with the Adjusted Probability of being PICS-Free at Follow-up.
These figures display the association between years of education and Clinical Frailty Scale score with the adjusted probability of being PICS-free at 3 months (left column) and 12 months (right column). For panels A and B the blue lines represent the association and blue shading represents the 95% confidence interval. For panels C and D, dots represent the point estimate and error bars the 95% confidence interval. The rug plot (just above the x-axes) shows the distribution of the exposure of interest. More years of education were associated with greater probability of being PICS-free (P<0.001 at 3 months, Panel A, and P<0.001 at 12 months, Panel B). Higher Clinical Frailty Scale scores, conversely, were associated with a lower probability of being PICS-free at 3 months (P=0.005, Panel C) and at 12 months (P=0.048, Panel D).
In the sensitivity analysis excluding 53 patients with a history of depression, education remained a significant predictor of being PICS-free, but the association with frailty was no longer statistically significant (see Table S4 in the supplemental digital content). The AHRQ Index of Socioeconomic Status score was not associated with being PICS-free (see Table S5 in the supplemental digital content).
DISCUSSION
In this multicenter cohort study of survivors of critical illness, we found that 6 out of 10 patients without preexisting cognitive impairment or disability developed one or more Post-Intensive Care Syndrome problems. Most patients with PICS had problems in a single domain, with cognitive impairment being most common, but disability in ADLs and depression also occurred frequently. Co-occurring PICS problems (i.e., problems in 2 or 3 domains) were present in 2 out of 10 patients. These data highlight the heterogeneous subtypes of PICS. Because the majority of patients included in this study were affected in only a single domain, our findings suggest that cognitive impairment, disability, and depression may be distinct sequelae of critical illness rather than part of a single unifying syndrome.
Over the last 15 years, investigators have studied cognitive, physical, and mental health function among survivors of critical illness, reporting significant proportions of these patients suffer from new or worsened impairments and disabilities, giving rise to the concept of PICS (1, 2, 6–9, 12, 17). To our knowledge, only one small cohort study has reported the co-occurrence of PICS problems. Maley et al., used a phone-based, patient-reported assessment of cognitive, physical, and mental health function among 43 survivors a median of 8 months after critical illness (22). At least one PICS problem was present in 84% (36/43) of patients. When this analysis was restricted to only patients who reported problems that were worse after critical illness, however, the overall prevalence of PICS decreased to 54%, nearly identical to the prevalence of PICS in the present study. They also reported that 2 or more PICS problems were present in 56% (24/43) of patients, but did not report the co-occurrence of problems that were worse after critical illness. In contrast, we report that 2 or more new PICS problems were present in 20% of our cohort. Thus, the different prevalence of co-occurring PICS problems between these studies may be because we considered only new PICS problems after critical illness.
We report that more years of education were associated with greater odds of being PICS-free. In studies of community-dwelling adults, those with more years of education have lower rates of dementia, disability, and depression (23–27). The exact mechanisms by which education may be protective from these problems are unclear. Education is associated with occupational attainment, greater income, better cognitive and critical thinking skills, and larger social/support networks that could represent greater resources to facilitate recovery (28). Our sensitivity analysis showing no association between socioeconomic status and freedom from PICS, however, suggests good outcomes after critical illness could be related to unmeasured education-related non-economic factors such as health behaviors (e.g., avoidance of cigarettes and heavy alcohol use, exercise, control of chronic disease), health literacy, or greater access to the health care system (28). Alternatively, because the RBANS is age-, but not education- adjusted, this finding could represent that those with greater years of education scored higher on the RBANS and therefore did meet our conservative definition of cognitive impairment. Finally, personality traits, such as the ability to persevere toward long-term goals (i.e., grit) that are associated with more years of education, may allow the those with more education to endure the road to recovery (29). These hypotheses should be evaluated in future long-term follow-up studies.
We also found that higher Clinical Frailty Scale scores were associated with lower odds of being PICS-free. Frailty is a state of heightened vulnerability characterized by diminished physiological reserve across multiple domains that results in the reduced ability to maintain and restore homeostasis in the setting of acute stress (30). In patients with critical illness, frailty is associated with greater mortality and subsequent disability (31–33). The association between greater frailty and lower odds of being PICS-free could reflect greater declines in cognitive, physical, and/or mental health by those with higher Clinical Frailty Scale scores during critical illness. Alternatively, if the declines in these domains were similar among patients across the fitness to frailty continuum, those with more severe frailty may possess reduced abilities to recover to their pre-illness status. These hypotheses need to be evaluated in future trials where trajectories of decline and recovery in each of the three PICS domains are measured using more frequent assessment than was available in the current study.
While we evaluated several modifiable risk factors for PICS (i.e., durations of sepsis, delirium, and mechanical ventilation), none were associated with being PICS-free at follow-up. Since collection of these data, however, interventions to reduce the modifiable risk factors of sedation and immobility have been shown effective. These interventions make up key components of the “ABCDEF bundle”. A growing body of evidence demonstrates the ABCDEF bundle to be associated with better short-term outcomes (i.e., lower in-hospital mortality, less delirium, and less immobility). Nevertheless, future studies are needed to determine the association between sedation, immobility and PICS problems and whether reducing these modifiable ICU-related risk factors for translates into improved outcomes for survivors.
Strengths of this investigation include enrollment of a geographically diverse cohort of medical and surgical critical illness survivors, that our cohort was 10-fold larger than the only other study to examine patterns of PICS problems, and that we achieved excellent long-term in-person follow-up. Moreover, we used a thorough three-step process to exclude patients from enrollment who had preexisting moderate or severe cognitive impairment, and we assessed participants for mild pre-illness cognition and disabilities using well-validated surrogate measures. We also prospectively collected a range of detailed clinical, physiologic, and pharmacologic parameters daily throughout the hospitalization.
Several limitations need to be acknowledged. First, given the emergent nature of critical illness, we were unable to directly assess participants’ cognitive function, disability in ADLs, and mental health prior to critical illness. Second, though we chose previously published definitions of clinically significant cognitive impairment, disability, and depression, these definitions are conservative and may underestimate PICS problems that are less overt yet still clinically important (12). Third, we did not assess for pre-existing anxiety or post-traumatic stress disorder (PTSD) and therefore did not include these mental health problems in our analyses that were focused on newly acquired PICS problems. Given that pre-critical illness mental health symptoms are strong risk factors for post-critical illness mental health symptoms (34, 35) future studies that account for pre-existing symptoms of anxiety and PTSD are needed to determine the effects of critical illness on these important mental health problems. Fourth, since it is unlikely that death and/or loss to follow-up occurred at random in this study, analyzing data only from survivors who were not lost to follow up could have resulted in survivor bias. Nevertheless, whether this bias represents unhealthy survivor bias (e.g., those with severe PICS problems could have been more likely to die or to be unable to participate in follow-up) or healthy survivor bias (e.g., those with no PICS problems resumed normal work and family activities and were therefore too busy to participate in follow-up) is unknown. Further study of the effects of survivorship bias on outcomes after critical illness is needed. Finally, as with any observational study, the possibility of residual confounding cannot be excluded. Nevertheless, we adjusted for several potential confounders in our multivariable analysis.
CONCLUSION
We found 6 out of 10 survivors of a critical illness had one or more PICS problems up to a year after ICU admission. Co-occurring PICS problems were present in 2 out of 10. More years of education was associated with being PICS-free and more severe frailty with lower odds of being PICS-free. Future work is needed to define better the specific subtypes of PICS, to identify the risk factors for co-occurring patterns of PICS, and to understand better the clinical, biological, and social factors related to the ability to withstand and recover successfully from critical illness. This understanding could facilitate the evaluation of interventions directed to improve outcomes for survivors of critical illness.
Supplementary Material
Acknowledgments
Dr. Pratik Pandharipande has received a research grant from Hospira Inc, in collaboration with the NIH. Dr. E. Wesley Ely has received honoraria from Pfizer, Abbott Laboratories, and Orion Pharma. Dr. Brummel has received honoraria for consulting from ArjoHuntleigh.
Funding/Support:
Department of Veterans Affairs Tennessee Valley Health Care System Geriatric Research, Education and Clinical Center (GRECC) and VA-MERIT, the National Institutes of Health under awards (K76AG054864, R01AG027472, KL2TR000446, R03AG040549, R01AG035117, R01HL111111, R01GM120484) and the Doris Duke Foundation.
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent those of the Department of Veterans Affairs, the National Institutes of Health, or Vanderbilt University Medical Center.
Drs. Pandharipande, Girard, Patel, Hughes, Jackson, Ely, and Brummel received support for article research from the National Institutes of Health (NIH). Dr. Pandharipande received other support from a research grant from Hospira Inc in collaboration with the NIH. Dr. Girard’s institution received funding from NIH AG034257 and NIH HL135144. Dr. Patel’s institution received funding from NIH/NIGMS R01 120484. Dr. Chandrasekhar’s institution received funding from hard money. Dr. Ely’s institution received funding from NIH and VA funding, and he received funding from Orion, Pfizer, and Abbott. Dr. Brummel received funding from ArjoHuntleigh.
Footnotes
Author contributions:
Drs. Marra and Brummel had full access to all study data, take responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: AM, NEB, PPP, TDG, EWE
Data acquisition, analysis and interpretation of the data: All authors
Statistical Analysis: JLT, RC
Drafting of the manuscript: AM, NEB
Critical revision of the article for important intellectual content: All authors
Final approval of the article: All authors
Obtaining Funding: EWE, PPP, TDG, NEB
Name of the institution(s) where the work was performed
Vanderbilt University Medical Center, Nashville, TN
Saint Thomas Hospital, Nashville, TN
Department of Veterans Affairs Medical Center, Tennessee Valley Healthsystem, Nashville, TN
Veterans Affairs Puget Sound Health Care System, Seattle, WA
George E. Wahlen Department of Veterans Affairs Medical Center, Salt Lake City, UT
Conflicts of interest:
Copyright form disclosure: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders' conference*. Crit Care Med. 2012;40(2):502–9. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 2.Elliott D, Davidson JE, Harvey MA, Bemis-Dougherty A, Hopkins RO, Iwashyna TJ, et al. Exploring the Scope of Post-Intensive Care Syndrome Therapy and Care: Engagement of Non-Critical Care Providers and Survivors in a Second Stakeholders Meeting. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 3.Cuthbertson BH, Rattray J, Campbell MK, Gager M, Roughton S, Smith A, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denehy L, Skinner EH, Edbrooke L, Haines K, Warrillow S, Hawthorne G, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17(4):R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott D, McKinley S, Alison J, Aitken LM, King M, Leslie GD, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15(3):R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 8.Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, et al. Psychiatric Symptoms in Acute Respiratory Distress Syndrome Survivors: A 1-Year National Multicenter Study. Crit Care Med. 2016;44(5):954–65. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry. 2013;21(9):887–97. doi: 10.1016/j.jagp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummel N, Sidiqi S, Pandharipande P, Thompson J, Vasilevskis E, Girard T, et al. Overlap of Cognitive, Physical and Mental Health Impairments in the Post-Intensive Care Syndrome. Crit Care Med. 2014;42(12):A358. [Google Scholar]
- 14.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–53. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 15.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT. BDI-II depression inventory manual. New York: Harcourt Brace; 1996. [Google Scholar]
- 17.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–79. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 20.Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–60. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. Creation of New Race-Ethnicity Codes and SES Indicators for Medicare Beneficiaries--Chapter 3. 2008 [updated 2008. Available from: https://archive.ahrq.gov/research/findings/final-reports/medicareindicators/medicareindicators3.html.
- 22.Maley JH, Brewster I, Mayoral I, Siruckova R, Adams S, McGraw KA, et al. Resilience in Survivors of Critical Illness in the Context of the Survivors' Experience and Recovery. Ann Am Thorac Soc. 2016;13(8):1351–60. doi: 10.1513/AnnalsATS.201511-782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 24.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenzuela MJ. Brain reserve and the prevention of dementia. Curr Opin Psychiatry. 2008;21(3):296–302. doi: 10.1097/YCO.0b013e3282f97b1f. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson S, Datta Gupta N. Identifying the effects of education on the ability to cope with a disability among individuals with disabilities. PLoS One. 2017;12(3):e0173659. doi: 10.1371/journal.pone.0173659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjelland I, Krokstad S, Mykletun A, Dahl AA, Tell GS, Tambs K. Does a higher educational level protect against anxiety and depression? The HUNT study. Soc Sci Med. 2008;66(6):1334–45. doi: 10.1016/j.socscimed.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Cutler M, Lleras-Muney A. Education and Health: Evaluating Theories and Evidence. Cambridge, MA: National Bureau of Economic Research; 2006. [Google Scholar]
- 29.Duckworth AL, Peterson C, Matthews MD, Kelly DR. Grit: perseverance and passion for long-term goals. J Pers Soc Psychol. 2007;92(6):1087–101. doi: 10.1037/0022-3514.92.6.1087. [DOI] [PubMed] [Google Scholar]
- 30.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med. 2017;196(1):64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186(2):E95–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty Before Critical Illness and Mortality for Elderly Medicare Beneficiaries. J Am Geriatr Soc. 2015;63(6):1121–8. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuthbertson BH, Hull A, Strachan M, Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30(3):450–5. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 35.Bienvenu OJ, Friedman LA, Colantuoni E, Dinglas VD, Sepulveda KA, Mendez-Tellez P, et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med. 2018;44(1):38–47. doi: 10.1007/s00134-017-5009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.