Abstract
Treatment-related side effects are a major clinical problem in cancer treatment. They lead to reduced compliance to therapy as well as increased morbidity and mortality. Well-known are the sequelae of chemotherapy on the heart, especially in childhood cancer survivors. Therefore, measures to mitigate the adverse events of cancer therapy may improve health and quality of life in cancer patients, both in short and long term. The renin angiotensin system (RAS) affects all hallmarks of cancer, and blockage of the RAS is associated with an improved outcome in several cancer types. There is also increasing evidence that inhibition of the RAS might be able to alleviate or even prevent certain types of cancer treatment related adverse effects. In this review, we summarize the potential of RAS inhibitors to mitigate cancer treatment related adverse events, with a special emphasis on chemotherapy-induced cardiotoxicity, radiation injury, and arterial hypertension.
Keywords: Angiotensin-converting enzyme inhibitors, Angiotensin receptor blockers Chemotherapy, Radiation therapy, Cardiotoxicity
Introduction
Cancer is a major public health issue with a projected number of 1,688,780 new cases and 600,920 cancer-related deaths in the United States in 2017 (1). The economic burden of cancer in the European Union rose continuously to €83.2 billion on health care expenditures and €19.1 billion on cancer drugs in 2014, production loss due to early death and lost working days not included (2).
Treatment-related side effects represent a major problem in oncology since they can severely interfere with the patient’s quality of life, require dose reductions and treatment delays, or even discontinuation of therapy. This reduces effectiveness of anti-cancer treatment and ultimately increases morbidity and mortality (3). Side effects also increase the number of emergency room visits and hospitalizations resulting in increased costs for healthcare systems and patients. Hence, smart prevention strategies might not only prolong patient survival, improve their quality of life, but also help to reduce healthcare costs (4).
The circulating renin-angiotensin system (RAS) plays a pivotal role in maintaining cardiovascular homeostasis as well as fluid and electrolyte balance. Additionally, a local RAS is expressed in many tissues and regulates cellular functions including growth and metabolism (5,6). Dysregulation of the local RAS is involved in the pathophysiology of several diseases, such as inflammation and fibrosis (7), and promotes cancer growth and dissemination (8,9). A recent meta-analysis demonstrated that the intake of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) is significantly associated with improved cancer progression free and overall survival (10).
Notably, angiotensin II (AngII) is also involved in the development of several cancer treatment-related side effects, such as cardiotoxicity (11), radiation-induced tissue injury (12), and muscle wasting (13–15). Hence, inhibition of AngII/angiotensin receptor type 1 (AT1R) signaling by renin-angiotensin inhibitors (RASi; i.e., direct renin inhibitors, ACEi, and ARBs) may not only improve the outcome of cancer treatment but may also help to treat or prevent certain adverse events. Notably, in addition to inhibiting AngII production, ACEi can also increase angiotensin (1–7) (Ang(1–7)) by blocking its breakdown through ACE. Ang(1–7) is known to counteract many effects of AngII/AT1R signaling (5,16). ARBs increase AngII levels by blocking the AT1R and thereby also contribute to Ang(1–7) generation from AngII via ACE2 (17,18). Thus, the beneficial effects of ACEi and ARBs may not only result from inhibiting AngII/AT1R signaling but may also partly be mediated by Ang(1–7) (17).
We have recently proposed that RASi-mediated improvement of drug delivery may allow for dose reductions of anti-cancer drugs without decreasing the therapeutic benefit, which could eventually result in a decreased number of side effects (9). In this review, we discuss the potential of RASi to prevent or improve tumor cachexia as well as cancer-treatment induced adverse events, such as cardiotoxicity, radiation injury, and arterial hypertension.
Chemotherapy-induced cardiotoxicity
Several anticancer agents, of which anthracyclines and trastuzumab are widely prescribed, can cause severe and even fatal cardiac side effects, with heart failure (HF) due to left ventricular dysfunction (LVD) being the most relevant (19). Of note, the presence of cardiotoxicity not only affects immediate and long term cardiac outcomes, but also limits the therapeutic options in case of disease recurrence. The term ‘cardiotoxicity’ encompasses all side effects affecting the heart, which span the entire cardiac domain, including detectable biomarkers, arrhythmia, structural changes, or clinical symptomatic heart disease. Currently, a general standard definition of cardiotoxicity is lacking, and definitions apply to the specific domain affected (e.g., left ventricular ejection fraction (LVEF) in HF) (11).
Cardiotoxicity following chemo- or targeted therapy can be divided in acute cardiotoxicity (immediately after administration), early-onset cardiotoxicity (within the first year of treatment) and late-onset cardiotoxicity (several years after chemotherapy) (20). The distinction between early- and late onset, however, is more or less artificial, as cardiotoxicity is rather a continuum where some injury occurs/manifests early and others not until later (19).
Late-onset cardiotoxicity, usually preceded by an early asymptomatic period, is of significant importance for pediatric cancer survivors (21) The incidence of anthracycline- and trastuzumab-induced overt HF depends on, amongst others, the cumulative dose, concomitant anti-cancer therapy and pre-existing cardiovascular disease. (11,20). For example, the overall incidence of echocardiographic LVD was 9% after a median period of 5.2 years or 1–3% for anti-HER2 targeted therapy (22,23). Though, incidences were much higher when anthracyclines and trastuzumab were concomitantly given, reaching up to 20% after 5 years follow-up (24).
The generation of reactive oxygen species (ROS)/oxidative stress represents a commonly recognized pathological mechanism and is a key player in anthracycline-mediated cardiotoxicity (Figure 1). On one hand, oxidative radicals are the consequence of the anthracyclines or its complexes with iron themselves. On the other hand, more recent evidence suggest that topoisomerase-2β in cardiomyocytes leads to increased oxidative stress via its effects on oxidative metabolism and mitochondrial functioning (11,19). Anthracyclines also appear to affect the neuregulin (NRG)-ErbB (better known as HER) receptor signaling, which is also targeted by trastuzumab. Acute exposure increases the expression of HER2, while chronic exposure inhibits HER4 expression, hence disrupting its normal signaling (11). The downstream cellular pathways of the HER2-HER4 heterodimer are responsible for cell performance and survival (25,26). This also explains why the cardiotoxicity of concomitant treatment with anthracyclines and trastuzumab is increased, since trastuzumab hampers the acute compensatory stress response (26).
Figure 1. Mechanisms of chemotherapy-induced cardiotoxicity.
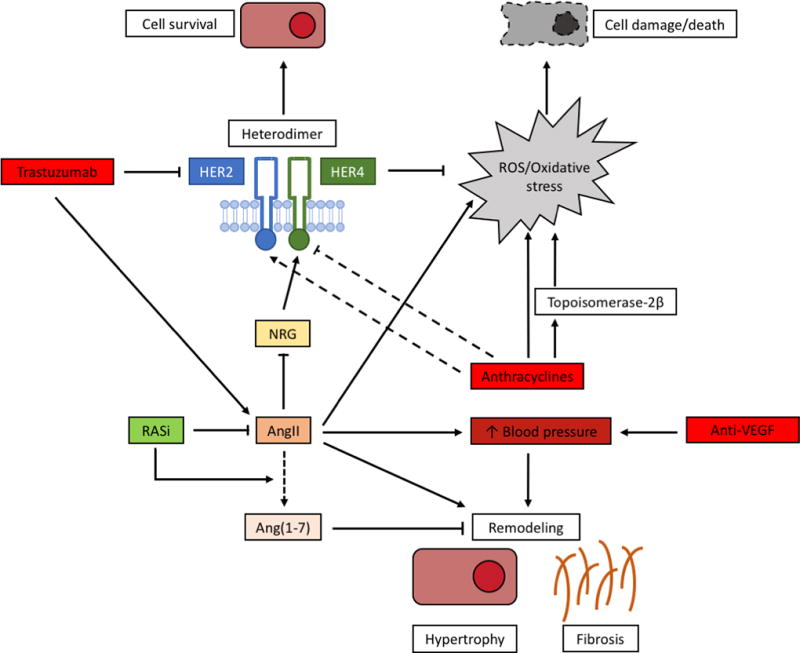
Anthracyclines induce significant oxidative stress, leading to cellular damage, via the formation of reactive oxygen species (ROS) and via the suppression of topoisomerase-2β. Anthracyclines may also affect HER2/HER4 signaling, where HER2 is induced after acute administration, but HER4 is reduced after chronic administration. Trastuzumab inhibits HER2 directly, preventing the formation of the cardioprotective HER2/HER4 heterodimer. Angiotensin II (AngII) is stimulated by trastuzumab and contributes to the formation of ROS. It is also able to inhibit HER2/HER4 signaling, via its inhibitory effects on the HER4 ligand neuregulin (NRG). The trophic effects of AngII lead to cardiac remodeling with cellular hypertrophy and fibrogenesis. This is also a consequence of increased arterial blood pressure (BP), that is provoked by inhibition of vascular endothelial growth factor inhibition (VEGFi). Inhibition of the renin angiotensin system (RAS) protects against the negative effects of AngII and stimulates the formation of cardioprotective Angiotensin (1–7) (Ang (1–7)).
When trastuzumab is given alone it does not cause cell death but rather cardiomyocyte dysfunction by disrupting HER2-mediated signaling, resulting in myocyte stunning or cell-cycle hibernation (19,27). Therefore, even though debated, trastuzumab-related cardiotoxicity is considered to be reversible (28,29). Beside the direct inhibition of the pro-survival NRG-HER signaling, trastuzumab increases AngII which is also a potent inhibitor of NRG. Additionally, AngII enhances ROS production via NADPH oxidase (25). In contrast, Ang(1–7) has cardioprotective effects in heart failure and counteracts the AngII/AT1R axis (30) (Figure 1).
Preclinical studies demonstrate that ACEi, ARBs as well as direct renin inhibitors can protect against chemotherapy-induced cardiotoxicity (31–36) However, clinical data using ACEi or ARBs at standard doses for heart failure reported positive as well as negative/inconclusive results for both substance classes in preventive setting (Table 1) (37–49). A meta-analysis showed that the prophylactic use of ACEi/ARBs is not able to attenuate the short to medium term development of LVD (defined by LVEF) or heart failure (Odds ratio 0.24 (95%CI, 0.03–1.73; p=0.16) (50). However, results tend to favor ACEi/ARBs and the lack of statistical significance is likely due to small sample sizes with relative short follow-up and low rate of heart failure (3.6% of all patients) (50).
Table 1.
Randomized controlled trials of RASi to protect against chemotherapy-induced cardiotoxicity
| Reference | CHT type, FU time |
Treatment (number of patients) |
Main findings | Outcome |
|---|---|---|---|---|
|
| ||||
| Primary prevention1 | ||||
|
| ||||
| Nakamae, 2005 (37) | CHOP, 7 days | Valsartan 80mg/d (20), Control (20) | Control: increased LVDd, BNP, ANP, QTc interval, QTc dispersion; Valsartan: prevented changes except of ANP elevation | Positive |
|
| ||||
| Georgakopoulos, 2010 (38) | Doxorubicin-based, 36 months | Enalapril (mean, 11mg/d) (43), Metoprolol (mean, 89mg/d) (42), Control (40) | No significant difference between the groups in echocardiographic parameters, and development of early/late cardiotoxicity or HF | Negative |
|
| ||||
| Cadeddu, 2010 (39) Dessì, 2011 (40) | Epirubicin-based, 18 months | Telmisartan 40mg/d (25), Placebo (24) | Diastolic impairment, reduced strain rate, and increased IL-6 and ROS in placebo group, but not in telmisartan group. Echocardiographic findings persisted till 12 months FU | Positive |
|
| ||||
| Liu, 2013 (41) | Anthracycline-based, 126 days | Candesartan 2.5mg/d + carvedilol 10mg/d (20), Placebo (20) | Attenuated decrease of LVEF in intervention group; increased LVEDD and LVESD in controls, not in intervention group; attenuated ECG changes and arrhythmias in intervention group; reduced troponin in intervention group | Positive |
|
| ||||
| Bosch, 2013 (42) | Intensive CHT for hematological malignancies, 6 months | Enalapril 20mg/d + carvedilol 50mg/d (45), Control (45) | Decreased LVEF only in control group; reduced incidence of combined event (death, HF, or final LVEF<45%) in intervention group | Positive |
|
| ||||
| Radulescu, 2013 (43) | Epirubicin-based, until 12 months after end of CHT | Perindopril 10mg/d (68), Control (68) | Significantly decreased LVEF only in controls; deterioration of LV diastolic function and prolongation of QTc in both groups | Inconclusive |
|
| ||||
| Janbabai, 2016 (44) | Anthracycline-based, 6 months | Enalapril 20mg/d (34), Placebo (35) | Systolic (including LVEF) and diastolic function only impaired in placebo group; troponin I and CK-MB higher in placebo group | Positive |
|
| ||||
| Gulati, 2016 (45) | Anthracycline-based +/− trastuzumab and radiation, 10–61 weeks | Candesartan 32mg/d + Metoprolol 100mg/d (30), Candesartan 32mg/d (32), Metoprolol 100mg/d (32), Placebo (32) | No interaction between candesartan and metoprolol; LVEF decline (primary outcome) was reduced by candesartan (but not by metoprolol) vs placebo; no effect of candesartan on secondary outcomes (RVEF, left ventricular global longitudinal strain, diastolic function, BNP, and troponin I) | Positive |
|
| ||||
| Boekhout, 2016 (46) | Trastuzumb (after anthracycline-based CHT), 92 weeks | Candesartan 32mg/d (103), Placebo (103) | No difference in LVEF and cardiac events between groups; NT-proBNP and hs-TNT not affected by candesartan | Negative |
|
| ||||
| Pituskin, 2017 (47) | Trastuzumab-based, 52 weeks | Perindopril 8mg/d (33), Bisoprolol 10mg/d (31), Placebo (30) | Primary outcome LV remodeling (LVEDVi) was not prevented by bisoprolol or perindopril; bisoprolol prevented reduction in LVEF; less trastuzumab interruptions due to LVEF drop in perindopril and bisoprolol; multivariate: perindopril and bisoprolol were independent predictors of maintained LVEF | Inconclusive |
|
| ||||
| Secondary prevention2 | ||||
|
| ||||
| Silber, 2004 (48) | Anthracycline-based, 34.6 months (mean) | Enalapril 0.15mg/kg/d (69), Placebo (66) | Enalapril had no effect on exercise performance (including MCI), but reduced LVESWS | Inconclusive |
|
| ||||
| Cardinale, 2006 (49) | High-dose CHT, 12 months | Enalapril 20mg/d (56), Control (58) | Reduction in LVEF and increase in end-systolic and diastolic volumes only in controls; incidence of cardiac events higher in controls; troponin I normalized within 3 months in enalapril (↑ troponin I at month 3: 0% vs 21%) | Positive |
Abbreviations: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CHOP, cyclophosphamide/doxorubicin/vincristine/prednisolone; CHT, chemotherapy; ECG, electrocardiogram; FU, follow-up; HF, heart failure; hs-TNT, high-sensitivity troponin T; LV, left ventricular; LVDd, LV end-diastolic diameter; LVEDVi, indexed LV end-diastolic volume; LVEF, LV ejection fraction; LVESWS, LV end-systolic wall stress; MCI, maximal cardiac index; NT-proBNP, N-terminal of prohormone BNP; RASi, renin-angiotensin system inhibitors; ROS, reactive oxygen species, RVEF, right ventricular ejection fraction.
Prophylactic setting
After detection of subclinical cardiotoxicity (e.g. ↑ troponin I, echocardiographic or ECG abnormalities)
Both ACEi and ARBs are corner stones in the treatment of heart failure, due to their capability to prevent cardiac remodeling and to reduce fibrogenesis (51,52). Hence, early treatment as soon as (subclinical) cardiotoxicity is detected (via echocardiography or biomarkers) is reasonable. Additionally, almost all cases of anthracycline-induced decline in LVEF are seen in the first 12 months following chemotherapy, implying that cardiotoxicity is a rather early phenomenon (22). Indeed, the sooner the ACE enalapril alone or in combination with the β-blocker carvedilol was given after detection of LVEF decline following chemotherapy, the larger the potential of full recovery of cardiac function. When initiated after 6 months, recovery was incomplete (53). Real-life data in older patients with follow up to 5 years confirm the beneficial effects of ACEi and β-blockers, where prompt start (i.e., within 6 months) was associated with lower cardiotoxicity risk and better cardiovascular outcomes (54).
The clinical guidelines of both the American Society of Clinical Oncology and the European Society of Cardiology recommend baseline and periodically surveillance of cardiac dysfunction. Screening includes cardiac biomarkers (troponins, natriuretic peptides) and cardiac imaging (echocardiography, cardiac magnetic resonance). It should be performed in case of clinical suspicion and in those patients at increased risk of developing cardiac dysfunction (20,55). Both organizations acknowledge the potential or RASi as part of a preventive strategy, but refrain to make a recommendation hereupon currently.
A proposed algorithm for anthracycline-related cardiotoxicity suggests enalapril in case of troponin I abnormalities and combined use of ACEi and β-blockers in case of LVD on echocardiography (19,56). However, this algorithm has not been validated. In general, treatment of chemotherapy-induced LVD and HF is similar to conventional HF therapy (52) and aims to reduce pathological left ventricle (LV) remodeling (57,58). ACEi and ARBs represent a mainstay since they can improve remodeling by reducing LV afterload and by directly antagonizing AngII/ angiotensin II receptor type 1 (AT1R)-mediated hypertrophy and fibrosis (58,59).
Taken together, ACEi/ARBs do have a clear potential to alleviate cardiotoxicity when given prophylactically or as soon as subclinical cardiotoxicity is detected. However, we are awaiting further larger studies. Little is known about the protective effects of long-term RASi use on cardiac outcome after chemotherapy (e.g., in childhood cancer survivors). Table 2, gives an overview of currently ongoing clinical trials.
Table 2.
Ongoing prospective trials of RASi to protect against cancer treatment-related side-effects
|
ClinicalTrials.gov identifier (Acronym study) |
Condition, treatment | Design | Intervention | Primary endpoint | Status |
|---|---|---|---|---|---|
|
| |||||
| Cardiotoxicity | |||||
|
| |||||
| NCT03127631 (RADICALPC) | Prostate cancer, androgen deprivation therapy | RCT | SOC | Composite of death, MI, Stroke, HF or Arterial Revascularisation | Recruiting |
| Behavioral interventions (nutrition, exercise, smoking cessation) + ASA + Statin + ACEi | |||||
|
| |||||
| NCT02907021 (SCHOLAR) | HER-2 positive breast cancer (non-metastatic) with moderate LV dysfunction | Single arm, Phase I | ACEi + BB | Development of cardiac dose-limiting toxicity | Not yet recruiting |
|
| |||||
| NCT02236806 (SAFE) | Breast cancer (non-metatstic), anthracyclines ± anti-HER2 | RCT, Phase III | Placebo | LVEF | Unknown |
| Bisoprolol | |||||
| Ramipril | |||||
| Bisoprolol + ramipril | |||||
|
| |||||
| NCT01904903 (SAFE-HEaRT) | HER-2 positive breast cancer with mild LV dysfunction, anti-HER-2 | Single arm, Phase II | ACEi + BB | Completion of therapy without cardiac events or worsening of cardiac function | Recruiting |
|
| |||||
| NCT03265574 (PROACT) | Breast cancer, adjuvant epirubicin | RCT, Phase III | SOC | Cardiac tropinin T relaese, cardiac function | Recruiting |
| Enalapril | |||||
|
| |||||
| NCT01968200 (ICOS-ONE) | Cancer, treatment with anthracyclines | RCT, Phase III | Enalapril (concomitant) | Occurence of cTn elevation | Active, not recruiting |
| Enalapril (after biochemical proven injury) | |||||
|
| |||||
| NCT01009918 | HER-2 positive breast cancer, trastuzumab | RCT, Phase III | Placebo | LVEF | Active, not recruiting |
| Carvedilol (extended release) | |||||
| Lisinopril | |||||
|
| |||||
| Radiotherapy-related toxicities | |||||
|
| |||||
| NCT01805453 (ASTER) | Newly-diagnosed glioblastoma, radiotherapy + temozolomide | RCT, phase II | Placebo | Steroid dose needed to control brain edema after radiotherapy | Active, not recruiting |
| Losartan | |||||
|
| |||||
| NCT01754909 | Lung cancer, radiotherapy | RCT, phase II | Placebo | Radiation pneumonitis | Recruiting |
| Enalapril | |||||
|
| |||||
| NCT01880528 | Lung cancer, radiotherapy | RCT | Placebo | Primary: adverse events of lisinopril -- Secondary: dyspnea, symptoms, QoL | Active, not recruiting |
| Lisinopril | |||||
|
| |||||
| NCT00004230 | Bone marrow or stem cell transplantation, following chemotherapy and radiotherapy | RCT, phase III | SOC | Lung injury | Completed |
| Captopril | |||||
|
| |||||
| Others | |||||
|
| |||||
| NCT02651415 (PARICCA) | Metastatic Colorectal cancer, regorafenib | Single arm, phase II | Perindopril | HFSR, AHT | Active, not recruiting |
Abbreviations: ACEi, angiotensin-converting-enzyme inhibitor; AHT, arterial hypertension; ASA, acetyl salicylic acid; BB, beta blocker; CHOP, cyclophosphamide/doxorubicin/vincristine/prednisolone; cTn, cardiac troponins; HF, heart failure; HFSR, hand-foot skin reaction; LV, left ventricular; LVEF, LV ejection fraction; MI, myocardial infarction; NSCLC, non-small cell lung cancer; QoL, quality of life; RASi, renin-angiotensin system inhibitors; RCT, randomized controlled trial; SOC, standard of care
Anti-VEGF therapy-induced arterial hypertension
Arterial hypertension represents a typical and mechanism-dependent ‘on target’ side effect of anti-vascular endothelial growth factor (VEGF) therapy and has also been proposed as a biomarker for treatment response (60–62). The induced afterload following arterial hypertension is a risk factor for the development of congestive HF, with a 2.69-fold increased risk for patients receiving VEGF receptor tyrosine kinase inhibitors (63). Thus treatment should start early and resolutely (20).
Several potential pathophysiological mechanisms have been proposed including capillary rarefication, downregulation of vasodilators (e.g., nitric oxide, prostacyclin), shift in pressure-natriuresis curve, and upregulation of vasoconstrictors (e.g., endothelin-1, thromboxanes) (62,64). The renin-angiotensin-aldosterone system seems not to play a significant role in the pathophysiology of anti-VEGF-mediated hypertension (62), but renin concentration and activity might even be suppressed in patients with anti-VEGF-induced hypertension (65). However, if anti-hypertensive medication is required, ACEi represent a preferred first-line option, especially in case of concomitant anti-VEGF-induced proteinuria (20,60,64,66). Moreover, treatment of VEGF-induced hypertension with RASi was associated with improved overall survival in metastatic renal cell carcinoma (67).
Notably, based on a small series, others hypothesized that ACEi might reduce the efficacy of bevacizumab by counteracting its antiangiogenic effect (68). This is not in line with other studies suggesting additive effects of RAS inhibition and anti-VEGF-based therapies (9,69). Unfortunately, a phase II study (ClinicalTrials.gov: NCT01705392) designed to investigate the ability of enalapril to prevent bevacizumab-induced hypertension compared to propranolol was suspended due to insufficient financial support. Hence, even though ACEi represent a first-line option, prospective trials are needed to determine which class of anti-hypertensive agents is most effective in preventing and treating anti-VEGF-related hypertension.
Radiation injury
Pathophysiologically, radiation can induce tissue injury through direct (radiation-DNA interaction) and indirect (production of ROS) DNA damage, and, if not repaired, eventually results in cell injury or death (70). There is some evidence that irradiation upregulates AngII expression in a dose dependent manner (71,72). The AngII/AT1R axis contributes to tissue damage and remodeling by upregulation of profibrogenic (e.g., transforming growth factor (TGF)-β) and proinflammatory pathways, and by production of ROS via activation of NADPH oxidase (12). Hence, inhibition of AngII/AT1R signaling represents an attractive target to prevent or reduce radiation-induced injury (Figure 2).
Figure 2. Mechanisms of radiation-induced tissue injury.
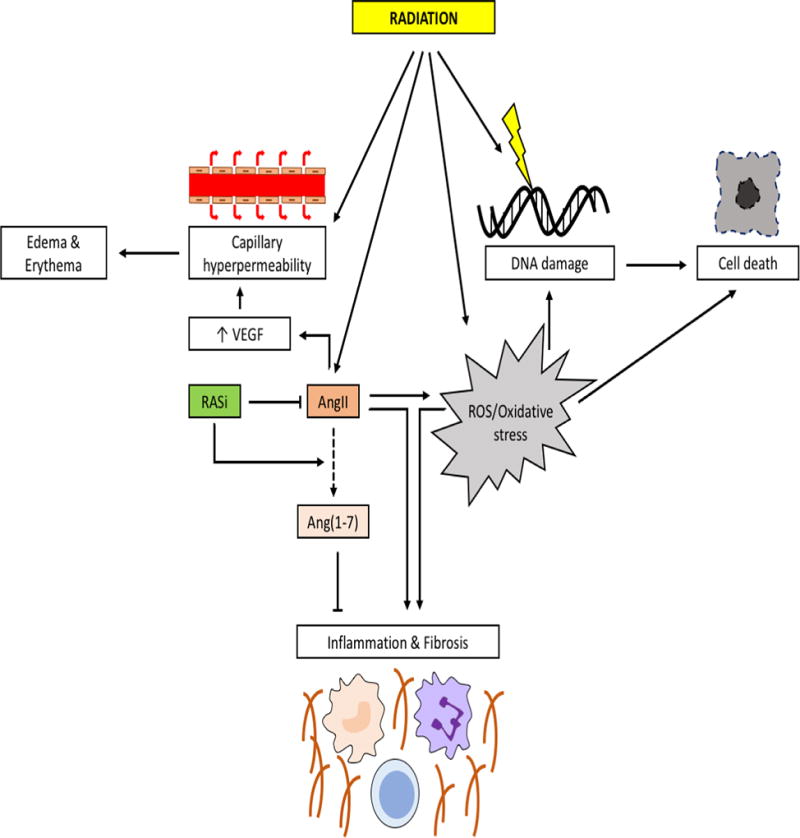
Radiation damages cellular DNA, both directly as well as indirectly via the formation of reactive oxygen species (ROS), that eventually result in cell death. This is augmented by the capability of radiation to upregulate angiotensin II (AngII) that contributes to the formation of ROS. The direct effects of radiation lead to capillary hyperpermeability that can lead to edema/erythema. This can be further augmented by AngII which increases vascular permeability via the stimulation of vacular endothelial growth factor (VEGF). Increased AngII itself stimulates inflammation and fibrosis of tissues. Inhibition of the renin angiotensin system (RAS) can reduce edema and lessens the effects on tissues. Moreover, induction of angiotensin (1–7) (Ang(1–7)) can counteract the effects of AngII.
Indeed, treatment with ACEi or ARBs has demonstrated some efficacy in preventing radiation-induced damage of normal tissue in different organs in preclinical studies (73–76) without compromising tumor response to radiation (77).
ACEi and ARBs might inhibit radiation-induced toxicity not only by their effects on AngII and AT1R but also by increasing Ang(1–7) (16,17), which indeed seems to have radioprotective effects (78,79). Noteworthy, both AngII and Ang(1–7) improved hematopoietic recovery after total body irradiation in mice (80).
Data from clinical trials are scarce and inconsistent. Several retrospective studies reported a decreased risk of radiation pneumonitis in lung cancer patients using ACEi/ARBs (81–86) while others found no association (87,88). Interestingly, in one study, a beneficial effect was only seen with ACEi and not with ARB (81).
As these retrospective studies only provide pooled analysis of different ACEi/ARB compounds, no conclusions on the effect of each drug within a RASi subclass can be drawn and no specifications on dosing can be made. However, one can assume that standard doses to treat cardiovascular diseases were used. Unfortunately, a large prospective study using captopril in radiation treated lung cancer patients was halted due to insufficient accrual (89).
ACEi use was also associated with reduced risk of radiation-induced proctitis in a retrospective study (90). A prospective non-interventional study found that statins alone and ACEi in combination with statins (but not ACEi alone) reduced gastrointestinal toxicity after pelvic radiation (91). In a small randomized controlled trial of 55 subjects undergoing total body radiation for hematopoietic stem cell transplantation, captopril at a maximum dose of 25mg tid had no significant effect on the risk of chronic renal failure, and on overall and pulmonary mortality (92).
Finally, radiation increases the permeability of the capillary wall and leads to endothelial dysfunction (93). The consequential edema is a frequent and threatening side effect when treating brain tumors. Two retrospective studies showed that the use of ACEi/ARBs was associated with reduced steroid doses for vasogenic edema in glioblastoma via blockage of VEGF production that is abundantly present in those tumors (94,95). The use of ACEi/ARBs is also protective for the adjacent normal brain tissue by preventing radiation necrosis (96), while preclinical evidence demonstrates the capability of an ARB to preserve cognition (97).
Taken together, ACEi/ARBs seem to be protective against the effects of radiation injury in several organ sites. However, prospective trials are needed for further validation. Trials currently testing RASi to prevent radiation injury are listed in table 2.
Cachexia
Cachexia is a multifactorial catabolic condition characterized by substantial weight and muscle loss (with or without fat loss) due to an underlying disease, that cannot be completely reversed by nutritional support (98,99). Cancer cachexia can reach different stages ranging from pre-cachexia, to cachexia, and refractory cachexia (99) and should be considered as a partially preventable cancer comorbidity (100). Cachexia has a high prevalence in cancer patients (30–60%), accounts for up to 20% of deaths, and represents an independent predictor of mortality, treatment response, and quality of life (101,102). AngII signaling contributes to muscle wasting by different mechanisms such as induction of muscle protein catabolism through up-regulation of the ubiquitine-proteasome proteolytic pathway (13), inhibition of protein synthesis in myotubes (15), and induction of apoptosis in myocytes (14). While the induction of skeletal myocyte apoptosis is mediated via AT1R (103), the effects of AngII on protein degradation in skeletal muscle are likely mediated via the angiotensin II receptor type 2 (AT2R) (13), known to counteract the effects of AngII via AT1R (5). This suggests that ACEi – by inhibiting AngII formation – may have a more potent protective effect in preventing cancer cachexia than ARBs, which only block AT1R.
Interestingly, in an observational study of older hypertensive women, continuous ACEi use significantly slowed decline in muscle strength (104).
Several preclinical studies have demonstrated that ACEi can control cancer cachexia in different rodent models (13,105,106), while imidapril had no beneficial effect on cardiac wasting in a rat hepatoma cancer cachexia model (107). In a subcutaneous murine colon cancer model, the direct renin inhibitor aliskiren had potent anti-cachexia effects. Aliskiren antagonized several mechanisms of cancer cachexia, namely RAS activation, inflammation, oxidative stress, and stimulation of the authophagy-lysosome as well as the ubiquitin-proteasome pathway (108).
In a phase III trial conducted by Ark Therapeutics, the ACEi imidapril attenuated weight loss in NSCLC and colorectal cancer, but not in pancreatic cancer; however, the trial missed its primary endpoint since the significance was gone in pooled analysis (http://www.apmhealtheurope.com/print_story.php?numero=L1135).
Other cancer treatment induced side effects
Many cancer patients develop chemotherapy-induced peripheral neuropathy with long lasting and refractory pain in a substantial percentage of patients. In a retrospective study analyzing results of a standardized panel testing sensory and pain sensation of the small fibers of the peripheral nerves (i.e., of the hand), ACEi/ARBs were associated with attenuated sensory loss. The authors suggest an angiotensin II receptor type 2 (AT2R)-mediated neuro-modulating effect over the small myelinated fibers of the glabrous skin (109).
An ongoing trial (NCT02651415) is currently investigating the potential of the ACEi perindopril to mitigate the occurrence of hand foot skin reactions – a well-known side effect of tyrosine kinase inhibitors – in colorectal cancer patients treated with regorafenib (Table 2).
Conclusions
The renin angiotensin system is, without doubt, closely intertwined with cancer. Available data suggest that concomitant use of RASi with standard cancer therapy may reduce morbidity and mortality of cancer patients. Moreover, RASi might also alleviate treatment-related side effects of cancer therapies, such as cardiotoxicity and radiation-induced tissue injury. However, knowledge on the role of RASi herein is currently limited to experimental studies, retrospective analysis, or small prospective studies. Consequently, to date, definite recommendations cannot be made. Additionally, most studies evaluated the role of ACEi and ARBs in this context and only little data is available for direct renin inhibitors – the third class of RASi.
The main body of research is related to the potential effects of RASi to reduce cardiotoxicity in anthracycline- or trastuzumab induced cardiotoxicity. There is a trend that RASi at standard doses could be useful in a prophylactic setting, but unequivocal evidence is lacking. The evidence for treating (sub)clinical HF with RASi is more substantial. Therefore, prophylactic treatment with RASi should be limited to selected high-risk patients. Once (sub)clinical HF is detected, treatment should be started promptly (20). Similarly, arterial hypertension should be treated aggressively and as early as possible in order to prevent the development of congestive HF (20).
Within the field of radiation-induced side effects, available data are scarce. Most studies currently investigating RASi in the setting of radiation are focusing on the prevention of radiation pneumonitis as this seems to be a promising approach.
Notably, by modulating the tumor microenvironment and improving drug delivery to tumors, RASi may also allow for dose reductions of anti-cancer drugs, including immunotherapy, without decreasing their therapeutic benefit, and this could ultimately decrease toxicity (9)
Finally, even though RASi may help to attenuate various side effects of cancer treatment, decisions for using these drugs are to be made on a case by case basis. For example, due to its effects on the glomerular filtration rate, RASi actually increase the risk on platinum-based nephrotoxicity (110).
Since RASi are already FDA-approved, have a well-known safety-profile and are relatively inexpensive, they are an appealing option to improve cancer-therapy substantially. Their future potential might even further grow in the quickly evolving landscape of cancer therapeutics. However, there is an obvious need for properly-powered randomized controlled trials to evaluate the full potential of RASi in preventing or improving certain cancer treatment-related adverse events. Studies with extended follow-up have the potential to alleviate the long-term side effects.
Prospective studies should also investigate if standard doses of RASi are required in the prevention setting or if even reduced doses have the potential to mitigate cancer treatment-induced adverse events.
Acknowledgments
M. Pinter is supported by an Erwin-Schroedinger Fellowship by the Austrian Science Fund (J 3747-B28). R.K. Jain is supported by NCI P01-CA080124, NCI R35-CA197743, NCI R01-CA208205, NCI U01-CA224173, and by the Bill and Melinda Gates Foundation (OPP1140482).
The authors want to express their gratitude to Drs. Jeffrey Clark and Melin J. Khandekar for their comments on the manuscript.
Conflict of interest
M.P. served as advisory board member of Bayer, BMS, and Eisai, and received travel support from Bayer and speaking fees from Bayer and BMS. He is also an investigator for Bayer, BMS, and Lilly.
R.K.J. received honorarium from Amgen, consultant fees from Enlight, Merck, Ophthotech, Pfizer, SPARC, and SynDevRx, owns equity in Enlight, Ophthotech, SynDevRx, and XTuit, and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund.
Abbreviations
- ACEi
angiotensin-converting enzyme inhibitors
- AngII
angiotensin II
- Ang(1–7)
angiotensin (1–7)
- ARB
angiotensin receptor blockers
- AT1R
angiotensin II receptor type 1
- AT2R
angiotensin II receptor type 2
- HF
heart failure
- LV
left ventricle
- LVD
left ventricular dysfunction
- LVEF
left ventricular ejection fraction
- NADPH
Nicotinamide adenine dinucleotide phosphate H
- NRG
neuregulin
- RAS
renin-angiotensin system
- RASi
renin-angiotensin system inhibitors
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jönsson B, Hofmarcher T, Lindgren P, Wilking N. The cost and burden of cancer in the European Union 1995–2014. Eur J Cancer. 2016;66:162–70. doi: 10.1016/j.ejca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–9. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 4.Nolan MT, Plana JC, Thavendiranathan P, Shaw L, Si L, Marwick TH. Cost-effectiveness of strain-targeted cardioprotection for prevention of chemotherapy-induced cardiotoxicity. Int J Cardiol. 2016;212:336–45. doi: 10.1016/j.ijcard.2016.02.137. [DOI] [PubMed] [Google Scholar]
- 5.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bader M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu Rev Pharmacol Toxicol. 2010;50:439–65. doi: 10.1146/annurev.pharmtox.010909.105610. [DOI] [PubMed] [Google Scholar]
- 7.Passos-Silva DG, Brandan E, Santos RAS. Angiotensins as therapeutic targets beyond heart disease. Trends Pharmacol Sci. 2015;36:310–20. doi: 10.1016/j.tips.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 8.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745–59. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 9.Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun H, Li T, Zhuang R, Cai W, Zheng Y. Do renin-angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients?: Evidence from a meta-analysis including 55 studies. Medicine (Baltimore) 2017;96:e6394. doi: 10.1097/MD.0000000000006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc Drugs Ther. Cardiovascular Drugs and Therapy. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins ME, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005;93:425–34. doi: 10.1038/sj.bjc.6602725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burniston JG, Saini A, Tan L-B, Goldspink DF. Angiotensin II induces apoptosis in vivo in skeletal, as well as cardiac, muscle of the rat. Exp Physiol. 2005;90:755–61. doi: 10.1113/expphysiol.2005.030908. [DOI] [PubMed] [Google Scholar]
- 15.Russell ST, Sanders PM, Tisdale MJ. Angiotensin II directly inhibits protein synthesis in murine myotubes. Cancer Lett. 2006;231:290–4. doi: 10.1016/j.canlet.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Tom B, Dendorfer A, Danser AHJ. Bradykinin, angiotensin-(1–7), and ACE inhibitors: how do they interact? Int J Biochem Cell Biol. 2003;35:792–801. doi: 10.1016/s1357-2725(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 17.Schindler C, Bramlage P, Kirch W, Ferrario CM. Role of the vasodilator peptide angiotensin-(1–7) in cardiovascular drug therapy. Vasc Health Risk Manag. 2007;3:125–37. [PMC free article] [PubMed] [Google Scholar]
- 18.Arumugam S, Thandavarayan RA, Palaniyandi SS, Giridharan VV, Arozal W, Sari FR, et al. Candesartan cilexetil protects from cardiac myosin induced cardiotoxicity via reduction of endoplasmic reticulum stress and apoptosis in rats: involvement of ACE2-Ang (1–7)-mas axis. Toxicology. 2012;291:139–45. doi: 10.1016/j.tox.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol. 2016;13:172–84. doi: 10.1038/nrclinonc.2015.171. [DOI] [PubMed] [Google Scholar]
- 20.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC. Eur Heart J. 2016;37:2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 21.Scholz-Kreisel P, Spix C, Blettner M, Eckerle S, Faber J, Wild P, et al. Prevalence of cardiovascular late sequelae in long-term survivors of childhood cancer: A systematic review and meta-analysis. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26428. [DOI] [PubMed] [Google Scholar]
- 22.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 23.Sodergren SC, Copson E, White A, Efficace F, Sprangers M, Fitzsimmons D, et al. Systematic Review of the Side Effects Associated With Anti-HER2-Targeted Therapies Used in the Treatment of Breast Cancer, on Behalf of the EORTC Quality of Life Group. Target Oncol. 2016;11:277–92. doi: 10.1007/s11523-015-0409-2. [DOI] [PubMed] [Google Scholar]
- 24.Bowles EJA, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: A “dual-hit”. Exp Clin Cardiol. 2011;16:70–4. [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen Z, Segers VFM, De Keulenaer GW. Basic Res Cardiol. Vol. 111. Springer; Berlin Heidelberg: 2016. ErbB2 signaling at the crossing between heart failure and cancer; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harbeck N, Ewer MS, De Laurentiis M, Suter TM, Ewer SM. Cardiovascular complications of conventional and targeted adjuvant breast cancer therapy. Ann Oncol Off J Eur Soc Med Oncol. 2011;22:1250–8. doi: 10.1093/annonc/mdq543. [DOI] [PubMed] [Google Scholar]
- 28.Pondé NF, Lambertini M, de Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO open. 2016;1:e000073. doi: 10.1136/esmoopen-2016-000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganz PA, Romond EH, Cecchini RS, Rastogi P, Geyer CE, Swain SM, et al. Long-Term Follow-Up of Cardiac Function and Quality of Life for Patients in NSABP Protocol B-31/NRG Oncology: A Randomized Trial Comparing the Safety and Efficacy of Doxorubicin and Cyclophosphamide (AC) Followed by Paclitaxel With AC Followed by Paclitax. J Clin Oncol. 2017;35:3942–8. doi: 10.1200/JCO.2017.74.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel VB, Lezutekong JN, Chen X, Oudit GY. Recombinant Human ACE2 and the Angiotensin 1–7 Axis as Potential New Therapies for Heart Failure. Can J Cardiol. 2017;33:943–6. doi: 10.1016/j.cjca.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Akolkar G, Bhullar N, Bews H, Shaikh B, Premecz S, Bordun K-A, et al. The role of renin angiotensin system antagonists in the prevention of doxorubicin and trastuzumab induced cardiotoxicity. Cardiovasc Ultrasound. 2015;13:18. doi: 10.1186/s12947-015-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monti M, Terzuoli E, Ziche M, Morbidelli L. The sulphydryl containing ACE inhibitor Zofenoprilat protects coronary endothelium from Doxorubicin-induced apoptosis. Pharmacol Res. 2013;76:171–81. doi: 10.1016/j.phrs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Sacco G, Bigioni M, Evangelista S, Goso C, Manzini S, Maggi CA. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. Eur J Pharmacol. 2001;414:71–8. doi: 10.1016/s0014-2999(01)00782-8. [DOI] [PubMed] [Google Scholar]
- 34.Toko H, Oka T, Zou Y, Sakamoto M, Mizukami M, Sano M, et al. Angiotensin II type 1a receptor mediates doxorubicin-induced cardiomyopathy. Hypertens Res. 2002;25:597–603. doi: 10.1291/hypres.25.597. [DOI] [PubMed] [Google Scholar]
- 35.Rashikh A, Pillai KK, Najmi AK. Protective effect of a direct renin inhibitor in acute murine model of cardiotoxicity and nephrotoxicity. Fundam Clin Pharmacol. 2014;28:489–500. doi: 10.1111/fcp.12054. [DOI] [PubMed] [Google Scholar]
- 36.Al-Harbi NO, Imam F, Nadeem A, Al-Harbi MM, Iqbal M, Rahman S, et al. Protection against tacrolimus-induced cardiotoxicity in rats by olmesartan and aliskiren. Toxicol Mech Methods. 2014;24:697–702. doi: 10.3109/15376516.2014.963773. [DOI] [PubMed] [Google Scholar]
- 37.Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer. 2005;104:2492–8. doi: 10.1002/cncr.21478. [DOI] [PubMed] [Google Scholar]
- 38.Georgakopoulos P, Roussou P, Matsakas E, Karavidas A, Anagnostopoulos N, Marinakis T, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol. 2010;85:894–6. doi: 10.1002/ajh.21840. [DOI] [PubMed] [Google Scholar]
- 39.Cadeddu C, Piras A, Mantovani G, Deidda M, Dessì M, Madeddu C, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010;160:487.e1–7. doi: 10.1016/j.ahj.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 40.Dessì M, Piras A, Madeddu C, Cadeddu C, Deidda M, Massa E, et al. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction. Exp Ther Med. 2011;2:1003–9. doi: 10.3892/etm.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Liu Z, Liu Y, Zheng Z, Liang X, Han Y, et al. [Preventive effect of low-dose carvedilol combined with candesartan on the cardiotoxicity of anthracycline drugs in the adjuvant chemotherapy of breast cancer] Zhonghua Zhong Liu Za Zhi. 2013;35:936–40. [PubMed] [Google Scholar]
- 42.Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted t. J Am Coll Cardiol. 2013;61:2355–62. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 43.Radulescu D, Buzdugan E, Ciuleanu TE, Todor N, Stoicescu L. Can the epirubicin cardiotoxicity in cancer patients be prevented by angiotensin converting enzyme inhibitors? J BUON. 2013;18:1052–7. [PubMed] [Google Scholar]
- 44.Janbabai G, Nabati M, Faghihinia M, Azizi S, Borhani S, Yazdani J. Effect of Enalapril on Preventing Anthracycline-Induced Cardiomyopathy. Cardiovasc Toxicol. 2017;17:130–9. doi: 10.1007/s12012-016-9365-z. [DOI] [PubMed] [Google Scholar]
- 45.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–80. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, et al. Angiotensin II-Receptor Inhibition With Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients With Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016;2:1030–7. doi: 10.1001/jamaoncol.2016.1726. [DOI] [PubMed] [Google Scholar]
- 47.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol. 2017;35:870–7. doi: 10.1200/JCO.2016.68.7830. [DOI] [PubMed] [Google Scholar]
- 48.Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–8. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–81. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 50.Gujral DM, Lloyd G, Bhattacharyya S. Effect of prophylactic betablocker or ACE inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy ± trastuzumab. Breast. 2018;37:64–71. doi: 10.1016/j.breast.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinic. J Am Coll Cardiol. 2016;68:1476–88. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 53.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. J Am Coll Cardiol. Vol. 55. Elsevier Inc.; 2010. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy; pp. 213–20. [DOI] [PubMed] [Google Scholar]
- 54.Wittayanukorn S, Qian J, Westrick SC, Billor N, Johnson B, Hansen RA. Prevention of Trastuzumab and Anthracycline-induced Cardiotoxicity Using Angiotensin-converting Enzyme Inhibitors or β-blockers in Older Adults With Breast Cancer. Am J Clin Oncol. 2017;0:1–10. doi: 10.1097/COC.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 55.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 56.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol Off J Eur Soc Med Oncol. 2012;23(Suppl 7):vii155–66. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 57.Tocchetti CG, Ragone G, Coppola C, Rea D, Piscopo G, Scala S, et al. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. Eur J Heart Fail. 2012;14:130–7. doi: 10.1093/eurjhf/hfr165. [DOI] [PubMed] [Google Scholar]
- 58.Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med. 2015;66:161–76. doi: 10.1146/annurev-med-070213-054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–32. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 60.de Jesus-Gonzalez N, Robinson E, Moslehi J, Humphreys BD. Management of antiangiogenic therapy-induced hypertension. Hypertens (Dallas, Tex 1979) 2012;60:607–15. doi: 10.1161/HYPERTENSIONAHA.112.196774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravaud A, Schmidinger M. Clinical biomarkers of response in advanced renal cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2013;24:2935–42. doi: 10.1093/annonc/mdt288. [DOI] [PubMed] [Google Scholar]
- 62.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010;30:591–601. doi: 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghatalia P, Morgan CJ, Je Y, Nguyen PL, Trinh Q-D, Choueiri TK, et al. Crit Rev Oncol Hematol. Vol. 94. Elsevier Ireland Ltd; 2015. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors; pp. 228–37. [DOI] [PubMed] [Google Scholar]
- 64.Small HY, Montezano AC, Rios FJ, Savoia C, Touyz RM. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: understanding and managing a new syndrome. Can J Cardiol. 2014;30:534–43. doi: 10.1016/j.cjca.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 65.Kappers MHW, van Esch JHM, Sluiter W, Sleijfer S, Danser AHJ, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertens (Dallas, Tex 1979) 2010;56:675–81. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 66.Izzedine H, Massard C, Spano JP, Goldwasser F, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer. 2010;46:439–48. doi: 10.1016/j.ejca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Penttilä P, Rautiola J, Poussa T, Peltola K, Bono P. Clin Genitourin Cancer. Vol. 15. Elsevier Inc.; 2017. Angiotensin Inhibitors as Treatment of Sunitinib/Pazopanib-induced Hypertension in Metastatic Renal Cell Carcinoma; pp. 384–390.e3. [DOI] [PubMed] [Google Scholar]
- 68.Emile G, Pujade-Lauraine E, Alexandre J. Should we use the angiotensin-converting enzyme inhibitors for the treatment of anti-VEGF-induced hypertension? Ann Oncol Off J Eur Soc Med Oncol. 2014;25:1669–70. doi: 10.1093/annonc/mdu197. [DOI] [PubMed] [Google Scholar]
- 69.Abd-Alhaseeb MM, Zaitone SA, Abou-El-Ela SH, Moustafa YM. Olmesartan potentiates the anti-angiogenic effect of sorafenib in mice bearing Ehrlich’s ascites carcinoma: role of angiotensin (1–7) PLoS One. 2014;9:e85891. doi: 10.1371/journal.pone.0085891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koukourakis MI. Radiation damage and radioprotectants: new concepts in the era of molecular medicine. Br J Radiol. 2012;85:313–30. doi: 10.1259/bjr/16386034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomic R, Jacobs E, Medhora M, Antonescu-turcu A, Swarajit G. Chest. Vol. 134. The American College of Chest Physicians; 2008. ROLE OF RENIN-ANGIOTENSIN SYSTEM IN RADIATION-INDUCED LUNG INJURY; p. p127001. [Google Scholar]
- 72.Cao S, Wu R. Expression of Angiotensin II and Aldosterone in Radiation-induced Lung Injury. Cancer Biol Med. 2012;9:254–60. doi: 10.7497/j.issn.2095-3941.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaggi JS, Seshan SV, McDevitt MR, Sgouros G, Hyjek E, Scheinberg DA. Mitigation of radiation nephropathy after internal alpha-particle irradiation of kidneys. Int J Radiat Oncol Biol Phys. 2006;64:1503–12. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 74.Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Medhora M, Hill RP. Targeting the Renin-angiotensin system combined with an antioxidant is highly effective in mitigating radiation-induced lung damage. Int J Radiat Oncol Biol Phys. 2014;89:722–8. doi: 10.1016/j.ijrobp.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robbins ME, Zhao W, Garcia-Espinosa MA, Diz DI. Renin-angiotensin system blockers and modulation of radiation-induced brain injury. Curr Drug Targets. 2010;11:1413–22. doi: 10.2174/1389450111009011413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Authors on behalf of ICRP. Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012;41:1–322. doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Kohl RR, Kolozsvary A, Brown SL, Zhu G, Kim JH. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440–5. doi: 10.1667/RR0707.1. [DOI] [PubMed] [Google Scholar]
- 78.Willey JS, Bracey DN, Gallagher PE, Tallant EA, Wiggins WF, Callahan MF, et al. Angiotensin-(1–7) Attenuates Skeletal Muscle Fibrosis and Stiffening in a Mouse Model of Extremity Sarcoma Radiation Therapy. J Bone Joint Surg Am. 2016;98:48–55. doi: 10.2106/JBJS.O.00545. [DOI] [PubMed] [Google Scholar]
- 79.Moore ED, Kooshki M, Metheny-Barlow LJ, Gallagher PE, Robbins ME. Angiotensin-(1–7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP kinase signaling. Free Radic Biol Med. 2013;65:1060–8. doi: 10.1016/j.freeradbiomed.2013.08.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodgers KE, Xiong S, DiZerega GS. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol. 2002;49:403–11. doi: 10.1007/s00280-002-0434-6. [DOI] [PubMed] [Google Scholar]
- 81.Alite F, Balasubramanian N, Adams W, Surucu M, Mescioglu I, Harkenrider MM. Decreased Risk of Radiation Pneumonitis With Coincident Concurrent Use of Angiotensin-converting Enzyme Inhibitors in Patients Receiving Lung Stereotactic Body Radiation Therapy. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 82.Bracci S, Valeriani M, Agolli L, De Sanctis V, Maurizi Enrici R, Osti MF. Renin-Angiotensin System Inhibitors Might Help to Reduce the Development of Symptomatic Radiation Pneumonitis After Stereotactic Body Radiotherapy for Lung Cancer. Clin Lung Cancer. 2016;17:189–97. doi: 10.1016/j.cllc.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 83.Harder EM, Park HS, Nath SK, Mancini BR, Decker RH. Angiotensin-converting enzyme inhibitors decrease the risk of radiation pneumonitis after stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e643–9. doi: 10.1016/j.prro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Jenkins P, Welsh A. Computed tomography appearance of early radiation injury to the lung: correlation with clinical and dosimetric factors. Int J Radiat Oncol Biol Phys. 2011;81:97–103. doi: 10.1016/j.ijrobp.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 85.Kharofa J, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–43. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Kharofa J, Gore E. Symptomatic radiation pneumonitis in elderly patients receiving thoracic irradiation. Clin Lung Cancer. 2013;14:283–7. doi: 10.1016/j.cllc.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Wang H, Liao Z, Zhuang Y, Xu T, Nguyen Q-N, Levy LB, et al. Do angiotensin-converting enzyme inhibitors reduce the risk of symptomatic radiation pneumonitis in patients with non-small cell lung cancer after definitive radiation therapy? Analysis of a single-institution database. Int J Radiat Oncol Biol Phys. 2013;87:1071–7. doi: 10.1016/j.ijrobp.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang LW, Fu XL, Clough R, Sibley G, Fan M, Bentel GC, et al. Can angiotensin-converting enzyme inhibitors protect against symptomatic radiation pneumonitis? Radiat Res. 2000;153:405–10. doi: 10.1667/0033-7587(2000)153[0405:caceip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 89.Small W, James JL, Moore TD, Fintel DJ, Lutz ST, Movsas B, et al. Utility of the ACE Inhibitor Captopril in Mitigating Radiation-associated Pulmonary Toxicity in Lung Cancer: Results From NRG Oncology RTOG 0123. Am J Clin Oncol. 2016;0:1–6. doi: 10.1097/COC.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alashkham A, Paterson C, Rauchhaus P, Nabi G. Can Angiotensin-Converting Enzyme Inhibitors Reduce the Incidence, Severity, and Duration of Radiation Proctitis? Int J Radiat Oncol Biol Phys. 2016;94:93–101. doi: 10.1016/j.ijrobp.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 91.Wedlake LJ, Silia F, Benton B, Lalji A, Thomas K, Dearnaley DP, et al. Evaluating the efficacy of statins and ACE-inhibitors in reducing gastrointestinal toxicity in patients receiving radiotherapy for pelvic malignancies. Eur J Cancer. 2012;48:2117–24. doi: 10.1016/j.ejca.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 92.Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, et al. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83:292–6. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J Am Coll Cardiol. 2013;61:2319–28. doi: 10.1016/j.jacc.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 94.Carpentier AF, Ferrari D, Bailon O, Ursu R, Banissi C, Dubessy A-L, et al. Steroid-sparing effects of angiotensin-II inhibitors in glioblastoma patients. Eur J Neurol. 2012;19:1337–42. doi: 10.1111/j.1468-1331.2012.03766.x. [DOI] [PubMed] [Google Scholar]
- 95.Kourilsky A, Bertrand G, Ursu R, Doridam J, Barlog C, Faillot T, et al. J Neurol. Vol. 263. Springer; Berlin Heidelberg: 2016. Impact of Angiotensin-II receptor blockers on vasogenic edema in glioblastoma patients; pp. 524–30. [DOI] [PubMed] [Google Scholar]
- 96.Chowdhary M, Okwan-Duodu D, Switchenko JM, Press RH, Jhaveri J, Buchwald ZS, et al. J Neurooncol. Vol. 136. Springer US; 2018. Angiotensin receptor blockade: a novel approach for symptomatic radiation necrosis after stereotactic radiosurgery; pp. 289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conner KR, Payne VS, Forbes ME, Robbins ME, Riddle DR. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. 2010;173:49–61. doi: 10.1667/RR1821.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 100.Muscaritoli M, Molfino A, Lucia S, Rossi Fanelli F. Cachexia: a preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit Rev Oncol Hematol. 2015;94:251–9. doi: 10.1016/j.critrevonc.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 101.Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305. doi: 10.1159/000356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315:1219–22. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalla Libera L, Ravara B, Angelini A, Rossini K, Sandri M, Thiene G, et al. Beneficial effects on skeletal muscle of the angiotensin II type 1 receptor blocker irbesartan in experimental heart failure. Circulation. 2001;103:2195–200. doi: 10.1161/01.cir.103.17.2195. [DOI] [PubMed] [Google Scholar]
- 104.Onder G, Penninx BWJH, Balkrishnan R, Fried LP, Chaves PHM, Williamson J, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet (London, England) 2002;359:926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 105.Chen S-Z, Xiao J-D. Rosiglitazone and imidapril alone or in combination alleviate muscle and adipose depletion in a murine cancer cachexia model. Tumour Biol. 2014;35:323–32. doi: 10.1007/s13277-013-1043-1. [DOI] [PubMed] [Google Scholar]
- 106.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. Int J cancer. 2013;133:1234–46. doi: 10.1002/ijc.28128. [DOI] [PubMed] [Google Scholar]
- 107.Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, et al. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur Heart J. 2014;35:932–41. doi: 10.1093/eurheartj/eht302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang C, Guo D, Wang Q, You S, Qiao Z, Liu Y, et al. Aliskiren targets multiple systems to alleviate cancer cachexia. Oncol Rep. 2016;36:3014–22. doi: 10.3892/or.2016.5118. [DOI] [PubMed] [Google Scholar]
- 109.Roldan CJ, Song J, Engle MP, Dougherty PM. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Modulate the Function of Myelinated Fibers after Chemotherapy: A Quantitative Sensory Testing Study. Pain Physician. 2017;20:281–92. [PubMed] [Google Scholar]
- 110.Komaki K, Kusaba T, Tanaka M, Kado H, Shiotsu Y, Matsui M, et al. BMC Cancer. Vol. 17. BMC Cancer; 2017. Lower blood pressure and risk of cisplatin nephrotoxicity: a retrospective cohort study; p. 144. [DOI] [PMC free article] [PubMed] [Google Scholar]


