Abstract
Introduction
Higher levels of moderate to vigorous physical activity improve all-cause mortality and cardiovascular events. However, the effect of running, a moderate to vigorous activity, in those with knee osteoarthritis (OA), a common arthritis that occurs with aging, a high risk group for mortality and cardiovascular events, is unclear. Therefore, we aimed to evaluate the association of self-selected running on OA symptom and structure progression in people with knee OA.
Methods
This nested cohort study within the Osteoarthritis Initiative (OAI) (2004–2014) included those over 50 years old with OA in at least one knee. Runners were defined using a self-administered questionnaire at the 96-month visit. At baseline and 48-months, symptoms were assessed and radiographs were scored for Kellgren-Lawrence (KL) grade (2–4) and medial Joint Space Narrowing (JSN) score (0–3). We evaluated the association of self-selected running with outcomes: KL worsening, medial JSN worsening, new knee pain, and improved knee pain over 48 months, adjusting for baseline age, sex, body mass index (BMI), KL score, contralateral KL score, contralateral knee pain, and injury. If data were not available at the 48 month visit, then they were imputed from the 36 month visit.
Results
1,203 participants had a mean age of 63.2 (7.9) years, BMI of 29.5 (4.6) kg/m2, 45.3% male, and 11.5% runners. Data from 8% of participants required imputation. Adjusted odds ratios for KL grade worsening and new frequent knee pain were 0.9 (0.6 – 1.3) and 0.9 (0.6 – 1.6) respectively. Adjusted odds ratio for frequent knee pain resolution was 1.7 (1.0 – 2.8).
Conclusions
Among individuals over 50 years old with knee OA, self-selected running is associated with improved knee pain and not with worsening knee pain or radiographically defined structural progression. Therefore, self-selected running, which is likely influenced by knee symptoms and may result in lower intensity and shorter duration sessions of exercise, need not be discouraged in people with knee OA.
Introduction
Aerobic physical activity, particularly moderate to vigorous intensity aerobic activity, has been recommended by the World Health Organization, for people of all ages [1] because there is clear evidence that moderate to vigorous intensity aerobic activity such as running improves overall mortality and cardiovascular events [2–4]. The groups at risk for these outcomes are the same as those for osteoarthritis (OA), the most common form of arthritis, with an age standardized global prevalence for symptomatic knee OA of 3.8% [5], contributing to more than 17.1 million years of life lived with disability annually [5]. Hence, many of the people who are recommended to participate in moderate to vigorous physical activity are also likely to have OA.
Running is a common activity included in the gamut of aerobic moderate to vigorous physical activity [6]. To our knowledge, the effect of running in those with knee OA has never been systematically addressed. Although there has been substantial effort made to determine whether running is harmful to a healthy knee joint [7–17], there has been little research focused on the effect of running to those with knee OA. Potential benefits of running in this population includes a lower body mass index (BMI), better proprioception and greater strength. Alternatively, it is possible that running might be harmful to those with knee OA as they may be at greater risk for incurring damage from excess loading from running. The purpose of this study was to evaluate whether running is harmful in those with knee OA using data from the Osteoarthritis Initiative (OAI), a multi-center observational study.
Methods
Study Design
This is an observational nested cohort study within the OAI inclusive of the 4 clinical sites, Memorial Hospital of Rhode Island (Pawtucket, RI,) Ohio State University (Columbus, Ohio), University of Pittsburgh (Pittsburgh, PA), and University of Maryland / Johns Hopkins University (Baltimore, MD). We obtained institutional review board approval at Baylor College of Medicine and all clinical sites. Written informed consent was obtained from all participants.
All publicly available data were accessed from OAI website (http://oai.epi-ucsf.org/datarelease/).
Study Timeline
The activity questionnaire was administered at the OAI 96 month visit, while the radiographs and knee pain questions were ascertained at the OAI baseline and 48 month visits.
Inclusion criteria
We included OAI participants who were ≥ 50 years old at OAI baseline, who had complete data on knee-specific pain and knee radiographs at the baseline and 36- or 48-month visits, and a modified version of the historical physical activity survey instrument at the 96-month visit. Participants were required to have radiographic OA (Kellgren-Lawrence (KL) grade ≥ 2) in at least one native knee at the time of OAI enrollment. Those knees with total arthroplasty at OAI baseline were excluded.
Historical Physical Activity Survey Instrument
The modified version of the historical physical activity survey instrument [18] was distributed to the participants prior to their 96 month visit, returned between September 12, 2012 and October 31, 2014. We adapted this questionnaire so that it could be administered as a take-home survey, similar to what has been done in the past by Chasan-Taber [19]. The questionnaire asked about 37 different leisure physical activities. One of these activities was “running or jogging”. The structure of the survey was that participants were asked to think about different age ranges in their life. During each age range, participants were asked to identify the top 3 activities they most frequently performed during each age range. For this study, we were interested in the age range of ≥ 50 years old. Participants who identified “running or jogging” as a top 3 most frequently performed physical activities was considered a runner. Participants were only asked to consider activities that were performed at least 10 times for at least 20 minutes each time. For each activity identified as a top 3 activity, the participants provided information on the number of years participated in the activity, months per year, and times per month. They were also asked whether they performed the activity on a competitive level.
Knee Radiographs
The largest number of funded radiographic readings with the longest follow-up occurred at the OAI baseline and 48-month visits. Thus we selected the baseline and 48-month follow up visits as the time points of interest for this study. If radiographs were missing from the 48-month visit, we used radiographs from the 36-month visit instead. At these visits, weight-bearing, posterior-anterior semi-flexed views of bilateral knees were obtained [20]. The radiographs were centrally scored [21] for KL grade (0–4) using the Osteoarthritis Research Society International Atlas [22], as well as for medial Joint Space Narrowing (JSN) OARSI grade (0–3). Only knees that had radiographic OA (KL grade ≥ 2) at the OAI baseline visit were included in this study. The reliability for these readings (read-reread) was good (weighted kappa for intra-rater reliability = 0.70 – 0.80 for KL grade and 0.74 – 0.75 for medial JSN) [21].
Knee Pain Assessment
At the OAI baseline and 48 month visits, participants were asked the following dichotomous questions to ascertain knee pain symptoms,: “During the last 12 months, have you had pain, aching, or stiffness in or around your right knee on most days for at least one month? By most days, we mean more than half the days of a month”[23]. The identical question was also asked regarding the left knee. If a response was missing from the 48 month visit, we used the response from the 36 month visit instead.
Total Knee Arthroplasty
A series of questions were administered to identify whether people had total knee replacements (TKRs) at the baseline, 12, 24, 36, and 48 month visits.
Covariates
Participants’ ages were calculated using their dates of birth as well as their baseline visit dates. BMI was calculated using weight and height measured at the baseline visit. Participants’ sex was self-reported. Participants were questioned about any history of knee injury during the prior year at the 12, 24, 36, and 48 month visits. Participants were asked about “injuries in the knee that limited ability to walk for at least two days.”[24] If an injury was reported at any of these visits, they were viewed as having an injury during the follow up of the first 48 months of the OAI. We only included injury that occurred during OAI follow up as a covariate because we were interested in controlling for injury that would have occurred after developing OA, not injury that would have occurred prior to the development of OA.
Outcome Definitions
We defined KL worsening as an increase in KL grade or receipt of a TKR between the baseline and 48 month visits. Medial JSN worsening was an increase in medial JSN score, including within OARSI grade worsening or receipt of a TKR between the baseline and 48 month visits. We chose to evaluate medial JSN worsening as most of the loading within the knee passes through the medial tibiofemoral compartment. New knee pain was defined as a knee transitioning from not having frequent knee pain at baseline to having had frequent knee pain at the 48 month visit or receipt of a TKR by the 48-month visit. Improved knee pain was defined as transitioning from baseline frequent knee pain to not having frequent knee pain.
Statistical Analysis
We performed knee-based logistic regression analyses, using generalized estimating equations to account for correlation between knees within a person, where the predictor was running during the age range of ≥ 50 years old. The outcomes were (1) new knee pain, (2) KL worsening, (3) medial JSN worsening, and (4) improving knee pain; adjusted analyses included covariates baseline age, sex, BMI, KL score, contralateral KL score, contralateral knee pain, and injury over the observation period. We adjusted for contralateral KL score because the grade of OA severity in one knee may influence the contralateral knee through off-loading to the opposite knee or OA severity in one knee may lead to OA in the other knee. Injury was included as a covariate for as it may accelerate OA worsening, and runners may have more knee injuries. Those who already had the pain outcomes were excluded from those respective analyses; for new knee pain, we excluded those who already reported frequent knee pain at baseline, for resolution of knee pain, we excluded those who did not report frequent knee pain at baseline. For structural outcomes of KL worsening and medial JSN worsening that were at the extremes of those variables, receipt of a TKR was considered progression of the outcome.
Sensitivity analyses
We performed three sensitivity analyses. First, because a higher percentage of men comprised the runners compared to the non-runners, the second set of sensitivity analyses were restricted to men only. There were insufficient numbers to run analyses on women only. Second, we excluded all people who required radiograph and pain scores imputation from the 36-month visit. And finally, we restricted the analyses to participants who were ages 50–55 years at baseline, to increase the likelihood that the exposure of running occurred after an established diagnosis of OA.
We tested for interactions of running with injury during the observation period, baseline KL score, and baseline BMI.
We used SAS version 9.4 to perform all analyses.
Results
In figure 1, we present the flow diagram for the participants who completed the historical physical activity survey instrument. In figure 2, we present the flow diagram picking up from figure 1 who were included in each of the logistic regression analyses. For the KL grade and JSN worsening analyses, all participants in the study are eligible for analyses. When evaluating the new knee pain or less knee pain analyses, 1132 knees were eligible for new knee pain while 676 were eligible for less knee pain (figure 2).
Figure 1.
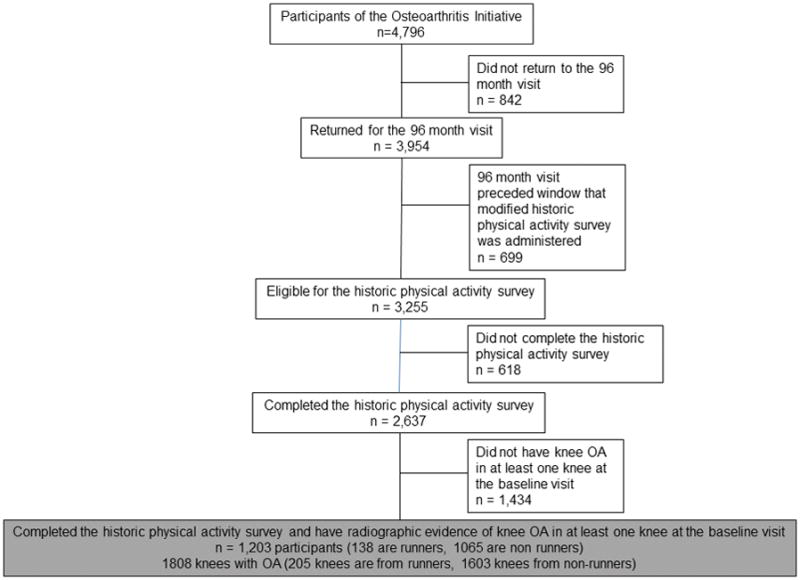
Flow diagram illustrating the selection of 1,203 participants who completed the historical physical activity survey instrument and had evidence of knee OA in at least one knee.
Figure 2.
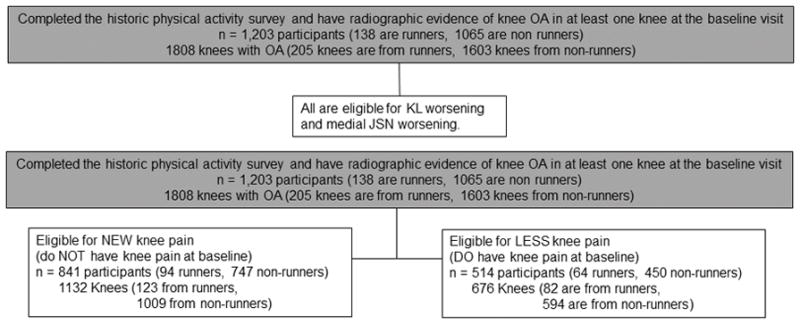
Flow diagram illustrating which of the 1,203 participants (contributing 1808 knees) from figure 2 were assessed for the outcomes of interest including KL worsening, medial JSN worsening, new knee pain, and less knee pain.
Of the 1,203 participants included in these analysis, 138 (11.5%) were runners. These participants contributed 1808 knees with OA, 205 were from runners and 1603 were from non-runners (table 1).
Table 1.
Baseline Characteristics of the whole cohort, runners, non-runners, and those who had imputed follow up radiographs.
| Participant Characteristics | All Participants (n = 1203) | Runners (n =138) | Non-Runners (n = 1065) | Imputed Follow up Radiographs (n = 101) |
|---|---|---|---|---|
| Age (years) | 63.2 (7.9) | 62.9 (7.3) | 63.2 (8.0) | 63.5 (7.7) |
| Sex (% Male) | 45.3% | 69.6% | 42.2% | 38.6% |
| BMI (kg/m2) | 29.5 (4.6) | 28.4 (4.0) | 29.6 (4.7) | 29.8 (5.0) |
|
| ||||
| Knee Based Characteristics | (n=1808 knees) | (n=205 knees) | (n=1603 knees) | (n=151 knees) |
|
| ||||
| KL Grade | ||||
| 2 | 63.8% | 61.5% | 64.1% | 55.0% |
| 3 | 29.3% | 29.3% | 29.3% | 40.4% |
| 4 | 7.0% | 9.3% | 6.7% | 4.6% |
| Medial JSN | ||||
| 0 | 35.3% | 33.7% | 35.5% | 25.8% |
| 1 | 35.8% | 35.1% | 35.9% | 36.4% |
| 2 | 24.2% | 25.4% | 24.0% | 34.4% |
| 3 | 4.7% | 5.9% | 4.6% | 3.3% |
| TKR by the 48 month visit | 4.3% | 3.9% | 4.3% | N/A |
| Injury that occurred between baseline to the 48 month visit during the OAI | 11.1% | 10.3% | 11.2% | 11.3% |
Some data were imputed within the overall sample. From 101 participants (of the 1,203 included in the study), 151 knees (of the 1808 included in the study) had radiograph follow up readings that were imputed from the 36 month visit readings to the 48 month readings. 2 follow up knee pain responses from 2 people were imputed from the 36 month visit responses to the 48 month responses. In total, data from 8% of the overall sample required imputation.
Baseline characteristics stratified by runner status are shown in Table 1. The mean age and BMI of all participants were 63.2 (7.9) years and 29.5 (4.6) kg/m2, with 45.3% male (table 1). Notably, 69.6% of runners were male, much higher than in the 42.2% in non-runners group, otherwise, the runners and non-runners were similar. From lowest to highest BMI tertile, 15.2%, 11.2%, and 8.1% were runners. The participants and knees that had follow up radiograph results imputed from the 36-month visit were similar to the overall participants though there were more knees with baseline KL grade 3 and who had some medial JSN (Table 1). Of participants who were identified as runners at age 50 years old or later, 74.6% ran for 6 or more years and 92.7% ran 5–12 months per year, 88.4% of runners ran more than 4 times per month, and 13.0% (18/138) participated in running competitively (table 2). The people who ran competitively, were similar to the broader running group, except that they had a lower BMI of 26.8 kg/m2 and the overall OA severity was somewhat higher with KL grades 2, 3 and 4 in this group being 44.8% (13/29), 37.9% (11/29) and 17.2% (5/29) respectively.
Table 2.
Self-reported Duration, Frequency of Running, and Participation on a Competitive Level Prevalences Among the Runners
| Number of Years | Prevalence (%) |
|---|---|
| 1–5 | 25.4% |
| 6–10 | 33.3% |
| 11–20 | 23.2% |
| >20 | 18.1% |
| Months Per Year | |
| 1–4 | 7.3% |
| 5–8 | 30.4% |
| 9–12 | 62.3% |
| Times Per Month | |
| 1–3 | 11.6% |
| 4–8 | 30.4% |
| >9 | 58.0% |
| Participated Competitively in Running | 13.0% |
Compared to non-runners, runners did not have increased odds for KL worsening, medial JSN worsening, or new knee pain (table 3). Results were unchanged when adjusting for age, sex, BMI, injury, ipsilateral KL score, contralateral KL score, and contralateral frequent knee pain (table 3). Adjusted odds ratios for worsening of KL grade and new frequent knee pain were 0.9 (0.6 – 1.3) and 0.9 (0.6 – 1.6) respectively. Adjusted odds ratio for resolution of frequent knee pain was 1.7 (1.0 – 2.8). Those who had resolution of frequent knee pain did not have statistically significantly different change in BMI between the baseline and follow up visit compared to those who did not (−0.01 v 0.01 kg/m2, p=0.8).
Table 3.
Results from logistic regression analyses where runner status is the predictor and the longitudinal outcomes are KL worsening, medial JSN worsening, new frequent knee pain and improvement of frequent knee pain.
| Prevalence of Outcome | Unadjusted Odds Ratios | Adjusted Odds Ratios* | |
|---|---|---|---|
| KL Worsening | |||
| Non-Runners | 307/1603 (19.2%) | Referent | Referent |
| Runners | 32/205 (15.6%) | 0.8 (0.5 – 1.2) | 0.9 (0.6 – 1.3) |
| Medial JSN Worsening | |||
| Non-Runners | 378/1603 (23.6%) | Referent | Referent |
| Runners | 40/205(19.5%) | 0.8 (0.5 – 1.2) | 0.8 (0.5 – 1.2) |
| New Frequent Knee Pain | |||
| Non-Runners | 293/1009 (29.0%) | Referent | Referent |
| Runners | 33/123 (26.8%) | 0.8 (0.5 – 1.3) | 0.9 (0.6 – 1.6) |
| Improvement of Frequent Knee Pain | |||
| Non-Runners | 232/594 (39.1%) | Referent | Referent |
| Runners | 41/82 (50.0%) | 1.6 (1.0 – 2.6) | 1.7 (1.0 – 2.8) |
adjusted for age, sex, BMI, injury, ipsilateral baseline KL score, contralateral baseline KL score, and contralateral frequent knee pain
The sensitivity analyses including men only and then only participants without imputed radiographic and symptom data only were similar to that of the main analyses. The sensitivity analyses including only participants ages 50–55 years at the time of enrollment into the OAI also resulted in similar findings to that seen in the whole group, though the number of events that occurred in these groups were too small to allow calculation of odds ratios (not shown).
For all four outcomes, KL worsening, medial JSN worsening, new frequent knee pain, and improvement in knee pain, there were no significant interactions between running and injury during the observation period, baseline KL score, and baseline BMI.
Discussion
This is the first study to our knowledge that has evaluated the effect of running on those with established knee OA. Contrary to what we expected, we found little evidence to suggest that running is harmful in this cohort, the OAI, an observational study not selected based on running status. Among individuals over 50 years of age with knee OA, running was not associated with longitudinal worsening knee pain or radiographically defined structural progression. Additionally, runners also had more improvement in knee pain compared to non-runners, suggesting that there may be a benefit to running from a knee health perspective in people who have knee OA.
Although we posited that it was possible that running might be harmful to those with knee OA as they may be at greater risk for incurring damage from excess loading from running, our findings did not support this possibility. We found that BMI was not different among runners compared to non-runners so this is not likely an important mediator of the possible benefits of running. Muscle strength is a complicated construct and there has been extensive data collected regarding this construct on OAI participants. Future study of a possible relationship between running and muscle strength may provide important insights into the merits of running. Perhaps running results in greater muscle strength that may lessen the impact felt by the knee from the ground reaction force that occurs in running. An additional possible reason for pain reduction in physically active individuals might also be resultant from the effect of muscle stimulation itself independent of muscle strength, similar in concept to the observed analgesic effect of electric stimulation in chronic pain syndromes [25, 26]. This may be particular pertinent given the recent evidence for a potential role of central sensitization in those with knee OA [27]. Additionally, proprioreception could be better among runners compared to non-runners with the idea that those who run may condition their sense of proprioreception and those with better proprioreception may then be better able to mitigate the stress that can occur within a knee that is participating in running. There were no measures of proprioreception within the OAI, but this could be of great interest for future studies of running as it relates to OA. Interestingly, the distribution of radiographic severity between runners and non-runners was very similar so it does not appear that there was not a selection bias for those with less severe knee OA to participate in running. There is an inherent likelihood that those who had knee pain with running were unlikely to run. Perhaps this is a protective mechanism, discouraging an activity that might be harmful to the knee. In this study, runners were self-selected. People ran voluntarily, not because it was mandated. Consequently, we can only evaluate the associations of self-selected running with knee outcomes and understand that we are unable to comment about the effect of mandated running on those with knee OA.
As in the National Health and Nutrition Examination Survey 1999–2006, a community based survey in the US [6], in our study a larger percentage of runners were males compared to non-runners. Although we adjusted for sex in our analyses, with such a large proportion of males in the runners group, it could be that this finding of running not being harmful might only apply to males. Sensitivity analyses confirmed the validity of these findings in men. Thus, the external validity of these findings may be more applicable to males. An effort should be made to replicate the findings in this study in a cohort that includes more female runners.
A strength of this study was the fact that it was performed within the OAI cohort, a well characterized cohort followed over several years. Standardized questionnaires were used to assess for symptoms and standardized, high quality radiographs were acquired at all clinical sites and centrally read.
There were some important limitations to our study. One is that running status was retrospectively assessed using a survey administered at the OAI 96-month visit. This time point occurred after all the outcome assessments due to the complicated logistics of including a new questionnaire into an existing longitudinal cohort. Additionally, we were not able to assure that knee OA pre-dated the running exposure because of the nature of the questions used to ascertain running status. Specifically, the survey instrument asked participants to consider physical activity when they were an age greater than or equal to 50 years old. The uncertainty arises in the following situation: if a participant’s OAI enrollment was age 70 (the time point when ROA was ascertained) and he only ran from ages 55 – 60 years old. Although the participant would answer affirmative to the fact that he ran at age ≥ 50 years old, the exposure would not have occurred after the confirmed diagnosis of ROA. To address this potential circumstance, we performed sensitivity analyses restricting the sample to only participants who were age 50 – 55 at the time of OAI enrollment. In this group of participants, we would have a much higher level of certainty that a diagnosis of OA predated the exposure of running. These analyses showed similar results to the main cohort.
Although ascertainment of running status in this study was not ideal, the participants were not aware of their radiographic OA status at the time that they completed the questionnaires nor were they aware of the specific hypotheses we planned on testing which would hopefully have reduced the risk for recall bias. Despite the imperfect manner in which running was assessed in our study, of those who were classified as runners in our study, most ran for more than 6 years, for more than half the months of a year and at least 4 times a month (table 2). We do know that 13% of the runners considered themselves to be competitive runners, but beyond that, we do not have information on running intensity, including running speed and distance. Therefore, we do not know if people mostly participated in gentle jogs, long distance marathons, sprints, or a combination of these. We also are unable to ascertain whether people stopped and started running over the observation period. Additional questions providing added dimensions about running would be of interest in future studies evaluating the influence of running on symptoms and structure progression in those with knee OA. We did find that the percentage of runners was highest in the lowest BMI tertile, adding construct validity to the definition of running used in this study. Although we were interested in testing whether there was a dose response of running exposure to the outcomes of interest, we were unable to run these analyses because the number of events in the running group was limited. Replication of our findings in a study that collected data on running in a prospective manner would be optimal. Also, readings of magnetic resonance imaging that have already been acquired as part of the OAI, will also likely be informative, including evaluation of soft tissue structures around the knee that also may have the potential to cause pain. Future evaluation of findings that may result from biomechanical differences, including differences that might be seen at the tibial spine and bony attrition, among those with knee OA who run and do not run may also prove to be informative. Such studies will require substantially more time and resources.
Existing literature to date on running and OA has focused on people who were free of OA at the beginning of the study [11–15, 28, 29]. Although our study does have limitations, it is unique in that we have focused on people who have established radiographic evidence of OA in at least one knee. Our study provides the first evidence to guide recommendations regarding whether people who have knee OA should be advised to run or not. This is particularly important given the broad recommendations by the World Health Organization, for people of all ages to participate in regular physical activity given the known cardiovascular and mortality benefits [1]. While we do feel that replication of our findings in other cohorts is important, our study is reassuring because among those who had knee OA who choose to run, running did not appear to be harmful. In fact, there was a beneficial effect of decreased pain symptoms in runners with knee osteoarthritis.
As mentioned previously, in this study, people ran voluntarily, not because it was mandated, important for interpretation of findings from our study. In conclusion, self-selected running, which is likely influenced by knee symptoms and may result in lower intensity and shorter duration sessions of exercise, need not be discouraged in people with knee OA. Future prospective studies of people with established knee OA participating in running, that request greater detail regarding duration and intensity of running, are warranted to replicate our findings.
Acknowledgments
Dr. Lo is supported by K23 AR062127, an NIH/NIAMS funded mentored award, providing support for design and conduct of the study, analysis, and interpretation of the data; and preparation and review of this work. Dr. Suarez-Almazor is supported by K24 AR053593, funded by NIH/NIAMS. This work is supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. The Osteoarthritis Initiative is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institutes of Health, or the Department of Veterans Affairs.
Disclosure of Potential Conflicts of Interests
None of the authors have a conflict of interest that could influence this work.
References
- 1.Organization, W.H. Global Recommendations on Physical Activity for Health. World Health Organization; Switzerland: 2010. [PubMed] [Google Scholar]
- 2.Blair SN, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262(17):2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Paffenbarger RS, Jr, et al. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 4.Paffenbarger RS, Jr, et al. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328(8):538–45. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 5.Cross M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 6.Dai S, et al. Participation in Types of Physical Activities Among US Adults--National Health and Nutrition Examination Survey 1999–2006. J Phys Act Health. 2015;12(Suppl 1):S128–40. doi: 10.1123/jpah.2015-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane N, Buckwalter JA. Exercise: a cause of osteoarthritis? Rheum Dis Clin N America. 1993;3:617–33. [PubMed] [Google Scholar]
- 8.Lane NE, Bloch DA, Wood PD, Fries JF. Aging, long-distance running, and the development of musculoskeletal disability. A controlled study. American Journal of Medicine. 1987;82(4):772–80. doi: 10.1016/0002-9343(87)90014-3. [DOI] [PubMed] [Google Scholar]
- 9.Lane NE, Bloch DA, Hubert HB, Jones H, Simpson U, Fries JF. Running, osteoarthritis, and bone density: initial 2-year longitudinal study. American Journal of Medicine. 1990;88(5):452–9. doi: 10.1016/0002-9343(90)90422-a. [DOI] [PubMed] [Google Scholar]
- 10.Lane NE, Michel B, Bjorkengren A, Oehlert J, Shi H, Bloch DA, Fries JF. The risk of osteoarthritis with running and aging: a 5-year longitudinal study. Journal of Rheumatology. 1993;20(3):461–8. [PubMed] [Google Scholar]
- 11.Lane NE, et al. Long-distance running, bone density, and osteoarthritis. Jama. 1986;255(9):1147–51. [PubMed] [Google Scholar]
- 12.Panush RS, Schmidt C, Caldwell JR, Edwards NL, Longley S, Yonker R, Webster E, Nauman J, Stork J, Pettersson H. Is running associated with degenerative joint disease? Jama. 1986;255(9):1152–4. [PubMed] [Google Scholar]
- 13.Chakravarty EF, et al. Long distance running and knee osteoarthritis. A prospective study. Am J Prev Med. 2008;35(2):133–8. doi: 10.1016/j.amepre.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettunen JA, et al. Lower-limb function among former elite male athletes. Am J Sports Med. 2001;29(1):2–8. doi: 10.1177/03635465010290010801. [DOI] [PubMed] [Google Scholar]
- 15.Kujala UM, Kettunen J, Paananen H, Aalto T, Battie MC, Impivaara O, Videman T, Sarna S. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis & Rheumatism. 1995;38(4):539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 16.Lo GH, et al. Is There an Association Between a History of Running and Symptomatic Knee Osteoarthritis? A Cross-Sectional Study From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2017;69(2):183–191. doi: 10.1002/acr.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams PT. Effects of running and walking on osteoarthritis and hip replacement risk. Med Sci Sports Exerc. 2013;45(7):1292–7. doi: 10.1249/MSS.0b013e3182885f26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriska AM, et al. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127(5):1053–63. doi: 10.1093/oxfordjournals.aje.a114881. [DOI] [PubMed] [Google Scholar]
- 19.Chasan-Taber L, et al. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155(3):282–9. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- 20.Radiographic (x-ray) manual (version 2.1, 8-23-2006) 2006 Dec 17; 2015]; Available from: https://oai.epi-ucsf.org/datarelease/OperationsManuals.asp.
- 21.Project 15 Test-Retest Reliability of Semi-quantitative Readings from Knee Radiographs. 2016 Nov 22; 2016 [cited 2017 February 3]; Available from: https://oai.epi-ucsf.org/datarelease/SASDocs/kXR_SQ_Rel_BU_descrip.pdf.
- 22.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 23.O’Reilly SC, Muir KR, Doherty M. Screening for pain in knee osteoarthritis: which question? Ann Rheum Dis. 1996;55(12):931–3. doi: 10.1136/ard.55.12.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Initiative, O. Knee Injury and Surgery. OAI Follow-up Visit Workbook. 2008 Available from: https://oai.epi-ucsf.org/datarelease/forms/FUWQ07_496.pdf?V01INJR12.
- 25.DeSantana JM, et al. Hypoalgesic effect of the transcutaneous electrical nerve stimulation following inguinal herniorrhaphy: a randomized, controlled trial. J Pain. 2008;9(7):623–9. doi: 10.1016/j.jpain.2008.01.337. [DOI] [PubMed] [Google Scholar]
- 26.DeSantana JM, et al. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10(6):492–9. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neogi T, et al. Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2016;68(3):654–61. doi: 10.1002/art.39488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kujala UM, Kaprio J, Sarna S. Osteoarthritis of weight bearing joints of lower limbs in former elite male athletes. Bmj. 1994;308(6923):231–4. doi: 10.1136/bmj.308.6923.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konradsen L, Hansen EM, Sondergaard L. Long distance running and osteoarthrosis. American Journal of Sports Medicine. 1990;18(4):379–81. doi: 10.1177/036354659001800408. [DOI] [PubMed] [Google Scholar]


