Abstract
Objective
To evaluate the epidemiology of hyperammonemia unrelated to liver failure in the critical care setting.
Design
Retrospective case series
Setting
Critically ill patients admitted to intensive care units at Mayo Clinic Rochester Minnesota (medical intensive care (ICU), two mixed medical-surgical ICUs, coronary care unit, or the cardio-surgical ICU) between 1st July 2004 and 31st October 2015.
Patients
Adult critically ill patients with hyperammonemia not related to acute or chronic liver failure. We excluded patients with diagnosis of moderate or severe liver disease, hyperbilirubinemia and patients who denied the use of their medical records.
Interventions
None
Measurements and Main Results
Of 3908 ICU patients with hyperammonemia, 167 (4.5%) had no evidence of acute or chronic liver failure. 101 (60.5%) patients were male with median age of 65.7 years (IQR 50–74.5) and median serum ammonia level of 68 mcg/dL (IQR 58–87). Acute encephalopathy was present in 119 (71 %) patients. Predisposing conditions included malnutrition 27 (16%), gastric bypass 6 (3.6%), total parenteral nutrition 4 (2.4%); exposure to valproic acid 17 (10%); status epilepticus 11 (6.6 %), high tumour burden 19 (11.3 %) and renal failure 82 (49.1%). Urea cycle defects were diagnosed in 7 patients (4.1 %). Hospital mortality was high (30 %) and median ammonia level was higher among the non-survivors (74 vs 67 mcg/dL, p = 0.05). Deaths were more likely in hyperammonemic patients who were older (p=0.016), had greater illness severity (higher APACHE III score, p<0.01), malignancy (p<0.01) and solid organ transplantation (p=0.04), while seizure disorder was more common in survivors (p=0.02). After adjustment, serum ammonia level was not associated with increased mortality.
Conclusions
Hyperammonemia occurs in a substantial minority of critically ill patients without liver failure. These patients have a poor prognosis, although ammonia level per se is not independently associated with mortality. Serum ammonia should be measured when risk factors are present, such as nutritional deficiencies and protein refeeding, treatment with valproic acid, high tumour burden and known or suspected urea cycle abnormalities.
Keywords: hyperammonemia, critical care, malnutrition, urea cycle disorders, valproic acid, mortality
Introduction
Hyperammonemia is a recognized complication of inadequate metabolic clearance of ammonia. It is commonly a consequence of liver failure, though recognition of hyperammonemia unrelated to hepatic dysfunction is increasing. [1–3]. The diagnosis of hyperammonemia is made by laboratory ammonium measurement, but can be suspected in patients with risk factors for this metabolic disorder. There are no pathognomonic bedside clinical signs and symptoms. Effective treatment rapidly reverses biochemical abnormalities, but without clinical awareness and timely intervention, hyperammonemia can be fatal[3] [4] [5] or cause serious neurological consequences (hyperammonemia-induced encephalopathy)[6] [7] [8].
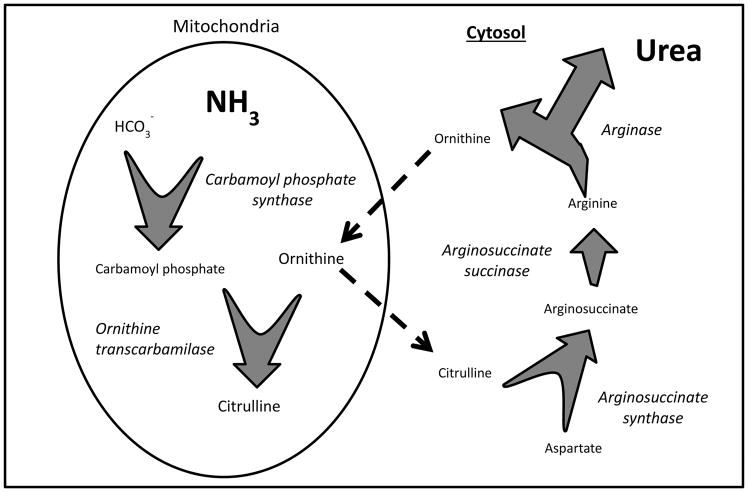
There have been several reviews on hyperammonemia in non-hepatic diseases [1, 2, 9] and critical care patients [10], but large observational studies on this topic are scant. Hyperammonemia results from a pathological imbalance between production of ammonium and its elimination via the urea cycle [11, 12]. Ammonia is generated from the deamination of amino acids and is metabolised into urea by mitochondrial and cytoplasmic enzymes of the urea cycle (Figure 1); urea is then excreted by the kidneys.
Figure 1. Urea cycle.
Within the mitochondria, an ATP-dependent reaction of bicarbonate and ammonia is catalyzed by carbamoyl phosphate synthase, which is the rate limiting step of the cycle. Carbamoyl phosphate and ornithine form citrulline in a reaction catalyzed by ornithine transcarbamylase. Citrulline enters the cytosol and, using further ATP, is metabolized to arginosuccinate, and in turn to arginine. The hydrolysis od arginine by arginase completes the urea cycle by releasing a urea molecule, and the ornithine re-enters the mitochondria.
Hyperammonemia may occur in the setting of enhanced ammonium production, decreased elimination, or both[11]. Case reports and case series have identified various factors associated with hyperammonemia, such as Roux-en-Y gastric bypass surgery, urea cycle disorders, malnutrition, high protein supplementation, total parenteral nutrition, solid organ transplantations, bone marrow and stem cell transplantation and certain anticonvulsants. [6, 13–16]
With the growing literature regarding hyperammonemia, the purpose of this study is to report the epidemiology of adult hyperammonemia not related to acute or chronic liver failure in a large consecutive sample of critically ill patients.
Materials and Methods
A retrospective observational cohort study was conducted on critically ill patients admitted to intensive care units (ICU) at Mayo Clinic Rochester Minnesota (medical ICU, two surgical ICUs, one mixed medical-surgical ICU, coronary care unit, and the cardio-surgical ICU) between 1st July 2004 and 31st October 2015. All adult ICU patients (≥18 years old) with laboratory confirmed hyperammonemia (ammonia > 50 mcg/dL) were included. Patients with moderate or severe liver disease based on Charlson score, patients with hyperbilirubinemia (bilirubin level > 2 mg/dl), and patients with diagnosed liver disease based on ICD-9 codes (supplemental document 1) were subsequently excluded. ICU DataMart was used to retrieve all pertinent clinical variables. Development, validation, and data security of ICU DataMart have been previously reported.[17] We used Mayo Clinic’s Advanced Cohort Explorer (ACE) as a search tool to identify patients appropriate for our study. This study was approved by Mayo Clinic Institutional Review Board. Acute brain failure was defined by Glasgow come scale ≤14 or positive Confusion Assessment Method (CAM-ICU) documented in the electronic medical record, as previously reported[18]. Malnutrition was defined as BMI less than 18.5, or serological evidence of micronutrient and vitamin deficiencies.
Continuous variables were reported as medians with interquartile range (IQR), and categorical variables are reported as percentages. To compare survivors and non-survivors, Pearson chi-square test was used for categorical variables and Wilcoxon rank-sum test was used for continuous variables. A p-value <0.05 was considered statistically significant. We used multivariate logistic regression model to evaluate if serum ammonia level was independently associated with hospital mortality. We used JMP Pro 10.0, a SAS institute statistical software for our primary data analysis.
Results
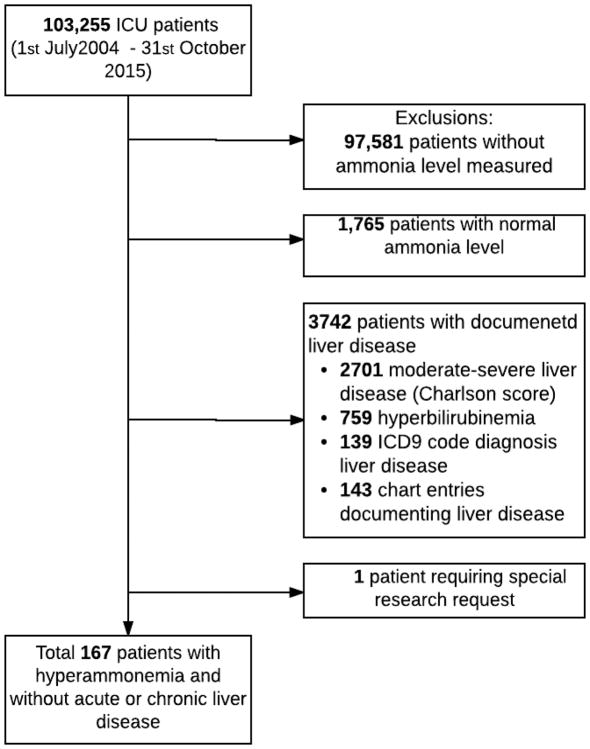
Between 1st July 2004 and 31st October 2015, 103,255 patients were admitted to the Mayo Clinic, Rochester ICUs. Of these, 97,581 (94,5 %) patients did not have an ammonia level measured and among the 5,674 (5,5%) patients that had measured ammonia 1,765 (31%) patients had normal levels. Of the 3,909 remaining patients with documented hyperammonemia, 3,742 (95.7 %) were excluded due to documented liver disease: 2,701 had moderate or severe liver disease based on Charlson score, 139 patients had liver disease noted by ICD 9 codes, 759 patients had hyperbilirubinemia (bilirubin level> 2 mg/dl), and following the manual record review, a further 142 patients were excluded because liver disease was noted in their medical records. One patient required a special research request, and thus was excluded. Therefore, 167 patients were identified as having hyperammonemia without associated liver disease, and manual chart review in those patients showed no evidence of documented liver failure. The final number of 167 patients represented a relative frequency of 1.6 per 1,000 ICU admissions and 4.5% of all patients with documented hyperammonemia (Figure 2).
Figure 2.
Study design
There were 101 (60.5%) male patients, the median age was 65.7 years (IQR 50–74.5) and median ammonium level was 68 microgram/dl (IQR 58–87). Fifty-one percent were older than 65 years and 72.8% were overweight (BMI >25). The majority of patients 119 (71 %) had acute brain failure (i.e. encephalopathy). Common predisposing conditions included nutritional disorders (malnutrition (16%), gastric bypass (3.6%), total parenteral nutrition (2.4%), valproic acid use (10%), status epilepticus (6.6 %), high tumour burden (11.3%) and renal failure (49.1%)). Metabolic disorders, such as urea cycle and fatty acid metabolism abnormalities were present in 7 (4.1%) patients with only 4 (2%) patients undergoing formal genetic testing for evaluation of enzyme deficiencies. Other potential causes of hyperammonemia not due to liver disease (upper and lower GI bleeding, urea cycle abnormality, treatment with carbamazepine, and solid organ transplantation) were present in smaller numbers of patients. (Table 1).
Table 1.
Characteristics of Patient Sample: 167 Consecutive Non-Hepatic Hyperammonemic Critical Care Patients
| Characteristic | Description | Number (%) |
|---|---|---|
|
| ||
| Gender | Female | 66 (39.5) |
|
| ||
| Age groups, yr | <35 | 14 (8) |
| 35–65 | 67 (40) | |
| >65 | 86 (51) | |
|
| ||
| Body Mass Index groups (kg/m2) | Underweight (<18.5) | 3 (1.9) |
| Normal (18.5–24.9) | 41 (25.3) | |
| Overweight (>25) | 118(72.8) | |
|
| ||
| Associated factors | Metabolic | |
| Carnitine palmitoyl transferase deficiency | 1 (0.6) | |
| Ornithine transcarbamylase deficiency | 3 (1.8) | |
| Other urea cycle abnormality | 3 (1.8) | |
| Nutrition/GI | ||
| Gastric bypass surgery | 6 (3.6) | |
| Total parenteral nutrition | 4 (2.4) | |
| Malnutrition | 27 (16.1) | |
| Upper GI bleeding | 8 (4.8) | |
| Lower GI bleeding | 2 (1.2) | |
| Drugs | ||
| Valproic Acid | 17 (10.2) | |
| Carbamazepine | 2 (1.2) | |
| Proton pump inhibitors | 58 (34.7) | |
| Renal failure*** | 82 (49.1) | |
| Generalized seizures | 11 (6.6) | |
| Solid organ transplantation | 4 (2.4) | |
| Aggressive Hematologic Tumors** | 19 (11.3) | |
Multiple myeloma, Myelodysplastic syndrome, Recurrent Burkitt’s lymphoma, Diffuse Large B-cell Lymphoma, Acute erythroblastic leukemia, Anaplastic large cell ALK-negative T-cell lymphoma, Relapsed T/NK cell acute lymphoblastic leukemia, Hodgkin’s lymphoma, NK/T cell Lymphoma, T-cell Lymphoma, Acute myeloid leukemia
Acute renal failure present in 44 patients (26.3%); chronic renal failure in 22 patients (13.2%); Acute-on-chronic renal failure in 16 patients (9.6%)
The median ICU and hospital LOS of patients in the cohort were 4.1 and 11.8 days, respectively. Hospital mortality occurred in 50 cases (30 %). Median ammonia was higher among non-survivors (74 vs 67 mcg/dL, p = 0.05). Deaths were more likely in hyperammonemic patients who were older (p=0.016), had greater acute illness severity (higher APACHE III score, p<0.01), malignancy (p<0.01) and solid organ transplantation (p=0.04), while seizure disorder was more common in survivors (p<0.01) (Table 2). After adjusting for baseline differences (age, APACHE, solid organ transplantation, malignancy, seizure disorder), ammonia level was not associated with increased mortality (OR 1.003; 95 %CI 0.99 – 1.01).
Table 2.
Comparison of Non-Hepatic Hyperammonemic Critical Care Patient Characteristics of Hospital Survivors and Non-Survivors
| Characteristic | Survivors (n=117) | Deceased (n=50) | p value |
|---|---|---|---|
|
| |||
| Age, median, yrs | 63(47–72) | 72(60–79) | 0.016 |
|
| |||
| Female sex, n (%) | 44(37) | 22(44) | 0.43 |
|
| |||
| Body Mass Index (kg/m2) groups | 0.23 | ||
| Underweight (<18.5) | 1(0.8) | 2(4) | |
| Normal (18.5–24.9) | 26(22) | 15(30) | |
| Overweight (>25) | 85(72) | 33(66) | |
|
| |||
| APACHE III score, median (IQR) | 68(58–85) | 92(73–127) | <0.01 |
| Serum ammonia, ug/dL median, (IQR) | 67(58–84) | 74(60–99) | 0.05 |
| Acute brain failure, n (%) | 87(74) | 32(64) | 0.17 |
|
| |||
| Potential causes | |||
| Metabolic | |||
| Carnitin palmitoyl transferase deficiency, n (%) | 1(0.8) | 0(0) | 0.51 |
| Ornithine transcarbamylase deficiency, n (%) | 1(0.8) | 2(4) | 0.16 |
| Other Urea cycle abnormality, n (%) | 1(0.8) | 2(4) | 0.16 |
| Nutrition/GI | |||
| Gastric bypass surgery, n (%) | 5(4.2) | 1(2) | 0.46 |
| Total parenteral nutrition, n (%) | 3(2.5) | 1(2) | 0.82 |
| Malnutrition, n (%) | 21(18) | 6(12) | 0.33 |
| Upper GI bleeding, n (%) | 6(5) | 2(4) | 0.73 |
| Lower GI bleeding, n (%) | 2(1.7) | 0(0) | 0.22 |
| Drugs | |||
| Valproate, n (%) | 14(12) | 3(6) | 0.24 |
| Carbamazepine, n (%) | 2(1.7) | 0(0) | 0.35 |
| Proton Pump Inhibitors, n (%) | 41(35) | 17(34) | 0.89 |
| Renal failure, n (%) *** | 53(45) | 29(58) | 0.13 |
| Generalized seizures, n (%) | 11(9) | 0(0) | 0.02 |
| Solid organ transplantation, n (%) | 1(0.8) | 3(6) | 0.04 |
| Aggressive hematologic tumors, n (%) ** | 6(5.1) | 13(26) | <0.001 |
|
| |||
| Treatment | |||
| Invasive mechanical ventilation, n (%) | 72(61) | 29(58) | 0.66 |
| Non-invasive Ventilation, n (%) | 47(40) | 18(36) | 0.61 |
| Lactulose, n (%) | 25(21) | 8(16) | 0.42 |
| Rifaximin, n (%) | 3(2.5) | 0(0) | 0.25 |
| Sodium Benzoate n (%) | 1(0.8) | 0(0) | 0.51 |
| Hemodialysis n (%) | 21(18) | 8(16) | 0.76 |
data are shown as median (IQR) or n (%)
Multiple myeloma, Myelodysplastic syndrome, Recurrent Burkitt’s lymphoma, Diffuse Large B-cell Lymphoma, Acute erythroblastic leukemia, Anaplastic large cell ALK-negative T-cell lymphoma, Relapsed T/NK cell acute lymphoblastic leukemia, Hodgkin’s lymphoma, NK/T cell Lymphoma, T-cell Lymphoma, Acute myeloid leukemia
Acute renal failure present in 44 patients (26.3%); chronic renal failure in 22 patients (13.2%); Acute-on-chronic renal failure in 16 patients (9.6%)
Discussion
In a large cohort of critically ill patients treated in a tertiary medical center, we identified a substantial minority of patients who had hyperammonemia in the absence of liver failure. The majority of these patients were symptomatic with acute encephalopathy. Common predisposing conditions included nutritional disorders, malignancies, certain medications and renal failure. Inborn metabolic errors were diagnosed in a minority of patients. The outcome was poor with high hospital mortality.
Predisposing nutritional factors, including malnutrition, gastric bypass and total parenteral nutrition were present in 37 patients (22%) of our hyperammonemic patients without documented liver disease. Hyperammonemia in the setting of high protein diets and malnutrition has long been recognized as a possible cause of death. Historical reports among hunter gatherers illustrate that diets wholly based on lean animal protein (without fat or carbohydrate) can be injurious[19, 20]. Malnutrition can lead to urea cycling in the gut[13] and refeeding syndrome[21]. High protein diets[14], somatic protein catabolism (burns, trauma, strenuous exercise, steroids), IV amino acid containing solutions including TPN[22, 23] and N-Acetylcysteine, GI bleeding[24], porto-systemic shunts unrelated to liver disease, blind loop intestinal bypass and bariatric surgery[3, 6, 25] have also been shown to be associated with hyperammonemia.
Urea cycle disorders were identified in 7 patients (4.1%). The urea cycle consists of five enzymes, including ornithine transcarbamylase (OTC), required for metabolism and excretion of ammonia. Genetic deficiencies in these enzymes typically result in hyperammonemia presenting roughly 72 hours after birth, when protein consumption overwhelms the dysfunctional urea cycle. Functional deficiencies in adulthood have now been recognized, where DNA analysis reveals normal enzyme levels, but functional enzymatic testing of liver biopsies demonstrates less than 1% activity[25]. OTC is necessary in the conversion of ornithine to citrulline in ammonia metabolism, and reports of functional OTC deficiencies are becoming increasingly prevalent in females after bariatric surgery presenting with hyperammonemia, Bariatric surgery is known to lead to hyperinsulinemia with resultant down-regulation of hepatic urea cycle enzymes [3]. Nutritional deficiencies are also common amongst bariatric surgery patients, and low zinc and arginine levels have been demonstrated to decrease OTC activity. It is postulated that this combination of mechanisms can cause hyperammonemia in some patients after bariatric surgery by creating a functional OTC deficiency. Adult-onset urea cycle abnormalities secondary to inherited genetic mutations are rare, but also need to be considered as significant metabolic stressors can unmask previously silent aberrations, leading to decompensation and development of hyperammonemia [2, 12, 26–28]. Notably, patients with true and functional urea cycle enzymatic deficiencies do not respond to traditional ammonia-lowering therapies. Lactulose and rifaximin target elimination of microbe-associated ammonia production, yet are unable to compensate for a lack of urinary ammonia excretion secondary to urea cycle disorders. Ammonia is a by-product of amino acid metabolism, and is transported from the liver as glutamate via glutamine synthetase. Once in the kidney, glutamate is converted back to ammonia via glutaminase, and is further eliminated as urea via the urea cycle. In hyperammonemia, excess ammonia combines with alpha-ketoglutarate and is forced back into glutamate. Similarly, excess ammonia can also be converted back into the amino acid glycine via glycine synthase.. Nitrogen scavengers, such as sodium phenylacetate and sodium benzoate can be used to bypass the urea cycle in refractory hyperammonemia[26]. Sodium phenylacetate conjugates with glutamine to form phenylacetylglutamine, and sodium benzoate binds glycine to form hippuric acid. Both products can be excreted by the kidneys without participation of the urea cycle. Our cohort included 6 patients with hyperammonemia and a history of gastric bypass surgery, thought to be related to a functional urea cycle disorder.
Drugs have been implicated in increased ammonia production, with valproic acid being the most widely reported example (acting by direct inhibition of hepatic N-acetyl glutamate synthase activity by valproyl- CoA[15]). Other medications have been associated with hyperammonemia, such as carbamazepine[29], topiramate[7], lamotrigine[30], primidone[8], gabapentin[31], salicylates, acetazolamide[32], chemotherapeutic drugs[33] (5-flurouracil, cytarabine, L-asparaginase[34]) and drugs of abuse, such as methamphetamine[35], but these associations are less common. Seventeen patients (10.2%) in our cohort had a history of taking valproic acid, reinforcing the importance of evaluating valproic acid use. Hyperammonemia can develop either from an acute overdose of valproic acid or with chronic use, and is thought to be secondary to the propionic acid metabolite[15] [36]. Propionic acid inhibits carbamoyl phosphate synthetise. If the activity is significantly reduced, accumulation of ammonia can occur. Similarly, carnitine deficiency can predispose to acquired urea cycle disorders in the setting of valproic acid use, and can be pre-existing or secondary to valproic acid administration [37] [38]. Valproic acid is a branched-chain carboxylic acid that undergoes hepatic metabolism via beta oxidation. Carnitine is a cofactor required for beta oxidation, and deficiency of this can lead to accumulation of ammonia, a metabolite of valproic acid metabolism. This accumulation of ammonia results in an increase in glutamate synthesis via alpha ketoglutarate, a molecule required for proper function of the Krebs cycle and the biosynthesis of carnitine. Treatment in this situation is largely focused on discontinuation of the offending agent and L-carnitine [39]. Only one patient (0.6%) was found to have carnitine palmitoyl transferase deficiency in our cohort.
Our analysis showed that patients who had solid organ transplantation and aggressive hematologic malignancies, together with hyperammonemia, were more likely to die (p=0.04, p<0.001, respectively). Solid organ transplantation can lead to increased production of ammonia. Some of examples are lung[16, 40] and renal[4] transplantation, pancreatectomy and islet transplantation[41], as well as bone marrow[5] and stem cell[42] transplantation. Haematological conditions such as myeloma[43] and chemotherapy [42] for haematological malignancies can also result in hyperammonemia. The high mortality in these cases could be secondary to the aggressiveness of the primary disease, but severe hyperammonemia after transplantation can be a fatal complication. [4] [5]
The proportion of hyperammonemia patients who had concurrent kidney disease was 49.1%. 44 patients had acute kidney disease, 22 had chronic kidney disease, and 16 had acute-on-chronic kidney injury. Other than increased severity of illness and comorbidities we do not have a good explanation for this observation. To our knowledge there are no studies to date suggesting a relationship between these two derangements, but this association may deserve further investigation.
Acute brain injury is present in majority of patients. None of the patients had evidence of cerebral herniation suggesting that in the absence of acute liver failure ammonia accumulation occurs over a longer period leading to encephalopathy (acute brain injury) but not necessarily an acute increase of intracranial pressure and cerebral herniation. We suggest considering ammonia level measurement if the risk factors are present and etiology of acute brain injury is unclear.
A limitation applicable to our study and all other studies assessing hyperammonemia is the concern for false positive ammonium values. Some evidence suggests that serum samples kept at room temperatures for long can undergo deamination in vitro, producing falsely high ammonia levels [2, 44, 45]. During the study period, ammonia was collected per institutional guidelines in which it is placed on ice immediately at the bedside and processed by no later than 60 minutes after collection. Another limitation is the large proportion of critically ill patients in whom serum ammonia was not measured. This could have resulted in an underestimation of hyperammonemia unrelated to liver disease in our cohort. Furthermore, since hyperammonemia was documented often in patients who died in the hospital, it is possible that early death (before ammonia measurement) could have led to further under-recognition. Additional limitation would be the possibility of occult/unrecognized liver disease. Formal investigations into urea cycle disorders are not often performed, thus limiting our ability to truly quantify the prevalence of such acquired conditions and their correlation with ammonia accumulation. Future studies could prospectively investigate symptomatic hyperammonemia in patients without evidence of liver disease, specifically evaluating the activity of urea cycle enzymes. Our study period spanned a course of eleven years, during which time the care of critically ill patients has changed dramatically. These differences could have an impact on outcomes in our retrospective analysis, but it is impossible to predict which variables may have been most greatly affected. Lastly, our study did not compare patients with hyperammonemia not due to liver disease and patients with normal ammonia level. This may provide additional valuable information and should be the target for future research.
Conclusion
Our study recognized several underlying conditions for hyperammonemia unrelated to hepatic dysfunction. Our study outlines alternative causes of hyperammonemia, and can be used to increase awareness of groups at risk, in whom a multifactorial diagnostic and treatment approach should be considered. Although the ammonia level per se was not independently associated with mortality in this small sample, the overall poor outcome of these patients is an important finding to increase clinician awareness and prognostication, and to help researchers design new studies to evaluate potential treatment and prevention approaches.
Supplementary Material
Acknowledgments
Dr. Amra Sakusic and Ognjen Gajic, MD MSc take full responsibility for the data, the analysis and interpretation, and the conduct of research. Dr. Sabov, Dr. McCambridge, Dr. Cook, Dr. Mukesh, Dr. Kashani, Dr. Rabinstein have made substantial contribution to conception and design, acquisition of data, analysis and interpretation. All the authors have provided the final approval of the version to be published. The authors declare that they have no conflict of interest. This publication was made possible, in part by funding from The Mayo Critical Care Research Committee and grants from the National Institute on Aging (U01 AG006786, P50 AG016574 and R01 AG034676.)
Footnotes
Financial disclosure and conflicts of interest: The authors declare that they have no conflict of interest. This publication was made possible, in part by funding from The Mayo Critical Care Research Committee and grants from the National Institute on Aging (U01 AG006786, P50 AG016574 and R01 AG034676.)
Copyright form disclosure: Dr. Gajic’s institution received funding from the Mayo Critical Care Research Committee and the National Institute on Aging (U01 AG006786, P50 AG016574 and R01 AG034676). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.LaBuzetta JN, Yao JZ, Bourque DL, et al. Adult nonhepatic hyperammonemia: A case report and diferential diagnosis. Amer J Med. 2010;123:885–891. doi: 10.1016/j.amjmed.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Walker V. Severe hyperammonaemia in adults not explained by liver disease. Annals Of Clinical Biochemistry. 2012;49(Pt 3):214–228. doi: 10.1258/acb.2011.011206. [DOI] [PubMed] [Google Scholar]
- 3.Acharya G, Mehra S, Patel R, et al. Fatal Nonhepatic Hyperammonemia in ICU Setting: A Rare but Serious Complication following Bariatric Surgery. Case Reports In Critical Care. 2016;2016:8531591–8531591. doi: 10.1155/2016/8531591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiberenge RK, Lam H. Fatal hyperammonemia after repeat renal transplantation. Journal Of Clinical Anesthesia. 2015;27(2):164–167. doi: 10.1016/j.jclinane.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Davies SM, Szabo E, Wagner JE, et al. Idiopathic hyperammonemia: a frequently lethal complication of bone marrow transplantation. Bone Marrow Transplantation. 1996;17(6):1119–11125. [PubMed] [Google Scholar]
- 6.Kromas ML, Mousa OY, John S. Hyperammonemia-induced encephalopathy: A rare devastating complication of bariatric surgery. World Journal Of Hepatology. 2015;7(7):1007–1011. doi: 10.4254/wjh.v7.i7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Ibañez A, Urrestarazu-Bolumburu E, Viteri-Torres C. Hyperammonemic encephalopathy related to valproate, phenobarbital, and topiramate synergism. Epilepsy & Behavior: E&B. 2011;21(4):480–482. doi: 10.1016/j.yebeh.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Katano H, Fukishimuga T, Karasawa K, et al. Primidone induced hyperammonemic encephalopathy in a patient with cerebral astrocytoma. J Clin Neurosci. 2002;9(1):79–81. doi: 10.1054/jocn.2001.1011. [DOI] [PubMed] [Google Scholar]
- 9.Laish I, Ben Ari Z. Noncirrhotic hyperammonaemic encephalopathy. Liver International: Official Journal Of The International Association For The Study Of The Liver. 2011;31(9):1259–1270. doi: 10.1111/j.1478-3231.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 10.Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132(4):1368–1378. doi: 10.1378/chest.06-2940. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann C. Mechanisms of hyperammonemia. Clinical Chemistry And Laboratory Medicine. 2002;40(7):653–662. doi: 10.1515/CCLM.2002.112. [DOI] [PubMed] [Google Scholar]
- 12.Walker V. Ammonia metabolism and hyperammonemic disorders. Advances In Clinical Chemistry. 2014;67:73–150. doi: 10.1016/bs.acc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Morsey MR, Madina H, Sharaf SA, et al. Hyperammonemia in marasmic children. J Trop Ped. 1994;40:97–99. doi: 10.1093/tropej/40.2.97. [DOI] [PubMed] [Google Scholar]
- 14.Bilsborough S, Mann N. A review of issues of dietary protein intake in humans. Int J Sport Nut Ex Met. 2006;16:129–152. doi: 10.1123/ijsnem.16.2.129. [DOI] [PubMed] [Google Scholar]
- 15.Aires CC, Van Crutchen A, Ijlst L, et al. New Insights into the mechanisms of valproate induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. Hepatology. 2011;55(2):426–434. doi: 10.1016/j.jhep.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Anwar S, Gupta D, Ashraf MA, et al. Symptomatic hyperammonemia after lung transplantation: lessons learnt. Hemodialysis International International Symposium On Home Hemodialysis. 2014;18(1):185–191. doi: 10.1111/hdi.12088. [DOI] [PubMed] [Google Scholar]
- 17.Herasevich Vitaly, Pickering Brian W, Dong Yue, et al. Informatics Infrastructure for Syndrome Surveillance, Decision Support, Reporting, and Modeling of Critical Illness. Mayo Clin Proc. 2010;85(3):247–254. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy DRS, Singh TD, Guru PK, et al. Identification of Acute Brain Failure using electronic medical records. Journal of critical care. 2016;34:12–16. doi: 10.1016/j.jcrc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefansson V. My Life with the Eskimo. New York: The Macmillan Company; 1913. [Google Scholar]
- 20.Stefansson V. The Fat of the Land. New York: MacMillan Co; 1956. [Google Scholar]
- 21.Welsh E, Kucera J, Perloff MD. Iatrogenic hyperammonemia after anorexia. Archives Of Internal Medicine. 2010;170(5):486–488. doi: 10.1001/archinternmed.2009.549. [DOI] [PubMed] [Google Scholar]
- 22.Cioccari L, Gautschi M, Etter R, et al. Further Concerns About Glutamine: A Case Report on Hyperammonemic Encephalopathy. Critical Care Medicine. 2015;43(10):e458–e460. doi: 10.1097/CCM.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 23.Pillai U, Kahlon R, Sondheimer J, et al. A rare case of hyperammonemia complication of high-protein parenteral nutrition. JPEN Journal Of Parenteral And Enteral Nutrition. 2013;37(1):134–137. doi: 10.1177/0148607112447815. [DOI] [PubMed] [Google Scholar]
- 24.Olde Damink SWM, Dejong CHC, Jalan R. Review article: hyperammonaemic and catabolic consequences of upper gastrointestinal bleeding in cirrhosis. Alimentary Pharmacology & Therapeutics. 2009;29(8):801–810. doi: 10.1111/j.1365-2036.2009.03938.x. [DOI] [PubMed] [Google Scholar]
- 25.Fenves AZ, Shchelochkov OA, Mehta A. Hyperammonemic syndrome after Roux-en-Y gastric bypass. Obesity (Silver Spring, Md) 2015;23(4):746–749. doi: 10.1002/oby.21037. [DOI] [PubMed] [Google Scholar]
- 26.Haberle J, Boddart N, Burlina A, et al. Suggested management guidelines for diagnosis and management of urea cycle disorders. Orph J Rare Dis. 2012;7(32):1–30. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helman G, Pacheco-Colón I, Gropman AL. The urea cycle disorders. Seminars In Neurology. 2014;34(3):341–349. doi: 10.1055/s-0034-1386771. [DOI] [PubMed] [Google Scholar]
- 28.Machado MCC, Pinheiro da Silva F. Hyperammonemia due to urea cycle disorders: a potentially fatal condition in the intensive care setting. Journal Of Intensive Care. 2014;2(1):22–22. doi: 10.1186/2052-0492-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambrosetto G, Riva R, Baruzzi A. Hyperammonemia in asterixis induced by carbamazipine. Acta Neurol Scand. 1984;69:186–189. doi: 10.1111/j.1600-0404.1984.tb07799.x. [DOI] [PubMed] [Google Scholar]
- 30.Fan CC, Huang MC, Liu HC. Lamotrigine might potentiate valproic acid-induced hyperammonemic encephalopathy. Neuro Biol Psych. 2008;32(7):1747–1748. doi: 10.1016/j.pnpbp.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Sechi G, Murgia B, Sau G, et al. Asterixis and toxic encephalopathy induced by gabapentin. Prog Neuro Biol Psych. 2004;28:195–199. doi: 10.1016/S0278-5846(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Ryu WS, Hwang YH, et al. Aggravation of ataxia due to acetazolamide induced hyperammonaemia in episodic ataxia. J Neurol Neurosurg Psychiatry. 2007;78(7):771–772. doi: 10.1136/jnnp.2006.105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nott L, Price TJ, Pittman K, et al. Hyperammonemia Encephalopathy: An important cause of neurological deterioration following chemotherapy. Leuk Lymph. 2007;48(9):1702–1711. doi: 10.1080/10428190701509822. [DOI] [PubMed] [Google Scholar]
- 34.Nussbaum V, Lubcke N, Findlay R. Hyperammonemia secondary to asparaginase: A case series. Journal Of Oncology Pharmacy Practice: Official Publication Of The International Society Of Oncology Pharmacy Practitioners. 2016;22(1):161–164. doi: 10.1177/1078155214551590. [DOI] [PubMed] [Google Scholar]
- 35.Lama M, Shannon S, Davin Q. Methamphetamine Intoxication Encephalopathy Associated With Hyperammonemia. Psychosomatics. 2016;57(3):325–329. doi: 10.1016/j.psym.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez M, Fagiolino P, Maldonado C, et al. Hyperammonemia Associated with Valproic Acid Concentrations. Biomed Research International. 2014 doi: 10.1155/2014/217269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coulter DL. Carnitine Deficiency - a Possible Mechanism for Valproate Hepatotoxicity. Lancet. 1984;1(8378):689–689. doi: 10.1016/s0140-6736(84)92209-8. [DOI] [PubMed] [Google Scholar]
- 38.Coulter DL. Carnitine, Valproate, and Toxicity. Journal of Child Neurology. 1991;6(1):7–14. doi: 10.1177/088307389100600102. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado C, Guevara N, Silveira A, et al. L-Carnitine supplementation to reverse hyperammonemia in a patient undergoing chronic valproic acid treatment: A case report. Journal of International Medical Research. 2017;45(3):1268–1272. doi: 10.1177/0300060517703278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C, Bain KB, Iuppa JA, et al. Hyperammonemia Syndrome after lung transplatnation: A single centre experience. Transplanatation. 2016;100(3):678–684. doi: 10.1097/TP.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 41.Navaneethan U, Venkatesh PGK. Idiopathic hyperammonemia in a patient with total pancreatectomy and islet cell transplantation. JOP: Journal Of The Pancreas. 2010;11(6):620–624. [PubMed] [Google Scholar]
- 42.Frere P, Canivet JL, Gennigens C, et al. Hyperammonemia after high dose chemotherapy and stem cell transplantation. Bone Mar Trans. 2000;26:343–345. doi: 10.1038/sj.bmt.1702485. [DOI] [PubMed] [Google Scholar]
- 43.Talamo G, Cavallo F, Zangari M, et al. Hyperammonemia and encephalopathy in patients with multiple myeloma. American Journal Of Hematology. 2007;82(5):414–415. doi: 10.1002/ajh.20808. [DOI] [PubMed] [Google Scholar]
- 44.Häberle J. Clinical practice: the management of hyperammonemia. European Journal Of Pediatrics. 2011;170(1):21–34. doi: 10.1007/s00431-010-1369-2. [DOI] [PubMed] [Google Scholar]
- 45.Howells JW, Short PA. The Importance of Clinical Context When Interpreting Serum Ammonia Levels: A Teachable Moment. JAMA Internal Medicine. 2015;175(12):1902–1903. doi: 10.1001/jamainternmed.2015.5772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.