Abstract
Objective
Benzodiazepine use may be associated with delirium in critically ill children. However, benzodiazepines remain the first line sedative choice in pediatric intensive care units (PICU). Objectives were to determine the temporal relationship between administration of benzodiazepines and delirium development, control for time-varying covariates such as mechanical ventilation and opiates, and evaluate the association between dosage of benzodiazepines and subsequent delirium.
Design
Retrospective observational study
Setting
Academic tertiary care PICU
Patients
All consecutive admissions from January-June 2015
Interventions
Retrospective assessment of benzodiazepine exposure in a population that had been prospectively screened for delirium
Measurements and Main Results
All subjects were prospectively screened for delirium throughout their stay, using the Cornell Assessment for Pediatric Delirium, with daily cognitive status assigned as follows: delirium, coma, or normal. Multivariable mixed effects modeling determined predictors of delirium overall, followed by subgroup analysis to assess effect of benzodiazepines on subsequent development of delirium. Marginal structural modeling was used to create a pseudo-randomized sample and control for time-dependent variables, obtaining an unbiased estimate of the relationship between benzodiazepines and next day delirium. The cumulative daily dosage of benzodiazepines was calculated to test for a dose-response relationship.
Benzodiazepines were strongly associated with transition from normal cognitive status to delirium, more than quadrupling delirium rates (OR 4.4,CI 1.7-11.1,p<0.002). Marginal structural modeling demonstrated OR 3.3 (CI 1.4-7.8), after controlling for time-dependent confounding of cognitive status, mechanical ventilation, and opiates. With every one-log increase in benzodiazepine dosage administered, there was a 43% increase in risk for delirium development.
Conclusions
Benzodiazepines are an independent and modifiable risk factor for development of delirium in critically ill children, even after carefully controlling for time-dependent covariates, with a dose-response effect. This temporal relationship suggests causality between benzodiazepine exposure and pediatric delirium and supports limiting the use of benzodiazepines in critically ill children.
Keywords: delirium, pediatric, benzodiazepines, critical care, intensive care, sedation, causality
INTRODUCTION
Delirium in critical illness has been linked to increased morbidity, mortality, and long term cognitive impairment(1–3). The pathophysiology of delirium remains incompletely understood, and involves a complex interplay between predisposing and precipitating influences(4). It is important to identify strategies to prevent delirium by focusing on specific modifiable risk factors. There is published evidence in the adult critical care literature establishing that sedatives -- particularly benzodiazepines -- contribute to the development of delirium(5–7). However, such evidence is lacking in the pediatric intensive care unit (PICU) population and benzodiazepines remain a first-line therapy for sedation in most PICUs(8). This represents a possible modifiable risk factor, as there are alternative sedative medications (albeit with other potential side effects) available for use in PICU patients(9).
Recent pediatric delirium studies have shown an association between benzodiazepine exposure and delirium(10–12), but fall short of establishing that benzodiazepines directly contribute to delirium development in children. It is important to assess for a temporal relationship before assuming a causal role. For example, a child may be prescribed benzodiazepines as a treatment for the symptoms of agitated delirium. Furthermore, there is an intricate relationship between benzodiazepine use, opiate use, mechanical ventilation, and delirium in the PICU. It is important to obtain an unbiased assessment of the relationship between benzodiazepines and next day delirium, as standard approaches to confounding may be biased by exposure to time-dependent covariates(13).
We designed this study to clarify the relationship between benzodiazepines and pediatric delirium. We hypothesized that there was an independent, temporal relationship between receipt of benzodiazepines and subsequent development of delirium in children, after controlling for time-dependent covariates. We further hypothesized that there was an association between dose of benzodiazepines administered and next-day diagnosis of delirium.
MATERIALS AND METHODS
Our study was conducted with approval from the Institutional Review Board at New York Presbyterian Hospital – Weill Cornell Medical Center. All patients admitted consecutively to our academic, urban, tertiary care PICU from January 2015 through June 2015 were included. This group is a part of a larger cohort of children included in a 12-month dataset. Children were prospectively screened for delirium each day throughout their PICU stay using the Cornell Assessment for Pediatric Delirium (CAPD). Each child was assigned a daily cognitive status of: delirium, coma, or normal (i.e.: delirium-free and coma-free (DFCF)). A status of ‘delirium’ was defined as a positive CAPD screen with diagnosis confirmed by the treating physician. A status of ‘coma’ was defined as being unarousable to verbal stimulation. A status of ‘normal’ was defined as a negative CAPD screen. This cohort was included in a prior publication describing the epidemiology and outcomes of pediatric delirium(11).
We reviewed the electronic medical record of enrolled patients to determine, in granular detail, the total dose of benzodiazepines and/or opiates each child received on a daily basis throughout the PICU stay. For benzodiazepines, we collected doses of lorazepam, midazolam, clonazepam and diazepam. For opiates, we collected doses of fentanyl, hydromorphone, morphine and oxycodone. We then converted all benzodiazepine medications to midazolam-equivalents in milligrams/kilogram/day(14). We converted all opiate medications to fentanyl-equivalents in micrograms/kilogram/day(15). A ‘transition to delirium’ was defined as a child who transitioned from normal status one day to delirious status the next day. All data regarding benzodiazepine and opiate exposure timing and dosing presented in this manuscript are novel, and have never been published before.
Based on extensive review of the adult and pediatric literature, we determined a priori that other variables that might predict transition to delirium would include: prior delirium (adult studies have shown that the strongest predictor of delirium in adults is delirium on the day prior), mechanical ventilation, and receipt of opiates(5,7,9–11,16).
Statistical Methods
Patient demographic and clinical characteristics as well as specific PICU characteristics were described as N (%) for categorical measures or as mean, standard deviation, median, and spread for continuous measures. Pearson’s Chi-square/Fisher’s Exact tests were used to explore the associations of day prior variables of interest and delirium. A multivariable mixed effects model was constructed to predict delirium by benzodiazepine exposure on the day prior to evaluation (yes/no), controlling for opiate exposure day prior (yes/no), cognitive status day prior (normal, comatose, or delirious), and mechanical ventilation (yes/no). To assess for temporality, a multivariable mixed effects model was then constructed to predict transition to delirium (i.e. delirium development after a normal day) by benzodiazepine exposure on the day prior to evaluation, while controlling for opiate exposure day prior and mechanical ventilation.
To minimize time-varying confounding, we then used a Marginal Structural Model (MSM). Marginal structural modeling allows for use of observational data in estimating causal effects of given exposures that vary with time(13). For example, a child who requires intubation is often sedated with opiates and benzodiazepines and is likely to be delirious. As noted by Pisani et al in their assessment of haloperidol and its effect on next-day delirium, MSM creates a pseudo-randomized sample with a main outcome model (likelihood of next day delirium, conditional on current day’s interventions) that has less time-varying confounding (17). We first constructed a weight model to calculate the probability of benzodiazepine exposure, adjusted for respective time-varying confounders (determined a priori) on the current day and prior day (Figure 1). Confounders in the weight model included opiate exposure, receipt of mechanical ventilation, cognitive status, and benzodiazepine exposure. Stabilized inverse probability of treatment weights (IPTWs) as defined by Robins et al (13) were obtained from the weight model and assessed for outliers. A subsequent logistic generalized estimating equation was constructed employing the weights and an adjusted odds ratio was obtained to assess the independent effect of benzodiazepine exposure on next day delirium.
Figure 1. Marginal structural model of association between time dependent variables and next-day delirium.
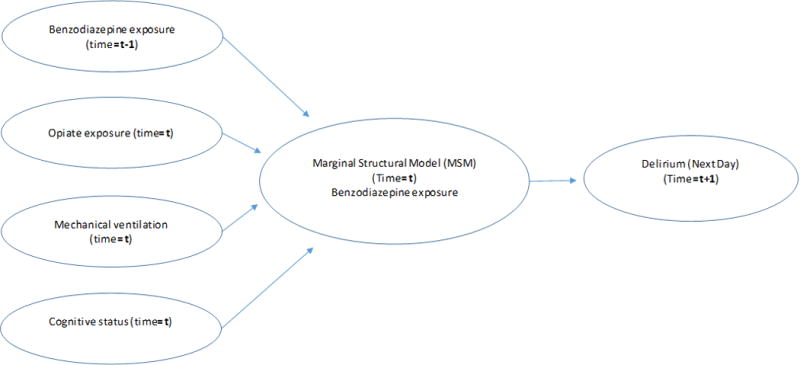
Schematic of confounding pathways used in weight modeling for this study. The treatment/interventions included were: cognitive status (delirium, coma, or normal), benzodiazepine exposure, opiate exposure, and mechanical ventilation (based on statistical method in Pisani, et al.) (17).
To assess for dose-response, a univariate mixed effects model was constructed to predict delirium by benzodiazepine dose received on the prior day, controlling for opiates and mechanical ventilation on that day. Midazolam and fentanyl equivalents were log-transformed for modelling and the predicted probability of delirium development was plotted to visualize the dose-response relationship. All tests were two-sided with statistical significance evaluated at the 0.05 alpha level. Analyses were performed in R versions 3.4.0 and 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Descriptives
Between January and June 2015, there were 580 consecutive admissions with a median PICU length of stay of three days. Table 1 shows the demographics of our study population. 2,291 PICU days were captured in this study. Children received benzodiazepines on 651 days (28% of total PICU days) and opiates on 1,017 days (44%). Children required invasive mechanical ventilation for 693 days (30% of total PICU days).
Table 1.
Patient characteristics (n=580)
| Descriptives | N (%) or mean (sd) | |
|---|---|---|
|
| ||
| Sex | Male | 310 (53.4%) |
| Age at admission (years) | <1 | 147 (25.3%) |
| 1-2 | 114 (19.7%) | |
| 3-5 | 114 (19.7%) | |
| 6-12 | 101 (17.4%) | |
| 13+ | 104 (17.9%) | |
|
| ||
| Admitting diagnosis category | ||
| Cardiac disease | 48 (8.3%) | |
| Hematologic/Oncologic disorder | 17 (2.9%) | |
| Infectious/Inflammatory | 60 (10.3%) | |
| Neurologic disorder | 137 (23.6%) | |
| Renal/Metabolic disorder | 36 (6.2%) | |
| Respiratory insufficiency/failure | 282 (48.6%) | |
|
| ||
| Pre-existing medical condition (yes) | 413 (71.2%) | |
|
| ||
| Probability of mortality1 | 2% (7%) | |
|
| ||
| Survived to hospital discharge | 565 (97.4%) | |
|
| ||
| PICU length of stay (days) | 5 (6.9) | |
|
| ||
| Hospital length of stay (days) | 6.9 (11.1) | |
|
| ||
| Delirium2 (ever) | 131 (22.6%) | |
|
| ||
| Coma2 (ever) | 58 (10.0%) | |
|
| ||
| Ever mechanically ventilated | 242 (41.7%) | |
|
| ||
| Benzodiazepines3 (ever) | 141 (24.3%) | |
|
| ||
| Opiates3 (ever) | 223 (38.4%) | |
|
| ||
| Number of benzodiazepine days4 | 4.8 (6.2) | |
|
| ||
| Number of opiate days4 | 4.9 (8.2) | |
Probability of mortality as determined by the Pediatric Index of Mortality-3 score.
Includes patients who were diagnosed with either delirium or coma during the PICU stay. Please see text for definition of coma and delirium.
Medication categories: includes patients who ever received a medication in this class.
Mean number of days exposed in those who received this medication class.
Description of the 580 admissions to the PICU during the study period.
Children were diagnosed with delirium on 487 of the 2,291 days (21%), and in coma for 237 days (10%; almost all due to deep pharmacologic sedation). For 68% of the days in the PICU, children were classified as having a normal mental status (1567 days). With respect to delirium subtypes, of the 487 days with delirium, hypoactive delirium was most common (255 days; 52%). The next most common subtype was mixed delirium (191 days; 39%), followed by hyperactive delirium (only 41 days; 8%).
Predictors of Delirium
In bivariate analyses we found that highly significant predictors of delirium in our sample included being delirious the day prior (65% vs. 27%; p<0.001), receipt of benzodiazepines the day prior (38% vs. 14%; p<0.001), receipt of opiates the day prior (30% vs. 14%; p<0.001), use of restraints (52% vs. 18%; p<0.001), and need for invasive mechanical ventilation (42% vs. 12%; p<0.001).
Multivariable mixed effects modelling (Table 2) determined that the strongest predictor of delirium was being delirious on the day prior, with an odds ratio of 8.2 (CI 5.7-11.7; p<0.001). Even after controlling for prior delirium status, receipt of benzodiazepines more than doubled a child’s chance of being delirious on the following day (OR 2.0, CI 1.4–2.9, p<0.001). As expected, need for invasive mechanical ventilation was highly and independently associated with delirium (OR 3.9, CI 2.6–5.9, p<0.001). Interestingly, opiate exposure was not independently associated with delirium.
Table 2.
Predictors of delirium in study cohort
| Multivariable mixed effects model | Marginal structural model (Adjusted for Time-Dependent Confounding) | ||
|---|---|---|---|
|
| |||
| Model Terms | OR (95% CI) | OR (95% CI) | |
| Cognitive status day prior | Coma | 0.85 (0.51, 1.41) | - |
| Delirium | 8.16 (5.67, 11.74)* | - | |
| Benzodiazepine exposure day prior | 2.02 (1.39, 2.93)* | 3.33 (1.43, 7.80)* | |
| Opiate exposure day prior | 1.09 (0.75, 1.59) | - | |
| Mechanical ventilation | 3.92 (2.59, 5.92)* | ||
significant at the 0.05 alpha level
Multivariable mixed effects modeling demonstrated that the strongest predictor of delirium was being delirious on the day prior. This does not take into account time-dependent confounding.
Marginal structural modeling was used to account for time-dependent confounders, including prior cognitive status, need for mechanical ventilation, and receipt of opiates. This created a pseudo-randomized sample, allowing for estimation of the causal effect of benzodiazepines on subsequent delirium. Benozdiazepines more than tripled delirium risk.
Temporal Relationship
As prior delirium was the strongest predictor of subsequent delirium, we then performed a subgroup analysis to include only days with normal cognitive status (i.e.: when benzodiazepines were administered to children who were not already delirious) (n=1540 days). This would allow determination of the effect of benzodiazepines on subsequent development of delirium. Benzodiazepines were highly and independently associated with transition from normal mental status to delirium, more than quadrupling delirium rates (OR 4.4, CI 1.7–11, p=0.002), when compared to a child with a normal cognitive status who did not receive benzodiazepines (Figure 2). We again noted that opiates did not prove to be a statistically significant predictor of transition to delirium.
Figure 2. Odds of developing new delirium after exposure to benzodiazepines.
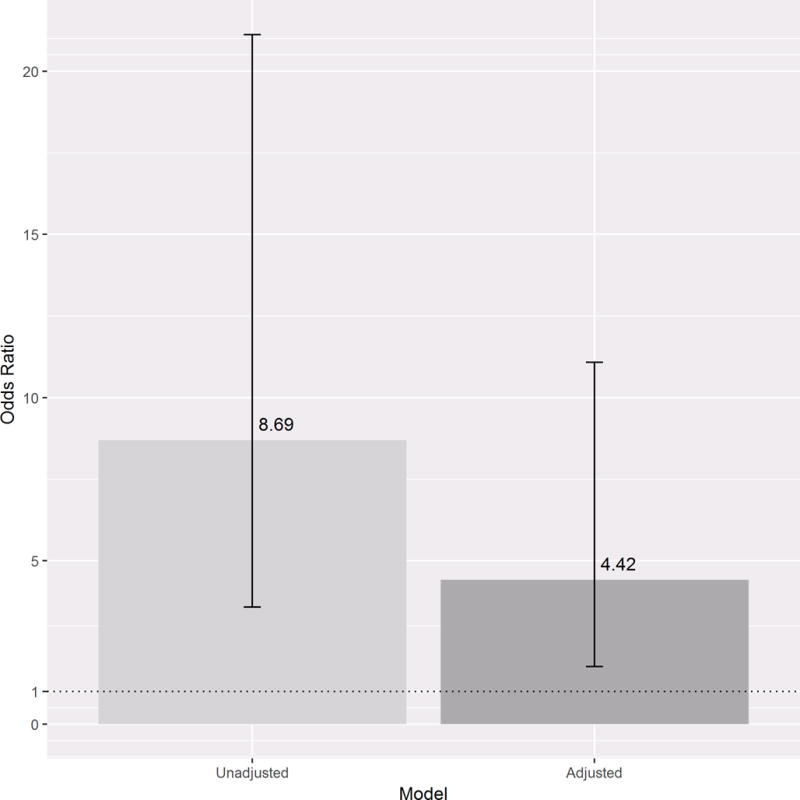
Subgroup analysis to include only days when patients had a normal cognitive status (n=1540 days with normal cognitive status and benzodiazepine exposure). The bars represent 95% confidence intervals for delirium development after exposure to benzodiazepines. On left, the unadjusted OR for delirium development after exposure to benzodiazepines is 8.7. On right, after controlling for mechanical ventilation and opiate exposure, benzodiazepines more than quadrupled risk of subsequent delirium.
The marginal structural model (MSM) created a pseudo-randomized sample, accounting for prior cognitive status, need for mechanical ventilation, and receipt of opiates when assessing the effect of benzodiazepines on subsequent delirium. This allowed for inclusion of children who were already delirious on the day when benzodiazepines were administered. The MSM (n=2291 days) demonstrated that benzodiazepines independently increased subsequent delirium risk by 333% (OR 3.33, CI 1.43–7.80) (Table 2).
Dose-Response Effect
A univariate model assessed the dose-response relationship between benzodiazepines (received on a day with normal cognitive status) and delirium development. It showed that for every one-log increase in benzodiazepine dose administered, there was a 43% increase in the risk for next-day transition to delirium (OR 1.43, CI 1.26–1.63; p<0.001). Figure 3 shows a graphical representation of benzodiazepine dosage, displaying that a child’s probability of transitioning to delirium increases drastically with increasing exposure. Even after controlling for receipt of opiates and mechanical ventilation, benzodiazepine dosage remained a significant predictor of transition to delirium (OR 1.25, CI 1.09–1.43, p=0.002).
Figure 3. Benzodiazepines and risk for delirium development.
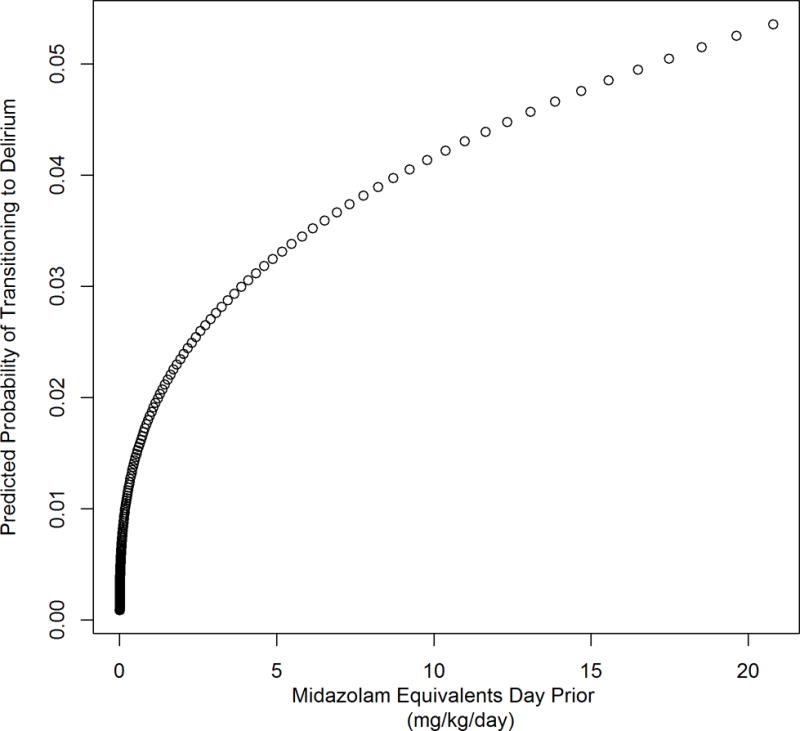
Probability of transitioning to delirium (%) increases with dose of benzodiazepine (mg/kg/day midazolam equivalents) given during the previous day.
DISCUSSION
Many studies in critically ill adults have shown a strong and independent relationship between benzodiazepines and delirium(7,18–20). As a result, there has been a paradigm shift in adult critical care such that benzodiazepines are avoided in most patients(1,21). However, in pediatric critical care units, benzodiazepines remain the first-line sedative choice(10).
This study is one of the first of its kind in pediatric critical care. Similar to the adult literature(7,18,20), we have shown that benzodiazepines are an independent predictor of subsequent delirium in critically ill children after controlling for multiple confounders including cognitive status. We have carefully structured a pseudo-randomized sample to control for time-varying confounders which demonstrated that benzodiazepines are temporally related to delirium (OR 3.3), and demonstrated an even stronger effect when benzodiazepines are given to children who are not yet delirious (OR 4.4). In the absence of a randomized controlled trial, these methods provide evidence for a causal interpretation of these observational data.
Moreover, we have shown a robust relationship between benzodiazepine dosage and predicted probability of delirium development. This is consistent with a recent pediatric study that showed an association between benzodiazepine dose and delirium risk, and between benzodiazepines and prolonged PICU stay(12).
Another important point to note is that opiate exposure was not independently associated with delirium. This may support the use of an ‘analgo-sedation’ approach in critically ill children. Endorsed by the Society of Critical Care Medicine (SCCM) for use in adults, analgosedation recommends an analgesic-first approach to critically ill patients, with optimization of pain control (utilizing both non-opioid and opioid analgesics, and taking advantage of the sedative side effects of the opiates), with minimization of additional sedation(1,21–23). Less sedation should improve recognition of pain, and optimize pain management. With better pain control, there is less need for sedation. With less sedation, there is less delirium and more opportunity for mobilization and weaning of mechanical ventilation. This paradigm shift has proven to be quite effective in changing the landscape in adult critical care(24,25). It is our hope that as similar data emerge in critically ill children, a comparable culture shift will take place(26).
This study has noteworthy strengths, including a large sample size, prospective assessment of daily cognitive status, granular data collection regarding medication exposure, and sophisticated statistical analyses that allow for control of time-dependent covariates. There are also important limitations. As a single center study, these findings may not be widely generalizable, and it will be important to replicate these findings in other institutions. Also, there are additional medications that may contribute to delirium, such as anticholinergics and steroids, that have not been included in this study design. Further research is needed to assess the relationship between delirium and these (and other) drug classes. We acknowledge the limitations of not accounting for all time-varying confounders, as well as not being able to account for unmeasured confounding. Finally, as an observational study, we must be cautious not to over-conclude (particularly with respect to the lack of independent opiate effect on delirium development; it is possible that the vast effect of benzodiazepines overshadowed an opiate component). Further multi-institutional interventional studies (perhaps comparing benzodiazepine-based sedation regimens to a benzodiazepine-sparing sedation approach) may be warranted.
CONCLUSION
In this study, we have shown an independent, temporal, and dose-response relationship between benzodiazepine exposure and subsequent pediatric delirium. With alternate sedation choices available, this is an important and modifiable risk factor. Adoption of a benzodiazepine-sparing approach in the pediatric intensive care unit may decrease delirium rates and improve the care we provide to critically ill children.
Acknowledgments
Supported by the Empire Clinical Research Investigator Program, and the Clinical Translational Science Center, grant number UL1-TR000457-06.
Copyright form disclosure: Dr. Greenwald received funding from Lubell & Rosen; Rissman, Barrett, Hurt, Donahue & McLain, PA; and Schenck, Price, Smith & King, LLP (all of the above from trial or deposition testimony), and he received funding from the Society of Critical Care Medicine (travel expenses). Dr. Silver disclosed off-label product use of medication in delirium. Dr. Traube’s institution received funding from ECRIP (Empire Clinical Research Investigator Program), and she received support for article research from the National Institutes of Health.
Footnotes
Name of institution where work performed: NY Presbyterian Hospital, Weill Cornell Medical College
Address for reprints: Reprints will not be ordered.
Disclosures: All authors have no relevant conflicts of interest to disclose.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Kalgi Mody, Mount Sinai School of Medicine.
Savneet Kaur, Weill Cornell Medical College.
Elizabeth A. Mauer, Weill Cornell Medical College.
Linda M. Gerber, Weill Cornell Medical College.
Bruce M. Greenwald, Weill Cornell Medical College.
Gabrielle Silver, Weill Cornell Medical College.
Chani Traube, Weill Cornell Medical College.
References
- 1.Barr J, Fraser GL, Puntillo K, et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Crit Care Med. 2013 Jan;41(1):278–80. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Jama. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 3.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010 Jul;38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldonado JR. Neuropathogenesis of Delirium: Review of Current Etiologic Theories and Common Pathways. Am J Geriatr Psychiatry. 2013 Dec;21(12):1190–222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015 Dec;41(12):2130–7. doi: 10.1007/s00134-015-4063-z. [DOI] [PubMed] [Google Scholar]
- 6.Skrobik Y, Leger C, Cossette M, et al. Factors Predisposing to Coma and Delirium: Fentanyl and Midazolam Exposure; CYP3A5, ABCB1, and ABCG2 Genetic Polymorphisms; and Inflammatory Factors. Crit Care Med. 2013 Apr;41(4):999–1008. doi: 10.1097/CCM.0b013e318275d014. [DOI] [PubMed] [Google Scholar]
- 7.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–6. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kudchadkar SR, Yaster M, Punjabi NM. Sedation, Sleep Promotion, and Delirium Screening Practices in the Care of Mechanically Ventilated Children: A Wake-Up Call for the Pediatric Critical Care Community. Crit Care Med. 2014 Jul;42(7):1592–600. doi: 10.1097/CCM.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver G, Traube C, Gerber LM, et al. Pediatric Delirium and Associated Risk Factors: A Single-Center Prospective Observational Study. Pediatr Crit Care Med. 2015 May;16(4):303–9. doi: 10.1097/PCC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traube C, Silver G, Reeder RW, et al. Delirium in Critically Ill Children: An International Point Prevalence Study. Crit Care Med. 2017 Apr;45(4):584–90. doi: 10.1097/CCM.0000000000002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traube C, Silver G, Gerber LM, et al. Delirium and Mortality in Critically Ill Children: Epidemiology and Outcomes of Pediatric Delirium. Crit Care Med. 2017 May;45(5):891–8. doi: 10.1097/CCM.0000000000002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith HAB, Gangopadhyay M, Goben CM, et al. Delirium and Benzodiazepines Associated With Prolonged ICU Stay in Critically Ill Infants and Young Children. Crit Care Med. 2017 Jun 1; doi: 10.1097/CCM.0000000000002515. [DOI] [PubMed] [Google Scholar]
- 13.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Nelson J, Chouinard G. Guidelines for the clinical use of benzodiazepines: pharmacokinetics, dependency, rebound and withdrawal. Canadian Society for Clinical Pharmacology. Can J Clin Pharmacol. 1999;6(2):69–83. [PubMed] [Google Scholar]
- 15.Patanwala AE, Duby J, Waters D, et al. Opioid Conversions in Acute Care. Ann Pharmacother. 2007 Feb;41(2):255–67. doi: 10.1345/aph.1H421. [DOI] [PubMed] [Google Scholar]
- 16.Patel AK, Biagas KV, Clarke EC, et al. Delirium in Children After Cardiac Bypass Surgery. Pediatr Crit Care Med. 2017 Feb;18(2):165–71. doi: 10.1097/PCC.0000000000001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisani MA, Araujo KLB, Murphy TE. Association of Cumulative Dose of Haloperidol With Next-Day Delirium in Older Medical ICU Patients. Crit Care Med. 2015 May;43(5):996–1002. doi: 10.1097/CCM.0000000000000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine Versus Nonbenzodiazepine-Based Sedation for Mechanically Ventilated, Critically Ill Adults: A Systematic Review and Meta-Analysis of Randomized Trials. Crit Care Med. 2013 Sep;41:S30–8. doi: 10.1097/CCM.0b013e3182a16898. [DOI] [PubMed] [Google Scholar]
- 19.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 20.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151–60. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 21.Barr J, Pandharipande PP. The Pain, Agitation, and Delirium Care Bundle: Synergistic Benefits of Implementing the 2013 Pain, Agitation, and Delirium Guidelines in an Integrated and Interdisciplinary Fashion. Crit Care Med. 2013 Sep;41:S99–115. doi: 10.1097/CCM.0b013e3182a16ff0. [DOI] [PubMed] [Google Scholar]
- 22.Breen D, Karabinis A, Malbrain M, et al. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497] Crit Care. 2005;9(3):R200. doi: 10.1186/cc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dale CR, Bryson CL, Fan VS, et al. A Greater Analgesia, Sedation, Delirium Order Set Quality Score Is Associated With a Decreased Duration of Mechanical Ventilation in Cardiovascular Surgery Patients. Crit Care Med. 2013 Aug 1; doi: 10.1097/CCM.0b013e31829a6ee7. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW. The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit Care Med. 2017 Feb;45(2):321–30. doi: 10.1097/CCM.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes-Daly MA, Phillips G, Ely EW. Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: Implementing PAD Guidelines Via the ABCDEF Bundle in 6,064 Patients. Crit Care Med. 2017 Feb;45(2):171–8. doi: 10.1097/CCM.0000000000002149. [DOI] [PubMed] [Google Scholar]
- 26.Smith HAB, Berutti T, Brink E, et al. Pediatric Critical Care Perceptions on Analgesia, Sedation, and Delirium. Semin Respir Crit Care Med. 2013 May 28;34(02):244–61. doi: 10.1055/s-0033-1342987. [DOI] [PubMed] [Google Scholar]


