Abstract
This past decade has witnessed a renewed interest in the function and biology of matrix-embedded osteocytes and these cells have emerged as master regulators of bone homeostasis. They secrete two very powerful proteins, sclerostin, a Wnt-inhibitor, that suppresses bone formation, and receptor-activator of NF-kB ligand (RANKL), a cytokine required for osteoclastogenesis. Neutralizing antibodies against these proteins are currently used for the treatment of osteoporosis. Recent studies however, ascribed yet another function to osteocytes: the control of hematopoiesis and the HSPC niche, directly and through secreted factors. In the absence of osteocytes there is an increase in HSC mobilization and abnormal lymphopoiesis whereas in the absence of Gsα signaling in these cells there is an increase of myeloid cells. How exactly osteocytes control hematopoiesis or the HSPC niche is still not completely understood. In this review we summarize the actions of osteocytes in bone and then analyze the effects of these cells on hematopoiesis. Future directions and gaps in current knowledge are further discussed.
Introduction
The skeleton is needed to protect vital organs, to serve as a major reservoir of mineral ions, to allow locomotion and, postnatally, to support hematopoiesis. Any pathological condition affecting the skeleton might compromise one or all of its functions. For example, osteopetrosis is characterized by defects in the bones and changes in hematopoiesis.
The importance of the bone microenvironment in controlling and maintaining the hematopoietic stem cell (HSC) niche, a specialized stromal microenvironment required to support HSCs, was described in 2003 when osteoblasts where first identified as niche-supporting cells (1). Over the last decade, however, the complexity and identity of the cells comprising the niche has greatly expanded to delineate an intricate and sophisticated network between bone marrow cells and different hematopoietic cell subtypes, as described below. Recently, new studies have identified osteocytes, the long forgotten cells of bone, as important regulators of bone metabolism and hematopoiesis (2, 3). This review will provide a summary of recent advancements in the understanding of osteocytes and bone-supporting niche cells and then discuss the role of osteocytes in hematopoiesis, both directly or through secreted factors or intermediate cells.
Osteocytes
Deeply encased within the hard mineralized matrix of bone, osteocytes have eluded and intrigued scientists for decades. Pioneers in the field of bone biology identified osteocytes as important regulators of bone metabolism but their impervious location delayed progress in unraveling the biology of these cells. Genetic studies linking human high bone mass diseases, sclerosteosis and Van Buchem disease, to sclerostin (4, 5), a protein made predominantly by mature osteocytes, have refocused the attention of scientists to these matrix-embedded cells. The last few years have brought a wealth of insight into osteocyte function and their molecular make-up and they have emerged as skeletal mechanosensors and central regulators of bone homeostasis and potentially fat metabolism (6). Osteocytes are terminally differentiated osteoblasts that, for mechanisms still largely unknown, remain entrapped within the matrix during active bone apposition. Two models have been proposed; self-entrapment or embedding by adjacent cells. One theory suggested that, on the mineralizing front, few osteoblasts lag behind their collagen synthesis and this “slow collagen production” dictates their entombment into the matrix. Alternatively, some studies suggest that the embedding is an active process in which the nascent osteocyte actively degrades the mineralized matrix to create the lacuna-canalicular system (7, 8). Whichever mechanism is responsible for inducing the osteoblast-to-osteocyte transformation the end result is a mature cell, the osteocyte, which displays a unique genetic makeup and a distinct morphology. Several molecules are required for a proper osteocyte “transformation”. For example, expression of E11 (or podoplanin) and matrix metalloproteinase 14 (MMP-14) are needed for the formation and elongation of the dendrites (7–9). In their absence, the lacuna-canalicular system is compromised and the result is osteopenia. The mature osteocyte resides within a lacuna and it is connected to adjacent osteocytes and to endosteal and periosteal osteoblasts via cellular extensions that travel within canaliculi. Through this quite extensive lacuna-canalicular system the osteocyte receives nutrients, signals and hormonal cues and communicates with the surrounding cells. Osteocytes near the endosteal surface can also communicate directly with bone marrow cells by extending some dendrites to the marrow space and by secreting molecules (and possibly macrovesicles) such as sclerostin, parathyroid hormone related peptide (PTHrP), prostaglandin E2 (PGE2), fibroblast growth factor-23 (FGF23) and RANKL (and possibly many more) capable of acting locally or on distant organs. Osteocytes express also a plethora of genes required for proper matrix mineralization and phosphate homeostasis such as phosphate-regulating neutral endopeptidase (Phex), dentin matrix protein 1 (DMP1), matrix extracellular phosphoglycoprotein (MEPE) and FGF23. Finally, they synthesize osteopontin (OPN), a phosphoprotein involved in mineralization and hematopoiesis (10).
Osteocytes and bone homeostasis
The identification of sclerostin as a potent inhibitor of bone formation (11), established the importance of osteocytes as regulators of bone formation. Sclerostin is a Wnt inhibitor that binds to and blocks LRP5/6 signaling. This glycoprotein requires the expression of LRP4 to anchor to the bone surface and to bind to LRP5/6 (12). Sclerostin is highly regulated by mechanical stimuli (an increase in load suppresses protein expression whereas a decrease in mechanical forces induces its expression) (13–15), PTH and several other factors, as evidenced in humans and animals (16, 17). Osteocytes are also key regulators of bone resorption by secreting both pro- and anti-osteoclastogenic factors, such as RANKL, macrophage-colony stimulating factor (M-CSF) and osteoprotegerin (OPG) (18–20). RANKL, together with M-CSF, is essential for proper osteoclastogenesis. Genetic ablation of RANKL in mice is characterized by severe osteopetrosis whereas its overexpression is associated with osteoporosis. Not surprisingly, RANKL neutralizing antibodies are currently used as anti-resorptive therapy for the treatment of osteoporosis. Osteocyte-derived RANKL, as is the case for sclerostin, participates in skeletal responses to mechanical cues and animal models, in which RANKL is ablated from osteocytes, are resistant to unloading-induced bone resorption (19). At the molecular level, both RANKL and sclerostin expression is controlled by PTH through mechanisms involving histone deacetylases 4 and 5 (HDAC4/5), as recently reported (21). Taken together, these pieces of evidence highlight the multifunctional role of osteocytes in regulating, in the adult skeleton, both bone formation and bone resorption (Fig. 2). Few decades ago, Harald Frost hypothesized the existence of a mechanism (named the “mechanostat”) capable of distinguishing between bone modeling (changes in shape) and remodeling (continuous replacement) and he identified the osteocyte as the “mechanostat” of bone (22). It is now clear that these cells are not only mechanosensors of bone, but also hormonal targets, as well as endocrine cells capable of controlling distant organs (for a comprehensive review see (23))
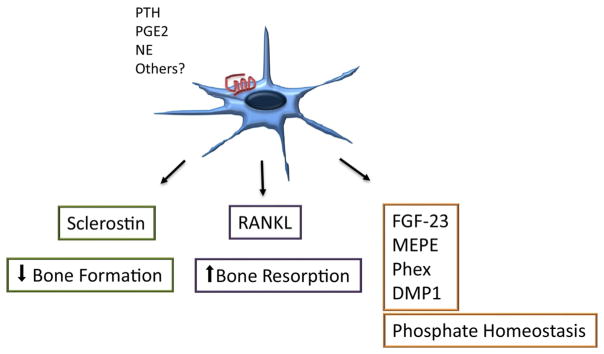
Figure 2. Osteocyte control of skeletal and mineral homeostasis.
The osteocyte expresses several membrane receptors including receptors for PTH, PGE2, NE and others and controls both osteoblast and osteoclast functions though sclerostin and RANKL. Osteocytes also secrete factors involved in phosphate homeostasis.
Hematopoiesis and the niche
Normal HSCs are located in special microenvironments or ‘niches’ within the bone marrow. In 1978 Schofield described the existence of signals between niche cells and HSC regulating HSC entry into cell cycle and differentiation programs (24). Since then, with the help of sophisticated technologies including in vivo imaging, whole mount immunofluorescence and multiparameter sorting of niche cells, multiple studies have shown the importance of the bone marrow microenvironment (BMM) for the normal physiology of HSC. The BMM represents a complex entity consisting of different cell populations, including endothelial cells, osteoblasts, osteocytes, adipocytes, neurons, mesenchymal stem cells and macrophages, as well as extracellular matrix proteins and physical factors such as oxygen content or mechanical forces essential for the maintenance, proliferation and differentiation of HSC (25) (26).
In mammals the location of hematopoietic stem and progenitor cells (HSPC) may depend on their maturation state (27) and/or their activity (28). To this day some controversies exist about the exact nature and function of the endosteal (1, 29, 30) versus the vascular niches (31–35) in the bone marrow, and how niche location of HSPC may correlate with function remains to be elucidated. However, more evidence for the essential role of the vascular niche for HSPC is accumulating, suggesting that HSCs exist close to arterioles and hematopoietic ‘stress’ leads to HSC proliferation and distribution away from arterioles (31, 36, 37). Low permeability of arterioles and low concomitant reactive oxygen species in their vicinity seem to lead to maintenance of the quiescence of HSCs, while high permeability of sinusoids and a high local concentration of reactive oxygen species around sinusoids leads to differentiation and migration of HSCs (38, 39). Sinusoids, in whose proximity 67% of HSCs were found (37), are radially distributed within the bone marrow, are lined with endothelial cells and are surrounded by perivascular cells. These perivascular cells, which are also found close to arterioles, predominantly consist of mesenchymal stromal cells (MSC), a heterogeneous population of cells defined by a set of different markers. Depending on the location in the BMM (periarteriolar or perisinusoidal) these MSCs are positive for nestin (40), neural-glial antigen (NG)-2 (35), leptin receptor (LepR) (35) or paired related homeobox-1 (Prx-1) (34). MSCs are characterized by their self-renewal ability and their ability to give rise to different lineages such as osteoblasts, chondrocytes, adipocytes etc. Depletion of nestin+ MSC, which reside in close proximity to adrenergic nerve fibers, from the bone marrow led to reduction of CD48−Lin−Sca-1+ c-Kit+ (LSK) cells and CD150+ CD48−LSK SLAM cells by almost 50%, partially due to mobilization to extramedullary sites (41). Different subsets of MSCs have been shown to be associated with HSCs (2) but, generally, MSCs are known to secrete HSC-supportive factors such as C-X-C Motif Chemokine Ligand 12 (CXCL12), angiopoietin, stem cell factor (SCF/Kit ligand) etc. However, differences according to MSC ‘type’ and ‘location’, i.e. arteriolar or sinusoidal, have been uncovered (42). For example, the use of stem cell factor (SCF-GFP) knock-in mice and various murine niche cell-specific conditional deletion models of SCF have demonstrated that SCF is primarily expressed by endothelial cells or leptin receptor-expressing perivascular cells, which regulate HSC number (35). Similarly, nestin-GFP cells (expressing GFP under the nestin promoter), which express high amounts of CXCL12, have been found in close proximity of HSCs (40). Nestin+ cells oscillate according to the circadian rhythm and, thereby, speak to the role of the sympathetic nervous system for the maintenance of the perivascular niche (43). Finally, endothelial cells themselves have been shown to support HSC maintenance by providing factors like CXCL12, SCF, angiopoietin, fibroblast growth factor (FGF) 2, Delta like 1 etc. (28, 44), and E-selectin deletion from endothelial cells increased HSCs quiescence and self-renewal, confirming an HSC-supportive role of E-selectin (45).
A fraction of HSPCs can also be found next to the endosteal bone surface, which lies at the interface between bone and bone marrow and which is lined primarily by osteoblastic cells. Evidence from ex vivo (46) culture systems, as well as in vivo experiments support the notion of regulation of HSC niche size and HSC function by these cells. PTH, a potent regulator of bone turnover, increased the number of osteoblastic cells (47) and led to a 2-fold expansion of HSCs, while conditional inactivation of BMP receptor type IA (BMPRIA) increased the number of spindle-shaped N-cadherin+ CD45− osteoblastic cells (48), concurrently leading to a ~2.4-fold expansion of HSC within the bone marrow. Furthermore, conditional ablation of the osteoblastic lineage after treatment with ganciclovir in a transgenic mouse model led to loss of the lymphoid, erythroid and myeloid progenitors, followed by a 3 to 10-fold decrease of the absolute number of phenotypic HSC (49). Additionally, osteoblastic cells maintain the quiescence and the repopulating activity of Tie2 receptor kinase-expressing HSC in vivo via the Tie2/Ang-1 signaling pathway (50).
The contribution of bone-degrading osteoclasts to hematopoiesis is far less studied, but a role of osteoclasts for mobilization of HSPC (51) and a reduction of the number and self-renewal ability of HSC after inhibition of osteoclast function by bisphosphonates has been demonstrated (52). Considering that both osteoblasts and osteoclasts have been identified as HSC supporting cells, it is not surprising that osteocytes too, play a role in hematopoiesis, as discussed below.
Lastly, other cell types, including bone marrow macrophages, nonmyelinating Schwann cells (53) and megakaryocytes (54) have also been reported to be important for maintenance of the endosteal HSC niche. Schwann cells and megakaryocytes have been proposed to control the activation of latent transforming growth factor-β (TGF-β), a key regulator of HSC dormancy and self-renewal. TGF-β comprises a large family of secreted poly-peptides that signal through cell surface serine/threonine kinase receptors. TGF-β is produced by a variety of cell types in the bone marrow and large quantities of latent TGF-β are deposited into the bone matrix. During bone remodeling active TGF-β is released from the matrix and acts as local recruiter of stromal ells and potential regulator of HSCs. Granulocyte colony-stimulating factor (G-CSF), the most commonly used mobilizing agent in clinical medicine, for example, resulted in ablation of endosteal osteoblasts, depletion of macrophages, inhibition of HSC-supportive cytokines and - as a consequence - mobilization of HSC into the peripheral blood (55). Furthermore, loss of macrophages led to enhanced CXCR4 antagonist- or GCSF-induced egress of HSC into the peripheral blood, partially due to loss of crosstalk between macrophages and nestin+ niche cells (56).
A non-cellular component of the BMM is the extracellular matrix (ECM) which is known to contain factors that support the adhesion, functionality and even the number of HSC. Almost 30 years ago heparin sulfate, produced by marrow-derived stromal cells, was shown to be essential for human hematopoietic blast colony-forming cells’ binding to the ECM in vitro (57). Furthermore, induced deletion of Ext1, a gene essential for the production of heparin sulfate, a major ECM component, in BM stromal cells of adult mice resulted in HSPC egress from the bone marrow and migration to the spleen while pharmacologic inhibition of heparin sulfate led to mobilization of more potent HSPCs (58). Tenascin-C, another ECM protein, expressed by stromal and endothelial cells of the BMM, is essential for the reconstitution of hematopoiesis after bone marrow ablation while promoting the proliferation of HSPC via integrin α9 in vitro (59). Finally, osteopontin (OPN), a matrix glycoprotein, produced by osteoblastic cells in response to different stimuli, negatively regulates the stem cell pool size through an increase of stromal Jagged1 and angiopoietin-1 and reduction of hematopoietic cell apoptosis (60). Calcium ions, the oxygen tension and the pH are among the extrinsic cues of the BM niche that can influence HSC behavior. Calcium-sensing receptor (CaR) is responsible for the homing of HSC to the endosteal region and for securing the effective adhesion of HSC to the ECM protein collagen (61). Further, it has been suggested that the endosteal niche is hypoxic whereas the more oxygenated vascular niche contains proliferative and differentiated hematopoietic progenitors (62, 63). Finally, the shear stress in bone marrow vessels was found to influence HSC status, as Runt-related transcription factor 1 (Runx1) was found to be upregulated in fetal CD41+ c-Kit+ hematopoietic progenitor cells leading to an increase of hematopoietic colony-forming potential in response to these forces (64). Though less extensively studied, the ‘elasticity’ of the ECM is a likely contributor to the maintenance of stem cell potential in the bone marrow niche (65).
Osteocytes and hematopoiesis
Considering that osteocytes orchestrate both osteoblast and osteoclast activities, it is not surprising that these cells directly, or indirectly, control the hematopoietic microenvironment. There are few mechanisms by which a cell embedded in the mineralized matrix can regulate HSCs and their niche; a) a direct effect on the niche, b) through the secretion of soluble factors capable of affecting the niche or c) by affecting intermediate cells capable of controlling the niche, such as perivascular cells, adipocytes, endosteal osteoblasts, osteoclasts or CAR cells (Fig. 1). Whereas the first mechanism requires direct contact between the osteocyte and the HSC, and therefore restricts this function to osteocytes near the endosteal surface, the other two do not. Indeed, several studies have described the role of osteocytes in hematopoiesis both via secreted factors and through direct cell-to-cell communication with other cells in the BM microenvironment. For example, mice lacking Gsα in osteocytes (Dmp1-Cre;Gsαfl/fl; Dmp1-GsαKO) are osteopenic (low bone mass) and develop a striking myeloproliferation characterized by increased granulocytes and platelets in the peripheral blood (PB) and in the bone marrow, as well as marked splenomegaly (3). BM transplantation from control donor mice into lethally irradiated Dmp1-GsαKO recipients demonstrated that the altered BM microenvironment of Gsα-deficient osteocytes was required for initiation and maintenance of the myeloproliferation and that the hematopoietic abnormalities were not intrinsic to the hematopoietic cells but rather imposed by the abnormal bone microenvironment. Ex vivo studies further demonstrated that the effect was mediated by osteocyte-derived granulocyte colony-stimulating factor (G-CSF). A similar phenotype (osteopenia and myeloid cell proliferation) is present in another mouse model in which Gsα is ablated in mature osteoblasts and/or osteocytes (Oc-Cre;Gsαfl/fl) (Divieti Pajevic unpublished data) whereas, when Gsα is eliminated in early osteo-progenitors (Osx-GsαKO), the mice have small spleens and develop B cell defects (66). Whereas the osteopenia is common to all these mouse models, the hematopoietic abnormalities have striking differences underlining the complexity of the functions and interactions between different bone cells and the bone marrow microenvironment. Animals lacking Gsα in osteocytes have significantly elevated serum G-CSF and increased HSC mobilization. G-CSF is widely used clinically to reduce chemotherapy-induced neutropenia, to treat isolated congenital neutropenic states and to mobilize hematopoietic stem cells prior to bone marrow transplantation. Indeed, HSC migration out of the BM niche is a critical process for clinical stem cell transplantation. The biology underlying HSC egress has been extensively studied; nevertheless the exact molecular mechanism, intracellular signaling events and cellular targets are still largely unknown. Recently, it was reported that administration of G-CSF induced changes in gene expression in osteocytes and disrupted their lacuna-canalicular network, suggesting a direct effect of the cytokine on these cells (2, 67, 68). Administration of G-CSF to wild type mice suppressed the expression of osteocytic markers such as SOST, DMP1, MEPE and PHEX implying that osteocytes might directly control the egress of HSCs possibly through the sympathetic nervous system (SNS). Furthermore, using a mouse model in which Dmp1-expressing cells are eliminated by diphtheria toxin injections (osteocyte-less mice), it was shown that ablation of osteocytes induces changes in lymphopoiesis and prevents HSC egress upon G-CSF administration (67). Nevertheless, evidence of a direct effect of G-CSF on osteocytes is lacking and the precise cellular mechanism(s) and signaling pathway involved have remained largely unexplored. Interestingly, osteocyte-less animals have marked osteopenia, osteoclast-mediated, and increased marrow adiposity suggesting that osteocytes might control hematopoiesis by osteoclasts or adipocytes-dependent mechanisms, as described above (and shown in Fig. 1). Indeed, marrow adipocyte negatively regulate hematopoiesis, as demonstrated by a decrease in HSCPs in adipocyte-rich marrow (69)
Figure 1. Mechanisms of the osteocyte-HSC interaction.
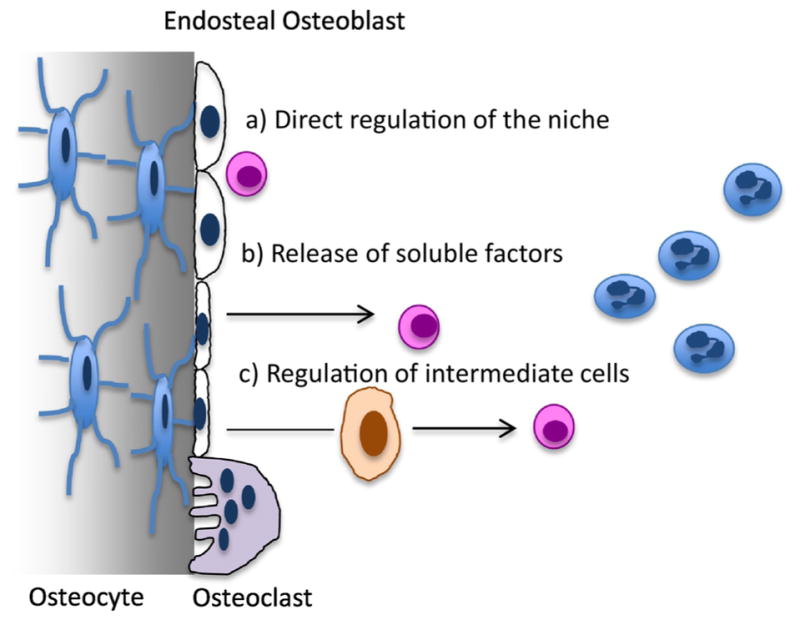
Osteocytes (stellate shaped blue cells on the left) can influence hematopoiesis a) via a direct influence on HSCs (pink round cells), b) indirectly through the release of soluble factors or c) via regulation of intermediate cells comprising the niche, such as endosteal osteoblasts, osteoclasts (purple cells) or others.
Further highlighting the complexity of the HSCs niche is the recent finding that expansion of the HSC niche, induced by PTH administration or by expression of a constitutively active (Ca) PTHR in 2.3Col1-positive cells, is not present when the receptor is expressed in osteocytes (70). Dmp1-CaPTHR animals have a skeletal phenotype very similar to 2.3Col1-CaPTHR mice, characterized by increased osteoblasts and trabecular bone (1, 70). Surprisingly, no changes in BM cellularity, HSCs or hematopoiesis are present in Dmp1-CaPTHR mice, demonstrating that neither the osteoblastic cell expansion nor osteocyte activation was sufficient to expand the HSCs (1, 70). One hypothesis is that PTH supports hematopoiesis by expanding and activating spindle-shaped Nestin+ cells (early osteoblast lineage), whereas it promotes bone anabolism by acting on all cells of the osteoblastic lineage, from progenitors to mature osteocytes, to bone lining cells. In support of the hypothesis that less differentiated cells of the osteolineage are the “constituents” of the niche comes from elegant work by Silberstein et al.(71), in which, by using a combination of micro-anatomical proximity and single cell RNA- seq they demonstrate that cells adjacent to HSPC are less differentiated osteoblasts, characterized by expression of angiogenin(72). One important osteocyte-derived molecule (and PTH-target) is sclerostin, a potent Wnt-inhibitor capable of suppressing bone formation. Sclerostin knock-out (Sost−/−) animals have increased bone volume and hyperostosis due to hyperactive osteoblasts. They display a high bone mass phenotype with reduced BM cavity volume and a relative decrease in BM cellularity. Similar to the Dmp1CaPTHR animals, the Sost−/− animals, despite the osteoblast expansion, have no differences in the frequency or absolute number of HSCs, common lymphoid progenitors (CLP), common myeloid/megakaryocyte erythroid progenitors (CMP/MEP), or granulocyte/monocyte progenitors (GMP) (73), suggesting that expansion of osteoblasts is not sufficient to affect HSCs. In these animals there is a defect in B cells and mice have a marked reduction in committed lymphoid and myeloid cells and a significant increase in B cell apoptosis driven by increased CXCL12 expression. Whether this hematopoietic abnormality is due to a direct osteocyte effect or through an intermediate cell type has to be determined.
Taken together, these studies recognize osteocytes as regulators of hematopoietic cells and identify G-CSF, sclerostin and OPN as possible factors. Despite these advancements in understanding the role of osteocytes further studies will be needed to completely characterize the role of these cells in hematopoiesis.
Closing remarks
In summary, these recent studies demonstrate a role for osteocytes in hematopoiesis, myeloid differentiation and lymphopoiesis. Additional studies, both in vitro and in vivo, are needed to elucidate the exact mechanisms, by which these cells regulate hematopoiesis, at the molecular and cellular level. For example, absence of Gsα signaling in osteocytes promotes myeloid cell proliferation (3), but the receptor(s) and downstream signaling pathway(s) are still unclear. As shown in Fig. 1 osteocytes can control hematopoiesis and myeloid cells either directly or indirectly via multiple mechanisms. As the field moves towards more sophisticated imaging and genetic tools, understanding of the interactions between bone-embedded osteocytes and the hematopoietic cells will become more clear.
Acknowledgments
Grant Supporters: This work was, in part, supported by the National Institutes of Health grants AR060221 to PDP and the LOEWE Center for Cell and Gene Therapy Frankfurt (CGT) (D.S.K.). The authors have no competing financial conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 2.Asada N, Katayama Y, Sato M, Mingawa K, Wakahashi K, Kawano H, Kawano Y, Sada A, Ikeda K, Matsui T, et al. Osteocytes embedded inside bone are essential for G-CSF-induced hematopoietic stem/progenitor mobilization. Blood. 2011;118:721. [Google Scholar]
- 3.Fulzele K, Krause DS, Barry K, Lotinun S, Baron R, Bonewald L, Feng JQ, Chen M, Weinstein LS, Scadden DT, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121(6):930–9. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16(3):319–27. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, Tuck P, Aronson JL, Liu X, Spatz JM, et al. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res. 2017;32(2):373–84. doi: 10.1002/jbmr.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 8.Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118(Pt 1):147–56. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 9.Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26(12):4539–52. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewad E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. Embo J. 2003;22(23):6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang MK, Kramer I, Huber T, Kinzel B, Guth-Gundel S, Leupin O, Kneissel M. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc Natl Acad Sci U S A. 2014;111(48):E5187–95. doi: 10.1073/pnas.1413828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 97(9):E1736–40. doi: 10.1210/jc.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, et al. The Wnt Inhibitor Sclerostin Is Up-regulated by Mechanical Unloading in Osteocytes in Vitro. The Journal of biological chemistry. 2015;290(27):16744–58. doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robling AG, Niziolek PJ, Baldridge L, KW C, MR A, IA, SM M, JG-H, TM B, SE H, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerosti. The Journal of biological chemistry. 2008;283(9):5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 16.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–83. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 17.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–58. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 19.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 17(10):1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris SE, MacDougall M, Horn D, Woodruff K, Zimmer SN, Rebel VI, Fajardo R, Feng JQ, Gluhak-Heinrich J, Harris MA, et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50(1):42–53. doi: 10.1016/j.bone.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wein MN, Liang Y, Goransson O, Sundberg TB, Wang J, Williams EA, O’Meara MJ, Govea N, Beqo B, Nishimori S, et al. Corrigendum: SIKs control osteocyte responses to parathyroid hormone. Nat Commun. 2017;8:14745. doi: 10.1038/ncomms14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 23.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34(5):658–90. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 25.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause DS, Scadden DT. A hostel for the hostile: the bone marrow niche in hematologic neoplasms. Haematologica. 2015;100(11):1376–87. doi: 10.3324/haematol.2014.113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2008;457(7225):92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Niu C, Ye L, Huang H, He X, Tong W-G, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 30.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–7. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 31.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Pitt LA, Tikhonova AN, Hu H, Trimarchi T, King B, Gong Y, Sanchez-Martin M, Tsirigos A, Littman DR, Ferrando AA, et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer Cell. 2015;27(6):755–68. doi: 10.1016/j.ccell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, O’Leary H, Fortney J, Gibson LF. Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood. 2007;110(9):3334–44. doi: 10.1182/blood-2007-01-068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koechlein CS, Harris JR, Lee TK, Weeks J, Fox RG, Zimdahl B, Ito T, Blevins A, Jung S-H, Chute JP, et al. High-resolution imaging and computational analysis of haematopoietic cell dynamics in vivo. Nature Communications. 2016;7:12169. doi: 10.1038/ncomms12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O, Lapidot T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21(11):1605–19. doi: 10.1089/ars.2014.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, Ledergor G, Jung Y, Milo I, Poulos MG, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532(7599):323–8. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macurthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19(3):214–23. doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 44.Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nature Reviews Immunology. 2017 doi: 10.1038/nri.2017.53. [DOI] [PubMed] [Google Scholar]
- 45.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Levesque J-P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18(11):1651–7. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 46.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87(2):518–24. [PubMed] [Google Scholar]
- 47.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 49.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–64. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 50.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/Angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 52.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117(5):1540–9. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–58. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321–6. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 55.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–28. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 56.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–71. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon MY, Riley GP, Clarke D. Heparan sulfate is necessary for adhesive interactions between human early hemopoietic progenitor cells and the extracellular matrix of the marrow microenvironment. Leukemia. 1988;2(12):804–9. [PubMed] [Google Scholar]
- 58.Saez B, Ferraro F, Yusuf RZ, Cook CM, Yu VW, Pardo-Saganta A, Sykes SM, Palchaudhuri R, Schajnovitz A, Lotinun S, et al. Inhibiting stromal cell heparan sulfate synthesis improves stem cell mobilization and enables engraftment without cytotoxic conditioning. Blood. 2014;124(19):2937–47. doi: 10.1182/blood-2014-08-593426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, Nagasawa T, Suda T, Kubota Y. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119(23):5429–37. doi: 10.1182/blood-2011-11-393645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201(11):1781–91. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 62.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104(13):5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459(7250):1131–5. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keung AJ, Healy KE, Kumar S, Schaffer DV. Biophysics and dynamics of natural and engineered stem cell microenvironments. Wiley Interdiscip Rev Syst Biol Med. 2010;2(1):49–64. doi: 10.1002/wsbm.46. [DOI] [PubMed] [Google Scholar]
- 66.Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci USA. 2008;105(44):16976–81. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asada N, Katayama Y, Sato M, Minagawa K, Wakahashi K, Kawano H, Kawano Y, Sada A, Ikeda K, Matsui T, et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12(6):737–47. doi: 10.1016/j.stem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Asada N, Sato M, Katayama Y. Communication of bone cells with hematopoiesis, immunity and energy metabolism. Bonekey Rep. 2015;4:748. doi: 10.1038/bonekey.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calvi LM, Bromberg O, Rhee Y, Weber JM, Smith JN, Basil MJ, Frisch BJ, Bellido T. Osteoblastic expansion induced by parathyroid hormone receptor signaling in murine osteocytes is not sufficient to increase hematopoietic stem cells. Blood. 2012;119(11):2489–99. doi: 10.1182/blood-2011-06-360933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silberstein L, Goncalves KA, Kharchenko PV, Turcotte R, Kfoury Y, Mercier F, Baryawno N, Severe N, Bachand J, Spencer JA, et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell. 2016;19(4):530–43. doi: 10.1016/j.stem.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goncalves KA, Silberstein L, Li S, Severe N, Hu MG, Yang H, Scadden DT, Hu GF. Angiogenin Promotes Hematopoietic Regeneration by Dichotomously Regulating Quiescence of Stem and Progenitor Cells. Cell. 2016;166(4):894–906. doi: 10.1016/j.cell.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res. 2012;27(7):1451–61. doi: 10.1002/jbmr.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]