Abstract
Anorexia nervosa (AN) is a psychiatric disorder characterized by inappropriate nutrient intake resulting in low body weight. Multiple hormonal adaptations facilitate decreased energy expenditure in this state of caloric deprivation including non-thyroidal illness syndrome, growth hormone resistance, and hypogonadotropic hypogonadism. Although these hormonal adaptations confer a survival advantage during periods of negative energy balance, they contribute to the long-term medical complications associated with AN, the most common of which is significant bone loss and an increased risk of fracture. In recent years, marrow adipose tissue (MAT) has emerged as an important potential determinant of the low bone mass state characteristic of AN. Unlike subcutaneous and visceral adipose tissue depots which are low in AN, MAT levels are paradoxically elevated and are inversely associated with BMD. In this review, we discuss what is known about MAT in AN and the proposed hormonal determinants of this adipose tissue depot.
Keywords: anorexia nervosa, marrow adipose tissue, bone mineral density
INTRODUCTION
Anorexia nervosa (AN) is a primary psychiatric disease characterized by the inability to maintain a normal body weight [1]. Individuals with anorexia nervosa maintain their low body weight by maintaining a state of negative energy balance either through restricting caloric intake and/or excessive energy expenditure through excessive exercise. Predominantly affecting women, the disease has a lifetime prevalence approaching 2.2% [2]. Importantly, the recovery rate is only approximately 50-60% and therefore this is a chronic disease for nearly half the women who are diagnosed [3, 4].
The negative energy balance characteristic of AN results in hormonal adaptations which minimize energy expenditure in the setting of restricted energy intake. These hormonal adaptations which include hypogonadotropic hypogonadism, hypercortisolemia and growth hormone resistance provide a survival advantage in the setting of decreased energy availability but when the decreased energy availability is prolonged, these adaptive mechanisms contribute to the medical complications associated with AN, the most common of which is significant bone loss [5]. Nearly 90 % of women with AN have bone mineral density (BMD) values more than 1 SD below comparably aged women and AN is associated with a significant increased risk of fracture [5, 6]. A prospective study of young women demonstrated a seven-fold increased risk of non-vertebral fractures in those with AN as compared to normal weight women [7]. Retrospective studies have also demonstrated a significantly increased risk of fractures with nearly 30% of girls and women with AN reporting a history of fracture [5, 8]. Therefore, understanding the determinants of this significant loss of bone mass and increased fracture risk is critical to reducing the morbidity associated with this chronic disease. In this review, we will discuss one potential determinant of bone mass in AN, marrow adipose tissue (MAT) and its association with BMD and other adipose tissue depots. The associations we will describe demonstrate a paradoxical association between MAT, peripheral adipose tissue depots and BMD in AN and this paradox suggests that the function of MAT may be fundamentally different than those of other adipose tissue depots.
Adipose tissue depots in AN
Body composition in AN
Body composition, and specifically body fat distribution, is significantly different in AN compared to normal-weight individuals. In adult women with AN, total body fat mass and percent total fat mass as measured by DXA are significantly lower compared to normal weight controls [9, 10]. and this difference is observed predominantly in the extremities. However, whereas percentage extremity fat (extremity fat/total fat mass) as measured by DXA is significantly lower in AN compared to normal-weight women, the percentage trunk fat (trunk fat/total fat mass) is similar in both groups [9, 10]. In addition, there are significant differences in body fat distribution when comparing adult women with AN to adolescents with AN. In contrast to adults, in adolescents with AN, although fat mass as measured by DXA is also significantly lower compared to normal-weight adolescent girls, extremity fat percentage is similar and percent trunk fat is significantly lower than controls [11]. This suggests that the mechanisms of fat distribution in adults versus adolescents with AN are different. Importantly, with the use of magnetic resonance imaging (MRI), subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) depots can be differentiated in cross-sectional images and in adult women with AN both SAT and VAT stores are lower when compared to normal weight controls [10, 12].
Body composition with weight-recovery
With weight recovery, adult women with AN gain significantly more trunk fat as compared to extremity fat. Short-term (< 9 months between low weight and recovery) weight recovery results in a significantly higher waist to hip ratio and significantly higher trunk/extremity fat ratio, as measured by DXA, as compared to control subjects [9, 10]. Those with the lowest percentage trunk fat at baseline have the greatest increase in percent trunk fat during recovery and cortisol has been shown to be associated with the change in trunk fat; both baseline urinary free cortisol levels and levels after weight gain are significantly correlated with the increase in trunk fat [9]. MRI measures of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) depots similarly demonstrate that VAT is significantly higher in AN after weight-recovery as compared to normal-weight women [10]. With longer-term weight recovery (12 months), the VAT/SAT ratio decreases and is similar to the ratio in normal-weight controls, suggesting that long-term weight maintenance is required for the normalization of body fat depots [13].
In adolescents, those who recover weight over a 12-month period have a significant increase in fat mass and the ratio of trunk/extremity fat but the ratio is similar to the trunk/extremity fat ratio in normal-weight controls [11]. Similar to adults, in adolescents with AN, those with the lowest percentage of trunk fat at baseline had the greatest increase in percent trunk fat with weight recovery, yet the hormonal correlates of trunk fat change differ in these two groups [11]. Unlike in adults, in adolescents with AN, urine free cortisol measurements did not correlate with changes in trunk fat but change in IGF-1 levels significantly correlated with change in trunk fat percentage as well as change in the trunk/extremity fat ratio [11]. This normalization of the trunk/extremity fat ratio after 12 months of weight recovery is similar to the normalization of body fat distribution observed in adults with AN after 12 months [11, 13]. Therefore, although the distribution of peripheral adipose tissue depots is significantly different in adolescents with active AN as compared to adults, with long-term weight recovery, there is a similar normalization of body fat distribution in both populations.
MAT in AN
The significant decrease in both SAT and VAT depots in AN compared to normal weight controls is expected [10, 12]. In contrast, the majority of studies have demonstrated increased MAT depots in AN compared to normal weight controls and inverse associations between MAT and subcutaneous adipose tissue stores [12, 14, 15]. In the one study reporting lower levels of MAT in women with AN as compared to controls, the patients were predominantly inpatients and therefore likely sicker and of a lower weight than the majority of patients with AN [16]. As the authors state, the findings likely represent atrophy or degeneration of bone marrow which has been previously described in very low weight individuals with AN [16-18]. A study of bone marrow specimens of 44 individuals with AN showed 50% had gelatinous degeneration and importantly the bone marrow findings correlated with the amount of weight loss [18].
MAT with recovery
With recovery, the bone marrow and MAT levels normalize. In a study comparing women with AN to women with a history of AN but who had recovered weight and menstrual cyclicity, we found that the women who had recovered from AN had similar MAT levels in the L4 vertebra as compared to the normal weight controls, whereas the women with AN had significantly higher levels of L4 MAT [19]. Similarly, in the study of individuals with AN who had bone marrow biopsies, a subset had a repeat biopsy after weight recovery which demonstrated normalization of the marrow [18]. Therefore, although the role of MAT remains unknown, the increase in MAT observed in AN appears to be a response to the low-weight state and reversible with weight recovery, suggesting that it may serve a function, currently unknown, during periods of nutrient deficiency.
MAT’s association with BMD in AN
In many populations, including healthy women, MAT is inversely associated with BMD [20, 21]. This relationship between MAT and BMD is also observed in AN [12]. Importantly, MAT is associated with parameters of decreased bone integrity [22], suggesting that the increased levels of MAT in AN may be a determinant of decreased bone strength. In adolescents with AN, L4 MAT has been inversely associated with high-resolution peripheral quantitative CT derived finite element analysis estimates of bone strength [23]. Whether the higher levels of MAT in this population directly contribute to the increased fracture risk is not known.
Potential determinants of MAT in AN (Table 1)
Table 1.
Potential hormonal determinants of marrow adipose tissue (MAT) in anorexia nervosa
| Hormone levels in anorexia nervosa (compared to normal weight individuals) | Association with bone | Association with MAT | |
|---|---|---|---|
| Cortisol | Elevated [24-27] | Inversely associated with BMD [27, 64] | No known association in AN |
| Preadipocyte factor-1 | Elevated [29] | Inversely associated with BMD [29] | Positive association with MAT in AN [29] |
| Estrogen | Low [64] | Duration of amenorrhea inversely associated with BMD [64] | Estrogen suppresses MAT in postmenopausal women (unknown in AN) [32] |
| Fibroblast growth factor 21 | Similar/low [38, 65, 66] | Inversely associated with parameters of bone microarchitecture [38] | Inverse association with MAT [39] |
| Ghrelin | Elevated [40-42] | Positive association with BMD in normal weight individuals but inverse association with BMD in AN [67, 68] | Stimulates marrow adipogenesis in rodent models [43] |
| Leptin | Low [45, 46] | Positively associated with BMD and parameters of bone microarchitecture [29, 47, 48] | Inverse association with MAT [29] |
| IGF-1 | Low [49] | Positively associated with BMD [53] | Inverse association with MAT in AN [29] |
| IGFBP2 | Elevated [49, 54, 55] | Inversely associated with BMD and osteocalcin (marker of bone formation) [29, 54] | Positive association with MAT [29] |
| Adiponectin | Majority of studies demonstrate elevated total adiponectin levels [58, 59] | Inversely associated with BMD in AN [69] | Adiponectin secreted by MAT in animal models [59] |
Cortisol
Cortisol, a counterregulatory hormone released during states of physiologic stress including starvation is increased in many women and adolescents with AN [24-27]. Therefore, cortisol has been postulated to be a hormonal determinant of MAT. However, frank hypercortisolism is not universally seen in this disorder. Furthermore, in a study using quantitative CT measuring intravertebral fat in women with AN and women with hypercortisolemia due to Cushing’s syndrome [14] we showed that although women with AN demonstrated significantly higher levels of intravertebral MAT as compared to controls, the women with Cushing’s syndrome did not [14]. Therefore, it is not likely that cortisol is an important hormonal determinant of MAT.
Preadipocyte factor (Pref)-1
Preadipocyte factor (Pref)-1 is a member of the epidermal growth factor family of proteins and a negative regulator of adipocyte and osteoblast differentiation [28]. Although Pref-1 inhibits adipocyte differentiation, we have demonstrated significant positive associations between Pref-1 and MAT in women with AN [19, 29]. Importantly, these positive associations were observed in cross-sectional studies and therefore it is not known whether the relationship between Pref-1 and MAT is causative. Longitudinal studies will be necessary to better elucidate the relationship between Pref-1 and MAT.
Estrogen
Estrogen may also be an important determinant of MAT. Estrogen suppresses adipocyte differentiation and therefore the estrogen deficiency due to hypogonadotropic hypogonadism characteristic of AN may contribute to MAT accumulation. In vitro studies demonstrate that 17-β estradiol decreases PPARγ-agonist induced adipocyte differentiation of human mesenchymal stem cells [30]. In murine models, estrogen deficiency, for example through ovariectomy, also enhances adipocyte infiltration of bone marrow and estrogen supplementation can prevent increases in marrow adiposity in these estrogen deficient animals [31]. Similarly, in women, marrow adipocyte volume/tissue volume (AV/TV) and adipocyte number increase after menopause and AV/TV decreases with estrogen replacement concomitant with an increase in BMD at the lumbar spine [32]. Whether estrogen is a determinant of MAT in AN is not known.
Fibroblast growth factor (FGF)21
Fibroblast growth factor (FGF)21 is a hormone secreted during states of starvation in both animal models and humans. In murine models, FGF21 is secreted early during starvation and is a regulator of ketogenesis, whereas in humans, FGF21 levels increase during late starvation (after 7-10 days of fasting) and circulating levels increase only after a peak in serum ketone levels [33-35]. Therefore, FGF21 appears to have divergent functions in mice as compared to humans. In one study evaluating the effects of FGF21 on bone, mice that transgenically overexpress FGF21 were found to have uncoupled bone turnover with increased bone resorption and decreased bone formation, coincident with increased levels of MAT [36], although a second murine study did not find a bone or MAT phenotype in diet-induced obese mice who were treated with recombinant human FGF21 [37]. In humans, we have shown that FGF21 levels are associated with worsened parameters of bone microarchitecture in AN [38] and hypothesized that MAT would be positively associated with FGF21 in AN. In a study of women with AN and normal-weight women, we found a significant inverse association between MAT of the L4 vertebra and FGF21 [39]. Whether this is a compensatory decrease in FGF21 in response to elevated MAT or an example of the divergent role of this hormone in mice as compared to humans is unknown.
Ghrelin
Ghrelin is an orexigenic hormone secreted by the fundal cells of the stomach. Ghrelin levels are significantly higher in girls and women with anorexia nervosa as compared to normal weight individuals as would be expected in a nutritionally deficient state [40-42]. In a rodent model, ghrelin has been shown to promote marrow adipogenesis but likely through a receptor other than the GHS1a receptor to which it is known to bind [43]. We measured MAT in women randomized to either four weeks of a GHS1a receptor agonist (relamorelin) (n=7) or placebo (n=11) and found that although L4 MAT was similar in both groups before treatment (p=0.28), there was a trend towards decreased L4 MAT in the women randomized to the GHS1a receptor agonist as compared to placebo (Figure 1). The women randomized to relamorelin, the GHS1a receptor agonist, had significantly lower levels of acyl ghrelin after four weeks of treatment as compared to the placebo group [44]. Whether this decrease in MAT was due to decreased levels of endogenous acyl ghrelin (which may mediate MAT promoting effects through a receptor other than the GHS1a receptor) or due to other changes mediated by the GHS1a receptor agonist is unknown but warrants further study.
Figure 1.
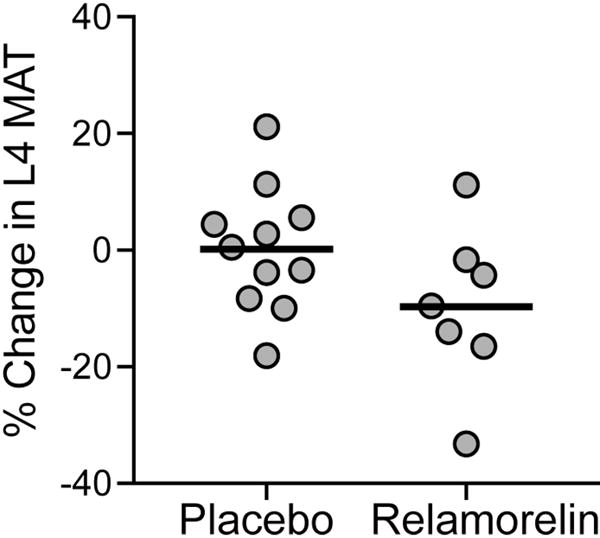
Marrow adipose tissue (MAT) in the L4 vertebra decreased more in women treated with four weeks of a GHS1a receptor agonist (relamorelin) as compared to those randomized to placebo. There was a trend towards significance (p=0.1).
Leptin
Levels of leptin, an adipokine secreted predominantly by SAT are low in AN [45, 46]. In AN, leptin is positively associated with both BMD [29, 47] and parameters of microarchitecture [48] and we have demonstrated an inverse association between L4 vertebral MAT and leptin in women with AN [29]. Importantly, as leptin is strongly associated with SAT in this population [29], it is unknown whether this association is observed simply because of the inverse association between MAT and SAT or whether the low levels of leptin are an independent hormonal determinant of the elevated levels of MAT.
IGF-1 and IGFBP2
IGF-1 is a nutritionally dependent hormone secreted by the liver in response to growth hormone. In states of starvation, including AN, IGF-1 levels are low [49]. Because IGF-1 is a known bone trophic hormone, [50-52] the low levels are likely an important contributor of low bone mass in AN, as evidenced by the fact that IGF-1 is positively associated with BMD in this population [53]. In contrast, levels of IGFBP2, a binding protein of IGF-1 are elevated in AN [49, 54, 55] and are inversely associated with a marker of bone resorption, osteocalcin [54]. We have found that MAT is positively associated with IGFBP2 and inversely associated with IGF-1 in women with AN [29]. In contrast, we demonstrated a positive association between IGF-1 and MAT in normal weight, healthy controls [29], although in a population inclusive of normal weight, overweight and obese women, MAT and IGF-1 were inversely associated [56]. This suggests that IGF-1 may have differential effects in various states of nutrient sufficiency. A better understanding of the association between MAT and growth hormone-IGF1 axis may allow for a better understanding of the role and function of MAT.
Adiponectin
Similar to MAT, levels of adiponectin, a hormone secreted by adipocytes, are paradoxically higher in normal weight individuals as compared to obese individuals [57] and total adiponectin levels are increased in AN in most studies [58, 59]. In animal models, adiponectin has been shown to be secreted by MAT [59]. Although adiponectin does not appear to be a hormonal determinant of MAT in animal models but instead a secretory product of MAT, it may explain the paradox of elevated levels of adiponectin in individuals with low levels of SAT and VAT, such as those with AN [60].
CONCLUSIONS
Despite having low levels of VAT and SAT, individuals with AN have increased MAT which is inversely associated with BMD. Importantly, these increased levels of MAT are reversible with weight recovery and therefore the increase is likely associated with weight loss and characteristic of the low-weight state. Although the role and function of MAT remains unknown, we hypothesize that it serves an important purpose given the fact that this adipose tissue depot increases during a state of decreased nutrient availability, a state which is typically characterized by utilization of fat stores. Importantly, although there is an inverse association between MAT and BMD in healthy populations [20, 21, 61, 62] and women with AN[12], there are disease states, including HIV, in which both MAT and BMD are decreased [63]. A better understanding of these disparate associations between BMD and MAT may provide further insight into the role of this adipose tissue depot. Further studies are needed to elucidate the determinants of MAT which will allow us to better understand the function of this adipose tissue depot.
Highlights.
Anorexia nervosa (AN) is psychiatric disorder characterized by low body weight
AN is associated with significant bone loss and an increased risk of fracture
Marrow adipose tissue (MAT) is a potential determinant of bone mass
Although sc and visceral fat stores are low in AN, MAT levels are elevated
Elevated MAT may be a determinant of low bone mass in AN
Acknowledgments
This project was supported by NIH grants R24 DK092759 (Klibanski), R03 DK106410 (Fazeli). The data presented for Figure 1 (change in marrow adipose tissue after treatment with a GHSR1a agonist or placebo) were acquired as part of an investigator-initiated study from Motus Therapeutics, formerly Rhythm Pharmaceuticals (Boston, MA) and were also supported by NIH grants 8 UL1 TR000170, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science and 1 UL1 TR001102. The funding sources did not have any role in the collection, management, analysis or interpretation of the data or the decision to submit the manuscript. Approval of the manuscript was not required prior to submission. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources, including the National Institutes of Health.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5) 5th. Washington, DC: 2013. [Google Scholar]
- 2.Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, Bulik CM, Kaprio J, Rissanen A. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164:1259–65. doi: 10.1176/appi.ajp.2007.06081388. [DOI] [PubMed] [Google Scholar]
- 3.Lowe B, Zipfel S, Buchholz C, Dupont Y, Reas DL, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. 2001;31:881–90. doi: 10.1017/s003329170100407x. [DOI] [PubMed] [Google Scholar]
- 4.Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, Edkins K, Krishna M, Herzog DB, Keel PK, Franko DL. Recovery From Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. J Clin Psychiatry. 2017;78:184–189. doi: 10.4088/JCP.15m10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–6. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 6.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–4. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. 1991;265:1133–8. [PubMed] [Google Scholar]
- 8.Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, Mendes N, Snelgrove D, Meenaghan E, Misra M, Klibanski A. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47:458–66. doi: 10.1002/eat.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr. 2001;73:865–9. doi: 10.1093/ajcn/73.5.865. [DOI] [PubMed] [Google Scholar]
- 10.Mayer L, Walsh BT, Pierson RN, Jr, Heymsfield SB, Gallagher D, Wang J, Parides MK, Leibel RL, Warren MP, Killory E, Glasofer D. Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr. 2005;81:1286–91. doi: 10.1093/ajcn/81.6.1286. [DOI] [PubMed] [Google Scholar]
- 11.Misra M, Soyka LA, Miller KK, Grinspoon S, Levitsky LL, Klibanski A. Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. Am J Clin Nutr. 2003;77:1361–7. doi: 10.1093/ajcn/77.6.1361. [DOI] [PubMed] [Google Scholar]
- 12.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer LE, Klein DA, Black E, Attia E, Shen W, Mao X, Shungu DC, Punyanita M, Gallagher D, Wang J, Heymsfield SB, Hirsch J, Ginsberg HN, Walsh BT. Adipose tissue distribution after weight restoration and weight maintenance in women with anorexia nervosa. Am J Clin Nutr. 2009;90:1132–7. doi: 10.3945/ajcn.2009.27820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayo-Smith W, Rosenthal DI, Goodsitt MM, Klibanski A. Intravertebral fat measurement with quantitative CT in patients with Cushing disease and anorexia nervosa. Radiology. 1989;170:835–8. doi: 10.1148/radiology.170.3.2916039. [DOI] [PubMed] [Google Scholar]
- 15.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiser F, Murtz P, Lutterbey G, Traber F, Block W, Imbierowicz K, Schilling G, Schild H, Liedtke R. Magnetic resonance spectroscopic and relaxometric determination of bone marrow changes in anorexia nervosa. Psychosom Med. 2001;63:631–7. doi: 10.1097/00006842-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Lambert M, Hubert C, Depresseux G, Vande Berg B, Thissen JP, Nagant de Deuxchaisnes C, Devogelaer JP. Hematological changes in anorexia nervosa are correlated with total body fat mass depletion. Int J Eat Disord. 1997;21:329–34. doi: 10.1002/(sici)1098-108x(1997)21:4<329::aid-eat4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Abella E, Feliu E, Granada I, Milla F, Oriol A, Ribera JM, Sanchez-Planell L, Berga LI, Reverter JC, Rozman C. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol. 2002;118:582–8. doi: 10.1309/2Y7X-YDXK-006B-XLT2. [DOI] [PubMed] [Google Scholar]
- 19.Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27:1864–71. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, Grunfeld C. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab. 2012;97:1337–46. doi: 10.1210/jc.2011-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Singhal V, Tulsiani S, Campoverde KJ, Mitchell DM, Slattery M, Schorr M, Miller KK, Bredella MA, Misra M, Klibanski A. Impaired bone strength estimates at the distal tibia and its determinants in adolescents with anorexia nervosa. Bone. 2018;106:61–68. doi: 10.1016/j.bone.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyar RM, Hellman LD, Roffwarg H, Katz J, Zumoff B, O’Connor J, Bradlow HL, Fukushima DK. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med. 1977;296:190–3. doi: 10.1056/NEJM197701272960403. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BT, Katz JL, Levin J, Kream J, Fukushima DK, Hellman LD, Weiner H, Zumoff B. Adrenal activity in anorexia nervosa. Psychosom Med. 1978;40:499–506. doi: 10.1097/00006842-197810000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, Neubauer G, Herzog DB, Klibanski A. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:4972–80. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 27.Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanski A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–6. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–52. doi: 10.1359/JBMR.040118. [DOI] [PubMed] [Google Scholar]
- 29.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95:407–13. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benvenuti S, Cellai I, Luciani P, Deledda C, Saccardi R, Mazzanti B, Dal Pozzo S, Serio M, Peri A. Androgens and estrogens prevent rosiglitazone-induced adipogenesis in human mesenchymal stem cells. J Endocrinol Invest. 2012;35:365–71. doi: 10.3275/7739. [DOI] [PubMed] [Google Scholar]
- 31.Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and Sirt1 in aging C57BL/6J mice. Biogerontology. 2009;10:747–55. doi: 10.1007/s10522-009-9221-7. [DOI] [PubMed] [Google Scholar]
- 32.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–30. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, Lee H, Catana C, Klibanski A, Patwari P, Steinhauser ML. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125:4601–11. doi: 10.1172/JCI83349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, Wan Y. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143–8. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Stanislaus S, Asuncion F, Niu QT, Chinookoswong N, Villasenor K, Wang J, Wong P, Boyce R, Dwyer D, Han CY, Chen MM, Liu B, Stolina M, Ke HZ, Ominsky MS, Veniant MM, Xu J. FGF21 Is Not a Major Mediator for Bone Homeostasis or Metabolic Actions of PPARalpha and PPARgamma Agonists. J Bone Miner Res. 2017;32:834–845. doi: 10.1002/jbmr.2936. [DOI] [PubMed] [Google Scholar]
- 38.Fazeli PK, Faje AT, Cross EJ, Lee H, Rosen CJ, Bouxsein ML, Klibanski A. Serum FGF-21 levels are associated with worsened radial trabecular bone microarchitecture and decreased radial bone strength in women with anorexia nervosa. Bone. 2015;77:6–11. doi: 10.1016/j.bone.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fazeli PK, Faje AT, Bredella MA, Polineni S, Martinez Salazar EL, Torriani M, Rosen C, Klibanski A. Fibroblast Growth Factor 21 Is Inversely Associated with Marrow Adiposity in Lean Women Endocrine Reviews. 2017;38 Endocrine Society Abstract. [Google Scholar]
- 40.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E347–56. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 41.Germain N, Galusca B, Le Roux CW, Bossu C, Ghatei MA, Lang F, Bloom SR, Estour B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am J Clin Nutr. 2007;85:967–71. doi: 10.1093/ajcn/85.4.967. [DOI] [PubMed] [Google Scholar]
- 42.Monteleone P, Serritella C, Martiadis V, Scognamiglio P, Maj M. Plasma obestatin, ghrelin, and ghrelin/obestatin ratio are increased in underweight patients with anorexia nervosa but not in symptomatic patients with bulimia nervosa. J Clin Endocrinol Metab. 2008;93:4418–21. doi: 10.1210/jc.2008-1138. [DOI] [PubMed] [Google Scholar]
- 43.Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T. Ghrelin and desoctanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234–42. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 44.Fazeli PK, Lawson EA, Faje AT, Eddy KT, Lee H, Fiedorek FT, Breggia A, Gaal IM, DeSanti R, Klibanski A. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. Journal of Clinical Psychiatry. 2018 Jan 2;79(1) doi: 10.4088/JCP.17m11585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, Ma Z, Vignati L, Bowsher R, Herzog D, Klibanski A. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3861–3. doi: 10.1210/jcem.81.11.8923829. [DOI] [PubMed] [Google Scholar]
- 46.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289:E373–81. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 47.Legroux-Gerot I, Vignau J, Biver E, Pigny P, Collier F, Marchandise X, Duquesnoy B, Cortet B. Anorexia nervosa, osteoporosis and circulating leptin: the missing link. Osteoporos Int. 2010;21:1715–22. doi: 10.1007/s00198-009-1120-x. [DOI] [PubMed] [Google Scholar]
- 48.Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46:458–63. doi: 10.1016/j.bone.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–7. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 50.Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest. 1980;66:709–19. doi: 10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hock JM, Centrella M, Canalis E. Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology. 1988;122:254–60. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- 52.Nakasaki M, Yoshioka K, Miyamoto Y, Sasaki T, Yoshikawa H, Itoh K. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone. 2008;43:869–79. doi: 10.1016/j.bone.2008.07.241. [DOI] [PubMed] [Google Scholar]
- 53.Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, Herzog D, Klibanski A. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. J Clin Endocrinol Metab. 1999;84:2049–55. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- 54.Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K. The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2000;85:200–6. doi: 10.1210/jcem.85.1.6321. [DOI] [PubMed] [Google Scholar]
- 55.Grinspoon S, Miller K, Herzog D, Clemmons D, Klibanski A. Effects of recombinant human insulin-like growth factor (IGF)-I and estrogen administration on IGF-I, IGF binding protein (IGFBP)-2, and IGFBP-3 in anorexia nervosa: a randomized-controlled study. J Clin Endocrinol Metab. 2003;88:1142–9. doi: 10.1210/jc.2002-021402. [DOI] [PubMed] [Google Scholar]
- 56.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 58.Housova J, Anderlova K, Krizova J, Haluzikova D, Kremen J, Kumstyrova T, Papezova H, Haluzik M. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90:1366–70. doi: 10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- 59.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheller EL, Burr AA, MacDougald OA, Cawthorn WP. Inside out: Bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte. 2016;5:251–69. doi: 10.1080/21623945.2016.1149269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93:2281–6. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96:782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 63.Huang JS, Mulkern RV, Grinspoon S. Reduced intravertebral bone marrow fat in HIV-infected men. AIDS. 2002;16:1265–9. doi: 10.1097/00002030-200206140-00009. [DOI] [PubMed] [Google Scholar]
- 64.Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68:548–54. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 65.Dostalova I, Kavalkova P, Haluzikova D, Lacinova Z, Mraz M, Papezova H, Haluzik M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3627–32. doi: 10.1210/jc.2008-0746. [DOI] [PubMed] [Google Scholar]
- 66.Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab. 2010;95:369–74. doi: 10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Misra M, Miller KK, Stewart V, Hunter E, Kuo K, Herzog DB, Klibanski A. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab. 2005;90:5082–7. doi: 10.1210/jc.2005-0512. [DOI] [PubMed] [Google Scholar]
- 68.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. J Clin Endocrinol Metab. 2008;93:1292–7. doi: 10.1210/jc.2007-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, Katzman DK, Klibanski A. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2046–52. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]


