Abstract
To investigate the prevalence and temporal trend of transmitted drug resistance (TDR), a nationwide cross-sectional survey was conducted among 5627 ART naïve newly diagnosed HIV-infected individuals in 2015 in China. Totally 4704 partial pol sequences were obtained. Among them, the most common HIV-1 circulating recombinant form (CRF) or subtype was CRF01_AE (39.0%), followed by CRF07_BC (35.6%), CRF08_BC (8.9%), and subtype B (5.5%). TDR mutations were found in 3.6% of the cases, with 1.1% harboring TDR to protease inhibitors (PIs), 1.3% having TDR to nucleoside reverse transcriptase inhibitors (NRTIs), and 1.6% to non-nucleoside reverse transcriptase inhibitors (NNRTIs). No significant difference was found in the prevalence of TDR, as compared with the results of another nationwide survey performed among ART naïve HIV-infected people in between 2004 and 2005, except in the 16–25 year-old group. In addition, four drug-resistant transmission clusters were identified in phylogenetic trees, accounting for 6.2% (9/145) of the individuals with TDR. Although the rate of TDR remained relatively low in the past 10 years in China, surveillance is still needed to monitor the trend of TDR and to optimize the first-line regimens.
Introduction
The epidemic of HIV/AIDS has kept rising in China since the first HIV case was reported in 1985. By the end of 2015, there were 557,423 individuals living with HIV/AIDS reported in China, of whom 382,129 were receiving antiretroviral treatment (ART)1. The scale-up of ART has led to dramatic reductions in HIV/AIDS-related morbidity and mortality2–4. HIV-infected people on suppressive ART have a low risk of onwards HIV transmission5,6. However, with the increasing coverage of ART, concerns for the emergence and transmission of HIV drug resistance are arising5,7–9.
The rates of transmitted drug resistance (TDR) vary throughout the world. The overall TDR prevalence was estimated to be less than 5% in sub-Saharan Africa and south/southeast Asia, with 2.8% and 2.9%, respectively. However, the estimated TDR rates were rather higher (more than 5%) in other regions, with 5.6% in upper-income Asian countries, 7.6% in Latin America/Caribbean, 9.4% in Europe, and 11.5% in North America10. It’s notable that upward trends in TDR continued to be reported in some regions10,11. In China, the overall prevalence of TDR among ART-naïve individuals in 2004 and 2005 was 3.8%12. The rate of TDR among 16–25 year-old ART-naïve newly diagnosed individuals in China was 3.6% in 2015 (unpublished data), however, there was no recent data on TDR among HIV-infected populations aged above 25 years old.
Since Chinese government officially launched the National Free Antiretroviral Treatment Program (NFATP) in 200313, the number of patients enrolled in this program has increased rapidly year by year. The free ART was estimated to have covered 59% of patients who met the criteria of ART initiation by 2015. During the past years, the first-line regimens had changed a lot as recommended by the national ART guidelines, with Didanosine (ddI) and stavudine (d4T) replaced by lamividine (3TC) and tenofovir (TDF), in 2005 and 2010, respectively. Furthermore, the rate of virological suppression was increased from 78% in 2004 and 2005 to more than 90% in 2013 among Chinese patients on ART. Here, we conducted a nationwide cross-sectional survey in 2015 to characterize TDR among ART-naïve newly diagnosed individuals, and to analyze the trends of TDR over different periods.
Results
Characteristics of surveyed populations
A total of 5627 individuals who were diagnosed as HIV positive during Apr. and Jun. 2015 in all the 31 provinces, autonomous regions and municipalities of mainland China were enrolled in this survey. The HIV strains of 4704 (83.6%) participants were successfully sequenced. Of these individuals, the median age was 38 years, 80.0% (3763) were male, and 82.0% (3859) were of Han nationality. More than half of the participants were heterosexuals (53.8%), followed by men who have sex with men (MSMs, 39.4%), and injection drug users (IDUs, 2.4%). According to the results of phylogenetic trees and blasting, the major subtypes and circulating recombinant forms (CRFs) were CRF01_AE (39.0%), CRF07_BC (35.6%), CRF08_BC (8.9%), and subtype B (5.5%). (Table 1).
Table 1.
Prevalence of TDR by various characteristics between 2004–2005 and 2015.
| Characteristics | 2004–2005 | 2015 | P Value | ||
|---|---|---|---|---|---|
| Number | TDR (%) | Number | TDR (%) | ||
| Total | 678 | 26 (3.8) | 4704 | 167 (3.6) | 0.71 |
| Age at diagnosis (yrs) | |||||
| 16–25 | 51 | 6 (11.8) | 862 | 28 (3.2) | 0.01 |
| 26–50 | 534 | 16 (3.0) | 2749 | 98 (3.6) | 0.51 |
| >50 | 93 | 4 (4.3) | 1003 | 39 (3.9) | 1.00 |
| Unknown | 0 | 0 (0.0) | 90 | 3 (3.3) | — |
| HIV risk exposure | |||||
| Sexual contact | 114 | 5 (4.4) | 4388 | 161 (3.7) | 0.88 |
| Heterosexual | — | — | 2533 | 103 (4.1) | — |
| MSM | — | — | 1855 | 58 (3.1) | — |
| IDU | 118 | 4 (3.4) | 115 | 2 (1.7) | 0.70 |
| Others or Unknown | 456 | 17 (3.7) | 201 | 4 (2.0) | 0.24 |
| Subtype | |||||
| 01_AE | 100 | 5 (5.0) | 1835 | 73 (4.0) | 0.81 |
| 07_BC | 47 | 1 (2.1) | 1675 | 47 (2.8) | 1.00 |
| 08_BC | 12 | 0 (0.0) | 418 | 13 (3.1) | 1.00 |
| B | 500 | 18 (3.6) | 261 | 12 (4.6) | 0.50 |
| Others | 19 | 2 (10.5) | 515 | 22 (4.3) | 0.21 |
The prevalence of TDR
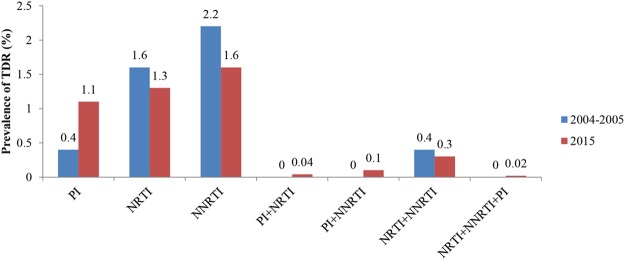
Among 4704 sequenced individuals, 167 (3.6%) were identified to harbor HIV strains with at least one drug resistance mutation (DRM). The prevalence of TDR to nonnucleoside reverse transcriptase inhibitors (NNRTIs) was 1.6%, followed by TDR to nucleoside reverse transcriptase inhibitors (NRTIs) at 1.3%, and to protease inhibitors (PIs) at 1.1%. The prevalence of dual-class TDR was 0.04% (PI and NRTI), 0.1% (PI and NNRTI), and 0.3% (NRTI and NNRTI). Only one individual (0.02%) was found to harbor triple-class TDR mutations (Fig. 1). The most frequent NNRTI-, NRTI- and PI-related drug resistant mutations (DRMs) were K103N/S, M184V and M46I/L, with the prevalence of 0.6%, 0.3% and 0.6% in the cases, respectively (Table 2).
Figure 1.
Prevalence of TDR by antiviral drug class between 2004–2005 and 2015.
Table 2.
HIV drug resistant mutations in 2004–2005 and 2015.
| TDR Mutations | 2004–2005 | 2015 | P Value | ||
|---|---|---|---|---|---|
| Number | TDR (%) | Number | TDR (%) | ||
| Total | 26 | 3.8 | 167 | 3.6 | 0.71 |
| PI | |||||
| M46I/L | 1 | 0.1 | 29 | 0.6 | 0.21 |
| I85V | 0 | 0 | 6 | 0.1 | 1.00 |
| G73C/S | 0 | 0 | 4 | 0.09 | 1.00 |
| L23I | 0 | 0 | 3 | 0.06 | 1.00 |
| I47V | 0 | 0 | 2 | 0.04 | 1.00 |
| F53L | 0 | 0 | 2 | 0.04 | 1.00 |
| I54L | 0 | 0 | 1 | 0.02 | 1.00 |
| I50V | 0 | 0 | 1 | 0.02 | 1.00 |
| V82L | 1 | 0.1 | 1 | 0.02 | 0.24 |
| N83D | 0 | 0 | 1 | 0.02 | 1.00 |
| I84V | 0 | 0 | 1 | 0.02 | 1.00 |
| N88D | 1 | 0.1 | 1 | 0.02 | 0.24 |
| NRTI | |||||
| M184V/I | 4 | 0.6 | 13 | 0.3 | 0.32 |
| T69D/N | 0 | 0 | 10 | 0.2 | 0.47 |
| K219R/Q | 2 | 0.3 | 9 | 0.2 | 0.92 |
| M41L | 2 | 0.3 | 8 | 0.2 | 0.82 |
| L210W | 0 | 0 | 6 | 0.1 | 1.00 |
| T215D/S | 3 | 0.4 | 6 | 0.1 | 0.17 |
| K70Q/R | 2 | 0.3 | 5 | 0.1 | 0.22 |
| K65R | 0 | 0 | 5 | 0.1 | 1.00 |
| D67N/G | 3 | 0.4 | 5 | 0.1 | 0.11 |
| K70R/E | 0 | 0 | 5 | 0.1 | 1.00 |
| V75M | 0 | 0 | 3 | 0.06 | 1.00 |
| Y115F | 0 | 0 | 1 | 0.02 | 1.00 |
| L74I | 0 | 0 | 1 | 0.02 | 1.00 |
| NNRTI | |||||
| K103N/S | 9 | 1.3 | 30 | 0.6 | 0.08 |
| V179F | 1 | 0.1 | 13 | 0.3 | 0.83 |
| K101E | 3 | 0.4 | 13 | 0.3 | 0.71 |
| G190A | 4 | 0.6 | 13 | 0.3 | 0.32 |
| V106M/A | 1 | 0.1 | 12 | 0.3 | 0.91 |
| Y181C | 1 | 0.1 | 7 | 0.1 | 1.00 |
| L100I | 0 | 0 | 1 | 0.02 | 1.00 |
| Y188H | 0 | 0 | 1 | 0.02 | 1.00 |
| P225H | 0 | 0 | 1 | 0.02 | 1.00 |
| M230L | 0 | 0 | 1 | 0.02 | 1.00 |
TDR in transmission routes, subtypes and age groups
The prevalence of TDR in heterosexuals, MSMs and IDUs was 4.1%, 3.1% and 1.7%, respectively. The prevalence of TDR of CRF01_AE, CRF07_BC, CRF08_BC and subtype B was 4.0%, 2.8%, 3.1% and 4.6%, respectively. There was no significant association between TDR and routes of transmission or subtypes in 2015, and there was no significant difference in TDR rates between the 16–25 year-old group and >25 year-old group (3.2% vs 3.6%, P = 0.48).
Comparison of TDR among different study periods
The rates of TDR in this study were compared with the results from another nationwide survey, carried out in 2004–2005 among ART naïve HIV-infected individuals. There was no significant difference in the rates of TDR among different routes of transmission or subtypes between the two study periods. However, the rate of TDR in the 16–25 year-old group was significantly higher in 2004–2005 (P = 0.01, Table 1). M184V was the most frequent NRTI-related mutation during 2004–2005, and the most frequent NNRTI-related mutation was K103N/S. PI-related mutations were rare and only found in 3 individuals. No significant difference was found in the prevalence of specific mutations between the two surveys (Table 2).
Phylogenetic analyses of the main subtypes
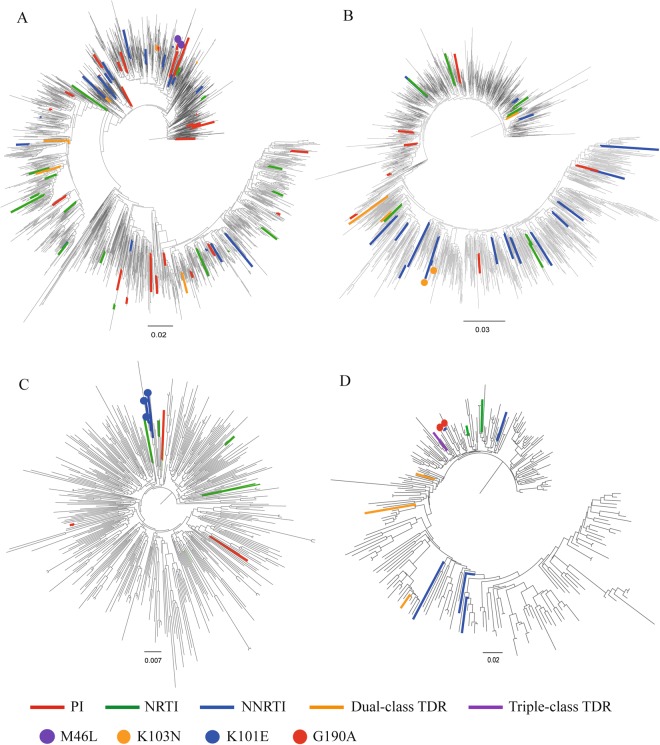
To explore the possible transmission relationship between individuals harboring drug-resistant strains, four phylogenetic trees were constructed based on sequences belonging to CRF01_AE (1835), CRF07_BC (1675), CRF08_BC (418), and subtype B (261), respectively (Fig. 2). Four drug-resistant transmission clusters including 9 sequences were identified in four phylogenetic trees. The DRMs in the clusters were M46L (cluster A), K103N (cluster B), K101E (cluster C), and G190A (cluster D). The presence of drug-resistant strains within drug-resistant transmission clusters accounted for 6.2% (9/145) of the total drug-resistant strains in this study.
Figure 2.
The maximum likelihood phylogenetic trees of 4198 main subtype sequences were constructed using GTR + Gamma substitution model in Fasttree. (A) phylogenetic tree of 1835 CRF 01_AE sequences. (B) phylogenetic tree of 1675 CRF 07_BC sequences. (C) phylogenetic tree of 418 CRF 08_BC sequences. (D) phylogenetic tree of 261 subtype B sequences. Nine sequences of the 4 drug-resistant transmission clusters are marked with different color dots.
Drug resistance-associated transmission network analysis
To explore the effect of TDR on viral transmission based on the transmission network, the 4704 sequences were divided into two groups: drug-resistant (167) and drug-sensitive (4537). The overall rate of clustering was 68.7%. The rate of clustering in drug-resistant strains was 60.5%, lower than in drug-sensitive strains (69.0%), P = 0.02. In addition, the degree was significantly lower in drug-resistant strains (P < 0.0003, Table 3).
Table 3.
Degree comparison for drug-resistant and drug-sensitive individuals in 2015.
| Drug resistance situation | Number | Inter-quartile range | Median | P Value |
|---|---|---|---|---|
| Total | 4704 | 7 | 2 | |
| Yes | 167 | 3 | 1 | |
| No | 4537 | 8 | 2 | <0.0003 |
Discussion
In this study, we investigated TDR among ART-naïve HIV-infected individuals in China who were newly diagnosed in 2015. The overall prevalence of TDR was 3.6%. Except a significant decreasing trend of TDR in the 16–25 year-old group, there was no significant difference in the rates of TDR in the whole study populations, or in any specific transmission routes and subtypes between the surveys conducted in 2004–2005 and 2015. In general, the prevalence of TDR remained low in China. This prevalence was comparable to other recent surveys in South America, South Africa and Asia, which showed 3.8% TDR in 2012 in Maranhão State, Northeast Brazil14, 4.7% in 2011 in rural KwaZulu-Natal, South Africa15, 4.8% during 1999 and 2012 in South Korea16, and 4.0% during 2008 and 2010 in Thailand17. In contrast, the prevalence of TDR in Europe and North America was much higher. A study carried out among HIV-infected individuals from 26 countries in Europe who were newly diagnosed between 2008 and 2010 revealed that the overall prevalence of TDR was 8.3%18. A sensitive sentinel mutation screening revealed that the rate of TDR in the United States was 13.6% during 2009 and 201119. Even higher rate of TDR was 22.5%, found in Metropolitan Washington, DC during 1994 and 201320. It is not surprising those countries like China has a lower rate of TDR, as the duration of access to ART was much shorter. In addition, HAART was applied from the beginning of the NFATP in China, as opposed to the situation in Europe and North America which experienced an extended period of much less effective regimens21. In 1987, zidovudine (AZT) was approved by US FDA (Food and Drug Administration) for use in patients with advanced HIV. Combination NRTI therapy was proven more efficacious in 1995. Triple drugs regimens were suggested to use during the Vancouver AIDS Conference in 1996 by IAS-USA (International Antiviral Society–USA)21.
The main transmitted DRMs were different between China and countries in Europe and North America. The most frequent NRTI-associated DRM found in our study was M184V, whereas were T215rev (revertant mutation) and M41L in Europe and North America18–20,22–28. The most frequent PI- associated mutation in our study was M46I/L, which is related to the high prevalence of CRF01_AE in China29. The most frequent PI-associated mutations were L90M and M46I/L in Europe and North America18–20,22–28. Consistent with that of Europe and North America, the most frequent NNRTI-associated DRM in China was K103N/S. The differences of the main types of DRM can be explained by the different ART regimens used and subtype distribution between regions. Firstly, zidovudine (AZT), lamivudine (3TC), tenofovir (TDF), abacavir (ABC), efavirenz (EFV), nevirapine (NVP), ritonavir-boosted lopinavir (LPV/r) were provided through the NFATP in China. Not all PIs are available and none of the integrase strand transfer inhibitors (INSTIs) are provided in China compared to Europe and North America. Secondly, the major subtypes in China are CRF01_AE and CRF07_BC, whereas it is subtype B in Europe and North America.
Four clusters containing HIV strains sharing the same DRM were found in the present study. The presence of drug-resistant strains within transmission clusters accounted for only 6.2% (9/145). This revealed that the prevalence of TDR was not concentrated in study populations. This finding is consistent with the low prevalence of TDR in China.
The degree and rate of clustering in transmission networks were significantly lower in drug-resistant strains. This may be explained by the lower replication capacity of the resistant virus. The resistant virus was transmitted only approximately 20% as frequently as expected according to a previous study30.
As with other observational studies, our study has limitations. Firstly, a proportion of the studied individuals might not be recently infected, and the drug-resistant strains in plasma become minor quasi-species after a period of infection. The Sanger sequencing method may underestimate the prevalence of TDR. Secondly, there would be a biased sampling since only 10% of individuals diagnosed in the second quarter of 2015 were randomly enrolled in this study.
In conclusion, the overall prevalence of TDR among recently diagnosed individuals in China remained at a low level in the recent 10 years. The prevalence of TDR was not concentrated in 2015. We suggest that effective measures are still needed to strengthen monitoring and guide ART usage.
Methods
Study population
We conducted a cross-sectional survey in 31 provinces, autonomous regions and municipalities of mainland China. Inclusion criteria for the subjects (1) were over 16 years old, (2) were permanent residents, (3) were diagnosed as HIV seropositive from April 2015 to June 2015, and (4) were ART naïve. Individuals who met the criteria were stratified by random sampling. The sampling ratio of each province was determined by the average number of HIV-infected individuals reported in 2011–2013. For provinces with fewer reported cases, higher sampling ratios were used to assure statistical confidence. The sampling ratios for provinces with >2000, 1200–2000, 800–1200, 250–800, and <250 cases were 5%, 10%, 12.5%, 15% and 20%, respectively. All patients provided written informed consent for participation in this study.
To explore the changes of TDR in the recent 10-year period in China, the data of a cross-sectional survey conducted in 28 provinces from September 2004 to October 2005 was introduced into the analysis. The data contained sequences and basic information of 676 ART-naïve individuals12.
Identification of transmitted drug resistance
Whole blood samples were collected at local Centers for Disease Control and Prevention (CDCs). For plasma samples, which were separated by centrifugation, HIV drug resistant test was carried out. The HIV pol region (protease 1–99 amino acids and reverse transcriptase 1–250 amino acids) was amplified by an in-house polymerase chain reaction protocol31. The nucleotide sequences for the pol region were assembled and edited in Sequencher version 4.10 and BioEdit version 7.0.9.1. Transmitted drug resistant mutations were identified using CPR 6.0 in the Stanford HIV Drug Resistance Database (https://hivdb.stanford.edu/).
HIV subtyping
For HIV subtyping, the edited sequences were aligned with reference sequences on HIV databases (http://www.hiv.lanl.gov/content/index). Phylogenetic trees were constructed using a neighbor-joining method with 1000 bootstrap replicates with MEGA6.0. The HIV subtypes and circulating recombinant forms (CRFs) were identified with a bootstrap equal to or above 70%. The results were checked using HIV Blast on HIV databases.
Phylogenetic analyses
To avoid the effect of convergent evolution, 43 codons for drug resistance surveillance were removed from all of the aligned sequences. The total length of the sequences was 910 bp. Four phylogenetic trees were respectively constructed based on the main circulating recombinant forms (CRFs) and subtypes predominating in China: CRF01_AE, CRF07_BC, CRF08_BC, and subtype B. Phylogenetic relationships were estimated using a maximum likelihood approach with a General Time Reversible + Gamma (GTR + η) model and 1000 replicates bootstrap in Fasttree. The criteria for identifying drug-resistant transmission clusters: (1) with a bootstrap above 90% and (2) at least two individuals in the same cluster carrying the same DRMs. These trees were edited and visualized with FigTree 1.4.3 and Adobe Illustrator CC 2014.
Transmission network analysis
For constructing the transmission network, genetic distances were first calculated using the Tamura-Nei 93 nucleotide substitution model (TN93) with HYPHY version 2.2.4. A putative transmission linkage was inferred if the genetic distance between two sequences was below 1.5%. This cutoff threshold was based on an evolutionary rate of 0.7%/year for HIV-1 pol within individuals32–34. The transmission network was then constructed and analyzed with Cytoscape version 3.2.0. The rate of clustering was defined as the percentage of individuals segregated into the network. The degree of each individual in the network was defined as the number of links with other individuals.
Statistical analyses
Categorical variables were compared using the χ2 test, Fisher exact tests, or logistic regression analysis. The statistical significance was defined as P < 0.05. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Ethics statement
Institutional review board (IRB) approval was granted by National Center for AIDS/STD Control and Prevention (NCAIDS), Chinese Center for Disease Control and Prevention (China CDC). All experimental protocols were approved by IRB at NCAIDS, China CDC, according to the international and Chinese ethical guidelines, and the methods were carried out in accordance with the approved guidelines. All patients were willing to provide informed consent for this research.
Acknowledgements
The authors would like to thanks the group for HIV molecular epidemiologic survey. Contributing members of the Group for HIV Molecular Epidemiologic Survey [name of contributor (facility of the contributor)]: Jianjun Wu (Anhui CDC); Hongyan Lu, Ruolei Xin (Beijing CDC); Guohui Wu (Chongqing CDC); Shouli Wu (Fujian CDC); Ailing Yu (Gansu CDC); Jin Yan (Guangdong CDC); Shujia Liang (Guangxi CDC); Xianguang Sun (Guizhou CDC); Jia Liu (Henan CDC); Tinghai Peng (Hubei CDC); Yiguang Bai (Hebei CDC); Peng Fu (Hainan CDC); Jie Zhang (Heilongjiang CDC); Xiaobai Zou (Hunan CDC); Haitao Yang, Xihui Zang (Jilin CDC); Xiaoqin Xu (Jiangsu CDC); Xu Kang (Liaoning CDC); Liping Liu (Jiangxi CDC); Yumei Wu (Neimenggu CDC); Lihua Zhao (Ningxia CDC); Shaohui Ma (Qinghai CDC); Ling Su (Sichuan CDC); Xiaoguang Sun (Shandong CDC); Hua Chen (Shanghai CDC); Wenhui Chang, Hua Li (Shaanxi CDC); Zidong Xve (Shanxi CDC); Minna Zheng (Tianjin CDC); Fengying Wang, Wen Yang (Xinjiang); Kagang Kari (Xizang CDC); Zhaohui Yang (Yunnan CDC); Jiafeng Zhang (Zhejiang CDC). Thanks to Dr. Edward C. Mignot, Shandong University, for linguistic advice. This study was supported by grants from the Guangxi Bagui Honor Scholars, Ministry of Science and Technology of China (2017ZX10201101), the National Natural Science Foundation (81471962) and 2016 annual major projects of Beijing Municipal Science & Technology Commission (D161100000416002). The fund providers had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
F.Y., Y.R., L.L., Y.S. and H.X. were responsible for study design and planning. Z.S., Y.R., L.L., Y.F. and H.X. contributed to writing the report. Z.S., Y.R., L.L., Y.F. and H.X. contributed to data analysis. Z.S., J.H., Y.L., Z.Z., J.Y., J.Z., W.X., F.L. and X.L. contributed to the experiments and data cleaning. The group for HIV molecular epidemiologic survey contributed to data collection. All authors read and approved the final version of the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuai Zhao and Yi Feng contributed equally to this work.
Contributor Information
Yiming Shao, Email: yshao08@gmail.com.
Hui Xing, Email: xingh@chinaaids.cn.
References
- 1.NCAIDS N, a. C. C. Update on the AIDS/STD epidemic in China and main response in control and prevention in December, 2015. Chin J AIDS STD 21:87.
- 2.May MT, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet (London, England) 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 3.Lessells RJ, Mutevedzi PC, Iwuji CC, Newell ML. Reduction in early mortality on antiretroviral therapy for adults in rural South Africa since change in CD4+ cell count eligibility criteria. Journal of acquired immune deficiency syndromes. 2014;65:e17–24. doi: 10.1097/QAI.0b013e31829ceb14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewden C, et al. Disease patterns and causes of death of hospitalized HIV-positive adults in West Africa: a multicountry survey in the antiretroviral treatment era. Journal of the International AIDS Society. 2014;17:18797. doi: 10.7448/IAS.17.1.18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hingankar NK, et al. Initial virologic response and HIV drug resistance among HIV-infected individuals initiating first-line antiretroviral therapy at 2 clinics in Chennai and Mumbai, India. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54(Suppl 4):S348–354. doi: 10.1093/cid/cis005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PloS one. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittkop L, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. The Lancet. Infectious diseases. 2011;11:363–371. doi: 10.1016/S1473-3099(11)70032-9. [DOI] [PubMed] [Google Scholar]
- 8.Bertagnolio S, Perno CF, Vella S, Pillay D. The Impact of HIV Drug Resistance on the Selection of First- and Second-Line ART in Resource-Limited Settings. The Journal of infectious diseases. 2013;207(Suppl 2):S45–48. doi: 10.1093/infdis/jit121. [DOI] [PubMed] [Google Scholar]
- 9.Phanuphak P, et al. Transmitted drug resistance and antiretroviral treatment outcomes in non-subtype B HIV-1-infected patients in South East Asia. Journal of acquired immune deficiency syndromes. 2014;66:74–79. doi: 10.1097/QAI.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee SY, et al. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS medicine. 2015;12:e1001810. doi: 10.1371/journal.pmed.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta RK, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet (London, England) 2012;380:1250–1258. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao L, et al. The prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in China. Journal of acquired immune deficiency syndromes. 2010;53(Suppl 1):S10–14. doi: 10.1097/QAI.0b013e3181c7d363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell research. 2005;15:877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 14.Moura ME, et al. Low rate of transmitted drug resistance may indicate low access to antiretroviral treatment in Maranhao State, northeast Brazil. AIDS research and human retroviruses. 2015;31:250–254. doi: 10.1089/aid.2014.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manasa J, et al. Increasing HIV-1 Drug Resistance Between 2010 and 2012 in Adults Participating in Population-Based HIV Surveillance in Rural KwaZulu-Natal, South Africa. AIDS research and human retroviruses. 2016;32:763–769. doi: 10.1089/aid.2015.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park M, et al. The trend of transmitted drug resistance in newly diagnosed antiretroviral-naive HIV/AIDS patients during 1999–2012 in South Korea. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2016;81:53–57. doi: 10.1016/j.jcv.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Sirivichayakul S, et al. Transmitted HIV drug resistance at the Thai Red Cross anonymous clinic in Bangkok: results from three consecutive years of annual surveillance. The Journal of antimicrobial chemotherapy. 2015;70:1146–1149. doi: 10.1093/jac/dku499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofstra LM, et al. Transmission of HIV Drug Resistance and the Predicted Effect on Current First-line Regimens in Europe. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;62:655–663. doi: 10.1093/cid/civ963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JF, et al. Sensitive sentinel mutation screening reveals differential underestimation of transmitted HIV drug resistance among demographic groups. Aids. 2016;30:1439–1445. doi: 10.1097/QAD.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 20.Kassaye SG, et al. Transmitted HIV Drug Resistance Is High and Longstanding in Metropolitan Washington, DC. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;63:836–843. doi: 10.1093/cid/ciw382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vella S, Schwartlander B, Sow SP, Eholie SP, Murphy RL. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. Aids. 2012;26:1231–1241. doi: 10.1097/QAD.0b013e32835521a3. [DOI] [PubMed] [Google Scholar]
- 22.Tostevin, A. et al. Recent trends and patterns in HIV-1 transmitted drug resistance in the United Kingdom. HIV medicine, 10.1111/hiv.12414 (2016). [DOI] [PMC free article] [PubMed]
- 23.Vega Y, et al. Epidemiological Surveillance of HIV-1 Transmitted Drug Resistance in Spain in 2004–2012: Relevance of Transmission Clusters in the Propagation of Resistance Mutations. PloS one. 2015;10:e0125699. doi: 10.1371/journal.pone.0125699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yebra G, et al. Different trends of transmitted HIV-1 drug resistance in Madrid, Spain, among risk groups in the last decade. Archives of virology. 2014;159:1079–1087. doi: 10.1007/s00705-013-1933-y. [DOI] [PubMed] [Google Scholar]
- 25.Descamps D, et al. National sentinel surveillance of transmitted drug resistance in antiretroviral-naive chronically HIV-infected patients in France over a decade: 2001–2011. The Journal of antimicrobial chemotherapy. 2013;68:2626–2631. doi: 10.1093/jac/dkt238. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt D, et al. Estimating trends in the proportion of transmitted and acquired HIV drug resistance in a long term observational cohort in Germany. PloS one. 2014;9:e104474. doi: 10.1371/journal.pone.0104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panichsillapakit T, et al. Prevalence of Transmitted HIV Drug Resistance Among Recently Infected Persons in San Diego, CA 1996–2013. Journal of acquired immune deficiency syndromes. 2016;71:228–236. doi: 10.1097/QAI.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burchell AN, et al. Increase in transmitted HIV drug resistance among persons undergoing genotypic resistance testing in Ontario, Canada, 2002–09. The Journal of antimicrobial chemotherapy. 2012;67:2755–2765. doi: 10.1093/jac/dks287. [DOI] [PubMed] [Google Scholar]
- 29.Hauser, A. et al. National molecular surveillance of recently acquired HIV infections in Germany, 2013 to 2014. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin22, 10.2807/1560-7917.es.2017.22.2.30436 (2017). [DOI] [PMC free article] [PubMed]
- 30.Leigh Brown AJ, et al. Transmission fitness of drug-resistant human immunodeficiency virus and the prevalence of resistance in the antiretroviral-treated population. The Journal of infectious diseases. 2003;187:683–686. doi: 10.1086/367989. [DOI] [PubMed] [Google Scholar]
- 31.Zhong P, et al. Genetic diversity and drug resistance of human immunodeficiency virus type 1 (HIV-1) strains circulating in Shanghai. AIDS research and human retroviruses. 2007;23:847–856. doi: 10.1089/aid.2006.0196. [DOI] [PubMed] [Google Scholar]
- 32.Wertheim JO, et al. The global transmission network of HIV-1. The Journal of infectious diseases. 2014;209:304–313. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldous JL, et al. Characterizing HIV transmission networks across the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55:1135–1143. doi: 10.1093/cid/cis612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hightower GK, et al. HIV-1 clade B pol evolution following primary infection. PloS one. 2013;8:e68188. doi: 10.1371/journal.pone.0068188. [DOI] [PMC free article] [PubMed] [Google Scholar]