Abstract
Pharmacological intervention using statins and PCSK9 inhibitors have become first-line therapy in the prevention of hypercholesterolemia and atherosclerosis. Currently, no agent is available for the primary prevention of atherosclerosis. However, there is an emerging hypothesis that atherosclerosis could be driven by inflammation. In this study, we tested whether pretreatment with an aqueous extract from sesame oil (SOAE), which showed potent anti-inflammatory properties without hypocholesterolemic actions, would prevent subsequent atherosclerosis development in a mouse model. RAW 264.7 macrophages and female low-density lipoprotein receptor knockout (LDLR−/−) mice were used for in vitro and in vivo studies, respectively. Plasma lipids, cytokines and atherosclerotic lesions were quantified at the end of the study. RNA was extracted from the liver and aortic tissues and used for gene analysis. Pre-treatment of SOAE prevented Ox-LDL uptake by RAW macrophages and further inflammation in vitro. SOAE pre-treatment significantly reduced atherosclerotic lesions and pro-inflammatory gene expressions in LDLR−/− mice as compared to control mice. No significant change in plasma cholesterol levels was observed. A significant reduction in plasma levels of TNF-α, IL-6, MCP-1 and VCAM1 was observed in the SOAE pre-treated animals. This is the first study that demonstrates that pre-treatment with anti-inflammatory agents, could delay/decrease atherosclerosis.
Introduction
Dyslipidemia and inflammation are associated with atherosclerosis1–4. Existing therapeutic approaches for the prevention and treatment of atherosclerosis heavily involve statins or PCSK9 antibodies to reduce hypercholesterolemia5,6. Very few, if any, anti-inflammatory agents have been tested for their effectiveness against atherosclerosis.
A cardioprotective diet and exercise have been considered important factors in the prevention and treatment of atherosclerosis. Cooking oil has a great influence on plasma lipid levels as well as on the progression of atherosclerosis7–10. Sesame oil (SO) is rich in both polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA), and has been shown to reduce high blood pressure and lower the amount of medication required to control hypertension11,12 in humans.
Others13,14 as well as our studies have demonstrated the ability of SO in reducing plasma lipid levels15,16 in vivo. In addition, our studies have also revealed that a diet rich in SO prevents inflammation and inhibits atherosclerotic lesion formation (by approximately 85%) in low-density lipoprotein-receptor-deficient (LDL-R−/−) male mice15,16. This finding led us to evaluate whether non-saponifiable components of edible oil that have been overlooked could have properties that would act in synergy with fatty acid components to not only inhibit atherosclerotic properties, but also promote regression. Subsequently, we demonstrated that an aqueous extract (SOAE) from SO was effective in inhibiting inflammation, both in vitro and in vivo17,18.
Our studies of SO/SOAE diet-fed LDL-R−/− mice showed significantly reduced atherosclerotic lesions as compared to high-fat diet-fed control animals15,16,18. The beneficial properties of SO/SOAE prompted us to test whether pre-treatment of SO/SOAE would have anti-inflammatory properties and as a result, decrease subsequent development of atherosclerosis. In addition, we also evaluated whether SO/SOAE pre-treatment would have an effect on lipid metabolism, reverse cholesterol transport (RCT), and scavenger receptors, all of which play a major role in the progression of atherosclerosis.
Results
In vitro studies
Pre-treatment of SOAE reduces Ox-LDL and Ac-LDL uptake by RAW 264.7 cells
As shown in Supplementary Fig. 1, oxidized-low-density lipoprotein/acetylated-low-density lipoprotein (Ox-LDL/Ac-LDL) uptake by macrophages was reduced with increasing concentration of SOAE pre-treatment for 2 hrs as compared to untreated control cells.
Pre-treatment of SOAE inhibits Ox-LDL-induced inflammation in RAW 264.7 macrophages
Macrophages were treated with Ox-LDL (25 µg/mL) for 24 h following 2 h pre-treatment by SOAE (50 and 250 µg/mL). RNA was isolated and gene expressions of tumor necrotic factor alpha (TNFα), Interleukin (IL)-1α, IL-1β, IL-6 and monocyte chemoattractant protein 1 (MCP-1) were analyzed. The cells responded well to the positive stimulus of 25 µg/mL of Ox-LDL as seen by significant induction of inflammatory gene expressions. Pre-treatment of SOAE even for 2 hrs significantly inhibited the inflammatory gene expressions in a dose-dependent manner (Supplementary Fig. 2A), whereas a slight but insignificant increase was observed in TNFα at higher concentrations (data not shown). In addition, no significant difference was observed either with lipid loading genes or cholesterol transport genes (data not shown). SOAE treatment without Ox-LDL did not induce any of the inflammatory cytokines. Polymerase chain reaction (PCR) products also corroborated with the gene expression studies (Supplementary Fig. 2B).
In vivo studies
Body weight analysis in mice
No significant changes in body/liver weight were observed in the SO/SOAE diet-fed animals, either at one month or following the two additional months of high-fat diet feeding (Supplementary Fig. 3A,B).
No significant changes in plasma lipid levels in SO/SOAE diet pre-treated animals
Plasma lipid profile analysis revealed no significant decrease in triglyceride (TRG), total cholesterol (TC), LDL-cholesterol, or very low-density lipoprotein (VLDL)-cholesterol in SO/SOAE diet pre-treated animals either prior to, or after, high-fat diet feeding. However, a slight but insignificant increase in high-density lipoprotein (HDL) cholesterol levels was observed after one month of SO/SOAE feeding in these animals compared to controls. Table 1 represents the plasma lipid profile of control and SO/SOAE diet-fed animals. In control animals, the cholesterol level was 1438.13 ± 132.37 mg/dL, whereas in the animals pre-treated with a SO/SOAE diet, the cholesterol levels were 1343.73 ± 79.8 mg/dL and 1466 ± 94 mg/dL, respectively. No significant change in triglyceride levels was observed. However, there was an increase in HDL levels after one month of feeding of both SO/SOAE and a reduction in TRG levels in SOAE-treated animals.
Table 1.
Plasma lipid levels in sesame oil diet-fed animals: Plasma lipid levels in mice fed one-month of SO/SOAE diet, followed by two-months of an atherogenic diet.
| Plasma lipids | One month treatment | One month followed by 2 month HF diet | ||||
|---|---|---|---|---|---|---|
| mg/dL | Control | SO | SOAE | Control to HF | SO to HF | SOAE to HF |
| TRG | 97.8 ± 20.9 | 107.7 ± 22.2 | 86.8 ± 9.7 | 247 ± 39.7* | 249 ± 39.79* | 266.5 ± 26.71* |
| TC | 145.3 ± 15.3 | 166 ± 19.9 | 171.8 ± 12.8 | 1438 ± 132.4* | 1343 ± 79.8* | 1466.3 ± 94.8* |
| LDLc | 87.3 ± 8 | 98.4 ± 10.37 | 102.85 ± 8.72 | 1310 0.16 ± 128* | 1219.16 ± 75.59* | 1371.83 ± 94.31* |
| HDLc | 38 ± 4 | 50.2 ± 7.29 | 51.71 ± 4.91 | 72.5 ± 8.19 | 73.5 ± 6.9 | 53 ± 3.68 |
| VLDLc | 19.56 ± 4.2 | 21.54 ± 4.44 | 17.37 ± 1.93 | 49.5 ± 7.94* | 49.96 ± 7.96* | 53.3 ± 5.34* |
| TC/HDL | 3.85 ± 0.15 | 3.37 ± 0.14 | 3.38 ± 0.12 | 21.11 ± 2.98 | 19.16 ± 2.21 | 42.77 ± 7.2 |
Values are represented as mean ± SD. *P < 0.05.
However, despite no changes in blood lipids, visual observation of plasma showed a clearer plasma in SO/SOAE diet pre-treated animals as compared to control animals (Supplementary Fig. 4). In lieu of the chemical lipid data, this probably reflected the need, perhaps an association of components of SO/SOAE with the lipoproteins for its actions in vivo.
Inhibition of atherosclerotic lesion formation in SO/SOAE pre-treated animals
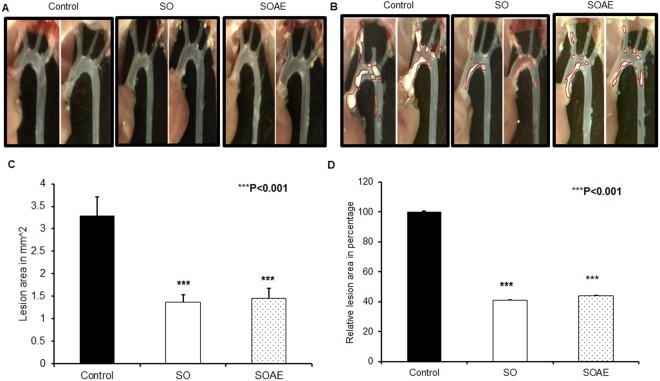
Atherosclerotic lesion quantification revealed a significant reduction in lesion area of SO/SOAE pre-treated animals as compared to controls. As represented in Fig. 1A, no lesions were observed in any group at the end of one month of feeding, whereas at the end of two months of feeding, control animals had significant lesion levels in the aortic arch (Fig. 1B) compared to SO/SOAE animals. Quantification analysis also showed a significant (***P < 0.001) reduction in lesion size/area of approximately 55–60% in SO/SOAE animals as compared to controls (Fig. 1C,D: mean ± SD (mm2), ***p < 0.001).
Figure 1.
Inhibition of atherosclerosis in SO/SOAE pre-treated animals. Representative images of atherosclerotic lesions in LDLR−/− mice in (A) one-month SO/SOAE pre-treated animals; (B) one-month SO/SOAE pre-treated followed by two-months high-fat diet animals. (C) Average lesion area of all animals from each group; (D) Relative (control set to 100) lesion area of animal groups. The values are expressed as mean ± SD (mm2). ***p < 0.0001.
Gene analysis of mouse livers
To evaluate the effect of pre-treatment by SO/SOAE, several pro-inflammatory genes (IL-1α, IL-1β, IL-6, MCP-1, myeloperoxidase (MPO) and TNF-α), anti-inflammatory genes (IL-4 and IL-10), lipid-loading genes [cluster differentiation (CD)36 and scavenger receptor (SR) A1], antioxidant genes [(catalase and manganese superoxide dismutase (MnSOD)], genes involved in RCT and lipid metabolism [ATP-binding cassette transporter (ABCA)1, ABCG1, SRB1, Niemann-Pick C1-like protein 1 (NPC1L1), Cytochrome P450 Family 7 subfamily A Member 1 (Cyp7A1)], nuclear receptor transcription factors [liver × receptor (LXR) and farnesoid × receptor (FXR)], genes associated with HDL [apolipoprotein A1 (APOA1) and paraoxonase 1 (PON1)], and a matrix metalloproteinase gene (MMP9) were analyzed by real-time PCR analysis (Table 2). The pattern of regulation of pro- and anti-inflammatory genes was consistent with cytokine array analysis. No significant difference was observed in genes involved in RCT and lipid metabolism. Similarly, no difference between pregnane × receptor (PXR) and Peroxisome proliferator-activated receptor-alpha (PPARα) expression was identified. In addition, we also evaluated the Flavin monooxygenase (FMO) 1–5 gene expressions, which play a major role in the formation of trimethylamine N-oxide (TMAO) and disease progression in these animals19. Surprisingly, there was a slight but insignificant reduction of these genes in SO/SOAE pre-treated animals.
Table 2.
Gene analysis from mice liver.
| Control | SO | SOAE | p value | ||
|---|---|---|---|---|---|
| Control Vs SO | Control Vs SOAE | ||||
| Pro-inflammatory genes | |||||
| IL-1 alpha | 1 | 0.681963 | 0.504738 | 0.140 | 0.820 |
| IL-1beta | 1 | 0.940317 | 0.451933 | 0.620 | 0.980 |
| IL-6 | 1 | 0.785231 | 0.32779 | 0.490 | 0.325 |
| MCP-1 | 1 | 2.659313 | 1.504779 | 0.152 | 0.170 |
| MPO | 1 | 1.26665 | 1.658319 | 0.680 | 0.650 |
| TNF-alpha | 1 | 0.735319 | 0.626319 | 0.330 | 0.060 |
| Anti-inflammatory genes | |||||
| IL-4 | 1 | 3.321804 | 0.6711 | 0.430 | 0.930 |
| IL-10 | 1 | 2.970702 | 1.393336 | 0.230 | 0.640 |
| Scavenger receptors | |||||
| CD36 | 1 | 0.650874 | 0.426833 | 0.740 | 0.360 |
| SRA1 | 1 | 0.886013 | 0.594106 | 0.580 | 0.510 |
| Antioxidant genes | |||||
| Catalase | 1 | 1.413807 | 1.185208 | 0.160 | 0.820 |
| Mn-SOD | 1 | 0.695595 | 1.301406 | 0.01* | 0.132 |
| RCT genes | |||||
| APOA1 | 1 | 1.885119 | 1.043672 | 0.550 | 0.450 |
| PON1 | 1 | 1.943017 | 1.249621 | 0.250 | 0.180 |
| ABCA1 | 1 | 3.371703 | 0.175983 | 0.900 | 0.836 |
| ABCG1 | 1 | 1.184474 | 0.949765 | 0.930 | 0.790 |
| SRB1 | 1 | 0.771615 | 0.899021 | 0.228 | 0.025 |
| NPC1L1 | 1 | 0.240529 | 0.069702 | 0.450 | 0.330 |
| Transcription factors | |||||
| LxR | 1 | 1.192538 | 0.625569 | 0.850 | 0.01* |
| FxR | 1 | 0.986115 | 0.423358 | 0.920 | 0.280 |
| PxR | 1 | 1.051423 | 0.774968 | 0.440 | 0.500 |
| Matrix metalloprotease | |||||
| MMP-9 | 1 | 0.488572 | 0.361897 | 0.290 | 0.527 |
| Flavin monooxygenases | |||||
| FMO1 | 1 | 0.841205 | 0.72209 | 0.820 | 0.420 |
| FMO2 | 1 | 1.180525 | 0.691214 | 0.900 | 0.140 |
| FMO3 | 1 | 1.210627 | 1.086112 | 0.560 | 0.180 |
| FMO4 | 1 | 0.598995 | 1.030688 | 0.070 | 0.850 |
| FMO5 | 1 | 0.886148 | 1.243417 | 0.070 | 0.125 |
*P < 0.05.
Gene expression in mouse aorta
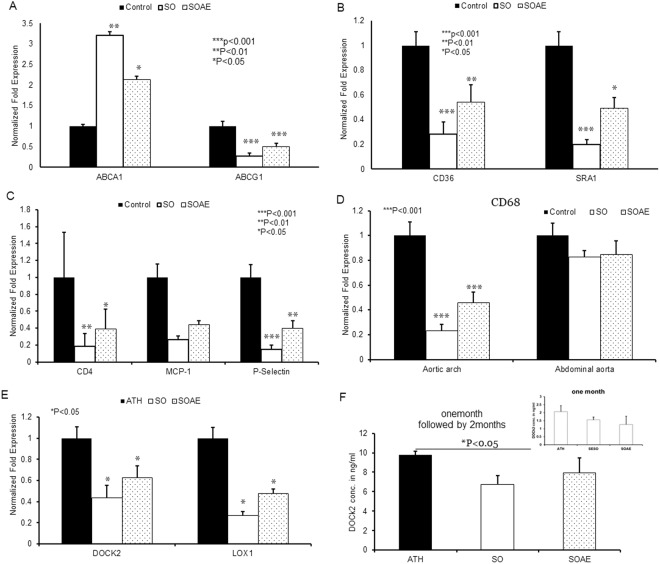
Aortic gene expressions were also analyzed using real-time PCR. No difference was observed in one-month SO/SOAE pre-treated animals as compared to control animals. However, following the two-month high-fat feeding, the results showed a significant increase in RCT ABCA1 gene expression and significantly reduced levels of ABCG1 expression in SO/SOAE pre-treated animals (Fig. 2A). Surprisingly, one-month pre-treatment with SO/SOAE significantly reduced the expression of monocyte/macrophage markers and scavenger receptors SRA1 and CD36 (Fig. 2B). In addition, a significant reduction was observed in inflammatory markers such as MCP-1, CD4 and P-selectin (Fig. 2C) in experimental animals as compared to control animals. A significant difference in the level of CD68 between control and experimental animals in the aortic arch segments was observed, whereas no such difference was observed in lesion-free abdominal aorta segments (Fig. 2D). SO/SOAE pre-treatment decreased CD68 levels in the aortic arch by 80% and 60% (p < 0.05), respectively. Dedicator of cytokinesis 2 (DOCK2) and lectin-like oxidized low-density lipoprotein (LOX1) also significantly reduced in SO/SOAE pre-treated animals (Fig. 2E). Protein levels by ELISA for DOCK2 also corroborated the gene expression studies (Fig. 2F). As shown in Fig. 2F, a significant reduction in plasma DOCK2 levels was observed in SO pre-treated animals, whereas a non-significant reduction was observed in SOAE pre-treated animals.
Figure 2.
Gene analysis from mouse aorta. mRNA level of several genes were analyzed in aortic tissue of LDLR−/− mice after one month of feeding with SO/SOAE diet, followed by 2 months of feeding with high-fat diet. Bar graph represents (A) RCT genes, (B) scavenger receptors, and (C) inflammatory genes analyzed from the aorta. (D) Relative level of CD68 mRNA in aortic arch and abdominal aorta (n = 8 from each group). (E) DOCK2 and LOX1 (F) DOCK2 ELISA. Values are represented as mean ± SD. *p < 0.05.
Gene expression in mouse peritoneal macrophages
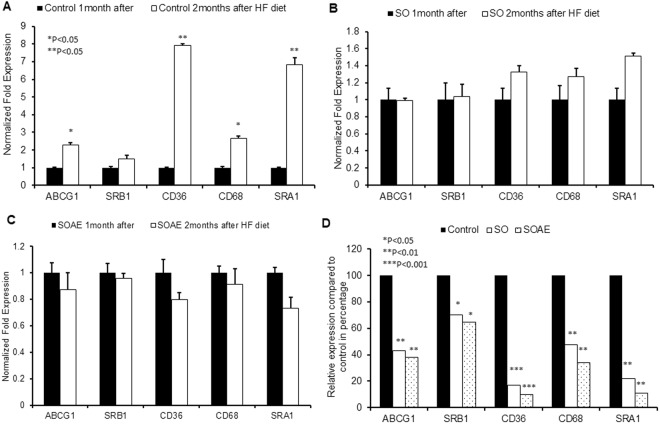
Peritoneal macrophage gene expressions were also analyzed in addition to the liver and aortic gene expressions, using real-time PCR. The results showed that SO/SOAE pre-treated animals had reduced levels of cholesterol transport gene expressions (ABCG1, SRB1, SRA1 and CD36) compared to control animals (Fig. 3A–C). A significant reduction (40–60%) in cholesterol uptake gene expression was observed in SO/SOAE animals, suggesting even the short-term pre-treatment of SO/SOAE may play a beneficial role in maintaining cholesterol homeostasis. Additionally, there was a significant increase in all cholesterol transport gene expressions at the end of the one-month treatment, suggesting accelerated lipid metabolism in the presence of SO/SOAE.
Figure 3.
Gene analysis from mice peritoneal macrophages. mRNA level of several genes were analyzed in peritoneal macrophages of LDLR−/− mice one month after SO/SOAE diet and followed by 2 months of feeding with high-fat diet. Bar diagrams represent RCT genes and Scavenger receptors (A) Control one month diet and followed by 2months HF diet (B) SO one month diet and followed by 2months HF diet (C) SOAE one month diet followed by 2months HF diet (D) Relative increase/decrease with respect to control in SO and SOAE pre-treated animals were analyzed from peritoneal macrophages (n = 8 from each group). Values are represented as mean ± SD. *P < 0.05.
Cytokine array
Cytokine array revealed that SO/SOAE pre-treatment induced minimal to significant changes, depending on the protein. Upregulation of growth-promoting cytokines was observed in SO/SOAE pre-treated animals as compared to controls, whereas several pro-inflammatory cytokines such as granulocyte-macrophage colony-stimulating factor (GMCSF), IL-6, MCP-1, TNF-α, TNFRI, TNFRII, and vascular cell adhesion molecule 1 (VCAM1) were significantly downregulated (Table 3). Anti-inflammatory cytokines, such as IL4 and IL-10, were upregulated immediately following the one-month SO/SOAE pre-treatment (data not shown).
Table 3.
Cytokine array: Comparison of changes in the level of inflammatory mediators in mouse plasma between pre-treated SO/SOAE animals and controls.
| Protein name | Protein symbol | Relative protein levels compared to ATH, fold difference | P value C vs SO | P value C vs SOAE | ||
|---|---|---|---|---|---|---|
| Control | SO | SOAE | ||||
| Tyrosine-protein kinase receptor | axl | 1 | 0.4149 | 0.4267 | 0.0124* | 0.0262* |
| B cell Attracting Chemokine-1 | BLC | 1 | 0.4791 | 0.4336 | 0.0019* | 0.0610 |
| CD30 ligand | CD30L | 1 | 0.5604 | 0.6153 | 0.0453* | 0.0052* |
| Cluster of differentiation 30 | CD30/TNFRSF8 | 1 | 0.5650 | 0.7813 | 0.9839 | 0.1208 |
| Cluster of differentiation 40 | CD40 | 1 | 0.6379 | 0.8003 | 0.5543 | 0.1949 |
| Cytokine responsive gene 2 | CRG-2 | 1 | 0.8479 | 0.7188 | 0.3718 | 0.6064 |
| Cuteaneous T-Cell Attracting Chemokine | CTACK | 1 | 0.8372 | 0.7293 | 0.2280 | 0.5573 |
| Chemokine 16 | CXCL16 | 1 | 0.3503 | 0.4235 | 0.4722 | 0.3235 |
| E-Selectin | E-Selectin | 1 | 0.9837 | 0.9848 | 0.8765 | 0.8410 |
| Low affinity immunoglobulin gamma Fc region receptor II-b | Fcg RIIB | 1 | 0.5797 | 0.8134 | 0.0741 | 0.3747 |
| Fractalkine | Fractalkine | 1 | 0.4488 | 0.5791 | 0.7109 | 0.7708 |
| Granulocyte colony stimulating factor | G-CSF | 1 | 0.9592 | 0.8227 | 0.8315 | 0.8108 |
| Granulocyte macrophage colony stimulating factor | GM-CSF | 1 | 0.6072 | 0.7116 | 0.0199* | 0.0226* |
| Hepatocyte growth factor receptor | HGF R | 1 | 0.8084 | 0.9227 | 0.5090 | 0.7133 |
| Intracellular adhesion molecule 1 | ICAM-1 | 1 | 0.7731 | 0.7365 | 0.2928 | 0.1603 |
| Interferon gamma | IFN-gamma | 1 | 0.4943 | 0.6302 | 0.0201 | 0.1071 |
| Insulin-growth factor binding protein 2 | IGFBP-2 | 1 | 0.9519 | 0.9933 | 0.7459 | 0.9591 |
| Insulin-growth factor binding protein 3 | IGF-BP-3 | 1 | 0.6091 | 0.6441 | 0.3863 | 0.2837 |
| Insulin-growth factor binding protein 5 | IGF-BP-5 | 1 | 0.8475 | 0.8614 | 0.3754 | 0.2825 |
| Inteleukin 2 | IL2 | 1 | 0.5927 | 0.5730 | 0.0129* | 0.0473* |
| Interleukin 3 | IL3 | 1 | 0.6250 | 0.7643 | 0.0014* | 0.0547 |
| Interleukin 3 receptor B | IL3 Rb | 1 | 0.7283 | 0.7316 | 0.5470 | 0.4356 |
| Interleukin 5 | IL5 | 1 | 0.5039 | 0.7117 | 0.0159 | 0.0063* |
| Interleukin 6 | IL6 | 1 | 0.5311 | 0.6715 | 0.0390* | 0.0153* |
| Interleukin 9 | IL9 | 1 | 0.4989 | 0.5826 | 0.0079* | 0.0029* |
| Interleukin 12 P40/P70 subunits | IL12-p40/p70 | 1 | 0.4908 | 0.8024 | 0.6243 | 0.0579 |
| Interleukin 12 P70 subunit | IL12-p70 | 1 | 0.6393 | 0.6336 | 0.0216* | 0.0744 |
| Interleukin 17 | IL17 | 1 | 0.5014 | 0.8513 | 0.0154* | 0.4477 |
| Leptin receptor | Leptin R | 1 | 0.6288 | 0.4419 | 0.0180* | 0.0813 |
| Leptin receptor | Leptin | 1 | 0.4129 | 0.8535 | 0.3866 | 0.5763 |
| Lymphocyte selectin | L-Selectin | 1 | 0.8507 | 0.6533 | 0.0157* | 0.0001* |
| Lymphotactin | Lymphotactin | 1 | 0.6955 | 0.6483 | 0.2066 | 0.0233* |
| Monocyte chemotactic protein 1 | MCP-1 | 1 | 0.4663 | 0.5434 | 0.0037* | 0.0102* |
| Monocyte chemotactic protein 5 | MCP-5 | 1 | 0.6026 | 0.6870 | 0.7321 | 0.7859 |
| Monokine induced by gamma interferon | MIG | 1 | 0.3939 | 0.8645 | 0.0001* | 0.4457 |
| Macrophage inflammatory protein 1 alpha | MIP-1-alpha | 1 | 0.9069 | 0.9083 | 0.9007 | 0.8884 |
| Macrophage inflammatory protein 3 beta | MIP-3-beta | 1 | 0.9786 | 0.8468 | 0.2460 | 0.6249 |
| Macrophage inflammatory protein 3 alpha | MIP-3-alpha | 1 | 0.7446 | 0.8197 | 0.2937 | 0.3140 |
| Matrix metalloproteinase 3 | MMP-3 | 1 | 0.9984 | 0.9609 | 0.9915 | 0.7725 |
| Platelet factor 4 | PF4 | 1 | 0.9058 | 0.8626 | 0.9482 | 0.3180 |
| Platelet selectin | P-Selectin | 1 | 0.7903 | 0.5439 | 0.9901 | 0.2998 |
| Resistin | Resistin | 1 | 0.9654 | 0.9619 | 0.4734 | 0.1207 |
| Stem cell factor | SCF | 1 | 0.8123 | 0.9165 | 0.0910* | 0.0171* |
| Stromal cell derived factor 1 alpha | SDF-1 alpha | 1 | 0.6650 | 0.7799 | 0.0014* | 0.0001* |
| Sonic hedgehog-N terminal domain | Shh-N | 1 | 0.9726 | 0.9027 | 0.9206 | 0.7007 |
| Thymus and activation-regulated chemokine | TARC | 1 | 0.5413 | 0.9009 | 0.0081* | 0.0607 |
| T Lymphocyte-Secreted Protein | TCA-3 | 1 | 0.9283 | 0.9559 | 0.0707 | 0.4547 |
| Thymus-Expressed Chemokine | TECK | 1 | 0.4709 | 0.5847 | 0.0708 | 0.0535 |
| Tissue inhibitor of metalloproteinase-1 | TIMP-1 | 1 | 0.9769 | 0.9920 | 0.7220 | 0.6062 |
| Tumor necrotic factor-alpha | TNF-alpha | 1 | 0.5126 | 0.5730 | 0.0093* | 0.0470* |
| TNF-related activation-induced cytokine | TRANCE | 1 | 0.9140 | 0.9003 | 0.5615 | 0.5172 |
| Soluble tumor necrotic factor receptor 1 | sTNF RI | 1 | 0.9708 | 0.7883 | 0.0008* | 0.0018* |
| Soluble tumor necrotic factor receptor 2 | sTNF RII | 1 | 0.1775 | 0.1892 | 0.0001* | 0.0001* |
| Thrombopoetin | TPO | 1 | 0.7166 | 0.6588 | 0.0244* | 0.0334* |
| Vascular cell adhesion molecule 1 | VCAM-1 | 1 | 1.0756 | 0.7386 | 0.2952 | 0.0015* |
| Vascular endothelial growth factor | VEGF | 1 | 0.4647 | 0.2821 | 0.0019* | 0.3102 |
Three samples from each group were analyzed by Ray-Bio® cytokine array analysis. The protein levels are expressed as fold-change between the groups. *P < 0.05.
Discussion
In the present study, we observed that pre-treatment with a SO/SOAE-supplemented diet effectively reduced subsequent atherosclerotic lesion formation in LDLR−/− female mice. No change in the plasma levels of TC, TRG, VLDLc, HDLc and LDLc was observed in these pre-treated animals as compared to atherosclerotic diet (control) animals. In our earlier studies with azelaic acid, we noted similar inhibition of atherosclerosis without appreciable changes in plasma lipid levels20. Based on the decreased number of CD68 positive macrophages in the atherosclerotic artery, we concluded that recruitment of leukocytes and reduced inflammation could have played a significant role in SO/SOAE’s mode of action, as opposed to lipid-lowering. A number of studies have demonstrated that knockdown of specific inflammatory mediator(s) affect atherosclerosis21,22. However, compensatory mechanisms and multiple interactions of inflammatory pathways could have affected the outcome. Our studies provide direct evidence for a role of pro-inflammatory cytokines in atherosclerosis and an alternative role for total or LDL-associated cholesterol in atherogenesis. We would like to exercise caution in the interpretation of our data, as lipids were increased in the high fat-fed state and the study did not measure changes in lipid classes or their distribution amongst subfractions. The study also did not measure specific pro- or anti-inflammatory proteins that could be associated with the lipoprotein classes.
Reduced mRNA levels of pro-inflammatory cytokine genes and scavenger receptor genes (CD36 and SRA1) were observed in SO/SOAE pre-treated animals liver tissue. Increased expression of antioxidant genes (catalase) and anti-inflammatory cytokine genes (IL-4 and IL-10) was also observed, suggesting that the pre-treatment had a significant influence on not only modulating cholesterol homeostasis but also upon lipid-loading and the generation of inflammation.
Enhanced RCT with increased expression of ABCA1 in SO/SOAE pre-treated animals was observed as compared to control animals. The increased ABCA1 and reduced ABCG1 in SOAE pre-treated animals might suggest that SOAE might promote enhanced RCT towards lipid-poor APOA1 rather than to intact HDL. This might be also due to the altered cytoskeleton which might promote increased binding of free APOA1 to the macrophage surface. These are mere speculations and need to be experimentally verified. It is to be pointed out that no significant changes in HDL were noted with SOAE; however, free and bound APOA1 were not measured. In contrast to ABC A1, ABC G1 appears to be promiscuous in its transport properties and it also appears to deliver cholesterol to LDL. Considering its complex actions, more studies are needed. No significant changes were observed in nuclear receptors such as LXR, FXR and Retinoid X receptor (RXR) in SO/SOAE pre-treated animals as compared to control animals.
Increased PON1 and APOA1 in SO/SOAE pre-treated animals suggests enhanced functionality of HDL as well as protection against oxidative stress-induced atherosclerosis. PON1 is synthesized in the liver and is thought to be exclusively associated with HDL23–26.
An insignificant reduction in MMP9 gene expression was observed in SO/SOAE pre-treated animals. MMP9 has been considered a major pathological factor in atherosclerosis due to its significant role in migration and proliferation of smooth muscle cells during plaque formation. Further, reduced plasma levels of MMP3 in SO/SOAE pre-treated animals suggest that one-month pre-treatment is quite effective in reducing matrix metalloproteinases. Evidence suggests that DOCK2 plays a major role in the phenotype modulation, migration and proliferation of smooth muscle cells (SMCs), a major event in lesion formation and in progression of atherosclerosis27–31. Further, these studies also suggest that targeting DOCK2 would be the best therapeutic approach to control disease progression. In our studies, we observed a significant reduction in DOCK2 expression in the aorta of SO/SOAE pre-treated animals, suggesting and confirming the ability of SO/SOAE in targeting DOCK2 by dietary approach.
A significant reduction in mRNA levels of CD36 and SRA1 was observed in aortic lesions of SO/SOAE pre-treated animals, suggesting the ability of both SO and its aqueous component (SOAE) to prevent foam cell formation. SO contains lignans such as sesamin, sesamolin and asarinin, which play an important role in promoting health32,33. In addition, sesamin and sesamolin have already shown antioxidant34, antiproliferative35,36, antihypertensive37,38, and neuroprotective activities39, as well as the ability to lower cholesterol levels40 and increase hepatic fatty acid oxidation enzymes41. Lignans of SO are known to form complexes with cholesterol and further prevent the latter’s absorption42,43. These lignans might also influence the reduced plasma lipids in SO treated animals42. The results of our studies also suggest the absence of these lignans in SOAE.
We also observed a decrease in the mRNA level of CD68 a monocyte/macrophage marker in SO/SOAE pre-treated animals as compared to controls. This decrease might suggest and confirms the reduced presence of foam cell-forming macrophages in SO/SOAE pre-treated animals atherosclerotic lesions (Fig. 2). Similarly, a significant reduction in levels of MCP-1 and CD4 was observed in SO/SOAE pre-treated animals as compared to control animals (Fig. 2), suggesting the possibility of reduction in additional chemotactic factors which might have contributed to the reduced occurrence of macrophages. The diminished levels of CD68 mRNA was also observed in the abdominal aortic segment, which did not contain any visible lesions.
Evidence suggests the upregulation of LOX-1 receptor under pro-atherogenic conditions44–49, and its presence in atherosclerotic lesions of both humans and animals44,48. According to studies from Mehta and Thakkar et al.50,51, LOX1 is a key player in the progression of atherosclerosis and targeting the LOX1 might be an effective therapeutic strategy. In our studies, both SO and SOAE pre-treatment significantly reduced the LOX1 expression in aortic lesions, suggesting its role in preventing the internalization of Ox-LDL followed by inflammation initiation, an early event which leads to a cascade of actions in disease progression.
Our in vitro studies suggest the pre-treatment of SOAE for 2 hr is able to inhibit the Ox-LDL-induced inflammation in RAW cells; similarly, in vivo studies also suggest the short-term pre-treatment is able to reduce inflammation and oxidative stress even in the presence of a high fat diet.
Our data suggest that SO/SOAE pre-treatment is able to inhibit subsequent development of atherosclerosis. To our knowledge, this is the first evidence that, despite no drastic changes in plasma lipid levels, atherosclerosis could be prevented and controlled by the manipulation of leukocyte recruitment and by reducing their inflammatory responses. These results prompted us to analyze the components of SOAE by LC-MS, select specific components, and further test their role against inflammation. Indeed, the components of SOAE in combination counteracted against LPS-induced inflammation in monocyte-derived macrophages52. Considering the fact that the lipophilic molecules of SO, such as lignans, α–tocopherol and phytosterols, have less feasibility to separate out into water (confirmed by the absence of these in SOAE by LC-MS analysis), our published studies17,18 suggest that apart from these lipophilic molecules, SO has other biologically active polar molecules that can counteract inflammation and atherosclerosis. However, the study should in no way be interpreted to suggest that ALL the inflammatory components reside in the aqueous extract. An aqueous extract is a simple extract and could be prepared and delivered easily. It could be concentrated and given as a pill or as a capsule. Sesame oil, despite its anti-inflammatory properties, is still an oil and contains huge amounts of lipids. A human dose of 30 ml is roughly over 250 calories. In a dyslipidemic situation, it would be undesirable to consume large quantities of fat.
Considering the plant-derived pharmaceutical nature of the extract, SOAE and related agents could be used in young and vulnerable subjects without long-term commitment to statins or other drugs. The combinational approach of SOAE and statins are our ongoing as well as future studies, as statins are potent lipid-lowering drugs.
Methods
Chemicals
All PCR primers and TrizolTM reagent were purchased from Invitrogen (Carlsbad, CA). DOCK2 Elisa kit was purchased from My Biosource (MBS9328886: San Diego, CA).
Cell culture
RAW 264.7 mouse macrophages (ATCC, Manassas, VA) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS).
Isolation and oxidation of lipoproteins
LDL was isolated from normal plasma by sequential ultracentrifugation using a Beckman TL-100 tabletop ultracentrifuge (Beckman, Palo Alto, CA)53,54. LDL was then dialyzed and filter-sterilized. Following estimation of protein concentration, LDL samples were subjected to oxidation with 5 µM copper in 1 ml of phosphate-buffered saline (PBS) at 37 °C. The formation of conjugated dienes was monitored at an optical density of 234 nm for about 18 h using SLM Amnico DB-3500 spectrophotometer (Urbana, IL). The degree of LDL oxidation was assessed by determination of peroxide content using leucomethylene blue (LMB) and thiobarbituric acid reactive substances (TBARS) assay. LDL was acetylated (Ac-LDL) using acetic anhydride. Electrophoretic mobility of modified lipoproteins was determined by agarose gel electrophoresis (Supplementary Fig. 5).
Preparation and analysis of SOAE
SOAE was prepared and used as described previously17.
Analysis of Ox-LDL/Ac-LDL uptake
RAW 264.7 cells were pre-treated with SOAE for 2 h followed by incubation with Ox-LDL (25 µg/ml) or Ac-LDL (10 µg/ml) for 24 h. Cells were stained with Oil Red O and images were recorded. To monitor the changes in genes involved in inflammation as well as in RCT, cells were harvested in Trizol™ for RNA isolation after 24 h of incubation.
Animals
Fifty, 4-week-old female LDLR−/− mice weighing 18–20 g were obtained from Jackson Laboratory (Bar Harbor, ME). After 2-weeks of acclimatization, six animals were sacrificed to obtain baseline parameters. The remaining animals were divided into three groups: group 1 was fed a normal diet (n = 14), group 2 (n = 15) was fed sesame oil reformulated a normal diet, and group 3 (n = 15) was fed an SOAE-supplemented normal diet for 1 month. After one month, 6, 7 and 7 animals were sacrificed from each group, respectively. The remaining animals were fed an atherogenic diet for 2 months following the pre-treatment period. The animals were regularly monitored, and a weekly record of body weight was maintained up to 90 days. All procedures were performed according to the protocol approved by The Institutional Animal Care and Use Committee of the University of Central Florida and all methods were performed in accordance with the relevant guidelines and regulations.
Diet
The normal Purina diet (TD.150278) with 1% soybean oil, normal diet reformulated with either 1% SO (TD.150515) or SOAE (prepared in-house) and an atherogenic diet with 0.2% cholesterol and 17% high fat (TD.04287) were purchased from Harlan Teklad (Madison, WI). Diets were stored at 4 °C to avoid oxidation.
Isolation of mouse peritoneal macrophages
Macrophages from the peritoneal cavity of all groups of animals were isolated by peritoneal lavage using 3 ml of cold saline and centrifugation at 1200 rpm for 5 min. Cells were utilized for RNA isolation.
Collection of plasma and organs
After 4 and 12 weeks, mice were fasted overnight and anesthetized with 1–2% isoflurane. Fasting blood samples were collected into EDTA tubes by heart puncture. Plasma was separated as described previously15 and stored at −80 °C. The liver was perfused with PBS, weighed, and the tissue used for RNA isolation.
Isolation and quantification of aortic lesions
Isolation of the aorta and quantification of aortic lesions was performed as described previously15,16,18. Lesion areas were marked on photographs. The lesion area was quantified using ImageJ software55. After imaging, aortas were saved for RNA extraction.
Plasma lipid analysis
Plasma lipid profiles of total cholesterol (TC), triglyceride (TRG), HDL cholesterol (HDLc), and LDL cholesterol (LDLc) were determined using a Cholestech L*D*X analyzer (Cholestech Corp, Hayward, CA).
cDNA synthesis and RT-PCR reaction
Total RNA from cells, liver and aortic tissue was isolated by using TrizolTM reagent. Total RNA of 1 µg was then reverse-transcribed into cDNA using the SuperscriptTM III First Strand Synthesis system (Invitrogen, Carlsbad, CA). cDNA (50 ng) samples were used to perform Quantitative real-time PCR by iQTM5 iCycler Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with SYBR Green (Invitrogen, Carlsbad, CA). Mouse oligonucleotide primers for RT-PCR were purchased from Invitrogen (Carlsbad, CA). Polymerase chain reaction (PCR) was carried out with ABCA1, ABCG1, SRB1, Cyp7a1, FMO1-5, NPC1L1, MCP-1, IL-1α, IL-1β, IL-6, IL-4, IL-10, IL-13, catalase, MnSOD, SRA1, CD36, LXR, PXR, FXR, MPO, CD68, DOCK2 and LOX1 with mouse-specific primers (Supplementary Table 1), resulting in a 200 bp fragment each for sample. As a reference gene, GAPDH primers were utilized. PCR was performed with an initial step of denaturation at 50 °C for 2 mins, 95 °C for 10 mins followed by 40 cycles of 95 °C for 20 s and 60 °C for 20 s. Melt curves were established for the reactions. Normalized fold expression was calculated by using 2−∆∆Ct method.
The entire reaction products were electrophoresed on 2% agarose gels.
Global Cytokine array
Plasma samples were analyzed by the global cytokine array by Ray Biotech Inc. (Norcross, GA) using RayBio®Mouse G Series Array 3 and 4 glass chip.
DOCK 2 ELISA
Fifty microliters of samples were analyzed using a sandwich ELISA kit (My Biosource Inc, CA) following manufacturer’s protocol. The absorbance was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA). Concentration of DOCK2 was expressed in ng/ml.
Statistics
Values are presented as means ± standard deviation (SD), and statistical analyses were performed by using student’s t-test, with p < 0.05 as the level of significance. Significance between groups was calculated by using two-tailed student’s t-test and Wilcoxon matched paired test using Prism Pad software (version V), with p < 0.05 considered significant.
Electronic supplementary material
Acknowledgements
This study was supported by National Institutes of Health Grant 5R01AT004106-05.
Author Contributions
First author C.A. wrote the manuscript, major role in performing dissection, experiments, acquiring data and analysis. All other co-authors K.B., M.D. and A.R. participated in the animal feeding, monitoring and maintaining of animals, euthanizing, harvesting organs and in experimental work. Corresponding author Dr. S.P. designed experiments, reviewed and finalized the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29849-x.
References
- 1.Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg D, Lewis A. Conner Memorial Lecture: oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.CIR.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198901053200122. [DOI] [PubMed] [Google Scholar]
- 5.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 6.Nissen SE, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 7.Mensink RP, Katan MB. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med. 1989;321:436–441. doi: 10.1056/NEJM198908173210705. [DOI] [PubMed] [Google Scholar]
- 8.Parks JS, Rudel LL. Effect of fish oil on atherosclerosis and lipoprotein metabolism. Atherosclerosis. 1990;84:83–94. doi: 10.1016/0021-9150(90)90077-V. [DOI] [PubMed] [Google Scholar]
- 9.Sanders TA. Olive oil and the Mediterranean diet. Int J Vitam Nutr Res. 2001;71:179–184. doi: 10.1024/0300-9831.71.3.179. [DOI] [PubMed] [Google Scholar]
- 10.Penumetcha M, Song M, Merchant N, Parthasarathy S. Pretreatment with n-6 PUFA protects against subsequent high fat diet induced atherosclerosis—Potential role of oxidative stress-induced antioxidant defense. Atherosclerosis. 2012;220:53–58. doi: 10.1016/j.atherosclerosis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355:97–104. doi: 10.1016/j.cccn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Sankar, D. Sesame oil helps to reduce dose of blood pressure lowering medicine., http://content.health.msn.com/content/article/64/72269.htm (accessed June 2005).
- 13.Kok T, et al. Induction of hepatic ABC transporter expression is part of the PPARalpha-mediated fasting response in the mouse. Gastroenterology. 2003;124(1):160–171. doi: 10.1053/gast.2003.50007. [DOI] [PubMed] [Google Scholar]
- 14.Satchithanandam S, et al. Effect of sesame oil on serum and liver lipid profiles in the rat. Int J Vitam Nutr Res. 1996;66:386–392. [PubMed] [Google Scholar]
- 15.Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S. Inhibition of atherosclerosis in low-density lipoprotein receptor -negative mice by sesame oil. J Med Food. 2006;9:487–490. doi: 10.1089/jmf.2006.9.487. [DOI] [PubMed] [Google Scholar]
- 16.Chandrakala AN, Selvarajan K, Litvinov D, Parthasarathy S. Anti-Atherosclerotic and Anti-Inflammatory Actions of Sesame Oil. Journal of Medicinal Food. 2015;205(18(1)):11–20. doi: 10.1089/jmf.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvarajan S, Chandrakala AN, Baputty RM, Parthasarathy S. Anti-inflammatory and anti-oxidant activities of the non-lipid (aqueous) components of Sesame oil: Potential use in atherosclerosis. Journal of Medicinal Food. 2015;18(4):1–10. doi: 10.1089/jmf.2014.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandrakala AN, et al. Water-soluble Components of Sesame Oil reduce inflammation and atherosclerosis. Journal of Medicinal Food. 2016;19(7):1–9. doi: 10.1089/jmf.2015.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett BJ, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvinov D, Selvarajan K, Garelnabi M, Brophy L, Parthasarathy S. Anti-atherosclerotic actions of azelaic acid, an end product of linoleic acid peroxidation, in mice. Atherosclerosis. 2010;209(2):449–454. doi: 10.1016/j.atherosclerosis.2009.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, et al. Hematopoietic knockdown of PPARδ reduces atherosclerosis in LDLR −/− mice. Gene Therapy. 2016;23(1):78–85. doi: 10.1038/gt.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Krauss RM, Chang JT, Teng BB. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J. Lipid Res. 2018;59:207–223. doi: 10.1194/jlr.M078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1995;15:1812–1818. doi: 10.1161/01.ATV.15.11.1812. [DOI] [PubMed] [Google Scholar]
- 24.Furlong CE, et al. Genetically determined susceptibility to organophosphorus insecticides and nerve agents: developing a mouse model for the human PON1 polymorphism. Neurotoxicology. 1998;19:645–650. [PubMed] [Google Scholar]
- 25.Ng CJ, et al. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med. 2005;38(2):153–163. doi: 10.1016/j.freeradbiomed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 26.Aviram M, et al. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler. Thromb. Vasc. Biol. 1998;18:1617–1624. doi: 10.1161/01.ATV.18.10.1617. [DOI] [PubMed] [Google Scholar]
- 27.Guo X, et al. Dedicator of Cytokinesis 2, A Novel Regulator for Smooth Muscle Phenotypic Modulation and Vascular Remodeling. Circ Res. 2015;116:e71–e80. doi: 10.1161/CIRCRESAHA.116.305863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–S89. [PubMed] [Google Scholar]
- 29.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 30.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 31.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwertner HA, Rios DC. Analysis of sesamin, asarinin, and sesamolin by HPLC with photodiode and fluorescent detection and by GC/MS: Application to sesame oil and serum samples. J. Am. Oil Chem. Soc. 2012;89:1943–1950. doi: 10.1007/s11746-012-2098-7. [DOI] [Google Scholar]
- 33.Wu WH. The contents of lignans in commercial sesame oils of Taiwan and their changes during heating. Food Chem. 2007;104:341–344. doi: 10.1016/j.foodchem.2006.11.055. [DOI] [Google Scholar]
- 34.Suja KP, Jayalekshmy A, Arumughan C. Free radical scavenging behavior of antioxidant compounds of sesame (Sesamum indicum L.) in DPPH˙ system. J. Agric. Food Chem. 2004;52:912–915. doi: 10.1021/jf0303621. [DOI] [PubMed] [Google Scholar]
- 35.Yokota T, et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007;98:1447–1453. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghafoorunissa S, Hemalatha S, Rao MVV. Sesame lignans enhance the antioxidant activity of vitamin E in lipid peroxidation systems. Mol. Cell. Biochem. 2004;262:195–202. doi: 10.1023/B:MCBI.0000038235.01389.a9. [DOI] [PubMed] [Google Scholar]
- 37.Lee CC, et al. Sesamin induces nitric oxide and decreases endothelin-1 production in HUVECs: Possible implications for its antihypertensive effect. J. Hypertens. 2004;22:2329–2338. doi: 10.1097/00004872-200412000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Nakano D, et al. Dietary sesamin suppresses aortic NADPH oxidase in DOCA salt hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2008;35:324–326. doi: 10.1111/j.1440-1681.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheng FC, Jinn TR, Hou RC, Tzen JTC. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int. J. Biomed. Sci. 2006;2:284–288. [PMC free article] [PubMed] [Google Scholar]
- 40.Visavadiya NP, Narasimhacharya AVRL. Sesame as a hypocholesteraemic and antioxidant dietary component. Food Chem. Toxicol. 2008;46:1889–1895. doi: 10.1016/j.fct.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Ashakumary L, et al. Sesamin, a sesame lignan, is a potent inducer of hepatic fatty acid oxidation in the rat. Metabolism. 1999;48:1303–1313. doi: 10.1016/S0026-0495(99)90272-X. [DOI] [PubMed] [Google Scholar]
- 42.Hirata F, et al. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis. 1996;122(1):135–136. doi: 10.1016/0021-9150(95)05769-2. [DOI] [PubMed] [Google Scholar]
- 43.Hirose N, et al. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J Lipid Res. 1991;32(4):629–638. [PubMed] [Google Scholar]
- 44.Chen M, Kakutani M, Minami M. Increased expression of lectin-like oxidized low-density lipoprotein receptor-1 in initial atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2000;20:1107–1115. doi: 10.1161/01.ATV.20.4.1107. [DOI] [PubMed] [Google Scholar]
- 45.Nagase M, Hirose S, Sawamura T, Masaki T, Fujita T. Enhanced expression of endothelial oxidized low-density lipoprotein receptor (LOX-1) in hypertensive rats. Biochem Biophys Res Commun. 1997;237:496–498. doi: 10.1006/bbrc.1997.7176. [DOI] [PubMed] [Google Scholar]
- 46.Kume N, et al. Inducible expression of LOX-1, a novel lectin-like receptor for oxidized low-density lipoprotein, in vascular endothelial cells. Circ Res. 1998;83:322–327. doi: 10.1161/01.RES.83.3.322. [DOI] [PubMed] [Google Scholar]
- 47.Murase T, et al. Fluid shear stress transcriptionally induces lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:328–333. doi: 10.1161/01.RES.83.3.328. [DOI] [PubMed] [Google Scholar]
- 48.Kataoka H, Miyamoto S. Expression of lectin-like oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.CIR.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 49.Chen M, et al. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and age. Biochem Biophys Res Commun. 2001;87:962–968. doi: 10.1006/bbrc.2001.5674. [DOI] [PubMed] [Google Scholar]
- 50.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovascular Research. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Thakkar S, et al. Structure-based Design Targeted at LOX-1, a Receptor for Oxidized Low-Density Lipoprotein. Scientific Reports. 2015;5:16740. doi: 10.1038/srep16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deme P, Narasimhulu CA, Pathasarathy S. Identification and evaluation of anti-inflammatory properties of aqueous components extracted from sesame (Sesamum indicum) oil. J Chrom B. 2018;1087–1088:61–69. doi: 10.1016/j.jchromb.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santanam N, Parthasarathy S. Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest. 1995;95:2594–2600. doi: 10.1172/JCI117961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandrakala AN, Sukul D, Selvarajan K, Sai-Sudhakar C, Parthasarathy S. Induction of Brain Natriuretic Peptide and Monocyte Chemotactic Protein-1 Gene Expressions by Oxidized Low-density Lipoprotein-Relevance to Ischemic Heart Failure. American Journal Physiology-Cell Physiology. 2012;302:C165–C177. doi: 10.1152/ajpcell.00116.2011. [DOI] [PubMed] [Google Scholar]
- 55.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.