Abstract
A complex and dynamic community of microorganisms, play important roles within the fish gastrointestinal (GI) tract. Of the bacteria colonizing the GI tract, are lactic acid bacteria (LAB) generally considered as favorable microorganism due to their abilities to stimulating host GI development, digestive function, mucosal tolerance, stimulating immune response, and improved disease resistance. In early finfish studies, were culture-dependent methods used to enumerate bacterial population levels within the GI tract. However, due to limitations by using culture methods, culture-independent techniques have been used during the last decade. These investigations have revealed the presence of Lactobacillus, Lactococcus, Leuconostoc, Enterococcus, Streptococcus, Carnobacterium, Weissella, and Pediococcus as indigenous species. Numerous strains of LAB isolated from finfish are able to produce antibacterial substances toward different potential fish pathogenic bacteria as well as human pathogens. LAB are revealed be the most promising bacterial genera as probiotic in aquaculture. During the decade numerous investigations are performed on evaluation of probiotic properties of different genus and species of LAB. Except limited contradictory reports, most of administered strains displayed beneficial effects on both, growth—and reproductive performance, immune responses and disease resistance of finfish. This eventually led to industrial scale up and introduction LAB-based commercial probiotics. Pathogenic LAB belonging to the genera Streptococcus, Enterococcus, Lactobacillus, Carnobacterium, and Lactococcus have been detected from ascites, kidney, liver, heart, and spleen of several finfish species. These pathogenic bacteria will be addressed in present review which includes their impacts on finfish aquaculture, possible routes for treatment. Finfish share many common structures and functions of the immune system with warm-blooded animals, although apparent differences exist. This similarity in the immune system may result in many shared LAB effects between finfish and land animals. LAB-fed fish show an increase in innate immune activities leading to disease resistances: neutrophil activity, lysozyme secretion, phagocytosis, and production of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α). However, some LAB strains preferentially induces IL-10 instead, a potent anti-inflammatory cytokine. These results indicate that LAB may vary in their immunological effects depending on the species and hosts. So far, the immunological studies using LAB have been focused on their effects on innate immunity. However, these studies need to be further extended by investigating their involvement in the modulation of adaptive immunity. The present review paper focuses on recent findings in the field of isolation and detection of LAB, their administration as probiotic in aquaculture and their interaction with fish immune responses. Furthermore, the mode of action of probiotics on finfish are discussed.
Keywords: lactic acid bacteria (LAB), finfish, probiotics, probiotic bacteria, fish immunity, aquaculture
Introduction
Optimal gastrointestinal (GI) functionality is essential for sustainable animal production. Effective functionality of the finfish GI tract and its gut microbiota play and important role in host health (Ringø et al., 2003; Round and Mazmanian, 2009), and several complex mechanisms are involved, and in the absence of gut microbiota, normal immune development, and function are impaired. Therefore it is crucial to increase our knowledge on beneficial gut bacteria, for example lactic acid bacteria (LAB) colonizing the GI tract, in the context of improved growth performance and health.
LAB are classified in phylum Firmicutes, class Bacilli, and order Latobacillales. They are Gram-positive, non-endosporing, with rod-shaped or coccid morphology, are catalase- and oxidase-negative and most of them are non-motile. The growth optimum of LAB is generally at pH 5.5–5.8, and they have complex nutritional requirements. They are divided into homofermentative and heterofermentative; homofermentative produce lactic acid from sugars, while heterofermentative produce lactic acid, acetic acid or alcohol, and carbon dioxide. A favorable trait of LAB is; they produce growth inhibition substances such as bacteriocins, hydrogen peroxide, diacyls, etc.; prevent proliferation of pathogenic—and spoilage bacteria in food (Alakomi et al., 2000; De Vuyst and Leroy, 2007), as well as adherence and colonization of pathogens in the digestive tract (Li et al., 2018).
LAB genera include rods; Carnobacterium, Dolosigranulum, and Lactobacillus, cocci; Aerococcus, Alloiococcus, Enterococcus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, and Vagococcus, and the coccoid or rod-shaped genus Weissella (Walter, 2008; Ventura et al., 2009; Fusco et al., 2015). They are isolated from different sources; e.g., plant material, fruits, dairy products, fermented meat, cavities of humans as well as the gastrointestinal (GI) tract of finfish (e.g., Ventura et al., 2009; Merrifield et al., 2014; Ringø et al., 2016).
The fish gut microbiota plays an important role in GI tract development, digestive function, mucosal tolerance, stimulating the host immune response, and protection against infections (e.g., Rawls et al., 2004, 2006; Gómez and Balcázar, 2008; German, 2009; Ray et al., 2012; Maiuta et al., 2013; Piazzon et al., 2017; Tarnecki et al., 2017; Li et al., 2018; Wang et al., 2018). Furthermore, host-microbe interactions are influenced by complex host genetics and environment. In a recent review, Lescak and Milligan (2017) suggested teleost as model organisms to understand host-microbe interactions, as traditional mammalian studies can be limited by isogenic strains, small sample sizes, limited statistical power and indirect characterization of gut microbiota from fecal samples.
As the GI tract in fish is one of the most important interfaces with the environment exposed to potential pathogens, it is of importance to evaluate the presence of beneficial bacteria such as LAB in the GI tract, as autochthonous bacteria rapidly colonize the digestive tract at early developmental larval stages of finfish (Ringø et al., 1996).
During the last 20 years, an impressive amount of knowledge has been published on LAB in finfish intestine, their potential as probiotics, pathogenicity and their effect on the immune system (Ringø and Gatesoupe, 1998; Ringø, 2004; Ringø et al., 2005, 2012a,b; Gatesoupe, 2008; Lauzon and Ringø, 2011; Merrifield et al., 2014; Ringø and Song, 2016; Zhou Z. et al., 2018). To avoid duplication, studies reviewed in the aforementioned reviews are not addressed in the present paper. The current review aimed to present an updated overview of recently published data on LAB, and on LAB data not mention in the aforementioned reviews on the topics; on LAB in the GI tract of finfish, antagonistic ability, health benefits as probiotics, pathogenicity, and on immunostimulation.
Lactic acid bacteria (LAB) in the gastrointestinal (GI) tract
The GI tract microbiota in endothermic animals as well as fish is divided into; the GI lumen microbiota (the allochthonous), and those that adhere to the mucosal surface (the autochthonous microbiota). In most studied showed in Table 1 have, however, characterized the allochthonous gut microbiota.
Table 1.
Lactic acid bacteria (LAB) in the gastrointestinal tract of finfish.
| LAB species isolated | Isolated from | “Segments” of the GI tract | References |
|---|---|---|---|
| LAB* | Tasmanian Atlantic salmon (Salmo salar) | Fecal content | Neuman et al., 2015 |
| Persian sturgeon (Acipenser persicus) | EI; auto and allo | Ovissipour et al., 2014 | |
| Beluga (Huso huso) | EI; allo | Adel et al., 2017 | |
| Oscar (Astronotus ocellatus) | EI; auto | Hoseinifar et al., 2016a | |
| Tilapia (Oreochromis niloticus) | EI; allo | Standen et al., 2016 | |
| Nile tilapia (Oreochromis niloticus) | EI; content | Boonanuntanasarn et al., 2017 | |
| Carnobacterium | Rainbow trout (Oncorhynchus mykiss) | DI; auto and allo | Lyons et al., 2017a |
| Rainbow trout | DI; auto and allo | Huyben et al., 2017 | |
| Rainbow trout | EI; auto | Bruni et al., 2018 | |
| Atlantic salmon (Salmo salar) | Fecal content | Zarkasi et al., 2016 | |
| Atlantic salmon | DI; content | Gajardo et al., 2017 | |
| Atlantic salmon | EI; content | Rudi et al., 2018 | |
| Turbot (Scophthalmus maximus) | EI; auto | Yang et al., 2018 | |
| Fine flounder (Paralichthys adspersus) | EI: content | Salas-Leiva et al., 2017 | |
| Northern snakehead (Channa argus) | EI: content | Miao et al., 2018 | |
| C. divergens | Rainbow trout | EI; allo | Bruni et al., 2018 |
| Lactobacillus | Rainbow trout | DI; auto and allo | Lyons et al., 2016 |
| Rainbow trout | PI; auto and allo | Bahramian and Parsa, 2017 | |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017a | |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017b | |
| Rainbow trout | DI; auto and allo | Huyben et al., 2017 | |
| Atlantic salmon | Fecal content | Zarkasi et al., 2016 | |
| Atlantic salmon | EI; Digesta samples | Dehler et al., 2017a | |
| Atlantic salmon | DI; Digesta samples | Dehler et al., 2017b | |
| Atlantic salmon | DI; allo | Zarkasi et al., 2016 | |
| Atlantic salmon | DI; content | Gajardo et al., 2017 | |
| Atlantic salmon | PI and DI; auto | Lavoie et al., 2018 | |
| Atlantic salmon | EI; auto and allo | Rimoldi et al., 2018 | |
| Arctic charr (Salvelinus alpinus) | PI; auto and allo DI; auto and allo | Nyman et al., 2017 | |
| Regal peacock (Aulonocara stuartgranti) | EI; allo | Mirzapour-Rezaee et al., 2017 | |
| Largemouth bass (Micropterus salmoides) | EI; content | Zhou M. et al., 2018 | |
| European sea bass (Dicentrarchus labrax) | EI; content | Torrecillas et al., 2017 | |
| Fine flounder | EI: content | Salas-Leiva et al., 2017 | |
| Gibel carp (Carassius auratus gibelio) | EI: content | Wu et al., 2018 | |
| Loach (Paramisgurnus dabryanus) | EI: auto and allo | Gao et al., 2017 | |
| Zebrafish (Danio rerio) | EI: content | Yang et al., 2017 | |
| Lb. aviarius | Tilapia | EI; auto and allo | Standen et al., 2015 |
| Lb. aviaries subsp. arafinosus | White sea bream (Diplodus sargus) | EI; auto and allo | Guerreiro et al., 2018a |
| Lb. brevis | Tilapia | EI; content | Del‘Duca et al., 2015 |
| Lb. crispatus/ Lb. amylovorus | Gilthead sea bream (Sparus aurata) | EI; auto and allo | Serra et al., 2018 |
| Lb. crispatus | White sea bream | EI; auto and allo | Guerreiro et al., 2018a |
| European sea bass | EI; auto and allo | Guerreiro et al., 2018b | |
| Lb. collinoides | Tilapia | EI; content | Del‘Duca et al., 2015 |
| Lb. coryniformis | Tilapia | EI; content | Del‘Duca et al., 2015 |
| Lb. farciminis | Tilapia | EI; content | Del‘Duca et al., 2015 |
| Lb. gallinarum | White sea bream | EI; auto and allo | Guerreiro et al., 2018a |
| Lb. johnsonii | European sea bass | EI; content | Torrecillas et al., 2017 |
| Lb. paracasei subsp. paracasei | Rainbow trout | EI; content | Popovic et al., 2017 |
| Lb. reuteri | Rainbow trout | EI; content | Huyben et al., 2018 |
| Lb. sakei | Rainbow trout | DI: auto and allo | Didinen et al., 2018 |
| Lactococcus | Rainbow trout | DI; auto and allo | Lyons et al., 2016 |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017a | |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017b | |
| Rainbow trout | DI; auto and allo | Huyben et al., 2017 | |
| Atlantic salmon | Fecal content | Zarkasi et al., 2016 | |
| Atlantic salmon | Digesta samples | Dehler et al., 2017b | |
| Atlantic salmon | DI; content | Gajardo et al., 2017 | |
| Atlantic salmon | EI; content | Rudi et al., 2018 | |
| Atlantic salmon | EI; auto and allo | Rimoldi et al., 2018 | |
| Arctic charr | PI; auto and allo DI; auto and allo | Nyman et al., 2017 | |
| Grass carp (Ctenopharyngodon idella) | NI | Tran et al., 2017 | |
| Gibel carp | EI: content | Wu et al., 2018 | |
| Northern snakehead | EI: content | Miao et al., 2018 | |
| Loach | EI: auto and allo | Gao et al., 2017 | |
| Zebrafish | EI: content | Yang et al., 2017 | |
| Zebrafish | EI: content | Zhou L. et al., 2018 | |
| L. garvieae | Pirarucu (Arapaima gigas) | EI; auto and allo | do Vale Pereira et al., 2017 |
| Turbot | EI; auto | Yang et al., 2018 | |
| L. lactis | Grass carp | EI; auto and allo | Dong et al., 2017 |
| L. garvieae | Rainbow trout | DI: allo | Didinen et al., 2018 |
| L. lactis subsp. cremoris | Rainbow trout | DI: allo | Didinen et al., 2018 |
| L. lactis subsp. lactis | Pirarucu | EI; auto and allo | do Vale Pereira et al., 2017 |
| L. piscium | European sea bass | EI; content | Torrecillas et al., 2017 |
| L. raffinolactis | Grass carp | EI; auto | Li et al., 2015 |
| Grass carp | EI; auto and allo | Dong et al., 2017 | |
| Leuconostocaceae | Rainbow trout | EI; auto and allo | Huyben et al., 2018 |
| Leuconostoc | Rainbow trout | DI; auto and allo | Lyons et al., 2016 |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017b | |
| Rainbow trout | DI; auto and allo | Huyben et al., 2017 | |
| Atlantic salmon | Digesta samples | Dehler et al., 2017b | |
| Atlantic salmon | DI; content | Gajardo et al., 2017 | |
| Atlantic salmon | EI; auto and allo | Rimoldi et al., 2018 | |
| Arctic charr | PI; auto and allo DI; auto and allo | Nyman et al., 2017 | |
| Tilapia | EI; auto and allo | Standen et al., 2015 | |
| Loach | EI: auto and allo | Gao et al., 2017 | |
| Pediococcus | Atlantic salmon | DI; content | Gajardo et al., 2017 |
| Atlantic salmon | PI and DI; auto | Lavoie et al., 2018 | |
| Turbot | EI; auto | Yang et al., 2018 | |
| P. acidilactici | Rainbow trout | DI: allo | Didinen et al., 2018 |
| Streptococcacceae | Rainbow trout | EI; auto and allo | Huyben et al., 2018 |
| Atlantic salmon | PI and DI; auto | Lavoie et al., 2018 | |
| Streptococcus | Rainbow trout | DI; auto and allo | Lyons et al., 2016 |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017a | |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017b | |
| Atlantic salmon | Fecal content | Zarkasi et al., 2016 | |
| Atlantic salmon | Digesta samples | Dehler et al., 2017a | |
| Atlantic salmon | Digesta samples | Dehler et al., 2017b | |
| Atlantic salmon | EI; auto and allo | Rimoldi et al., 2018 | |
| European sea bass | EI; content | Torrecillas et al., 2017 | |
| Turbot | EI; auto | Yang et al., 2018 | |
| Fine flounder | EI: content | Salas-Leiva et al., 2017 | |
| Pirarucu | EI; auto and allo | do Vale Pereira et al., 2017 | |
| Northern snakehead | EI: content | Miao et al., 2018 | |
| S. luteciae | Rainbow trout | DI; auto and allo | Huyben et al., 2017 |
| Arctic charr | PI; auto and allo DI; auto and allo | Nyman et al., 2017 | |
| S. sobrinus | Rainbow trout | DI; auto and allo | Huyben et al., 2017 |
| Arctic charr | PI; auto and allo DI; auto and allo | Nyman et al., 2017 | |
| Enterococcus | Rainbow trout | DI; auto and allo | Lyons et al., 2016 |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017a | |
| Atlantic salmon | EI; auto and allo | Rimoldi et al., 2018 | |
| Turbot | EI; auto | Yang et al., 2018 | |
| Zebrafish | EI: content | Yang et al., 2017 | |
| Zebrafish | EI: content | Zhou L. et al., 2018 | |
| E. faecalis | Mrigal (Cirrhinus mrigala) | EI; allo | Shahid et al., 2017 |
| E. faecium | European sea bass | EI; content | Torrecillas et al., 2017 |
| Tilapia | EI; auto and allo | Standen et al., 2015 | |
| Pirarucu | EI; auto and allo | do Vale Pereira et al., 2017 | |
| Vagococcus | Rainbow trout | DI; auto and allo | Lyons et al., 2017a |
| Atlantic salmon | DI; content | Gajardo et al., 2017 | |
| Atlantic salmon | EI; content | Rudi et al., 2018 | |
| Fine flounder | EI: content | Salas-Leiva et al., 2017 | |
| Weissella | Rainbow trout | DI; auto and allo | Lyons et al., 2016 |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017a | |
| Rainbow trout | DI; auto and allo | Lyons et al., 2017b | |
| Atlantic salmon | Digesta samples | Dehler et al., 2017b | |
| Atlantic salmon | DI; content | Gajardo et al., 2017 | |
| Atlantic salmon | EI; content | Rudi et al., 2018 | |
| Atlantic salmon | EI; auto and allo | Rimoldi et al., 2018 | |
| Rohu (Labeo rohita) | EI; allo | Shahid et al., 2017 | |
| Tilapia | EI; auto and allo | Standen et al., 2015 | |
| Common snook (Centropomus undecimalis)—larvae | Whole larvae | Tarnecki and Rhody, 2017 | |
| Fine flounder | EI: content | Salas-Leiva et al., 2017 | |
| W. paramesenteroides | Pirarucu | EI; auto and allo | do Vale Pereira et al., 2017 |
| Bifidobacterium | Nile tilapia | EI: content | Boonanuntanasarn et al., 2017 |
A no further information was given; EI, entire intestine without pyloric caeca; PI, posterior intestine; DI, distal intestine; auto, autochthonous; allo, allochthonous; NI, no information.
During the last decades, numerous investigations on the isolations of LAB in finfish have been carried out. According to Merrifield et al. (2014) members belonging to Lactobacillus, Lactococcus, Leuconostoc, Enterococcus, Streptococcus, Carnobacterium, Pediococcus, and Weissella genera are indigenous species in finfish. In this subsection, results of some investigations published the last 3 years are presented. Readers with special interest in studies not described in the text are recommended to have a closer look at the original papers.
LAB
In numerous studies, counts of presumptive LAB has been revealed, but without going into further identification. In their study of Persian sturgeon (Acipenser persicus L.) larvae fed tuna viscera protein hydrolysate, Ovissipour et al. (2014) reported that culturable LAB counts in the intestinal contents was significantly (P < 0.05) higher when the larvae were fed fish protein hydrolysate at the highest inclusion level, 347g kg−1, compared to control fed larvae. However, the log LAB counts were only ~3.0 compared to log levels of total counts; ~5.0. In their comprehensive review devoted to dietary effect on gut microbiota of finfish, Ringø et al. (2016) revealed an overview on gut microbiota due to seasonal variations. It is also worth mentioning that seasonal variations of Lactobacillus and putative pathogenic bacteria density occurs in aquaculture system (Resende et al., 2015). Neuman et al. (2015) evaluated the effect of diets, smolt-, summer, and growing diets, on fecal microbiota of farmed Tasmanian Atlantic salmon (Salmo salar L.) and revealed a decrease in LAB numbers during rearing from November to May. Furthermore, Hoseinifar et al. (2016a) revealed that increasing supplementation of xylooligosaccharide significantly increased population level of presumptive gut LAB in Oscar (Astronotus ocellatus).
carnobacterium
Genus Carnobacterium belongs to the family Carnobacteriaceae within the order of Latobacillales and consists currently of 10 species of which; Carnobacterium (piscicola) maltaromaticum, C. mobile, Carnobacterium divergens, C. alterfunitum, and C. inhibens have been isolated from finfish intestine. The first study to isolate carnobacteria from GI tract of finfish, wild Atlantic salmon (S. salar L.), was carried out by Strøm (1988). She initially identified the bacterium as Lactobacillus plantarum Lab01, but later Ringø et al. (2001), reclassified the bacterium as C. divergens.
During the last 3 years, have several studies revealed genus Carnobacterium in finfish intestine (Table 1). As the distal intestine (DI) is considered to be the primary site of intestinal absorption of macromolecules in salmonids (Ringø et al., 2003; Desai et al., 2012), Lyons et al. (2017a) “investigated the diversity of allochthonous and autochthonous bacteria in DI of rainbow trout (Oncorhynchus mykiss) by next generation sequencing (NGS) and revealed that carnobacteria were the most prevalent of the autochthonous LAB genera (6.2%), and 4.15% of the allochthonous bacteria belonged to genus Carnobacterium.” In an investigation evaluated the dietary effect of black soldier fly (Hermetia illucens) by DGGE, Bruni et al. (2018) reported Carnobacterium sp., and that C. divergens were one of the dominant bacterial species in the insect-fed groups vs. control fed fish.
lactobacillus
Lactobacillus are acid-tolerant facultative anaerobes, and they are either homo- or heterofermentative. Kraus (1961) carried out the first study revealing that fish, herring (Clupea harengus L.), contained lactobacilli in the GI tract. Since this pioneer study was carried out, have several reviews revealed Lactobacillus species in the GI tract of several finfish species (e.g., Ringø and Gatesoupe, 1998; Ringø, 2004; Ringø et al., 2005; Gatesoupe, 2008; Lauzon and Ringø, 2011; Merrifield et al., 2014).
Table 1 show that Lactobacillus spp., Lb. aviarius, Lb. aviaries subsp. arafinosus, Lactobacillus brevis, Lb. crispatus/Lb. amylovorus, Lb. crispatus, Lb. collinoides, Lb. coryniformis, Lb. farciminis, Lb. gallinarum, Lb. johnsonii, Lb. reuteri, and Lb. sakei have been reported in the GI tract of several finfish species during the last 3 years. Characterization of the DI microbiome of rainbow trout from both farm and aquarium settings were investigated by Lyons et al. (2016). Differences were noted in the microbial community within the intestine of both populations, Phylum Firmicutes was slightly more prominent in the aquarium reared fish, and within principal OTUs were identified as Lactobacillus, Acetanaerobacterium, Catellicoccus, Streptococcus, Lactococcus, Leuconostoc, Enterococcus, Weissella, and Bacillus. Bahramian and Parsa (2017) revealed that culturable Lactobacillus spp. was reduced in the GI tract of rainbow trout fed diets supplemented with essential oil of Pistacia atlantica subsp. kurdica. In the study of Lyons et al. (2017a), the authors revealed that Lactobacillus was present in very low abundance (0.1%), but a higher proportion (1.15%) of Lactobacillus was displayed by the allochthonous microbiota in the DI of rainbow trout.
An interesting topic within gut bacterial adherence and colonization is; to how increase the relative abundance of beneficial Lactobacillus. In a recent study, (Liu W. et al., 2017) evaluated the effect of gut adhesive Lactobacillus strains and the combined effect of short chain fucto-oligosaccharides (scFOS) on growth performance, gut adhesive bacteria and disease resistance of juvenile tilapia, and concluded that scFOS increased the relative abundance of the Lactobacillus strains.
The effect of chromic oxide (Cr2O3), one of the most widely used indicators for determination of nutrient digestibility in fish (Austreng, 1978; Ringø and Olsen, 1994), is less investigated in finfish studies. In three studies using Arctic charr (Salvelinus alpinus L.), Ringø (1993a,b, 1994) revealed that inclusion of 1% (Cr2O3) increased population level of culturable Lactobacillus and Streptococcus. In contrast, Serra et al. (2018) using the DGGE method to evaluate the gut microbiota of gilthead seabream (Sparus aurata) juvenile showed no effect of 0.5% inclusion level of Cr2O3 on number of operational taxonomic units, microbiota richness, diversity and similarity indices. The authors suggested that the difference between their results and Ringø's may be due to different inclusion level and the sharpening of the GI tract of the fish species.
lactococcus
The genus Lactococcus is included within the family Streptococcacceae, and was described for the first time in 1985 after the division of genus Streptococcus, which included a group of microorganisms known as lactic streptococci represented by agents isolated from plant material, dairy cattle, and milk products (Schleifer et al., 1985). Lactococcus produce L (+) lactate from glucose as opposed to Leuconostoc produce D (–) lactate from glucose. One of the first studies isolating genus Lactococcus from finfish, common carp (Cyprinus carpio), was revealed by Cai et al. (1999), but later the genus has been isolated from the GI tract of several finfish species (Merrifield et al., 2014), and during the last years, numerous studies have revealed Lactococcus spp., L. lactis garvieae, L. lactis subsp. cremoris, L. piscium, and L. raffinolactis in the GI tract of finfish (Table 1). In their study with turbot (Scophthalmus maximus); autochthonous microbiota in the entire intestine, Yang et al. (2018) revealed that dietary stachyose significantly elevated the abundance of Lactococcus as well as Carnobacterium, Pediococcus, and Enterococcus. Li et al. (2015) used culture-dependent and culture-independent techniques to investigate the autochthonous bacterial communities in the whole intestine of grass carp (Ctenopharyngodon idellus) (Valenciennes) and revealed seven culturable strains showing high similarity (99%) to L. raffinolactis and one OUT similar to L. raffinolactis. Lyons et al. (2017a) revealed that both autochthonous and allochthonous Lactococcus was present in very low abundance (0.2 and 0.23%, respectively) in the DI of farmed rainbow trout.
leuconostoc
Leuconostoc spp. are generally ovoid cocci often forming chains; are resistant to vancomycin and are catalase-negative. All Leuconostoc species are heterofermentative, produce D (–) lactate from glucose and are able to produce dextran from sucrose, and are generally slime-producers. Species of genus Leuconostoc are isolated from different sources (Carr et al., 2002) as well as from the GI tract of finfish (Merrifield et al., 2014). Since 2016, genus Leuconostoc, both autochthonous and allochthonous, has been reported in the intestine of rainbow trout, Atlantic salmon and Arctic charr (Table 1).
pediococcus
Pediococcus usually occur in pairs or tetrads, and divide along two planes of symmetry, and they are purely homofermentative. To our knowledge, the first studies to isolate Pediococcus from intestine of finfish was carried out in the late 90's by Cai et al. (1999) and Halami et al. (1999). During the last 3 years, only one study has revealed Pediococcus in the intestine of finfish, turbot, evaluating the effect of dietary stachyose; a significant higher abundance of Pediococcus was revealed in fish fed diet added 5% stachyose (Yang et al., 2018).
streptococcus
This genus has been subjected to important changes, as several species have been reclassified into genera Lactococcus, Enterococcus, and Vagococcus, based on biochemical characteristics and by molecular methods (Schleifer and Kilpper-Bälz, 1984; Schleifer et al., 1985; Collins et al., 1989). Species within genus Streptococcus have been isolated from several finfish species (Merrifield et al., 2014).
An overview of streptococci species revealed in the intestine of finfish since 2016 and until today is presented in Table 1. Lyons et al. (2017a) revealed that autochthonous Streptococcus was present in low abundance (2.3%) in the DI of farmed rainbow trout, but a slightly higher abundance (2.89%) was noticed by the allochthonous microbiota.
enterococcus
Modern classification techniques of Enterococci resulted in the transfer of some members of genus Streptococcus, Lancefield's group D streptococci, to the new genus Enterococcus. Recently, Lyons et al. (2017a) revealed that autochthonous Enterococcus was present in low abundance (1.72%) in the DI of farmed rainbow trout. In addition to Enterococcus spp., E. faecalis and Enterococcus faecium were isolated from the GI tract of mrigal (Cirrhinus mrigala) (Shahid et al., 2017) and European sea bass (Dicentrarchus labrax) (Torrecillas et al., 2017), respectively.
vagococcus
Collins et al. (1989) proposed that on the basis of the present sequence data and earlier chemotaxonomic studies that the motile group Lancefield group N cocci strains be classified in a new genus Vagococcus. The first study isolated Vagococcus (Vagococcus fluvialis) from finfish intestine was displayed by González et al. (2000). Recently Lyons et al. (2017a) revealed that autochthonous Vagococcus was present in low abundance (1.74%) in the DI of farmed rainbow trout, while the abundance of allochthonous Vagococcus was 0.72%.
weissella
Genus Weissella belongs to Leuconostocaceae family and are obligate heterofermentative, producing CO2 from carbohydrate metabolism with either D (–), or a mixture of D (–)—and L (+)—lactic acid and acetic acid as major end products from sugar metabolism. According to the review of Fusco et al. (2015), there are 19 Weissella species known. The first study revealing Weissella (W. confusa) from the intestinal tract of fish, seabass (Lates calcarifer), was carried out by Rengpipat et al. (2014). During the last 3 years, several studies have revealed Weissella in the digestive tract of finfish (Table 1). For example, Lyons et al. (2017a) revealed that both autochthonous and allochthonous Weissella was present in very low abundance (0.1 and 0.39%) in the DI of farmed rainbow trout.
bifidobacterium
Bifidobacterium are commonly reported in the GI tract of endothermic animals, but they are only been isolated in few studies from the digestive tract of finfish (Merrifield et al., 2014). Recently, Boonanuntanasarn et al. (2017) revealed increased population level of Bifidobacterium spp. by feeding Nile tilapia (Oreochromis niloticus) fingerlings fed inulin and Jerusalem artichoke (Helianthus tuberosus).
Antibacterial effects of LAB; bacteriocins produced by LAB
Massive growth and intensification in aquaculture during the last decades has been associated with numerous problems; fish diseases caused by pathogenic bacteria being one of them (Sahoo et al., 2016). An array of conventional and advanced prophylactic or curative measures have been put forward to dispose of bacterial fish diseases, e.g., use of antibiotics (Burridge et al., 2010), vaccines (Gudding and Van-Muiswinkel, 2013), disinfectants, feed additives, dietary supplements, herbal immunostimulants (Newaj-Fyzul and Austin, 2014), prebiotics (Ganguly et al., 2012), and probiotics (e.g., Verschuere et al., 2000; Kesarcodi-Watson et al., 2008; Nayak, 2010; Pandiyan et al., 2013; Dawood and Koshio, 2016). The commonly use of disinfectants and antimicrobial agents as growth promotors and in disease control in aquaculture, increased the concern about the indiscriminate use due to the selective pressure on the intestinal microorganisms and development of antibiotic resistant bacteria (Cabello, 2006; Kolndadacha et al., 2011; Romero et al., 2012). As a natural consequence, there was seek for novel antibacterial compounds (preferably proteinaceous) with prophylactic or therapeutic potential and for which pathogens may not develop resistance (Patil et al., 2001; Sahoo et al., 2016).
The antibacterial agents are antibiotics, bacteriocins, lysozymes, proteases, siderophores, and/or hydrogen peroxide and acidic pH by organic acids production (De Vuyst and Leroy, 2007; Bindiya et al., 2015; Mukherjee et al., 2016).
Bacteriocins, are ribosomal-synthesized antimicrobial peptides, and LAB are the most common producers (Zacharof and Lovitt, 2012; Silva et al., 2018). They are small cationic molecules of 30–60 amino acids, form amphiphilic helices and are stable at 100°C for 10 min. During the last decade probiotic LAB with antimicrobial potential has achieved interest in aquaculture (Muñoz-Atienza et al., 2013), and the use of bacteriocins as supplements or adjuncts could be an eco-friendly approach to alleviate antibiotic overuse and resistance (Lagha et al., 2017).
Fish could be a potential source of bacteriocin-producing (bacteriocinogenic) bacteria and extensive screening of gut associated microorganisms may be taken up to avoid the use of antibacterial drugs in aquaculture (Sahoo et al., 2016). Reports indicated that the LAB isolated from diverse fish species, other aquatic organisms, culture water and sediments possess antagonistic activity against the fish pathogens (Balcázar et al., 2007a,b; Sugita et al., 2007; Ringø, 2008; Shahid et al., 2017). Hence, the potential use of bacteriocinogenic LAB as probiotics and bio-protective agents has received growing attention during the last decade (e.g., Gillor et al., 2008; Satish Kumar et al., 2011; Heo et al., 2012). According to Elayaraja et al. (2014), genera Lactobacillus, Lactococcus, Streptococcus, Pediococcus, Oenococcus, Enterococcus, Leuconostoc, and Carnobacterium produce a variety of bacteriocins. Numerous investigations on isolation and characterization of bacteriocins and bacteriocinogenic LAB from different sources are available, however, lesser research has been done on bacteriocins of LAB from fish (Gómez-Sala et al., 2015).
This section will present an overview on the beneficial attributes that might be associated with the use of bacteriocins and bacteriocinogenic LAB in aquaculture, diverse classes of bacteriocins produced by LAB, methods to characterize bacteriocins and an update on the efficacy of LAB against fish pathogens.
Benefits associated with the LAB and bacteriocins produced by LAB
Interest on bacteriocinogenic bacteria, especially LAB, has achieved huge impetus due to its potential as both, probiotics and therapeutic antibiotics (Gillor et al., 2008; Cotter et al., 2013; Perez et al., 2014). Bacteriocins have several positive attributes that made them especially attractive for application in various sectors including aquaculture (Perez et al., 2014).
LAB and its metabolites are generally regarded as safe for human consumption, as they are found or used in food and fermented food products (FAO/WHO, 2002). Thus, aquatic organisms produced with application of LAB or bacteriocins thereof could be considered as safe for human consumption.
LAB bacteriocins are tolerant to high thermal stress and their activity over a wide pH range are well-known. Therefore, if applied as aquafeed supplement, efficacy of the bacteriocins from LAB is expected to be retained within the fish GI tract.
Bacteriocins forms pores in the target membrane of bacteria, even at extremely low concentrations.
These microbial metabolites are colorless, odorless, and tasteless, and therefore, do not interfere with acceptability of the diet if used as a supplement.
To our knowledge, there are no documentation on the development of resistant bacteria.
Bacteriocins usually have low molecular weight (rarely over 10 kDa), and they undergo posttranslational modification. Being proteinaceous, they can be easily degraded by the proteolytic enzymes of the host (Zacharof and Lovitt, 2012). Therefore, bacteriocin fragments do not live long either in the host or in the environment, thus minimizing the opportunity of target strains to interact with the degraded fragments and development of resistance.
Bacteriocins are ribosomally synthesized and produced during the primary phase of growth unlike antibiotics, which are usually secondary metabolites (Beasley and Saris, 2004). Bacteriocins generally restrict their activity to the strains of species closely related to the producer strain (Lisboa et al., 2006; Bakkal et al., 2012); compared to antibiotics having wider activity spectrum (broad-spectrum).
Not only antagonistic against some fish pathogens, bacteriocin has also been reported to be an important molecule in quorum sensing process (Czaran et al., 2002; Gobbetti et al., 2007). In fact, quorum sensing has been believed to be responsible for the expression of genes that code for bacteriocins in LAB. To outcompete the related species, sensing of its own growth enables the LAB to switch on bacteriocin production when competition for nutrients is likely to become more severe (Eijsink et al., 2002).
Classes of bacteriocins produced by LAB
Gram-positive bacteria account for the majority of bacteriocins recorded per se (Rather et al., 2017), although bacteriocins are also revealed in Gram-negative (Sahoo et al., 2016). Among the Gram-positive bacteria, bacteriocins produced by LAB have gained particular attention nowadays. However, to deal with, firstly we need to see the classes of bacteriocins produced by diverse bacteria and then bacteriocins produced by LAB may be narrowed down.
Bacteriocin classification is an ongoing subject of debate, and therefore, proper classification is yet to be established (Desriac et al., 2010). A variety of criteria or their combinations are proposed as the basis for bacteriocin classification. For example, the producer bacterial family, molecular weights, amino acid composition, sequence homologies, primary structures, organization of the gene cluster (Hammami et al., 2010), mechanism of action and Gram designation. Bacteriocins were primarily divided into four classes (Klaenhammer, 1993). The Class I bacteriocins are called lantibiotics, represented by nisin and lactocin, gathers very low molecular weight (< 5 kDa) thermostable peptides, characterized by the post-translational modification and presence of lanthionine or derivatives. The Class II bacteriocins consist of small thermostable peptides (< 10 kDa) divided into three subclasses: IIa (pediocin and enterocin), IIb (lactocin G) and IIc (lactocin B). They are usually non-modified peptides, cationic, hydrophobic and often amphiphilic reflecting their ability to act on target cells by permeabilizing the cell membrane. Class IIa bacteriocins, the mostly studied LAB bacteriocins possessed strong antimicrobial properties against a broad range of Gram-positive spoilage and food-borne pathogens (Sahoo et al., 2016). The Class III bacteriocins having high molecular weight (>30 kDa), thermolabile peptides such as the helveticin J, while in the Class IV we can find large complexes of peptides with carbohydrates or lipids. Cotter et al. (2005) suggested a new classification; dividing bacteriocins into two categories: lantibiotics (Class I) and not containing lanthionine lantibiotics (Class II), while high molecular weight thermolabile peptides formally recognized under the above class III, would be separately re-classified as “bacteriolysins,” i.e., hydrolytic polypeptides. Thus, finally bacteriocins are divided into three major classes according to their genetic and biochemical characteristics (Drider et al., 2006). Consequently, different types of bacteriocins produced by the LAB are now classified (Table 2) as: Class I or Lantibiotics (<5 kDa), Class II or Non-Lantibiotics (usually <10 kDa) and Class III bacteriocins (generally > 30 kDa) (Ghosh et al., 2014).
Table 2.
Different classes of bacteriocins produced by the LAB.
| Classes | Characteristic features | Bacteriocins produced | Typical producer organism | References |
|---|---|---|---|---|
| Class I: Lantibiotics | Lantibiotics, small (<5 kDa) peptides containing lanthionine and b-methyllanthionine | Nisin, lactocin, mersacidin | Lb. lactis subsp. lactis | Parada et al., 2007 |
| Class II: Non-lantibiotics | Small (<10 kDa), heat-stable, non-lanthionine-containing peptides | |||
| Class IIa | Heat stable, non-modified, cationic, hydrophobic peptides; contain a double–glycine leader peptide; pediocin-like peptides | Pediocin PA1, sakicin A, leucocin A | Lc. gelidum | Todorov, 2009 |
| Class IIb | Require synergy of two complementary peptides; mostly cationic peptides | Lactococcin G, plantaricin A, enterocin X | E. faecium | Perez et al., 2014 |
| Class IIc | Affect membrane permeability and cell wall formation | Acidocin B, entereocin P, reuterin 6 | Lb. acidophilus | Šušković et al., 2010 |
| Class III: Large heat labile bacteriocins | Heat sensitive peptides, large molecular mass (>30 kDa) | Lysostaphin, enterolysin A, helveticin J | Lb. helveticus | Cotter et al., 2005 |
Screening and characterization of bacteriocins produced by LAB
Bacteriocins are ribosomally synthesized peptides, which are usually synthesized as inactive precursors of peptides having an N-terminal sequence and later modified to attain an active state (Todorov, 2009; Perez et al., 2014). The activity of bacteriocins produced by different LAB is not uniform and constant, and depends on the physico-chemical composition of the microbial growth media (Balciunas et al., 2013). For aquaculture application of either bacteriocinogenic LAB or their bacteriocins, screening of efficient organism is a prerequisite. Bacteriocinogenic potential of a strain can be studied either by culture-dependent methods or by molecular methods employing PCR amplification of known bacteriocin structural genes. Initial screening to detect and determine the antibacterial activities of bacteriocinogenic strains can be done by an agar spot test (Schillinger and Lücke, 1989) or by agar well diffusion assay (Srionnual et al., 2007); using some indicator strains, e.g., Lb. sakei ssp. sakei JCM 1157T and Listeria monocytogenes ATCC 19111 (Lin et al., 2012). Then, antibacterial activity of the crude bacteriocin or bacteriocin like inhibitory substance (BLIS) may be further confirmed and optimized by characterization of the cell-free supernatants through pH and temperature adjustments, and proteinase-K treatment (Lin et al., 2012). For molecular detection of bacteriocinogenic potential, PCR amplification of known bacteriocin structural genes can be performed using the specific primers. For example, enterocin structural genes may be amplified with specific primers like EnterA-F/EnterA-R for detection of enterocin A (entA), EntB3/EntB5 for enterocin B (entB), EntP1/EntP2 for enterocin P (entP), and so on (Almeida et al., 2011; Gómez-Sala et al., 2015).
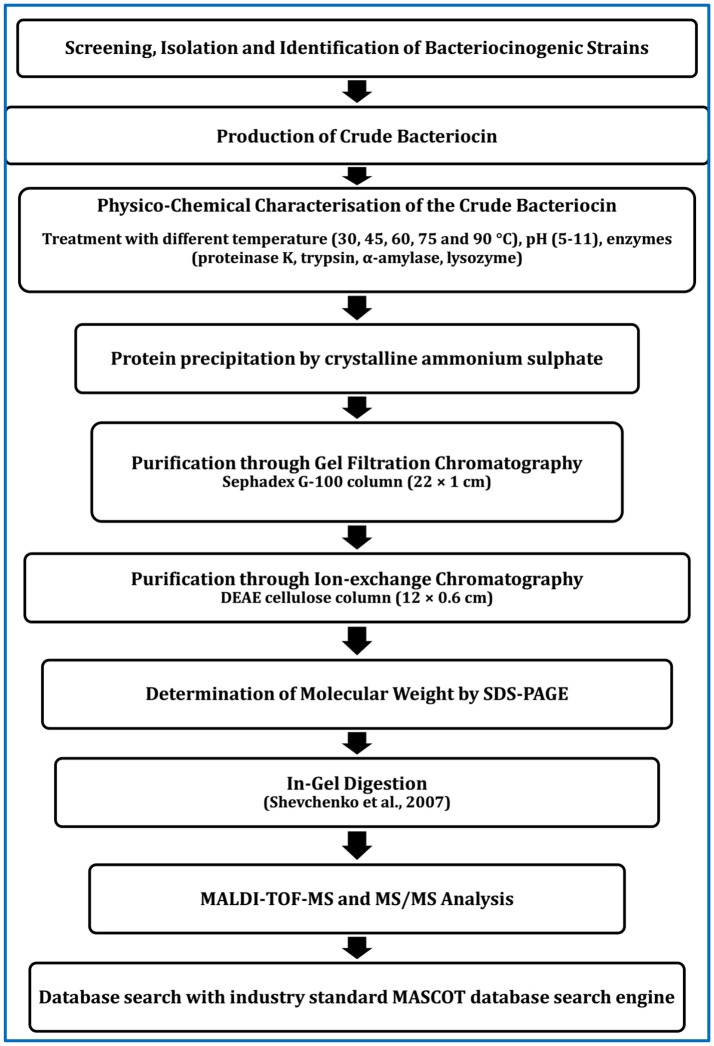
For application of bacteriocinogenic LAB as probiotics, screening and determination of potent LAB strain would be sufficient. However, for application of purified bacteriocin as feed supplement, production of pure bacteriocin and determination of molecular mass seem to be essential. Purification can be done by several steps as depicted in Figure 1: ammonium sulfate precipitation, gel filtration chromatography followed by ion-exchange chromatography. The active fraction that would display maximum antibacterial activity should be collected and used for further studies. The purity, homogeneity and molecular size of BLIS can be determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Srionnual et al., 2007). The molecular mass of the purified bacteriocin can be determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) using a mass spectrometer and database search through Mascot search engine (Lin et al., 2012).
Figure 1.
Scheme for purification of bacteriocins produced by LAB or other bacteria.
As per as aquaculture application is concerned, the use of purified bacteriocins is still a question mark, as the major apprehension would be administration of the compounds to the farmed fish that are aquatic. Numerous studies have recommended the bacteriocinogenic strains to be used as aquaculture probiotics (Irianto and Austin, 2002; Gatesoupe, 2008; Issazadeh et al., 2012). This is indeed a more reasonable and practical approach than direct application of purified bacteriocins in consideration of the fact that the probiotic strains are live cultures and thus able to ultimately establish themselves in the hosts and the aquatic environment (Rather et al., 2017).
Activity of bacteriocinogenic LAB against fish pathogens: an update
It has been predicted that application of bacteriocins/BLIS from LAB or bacteriocinogenic LAB might not only effective in preventing diseases, but also minimize the risks of using broad-spectrum antibiotics in aquaculture. In aquaculture, numerous studies have indicated the potential use of bacteriocinogenic LAB as biocontrol agents against pathogens (e.g., Gillor et al., 2008; Desriac et al., 2010; Satish Kumar et al., 2011; Heo et al., 2012). Apart from LAB of fish origin, LAB from non-fish sources has also been tested to accomplish health benefits or disease prevention and achieved experimental success. For example, administration of the human probiotic, Lactobacillus rhamnosus 53101, reduced mortalities from 52.6 to 18.9% (109 cells/g of feed) and to 46.3% (1012 cells/g of feed) in rainbow trout following challenge with Aeromonas salmonicida (Nikoskelainen et al., 2001). Furthermore, LAB-produced bacteriocins have been applied as bio-preservatives in marine food products and have shown to control pathogenic and spoilage microorganisms (Calo-Mata et al., 2007; Yin et al., 2007; Diop et al., 2009; Chahad et al., 2012).
To avoid harmful effects on the host fish as well as on the indigenous microbiota, use of autochthonous bacteria or their metabolites might be preferred to use vs. allochthonous. In aquaculture, the justification of using LAB or bacteriocinogenic LAB isolated from the autochthonous microbiota is based on the fact that the producer bacterial strains occupy more or less the same ecological niche with the pathogens and hosts of concern (Prasad et al., 2005; Zai et al., 2009). Antagonistic activity of LAB isolated from fish intestine against fish pathogens i.e., furunculosis, columnaris, peduncle disease, streptococcosis have been documented (e.g., Gutowska et al., 2004; Ringø et al., 2005; Sugita and Ito, 2006; Sahoo et al., 2016; Banerjee and Ray, 2017). Although, bacteriocins characterized from fish—and aquatic bacteria are scarce (Table 3), most of the characterized bacteriocins of aquatic origin that have antagonistic activity against many bacterial pathogens are isolated from marine aquaculture, while few from freshwater (Sahoo et al., 2016).
Table 3.
Bacteriocins from LAB characterized and identified from aquatic resources.
| Bacteriocinogenic LAB | Source | Bacteriocin/BLIS (Molecular weight) | Antagonistic to pathogens | References |
|---|---|---|---|---|
| E. faecium | Mangrove | Enterocin (5 kDa) | Listeria monocytogenes, Lb. plantarum, Listeria innocua, E. faecalis, Salmonella typhi, Salmonella paratyphi | Annamalai et al., 2009 |
| E. faecium ALP7 P. pentosaceus ALP57 | Marine shellfish | Enterocin B P Ediocin PA-1/AcH (<10 kDa) | Listeria innocua, Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, other LAB | Pinto et al., 2009 |
| Lb. acidophilus | Gut of marine prawn (Penaeus monodon) | Bacteriocin (2.5 kDa) | Lb. bulgaricus, Salmonella enteric serovar typhimurium, Staphylococcus aureus, Bacillus subtilis, Salmonella enterica serovar paratyphi ‘B', Escherichia coli, Klebsiella sp., Serratia marcescens, Pseudomonas aeruginosa | Karthikeyan and Santhosh, 2009 |
| E. faecium PE2-2 | Sword fish | Enterocin A | Listeria sp., Enterococcus sp., Staphylococcus sp. | Valenzuela et al., 2010 |
| Lb. lactis | Marine sediments (Chennai Harbor, India) | Bacteriocin (94 kDa) | B. subtilis, Staph. aureus, E. faecalis, P. aeruginosa | Rajaram et al., 2010 |
| E. faecium MC13 | Gray mullet (Mugil cephalus) | Enterocin (2.148 kDa) | V. parahaemolyticus, Vibrio harveyi, A. hydrophila | Satish Kumar et al., 2011 |
| Lb. fermentum | Gray mullet (gut), prawn (Penaeus monodon) (muscle) | Bacteriocin (18 kDa) | V. parahaemolyticus, L. monocytogenes, Listeria sp., Staph. aureus | Indira et al., 2011 |
| L. lactis PSY2 | Marine perch fish (Perca flavescens) | Bacteriocin PSY2 | Arthrobacter sp., Acinetobacter sp., Bacillus subtilis, E. coli, L. monocytogenes | Sarika et al., 2012 |
| E. thailandicus B3-22 | Gray mullet | BLIS (6.3 kDa) | L. garvieae | Lin et al., 2012 |
| Lactobacillus casei AP8 | Persian sturgeon (Acipenser persicus) (gut) | BLIS AP8 (5 kDa) | E. coli, Listeria spp., Salmonella spp., Staph. aureus, A. hydrophila, V. anguillarum, B. cereus | Ghanbari et al., 2013 |
| Lb. plantarum H5 | Persian sturgeon (gut) | BLIS H5(3 kDa) | E. coli, Listeria spp., Salmonella spp., Staph. aureus, A. hydrophila, V. anguillarum, B. cereus | Ghanbari et al., 2013 |
| Lb. brevis FPTLB3 | Mrigala (Cirrhinus mrigala) | BLIS (54 kDa) | E. coli, E. faecalis, Lb. casei, Lb. sakei, Staph. aureus | Banerjee et al., 2013 |
| Lb. fermentum strain SBS001 | Estuarine water | Bacteriocin (78 kDa) | Klebsiella oxytoca, P. aeruginosa, E. coli | Singh et al., 2013 |
| E. faecalis | Marine environment | Bacteriocin (94 kDa) | E. faecalis, Staph. aureus, B. subtilis | Vadanasundari et al., 2013 |
| Lb. murinus AU06 | Marine sediments | BLIS (21 kDa) | Micrococcus sp., Staph. aureus, P. aeruginosa, E. coli | Elayaraja et al., 2014 |
| L. lactis PSY2 | Mucus and scales of marine fish (viz., Platax sp., Perca sp. and Tuna sp.) | Bacteriocin PSY2 | L. monocytogenes | Sarika et al., 2017 |
It has been predicted that Pscicocin V1a and Pscicocin V1b isolated from C. piscicola CS526 and C. piscicola V1, respectively could prevent haemorrhagic septicaemia caused by Pseudomonas sp. (Bhugaloo-Vial et al., 1996). In another report, Phocaecin PI80 bacteriocin produced by Streptococcus phocae PI80 isolated from the gut of Indian white shrimp (Peneaus indicus) has been documented that might prevent Vibrio septicaemia caused by Vibrio sp. (Kumar and Arul, 2009). Likewise, BLIS AP8 from Lactobacillus casei AP8 and bacteriocin like inhibitory (substance) H5 from Lb. plantarum H5 might be effective against haemorrhagic septicaemia and Vibrio septicaemia (Ghanbari et al., 2013), although their mode of action is yet to be confirmed. In addition, the bacteriocin-producing LAB from aquatic organisms including fish include enterocin P produced by E. faecium isolated from turbot (Arlindo et al., 2006), nisin F produced by L. lactis from freshwater catfish (Clarias gariepinus) (De Kwaadsteniet et al., 2008), and divercins and piscicocins produced by Carnobacterium spp. (Desriac et al., 2010).
Although several reports have shown promising results regarding the aquaculture potential of bacteriocinogenic LABs or their bacteriocins from aquatic sources, subsequent studies are still needed to substantiate its viability in field condition with large number of organisms (Rather et al., 2017). Moreover, application strategy of the bacteriocins from LAB maintaining its effectiveness should be standardized so as to explore its potential in the disease prevention and sustainability of the aquaculture industry.
LAB as probiotic
During the last years, numerous LAB strains have been used as probiotics in finfish aquaculture due to their health beneficial effect (Table 4). According to Belicova et al. (2013) an organism should be defined as probiotic when it is non-pathogenic, reveal antibacterial activities toward potential pathogens, tolerate low pH, high concentrations of conjugated, and de-conjugated bile salts, be accepted by the immune system, and not result in formation of antibodies. In addition, the probionts must not transfer antibiotic resistance genes to pathogens through horizontal gene transfer.
Table 4.
An overview on LAB used as probiotic in finfish aquaculture.
| Probiotic | Doses and administrationduration | Fish species | Parameters examined | References |
|---|---|---|---|---|
| Lb. plantarum | 2 × 107 CFU g−1–72 days | Rainbow trout (Oncorhynchus mykiss) | Growth performance and immune parameters | Soltani et al., 2017b |
| 10 × 109 CFU/kg–90 days | European sea bass (Dicentrarchus labrax) | Growth performance and serum biochemical parameters | Piccolo et al., 2015 | |
| 108 CFU g−1–60 days | Rainbow trout | Serum biochemical as well as immune responses | Kane et al., 2016 | |
| 1.2 × 106, 0.9 × 106, and 0.56 × 106 cfu/g–80 days | Common carp (Cyprinus carpio) | Growth performance, Immune parameters, disease resistance | Soltani et al., 2017a | |
| 1.81 × 107 CFU g−1–58 days | Nile tilapia | Growth performance, haemato-immunological parameters and gut microbiota | Yamashita et al., 2017 | |
| 108 CFU g−1–28 days | Nile tilapia | Intestinal microbiota, growth performance and resistance against Cd exposure | Zhai et al., 2017 | |
| Growth performance and resistance against waterborne aluminum exposure | Yu et al., 2017 | |||
| Heat killed Lb. plantarum | 0.01, 0.1, 1 and 2 g kg−1–56 days | Red sea bream (Pagrus major) | Growth performance, immune parameters and antioxidant defense | Dawood et al., 2015 |
| Lb. plantarum + B. subtilis + P. aeruginosa | 0.5 × 108 CFU g−1–60 days | Rohu (Labeo rohita) | Immune parameters, antioxidant defenses and disease resistance | Giri et al., 2014 |
| Lb. plantarum + L. lactis | log10 7.0 CFU/g–30 days | Olive flounder (Paralichthys olivaceus) | Immune parameters and disease resistance | Beck et al., 2015 |
| Lb. plantarum +LMWSA | 108 CFU g−1–60 days | Nile tilapia (Oreochromis niloticus) | Growth performance, immune parameters and disease resistance | Van Doan et al., 2016c |
| Lb. plantarum + Jerusalem artichoke | 108 CFU g−1–12 weeks | Pangasius catfish (Pangasius bocourti) | Growth performance, immune parameters and disease resistance | Van Doan et al., 2016a |
| Lb. plantarum + Eryngii mushroom (Pleurotus eryngii) | 108 CFU g−1–90 days | Pangasius catfish | Growth performance, immune parameters and disease resistance | Van Doan et al., 2016b |
| Lb. acidophilus | 1.5 × 108, 3 × 108 and 6 × 108 CFU g−1–70 days | Black swordtail (Xiphophorus helleri) | Growth performance, mucosal immunity and intestinal microbiota | Hoseinifar et al., 2015c |
| 1.5 × 108, 3 × 108 and 6 × 108 CFU g−1–56 days | Gold fish (Carassius auratus gibelio) | Skin mucus protein profile and immune parameters, appetite and immune related genes expression | Hosseini et al., 2016 | |
| 106 CFU g−1–15 days | Nile tilapia | Immune related genes expression and disease resistance | Villamil et al., 2014 | |
| Lb. acidophilus + B. cereus + Clostridium butyricum | 1.0 × 109 CFU g−1–60 days | Hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus ♀) | Growth performance, digestive and antioxidant enzymes activities | He et al., 2017 |
| Lb. casei | 5 × 106, 5 × 107 and 5 × 108 CFU g−1–60 days | Shirbot (Barbus gryprus) | Growth performance and digestive enzymes activity | Mohammadian et al., 2017 |
| 1.0 × 108 cells/g–28 days | Zebrafish (Danio rerio) | Reproductive performance and related genes expression | Qin et al., 2014 | |
| Lb. casei + apple cider vinegar | 108 CFU g−1–56 days | Common carp | Serum and mucus immune parameters, immune and antioxidant defense related genes expression | Safari et al., 2017 |
| Lb. paracasei | 106 CFU g−1–66 days | Rainbow trout | Growth performance and intestinal microbiota | Lopez Cazorla et al., 2015 |
| Lb. delbrueckii | 1 × 105, 1 × 106, 1 × 107 and 1 × 108 CFU g−1 | Common carp | Intestinal immune parameters, immune related genes expression, antioxidant defense, disease resistance | Zhang C.-N. et al., 2017 |
| Lb. delbrueckii ssp. bulgaricus | 5 × 107 CFU g−1–60 days | Shirbot | Immune parameters and disease resistance | Mohammadian et al., 2016 |
| Lb. rhamnosus | 103,105 and 106 CFU/g–63 days | European eel (Anguilla anguilla) | Sperm quality and quantity, expression of genes related to spermatogenesis | Vílchez et al., 2015 |
| 1 × 102, 1 × 104 and 1 × 106 cells g−1–56 days | Red sea bream | Plasma and mucus parameters | Dawood et al., 2017 | |
| 107 and 108 CFU g−1–56 days | Rainbow trout | Intestinal microbiota and histology, biochemical parameters, and antioxidant defense | Topic (Popovic et al., 2017) | |
| Lb. rhamnosus+ Lb. lactis | 106 × cell/g–56 days | Red sea bream | Immune parameters and antioxidant defense | Dawood et al., 2016b |
| P. acidilactici | 106 CFU/g–10 days | Zebrafish | Expression of genes related to male and sperm quality | Valcarce et al., 2015 |
| 1 g kg−1–56 days | Green terror (Aequidens rivulatus) | Innate immune parameters and resistance to hypoxia stress | Neissi et al., 2013 | |
| P. acidilactici+ galactooligosaccharide (GOS) | 7.57 log CFU g−1–56 days | Rainbow trout | Growth performance, immune parameters and disease resistance | Hoseinifar et al., 2015a,b, 2016a |
| P. acidilactici+ GOS | 7.57 log CFU g−1–56 days | Common carp | Immune parameters and related genes expression | Modanloo et al., 2017 |
| P. pentosaceus | 6 × 1010, 1.6 × 1011, 1.6 × 1012 and 3.2 × 1012 cells g−1–56 days | Red sea bream | Skin mucus and serum immune parameters, resistance to low-salinity stress | Dawood et al., 2016a |
| 2 × 107, 2 × 108 and 2 × 109 CFU g−1–56 days | Siberian sturgeon | Intestinal and body composition | Moslehi et al., 2016 | |
| 109 CFU g−1–21 days | Orange-spotted grouper (Epinephelus coioides) | Growth performance, immune related genes expression and disease resistance | Huang J.-B. et al., 2014 | |
| W. cibaria | 1.18 × 107 CFU g−1–45 days | Brazilian native surubins | Growth performance, haemato-immunological parameters and intestinal histomorphology | Jesus et al., 2017 |
| Lc. mesenteroides+ E. faecalis+ Lb. fermentum | 105, 107 and109 CFU g−1–56 days | Javanese carp (Puntius gonionotus) | Growth performance, intestinal microbiota and body composition | Allameh et al., 2016 |
| L. lactis WFLU12 | 109 CFU g−1–56 days | Olive flounder | Growth performance, immune parameters and disease resistance | Nguyen et al., 2017 |
| E. faecium | 107 CFU/g–35 days | Javanese carp | Digestive enzymes activity, intestinal short chain fatty, disease resistance | Allameh et al., 2015 |
| E. gallinarum L-1 | 106, 107, and 108 cfu mL−1–28 days | Sea bream, European sea bass, meager (Argyrosomus regius) and red porgy (Pagrus pagrus) | Immune parameters and peroxidase content | Román et al., 2015 |
| E. casseliflavus | 107, 108, and 109 CFU g−1–56 days | Rainbow trout | Intestinal microbiota, humoral immune parameters and disease resistance | Safari et al., 2016 |
Considering the potential of LAB as feed additive in aquaculture there is extensive literatures available. The researchers investigated possible effects on growth performance, feed utilization, digestive enzymes activity, immune response, and disease resistance. Despite some contradictory results, most of the studies revealed beneficial effects on measured parameters. This section present an overview on available literatures regarding LAB administration as probiotic in aquaculture. To avoid overlap with previous reviews, we have focused on the papers published from 2014. Readers with special interests on previous studies, are referred to the reviews of Ringø and Gatesoupe (1998), Nayak (2010), Carnevali et al. (2014), Castex et al. (2014), De et al. (2014), Lauzon et al. (2014), Merrifield et al. (2014), Ringø et al. (2014) and Hoseinifar et al. (2016c).
lactobacillus spp.
lactobacillus plantarum
Within lactobacilli, Lb. plantarum is the most studied strain. Piccolo et al. (2015) evaluated the effects of dietary Lb. plantarum on performance and serum biochemical parameters of European sea bass. The inclusion level was 10 × 109 CFU/kg and fishes were fed on the probiotic supplemented diet for 90 days and probiotic feeding revealed noticeable effect on growth performance vs. control. Regarding serum biochemical parameters only total cholesterol and triglycerides were studied, but a significantly increased following probiotic administration was revealed. In a 72-days feeding trial, Soltani et al. (2017a) fed rainbow trout (vaccinated to yersiniosis) a probiotic diet containing Lb. plantarum, 2 × 107 CFU g−1. At the end of the trial, the vaccinated fish fed the probiotic diet had noticeably higher lysozyme and alkaline phosphatase compared to the other treatments. Besides, improved growth performance was noticed in the vaccine + probiotic treatment vs. the others. However, no significant difference among different treatments in case of hameato-immunological parameters as well as LAB levels in intestinal microbiota were revealed. The authors concluded that administration of probiotics following vaccination can be considered as beneficial by increasing vaccines efficacy. The same research group, Kane et al. (2016), evaluated the effects of 108 CFU g−1 of Lb. plantarum on serum biochemical as well as immune responses in rainbow trout treated with streptococcosis/lactococosis vaccine, and revealed that feeding Lb. plantarum to immunized fish resulted in significant increase of immune parameters such as lysozyme, alternative complement activities, antibody titer, total leukocytes and lymphocytes, and serum biochemical parameters. Moreover, Soltani et al. (2017b) supplemented a common carp diet with different levels (1.2 × 106, 0.9 × 106, and 0.56 × 106 CFU/g) of Lb. plantarum, and after 80 days feeding; significantly improved growth performance and immune parameters compared to the control treatment was noticed. However, probiotic administration had no significant effect on liver enzymes level. The challenge test showed that probiotic fed fish had higher resistance against Aeromonas hydrophila. When discussing the effect of probiotic toward disease resistance, Fečkaninová et al. (2017) reviewed and highlighted the potential of LAB to protect against different Aeromonas spp. in salmonid aquaculture.
The possible effects of Lb. plantarum on growth performance, haemato-immunological factors, intestinal microbiota and histology as well as disease of Nile tilapia was studied by Yamashita et al. (2017). Interestingly, dietary administration of Lb. plantarum increased LAB level and decreased Vibrionaceae counts in intestinal microbiota. Besides, feeding on probiotic improved growth performance and feed utilization, while no significant difference was observed pre-challenge, but probiotic fed fish showed improved hematological parameter post-challenge. On the other hand, histological evaluations, intestinal epithelium structure, revealed no significant difference between probiotic treatment and control fed fish. In a study using Nile tilapia, Zhai et al. (2017) evaluated the protective effects of 108 CFU g−1 Lb. plantarum against cadmium exposure. The study included four treatments; control, probiotic, Cd exposure and Cd exposure + probiotic. The exposure with Cd drastically decreased the richness of intestinal microbiota. However, feeding with probiotic reversed the changes were revealed. In addition, the highest growth performance was noticed in fish fed probiotics. The protective effects of Lb. plantarum against waterborne aluminum exposure of tilapia by Yu et al. (2017), revealed that fish fed Lb. plantarum CCFM639 significantly increased growth performance and alleviated aluminum damages. The effect of different levels of Lb. plantarum (1 × 107, 1 × 108, and 1 × 109 CFU g−1) on growth performance and immune parameters in Siberian sturgeon (Acipenser baerii) were investigated by Pourgholam et al. (2016). Compared to control treatment, significant increase of innate immune parameters were noticed in probiotic fed fish, and the highest level of immunity was observed in fish fed 1 × 108 CFU g−1 probiotic as well as improvements of growth performances.
Dietary administration of head-killed probiotic has been suggest as efficient and safe feed additive in aquaculture (Yan et al., 2016). Beside working on live Lb. plantarum, the efficacy of dead Lb. plantarum was evaluated by Dawood et al. (2015). Red sea bream (Pagrus major) with average weight of 11g were fed different levels (0.01, 0.1, 1, and 2 g kg−1) of heat killed Lb. plantarum for 56 days. The results revealed improved growth performance, immune parameters as well as antioxidant defense. The author displayed that 1 g kg−1 was the best inclusion level of heat killed Lb. plantarum for Red sea bream. However, as there is limited information available on the use of dead or inactivated probiotics on other species, this topic merits further investigations.
A review of the literature showed that, Lb. plantarum has been used as multi-strain probiotic and in combination with Bacillus subtilis VSG1 and Pseudomonas aeruginosa VSG2 (Giri et al., 2014), and feeding rohu (Labeo rohita) a multi-strain probiotic supplemented diet increased immune parameters, antioxidant defenses as well as disease resistance. The study also revealed that multi-strain administration was more efficient than single administration. In a study with olive flounder (Paralichthys olivaceus) fed Lb. plantarum FGL0001 and Lac. lactis BFE920 as multi-strain probiotics (Beck et al., 2015), the authors observed higher immune parameters and disease resistance in fish fed multi-strain probiotic vs. individual probiotic.
Gibson and Roberfroid (1995) proposed the synbiotics (a combination of pro- and prebiotics) concept; “characterize some colonic foods with interesting nutritional properties that make these compounds candidates for classification as health-enhancing functional ingredients.” This concept is well used in endothermic studies (e.g., DuPont and DuPont, 2011; Ford et al., 2014) as well as in fish (Ringø and Song, 2016). Van Doan et al. (2016a) evaluated combined administration of low molecular weight sodium alginate (LMWSA) as prebiotic with Lb. plantarum in Nile tilapia diet, and concluded that co-application increased the immunomodulatory effect as well as disease protecting effects of Lb. plantarum. Similar results were observed when Jerusalem artichoke (Van Doan et al., 2016b) or Eryngii mushroom (Pleurotus eryngii) (Van Doan et al., 2016c) were used in combination with Lb. plantarum in a diet fed to Pangasius catfish (Pangasius bocourti).
lactobacillus acidophilus
Lactobacillus acidophilus has been used as common probiotic in aquatic animals. Hoseinifar et al. (2015c) addressed the effects of different dose of Lb. acidophilus (1.5 × 108, 3 × 108, and 6 × 108 CFU g−1) on intestinal microbiota, mucosal immune parameters as well as stress resistance in black swordtail (Xiphophorus helleri). At the end of feeding trial, the probiotic strain successfully colonized the intestine and the dose of LAB significantly increased. Probiotic treatment, also increased growth performance as well as skin mucus immunity. Swordtail fish fed with Lb. acidophilus showed significantly higher resistance when exposed to salinity stress test. In another study with ornamental fish, Hosseini et al. (2016) investigated possible effects of Lb. acidophilus as probiotic on protein profile and immune parameters of skin mucus as well as ghrelin gene expression of gold fish (Carassius auratus gibelio). Dietary probiotic affected protein profile and improved immune parameters. Interestingly, feeding on probiotic suppressed appetite related gene, while, immune related genes were up-regulated by probiotic treatments. These studies highlighted the potential of Lb. acidophilus as probiotic for ornamental fish.
Furthermore, in a study with Nile tilapia, Villamil et al. (2014) evaluated possible effects of Lb. acidophilus on the expression of immune related genes as well as resistance against A. hydrophila. The results showed up-regulation of IL-1β and transferrin in spleen and kidney. Also, feeding on probiotic supplemented diet resulted in higher protection against disease. Furthermore, the author reported that extracellular products (ECPs) of Lb. acidophilus inhibited the growth of different fish pathogens under in vitro conditions. He et al. (2017) carried out a study on hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus ♀) fed either single Lb. acidophilus LAG01 or in combination with B. cereus BC-01, Clostridium butyricum CBG01 for 60 days. Feeding on either Lb. acidophilus or combination of three strains remarkably increased growth performance. Similar results were observed in case of digestive- and antioxidant enzymes activities. However, no statistical significant difference were revealed between mono or multi-strain probiotic supplementation.
lactobacillus casei
In a 60-days feeding trial with shirbot (Barbus gryprus) fed four experimental diets with varying dose (5 × 106, 5 × 107, and 5 × 108 CFU g−1) of Lb. casei, the results revealed higher performance in probiotic fed fish (Mohammadian et al., 2017). Furthermore, chymotrypsin and trypsin activities in probiotic groups were remarkably higher compared to the control. Safari et al. (2017) showed beneficial effects of Lb. casei on innate immune parameters (either serum or skin mucus) as well as expression of selected immune and antioxidant defense related genes. Moreover, the authors revealed that combined administration of probiotic with apple cider vinegar improved efficacy of the probiotic supplementation. This study highlighted the importance of additional research on evaluation of other feed additives (e.g., medicinal herbs and prebiotics) to be used in combination with probiotics, a topic being less investigated in fish (Ringø and Song, 2016).
Zebrafish (Danio rerio) has been suggested as model organism in human and animal studies (Penberthy et al., 2002; Hoseinifar et al., 2017). The possible effects of Lb. casei as probiotic on reproductive performance and maternal immunity of zebra fish was studied by Qin et al. (2014). Zebrafish fed the probiotic diet for 28 days displayed remarkably improved reproductive parameters such as egg ovulation, fertilization, and hatching rate. Furthermore, feeding on probiotic noticeably increased the expression of selected genes related to reproduction (eptin, kiss2, gnrh3, fsh, lh, lhcgr, and paqr8).
lactobacillus paracasei
In a study using rainbow trout (31.25 ± 3.43 g), Lopez Cazorla et al. (2015) tested Lb. paracasei subsp. tolerans F2 as probiotic on growth performance and intestinal microbiota. This probiotic was originally isolated from the digestive tract of Ramnogaster arcuate (Osteichthyes, Clupeidae). The results revealed significant effects on growth performance parameters and LAB dose in intestinal microbiota of probiotic fed fish was significantly higher vs. control.
lactobacillus delbrueckii
The effects of dietary Lb. delbrueckii (1 × 105, 1 × 106, 1 × 107, and 1 × 108 CFU g−1) on immune parameters as well as protection against A. hydrophila in carp was studied by Zhang C.-N. et al. (2017) and revealed improved intestinal immune parameters. Furthermore, probiotic feeding affected immune related genes expression; down-regulation of TNF-α, IL-8, IL-1β, and NF-κBp65 and up-regulation of IL-10 and TGF-β genes. Moreover, fish fed with 1 × 106 CFU g−1 Lb. delbrueckii showed increased antioxidant defense both at gene expression and enzyme levels. The challenge test showed higher protection against A. hydrophila infection. Mohammadian et al. (2016) used a Lb. delbrueckii ssp. bulgaricus isolated from shirbot intestine and supplemented the diet with the probiotic at rate of 5 × 107 CFU g−1. At the end of feeding trial, 60 days, immune parameters as well as resistance against A. hydrophila were measured. Evaluation of immune response and disease resistance revealed higher immune parameters (lysozyme, complement, and respiratory burst activities) as well as survival rate after challenge test (Mohammadian et al., 2016).
lactobacillus rhamnosus
In a study using European eel (Anguilla anguilla), Vílchez et al. (2015) administered three dose (103,105, and 106 CFU/g) of Lb. rhamnosus in the diet and monitored possible effects on spermatogenesis process. After 63 days of oral administration, up-regulation of genes related to reproduction such as activin, androgen receptors α and β (arα and arβ), progesterone receptor 1 (pr1), bone morphogenetic protein 15 (bmp15), and FSH receptor (fshr) was noticed. These changes at molecular levels were corresponded with observed changes in sperm quality and quantity. The authors concluded that Lb. rhamnosus confers the spermatogenesis process in European eel. Dawood et al. (2016b) also conducted an investigation on the effects of Lb. rhamnosus (either single e or combined with Lb. lactis) on growth performance and immune parameters of red sea bream, and displayed increased immune parameters and antioxidant defense in fish fed supplemented diet; higher effect was revealed when the two strains was used simultaneously. Similar effects were observed on growth performance and feed utilization. Moreover, probiotic administration decreased total cholesterol and triglycerides levels. The same research group, evaluated in another study the effects of varying dose (1 × 102, 1 × 104, and 1 × 106 cells g−1) of Lb. rhamnosus on red sea bream (Dawood et al., 2017), showed significant increase of plasma and mucus parameters (total protein, mucus myeloperoxidase activity, and mucus secretion), and concluded the results to be a sign for beneficial effects on host physiological responses. In a study with rainbow trout, Popovic et al. (2017) investigated the effect of dietary Lb. rhamnosus (107 and 108 CFU g−1) on intestinal microbiota and histology, biochemical parameters, and antioxidant defense in a 6-weeks feeding trial. While probiotic feeding had no significant effects on antioxidant defense, biochemical parameters were affected. Moreover, histological investigations revealed improvement of microvilli length in the proximal intestine (PI) as well as enhanced number of goblet cells in PI and distal intestine of probiotic fed fish. The authors concluded that Lb. rhamnosus was a promising feed additive, capable of improving rainbow trout health (Popovic et al., 2017).
pediococcus spp.
pediococcus acidilactici
During the past years there was increasing interests toward administration of Pediococcus spp. as probiotic in aquaculture and most of the studies have focused on P. acidilactici; the commercial product named Bactocell. For instance, the possible effects of dietary P. acidilactici (106 CFU/g) was assessed on sperm quality in zebrafish (Valcarce et al., 2015). After 10 days treatment of zebrafish male with probiotic, remarkable up-regulation of selected genes related to male and sperm quality was noticed. Hoseinifar et al. (2015a,b, 2016b) studied the effects of single or combined administration of P. acidilactici and galactooligosaccharide in rainbow trout. While single administration had no significant effects on growth performance, combined administration remarkably improved growth performance parameters. Also, feeding on supplemented diet remarkably increased immune response and resistance against Streptococcus iniae. Similar results were observed in a study using common carp (Modanloo et al., 2017). Furthermore, in a study with ornamental fish, green terror (Aequidens rivulatus), Neissi et al. (2013) studied the effects 0.1% inclusion of commercial P. acidilactici and revealed remarkable increase of the innate immune parameters as well as improvement of stress indicators following exposing fish to hypoxia stress.
pediococcus pentosaceus
Recently, Pediococcus pentosaceus has received attention as probiotic, but still limited information on the use of this strain is available. In a 56 days study, the effects of different dose (1.6 × 1010, 1.6 × 1011, 1.6 × 1012, and 3.2 × 1012 cells g−1) of inactivated P. pentosaceus was evaluated in red sea bream (Dawood et al., 2016a). Dietary administration of inactivated probiotic noticeably increased growth performance as well as mucus secretion. Also, skin mucus and serum immune parameters showed increment following treatment with probiotic. Furthermore, fish fed the probiotic supplemented diets had remarkably higher low-salinity stress resistance. Based on these results the authors suggested that inactivated P. pentosaceus as efficient and safe probiotic. Likewise, Moslehi et al. (2016) reported modulation of intestinal microbiota as well as body composition in Siberian sturgeon following dietary administration of a P. pentosaceus strain isolated from Persian sturgeon intestine. Furthermore, Huang J.-B. et al. (2014) addressed the effect of P. pentosaceus as probiotic in orange-spotted grouper (Epinephelus coioides). The probiotic bacteria was originally isolated by the authors from cobia (Rachycentron canadum) intestine. The strain showed antagonistic effects against pathogens under in vitro conditions and in an in vivo experiment, dietary administration of P. pentosaceus significantly increased growth performance, immune related genes expression as well as disease resistance.
weissella spp.
There is relatively limited information available about efficacy of Weissella species as probiotic in aquaculture. In recent study with Brazilian native surubins (43.3 g), the effects of dietary Weissella cibaria (1.18 × 107 CFU g−1) was investigated on performance, haemato-immunological parameters and intestinal histomorphology (Jesus et al., 2017). Regarding the hematological parameters, most of the parameters remained unaffected, except red blood cells, thrombocyte and lymphocyte counts which were higher in probiotic fed fish. Evaluation of immune parameters revealed higher phagocytosis, agglutination titer, and total Ig in probiotic groups compared with control. Feeding on probiotic supplemented diets significantly improved intestinal histology as observed increased height and width and number of villi as well as mucus producing goblet cells counts per villi. These results highlighted the potential of Weissella spp. to be used as a novel probiotic in aquaculture.
leuconostoc spp.
To our knowledge, possible effects of Leuconostoc as probiotic has only been investigated in one study. Allameh et al. (2016) supplemented Javanese carp (Puntius gonionotus) diet with either single Lc. mesenteroides or in combination with E. faecalis and Lb. fermentum as multi-strains probiotics. Interestingly, growth performance of fish fed single Lc. mesenteroides was better than those fed multi-strains probiotic. However, no significant effect was noticed in body composition.
lactococcus spp.
Nguyen et al. (2017) isolated L. lactis WFLU12 from intestine of wild marine fishes and based on in vitro probiotic effects selected the strain to be used in olive flounder diet. Interestingly, inclusion of this host-associated probiotic caused improvement of immune responses and protection against Streptococcus parauberis infection. Besides, probiotic fed fish showed improved growth performance and feed utilization. These results highlighted the importance of isolation of host-associated probiotic, a topic that merits further investigations.
enterococcus spp.
Enterococcus spp. and especially E. faecium are among the most studied probiotics in aquaculture, and from 2014 there are some reports available. For instance, Allameh et al. (2015) studied possible effect of oral administration of E. faecium on physiological responses of Javanese carp. Fish were fed on a single dose (107 CFU/g) for 5 weeks and at the end of the rearing period; significant increase of digestive enzymes activity as well as intestinal short chain fatty acid production (propionic and butyric acid) were noticed in the probiotic group. These improvements were in line with increased protection against A. hydrophila challenge. In accordance, elevation of cell-mediated immune response following oral administration of E. faecium has been reported by Matsuura et al. (2017). Besides the results on E. faecium, there are interests toward other species of this genus. Enterococcus gallinarum L-1 was used as potential probiotic in different species including gilthead sea bream, European sea bass, meager (Argyrosomus regius) and red porgy (Pagrus pagrus) diets (Román et al., 2015). The strain was originally isolated from gilthead sea bream intestine and the authors tested different forms; live or inactivated with heat or U.V. The authors reported no immunostimulatory effects of E. gallinarum in meager, however, immune stimulation was noticed in sea bream, sea bass and red porgy leucocytes. The immunostimulatory effects were increased along with elevation of probiotic level in diet; highest dose in the 108 CFU mL−1 treatment. Furthermore, Safari et al. (2016) isolated Enterococcus casseliflavus from rainbow trout intestine and evaluated its probiotic potential in rainbow trout. The probiotic strain was orally administered at rate of 107, 108, and 109 CFU g−1 for 8 weeks. At the end of feeding trial, significant change was noticed in LAB counts in the intestinal microbiota. This change was in line with remarkably increase of humoral immune parameters. Also, probiotic fed fish had significantly higher resistance when exposed to experimental challenge with S. iniae. Based on these results the authors suggested this host-associated strain as beneficial probiotic for rainbow trout culture.
When discussing the use of probiotics, it is of interest to notice that Lb. rhamnosus GG outcompete vancomycin-resistant E. faecium via mucus-binding pili (Tytgat et al., 2016), and the finding of He et al. (2017) using Lb. rhamnosus GG and its mutant (PB22) lacking SpaCBA pili to investigate the influence of pili on spatial distribution. LGG showed a mucosa type distribution, while PB22 revealed a hybrid distribution and the disease protection was accordingly improved.
However, prior to use of probiotics; injury to the mucosa and epithelial cells should be investigated in details as Lb. plantarum originally isolated from traditional Sabalan Iranian cheese from sheep raw milk resulted in damaged epithelial cells and disorganized microvilli of beluga (Huso huso) (Salma et al., 2011), while LGG induced injury to the mucosa of zebrafish (He et al., 2017).
Pathogenic LAB
In addition to probiotic, some pathogenic LAB are also documented (Ringø and Gatesoupe, 1998; Leisner et al., 2007; Michel et al., 2007). This sub-chapter focus on pathogenic LAB in aquaculture (Table 5), and the treatments (Table 6).
Table 5.
Pathogenic LAB in aquaculture.
| Pathogenic LAB species | Studied species | References |
|---|---|---|
| S. agalactiae | Silver pomfret (Pampus argenteus) Red tilapia (Oreochromis niloticus) Golden pompano (Trachinotus blochii) Barcoo grunter (Scortum barcoo) Hybrid tilapia (O. niloticus × O. aureus) |
Duremdez et al., 2004 Musa et al., 2009 Amal et al., 2012 Liu et al., 2014 Al-Harbi, 2016 |
| S. iniae | Hybrid striped bass (Morone chrysops × Morone saxatilis) Rabbitfish (Siganus canaliculatus) Sea bass (Dicentrarchus labrax) Japanese flounder (Paralichthys olivaceus) Barramundi (Lates calcarifer) Hybrid tilapia |
Stoffregen et al., 1996 Yuasa et al., 1999 Colorni et al., 2002 Nguyen et al., 2002 Bromage et al., 1999 Al-Harbi, 2011 |
|
S. dysgalactiae S. parauberis S. uberis |
Sturgeon (Acipenser schrenckii) Wild striped bass (Morone saxatilis) Mandarin fish (Siniperca chuatsi) |
Yang and Li, 2009 Haines et al., 2013 Luo et al., 2017 |
| Enterococcus sp. | Yellow tail (Seriola quinqueradiata) | Kusuda and Salati, 1993 |
| Turbot (Scophthalmus maximus) | Nieto et al., 1995 | |
| Nile tilapia (Oreochromis niloticus) | Plumb and Hanson, 2010 | |
| L. garvieae | Red sea wrasse (Coris aygula) | Colorni et al., 2003 |
| Nile tilapia and Pintado (Pseudoplathystoma corruscans) | Evans et al., 2009 | |
| Rainbow trout (Oncorhynchus mykiss) | Aguado-Urda et al., 2011; Reimundo et al., 2011 | |
| Gray mullet (Mugil cephalus) | Chen et al., 2002 | |
| Catfish (Silurus glanis) | Ravelo et al., 2003 | |
| Freshwater prawn (Macrobrachium rosenbergii) | Shih-Chu et al., 2001 | |
| Carnobacterium sp. | Rainbow trout Striped bass and channel catfish Salmon Lake white fish |
Hiu et al., 1984; Baya et al., 1991; Starliper et al., 1992; Toranzo et al., 1993b Baya et al., 1991; Toranzo et al., 1993a Michel et al., 1986 Loch et al., 2008 |
Table 6.
Treatments for pathogenic LAB in aquaculture.
| LAB species/treatments | Type of treatments | Studied species | References |
|---|---|---|---|
| Streptococcus agalactiae | |||
| Vaccine | Feed-based recombinant | Tilapia (Oreochromis sp.) | Nur-Nazifah et al., 2014 |
| Oral DNA | Nile tilapia (Oreochromis niloticus) | Huang L. Y. et al., 2014; Ma et al., 2017; Zhu et al., 2017 | |
| FbsA and α-enolase | Nile tilapia | Yi et al., 2014 | |
| SAΔphoB live attenuated vaccine | Golden pompano (Trachinotus ovatus) | Cai et al., 2017 | |
| PLGA-LrrG protein microparticle | Nile tilapia | Ke et al., 2017 | |
| GapA protein | Nile tilapia | Zhang Z. et al., 2017 | |
| Medical herbs | Sophora flavescens root extract | Nile tilapia | Wu et al., 2013 |
| Liposome-encapsulated cinnamaldehyde | Zebrafish (Danio rerio) | Faikoh et al., 2014 | |
| Essential oils | Nile tilapia | Brum et al., 2017 | |
| Excoecaria agallocha leaf extracts | Nile tilapia | Laith et al., 2017 | |
| Probiotics | B. subtilis | Tilapia | Ng et al., 2014; Liu et al., 2017 |
| B. pumilus | Nile tilapia | Srisapoome and Areechon, 2017 | |
| Saccharomyces cerevisiae | Nile tilapia | Pinpimai et al., 2015 | |
| Lb. rhamnosus | Nile tilapia | Pirarat et al., 2015 | |
| Prebiotics | β-glucan | Nile tilapia | Pilarski et al., 2017 |
| Cordyceps militaris spent mushroom substrate | Nile tilapia | Van Doan et al., 2017a,b | |
| Probiotics + Prebiotics | Kefir + low molecular weight sodium alginate | Nile tilapia | Van Doan et al., 2017b |
| Cordyceps militaris spent mushroom substrate + Lb. plantarum | Nile tilapia | Van Doan et al., 2017a | |
| Streptococcus iniae | |||
| Vaccine | Formalin-killed cells Live S. iniae mutant strain Lb. lactis BFE920-SiMA feed vaccine DNA vaccines (pEno) |
Nile tilapia Nile tilapia Olive flounder (Paralichthys olivaceus) Nile tilapia |
Klesius et al., 2000 Wang et al., 2014 Kim et al., 2016 Kayansamruaj et al., 2017 |
| Medical herbs | Inositol Essential oils Aloe vera Spirulina platensis |
Peres et al., 2004 Soltani et al., 2014 Gabriel et al., 2015 Adel et al., 2016 |
|
| Probiotics and prebiotics | Grobiotic™AE B. subtilis and Lb. acidophilus |
Hybrid striped bass (Morone chrysops x M. saxatilis) Nile tilapia |
Li and Gatlin, 2004 Aly et al., 2008 |
| L. lactis | Olive flounder | Kim et al., 2013 | |
| B. subtilis, S. cerevisiae and Aspergillus oryzae | Nile tilapia | Iwashita et al., 2015 | |
| E. casseliflavus | Rainbow trout (Oncorhynchus mykiss) | Safari et al., 2016 | |
| Nucleotides | Oligonucleotides | Hybrid striped bass | Li et al., 2004 |
| Nucleotides | Rainbow trout | Tahmasebi-Kohyani et al., 2011 | |
| Vitamins | Vitamin E | Nile tilapia | Lim et al., 2009 |
| Vitamin A | Nile tilapia | Guimarães et al., 2014 | |
|
S. dysgalactiae S. parauberis S. uberis |
Not available | Not available | Not available |
| Enterococcus faecalis | |||
| Medical plants | Tamarindus indica and Emblica officinalis leaves, Allium sativum bulb, and Syzygium aromaticum bud extracts | Nile tilapia, freshwater catfish (Clarias batrachus) and Asian stinging catfish (Heteropneustes fossilis) | Rahman et al., 2017 |
| Lactococcus garvieae | |||
| Vaccine | Autogenous formalin-inactivated Inactivated vaccine Ichtiovac-Lg Bivalent vaccine Subunit vaccines |
Tilapia and rainbow trout Rainbow trout Rainbow trout and Olive flounder |
Bercovier et al., 1997 Vendrell et al., 2007 Bastardo et al., 2012 Ra et al., 2009 |
| Medical herbs | Essential oils Mushroom extracts Stinging nettle Extract of noni leaves Huanglian Jiedu decoction |
Rainbow trout Rainbow trout Rainbow trout Freshwater prawn (Macrobrachium rosenbergii) Gray mullet (Mugil cephalus) |
Soltani et al., 2015 Baba et al., 2015 Saeidi Asl et al., 2017 Marisa Halim et al., 2017 Choi et al., 2014 |
| Antibiotics | Lincomycin, tetracycline chloramphenicol Erythromycin, lincomycin, and oxytetracycline Erythromycin, oxytetracycline, and amoxicillin | Yellow tail (Seriola quinqueradiata) Yellow tail | Aoki et al., 1990 Kawanishi et al., 2005 Vendrell et al., 2006 |
| Carnobacterium sp. | Not available | Not available | Not available |
streptococcus
Streptococcus spp. is the most common pathogen in aquaculture, and up to date, several species within this genus have been reported as important pathogens of fish, such as silver pomfret (Pampus argenteus) (Duremdez et al., 2004), red tilapia (O. niloticus) (Musa et al., 2009), golden pompano (Trachinotus blochii) (Amal et al., 2012), barcoo grunter (Scortum barcoo) (Liu et al., 2014), and hybrid tilapia (O. niloticus × O. aureus) (Al-Harbi, 2016).
Infection of Streptococcus agalactiae led to persistent high mortality with a distinctive swollen belly, eye hemorrhages, corneal opacity, exophthalmia, hemorrhage, enlarged liver and congestion of the kidney and spleen (Duremdez et al., 2004; Amal et al., 2012; Liu et al., 2014; Al-Harbi, 2016). To deal with this bacterial strain, several type of vaccines have been developed, which include formalin-killed cells and concentrated extracellular products of a single isolate of S. agalactiae vaccine (Evans et al., 2004), feed-based recombinant vaccine encoding cell wall surface anchor family protein of S. agalactiae (Nur-Nazifah et al., 2014), oral DNA vaccine (Huang L. Y. et al., 2014; Ma et al., 2017; Zhu et al., 2017), FbsA and α-enolase (Yi et al., 2014), SAΔphoB live attenuated vaccine (Cai et al., 2017), PLGA-LrrG protein micro-particle vaccine (Ke et al., 2017), and GapA protein vaccine (Zhang Z. et al., 2017). In addition to vaccines, many functional feed additives have been proved to protect fish and shellfish against S. agalactiae such as Ku shen (Sophora flavescens) root extract (Wu et al., 2013), liposome-encapsulated cinnamaldehyde (Faikoh et al., 2014), B. subtilis and B. pumilus Ng et al., 2014; Liu H. et al., 2017; Srisapoome and Areechon, 2017, yeast (Saccharomyces cerevisiae) (Pinpimai et al., 2015), Lb. rhamnosus (Pirarat et al., 2015), essential oils (Brum et al., 2017), buta–buta (Excoecaria agallocha) leaf extracts (Laith et al., 2017), β-glucan (Pilarski et al., 2017), kefir, low molecular weight sodium alginate, and Lb. plantarum Van Doan et al., 2016a,b, 2017b, and scarlet caterpillar (Cordyceps militaris) spent mushroom substrate and Lb. plantarum (Doan et al., 2017; Van Doan et al., 2017a). S. iniae is another Streptococcus species that cause disease outbreaks in different fish species (Agnew and Barnes, 2007), hybrid striped bass (Morone chrysops × Morone saxatilis) (Stoffregen et al., 1996), rabbitfish (Siganus canaliculatus) (Yuasa et al., 1999), European sea bass (Colorni et al., 2002), Japanese flounder (Nguyen et al., 2002), barramundi (L. calcarifer) (Bromage et al., 1999), and hybrid tilapia (Al-Harbi, 2011). Infection of this bacterium has led to vast economic losses in the world aquaculture industry of ~150 million US$, annually (Shoemaker et al., 2001; Al-Harbi, 2011). Huge effort has been contributed to deal with this bacterium which include vaccine (Klesius et al., 2000; Wang et al., 2014; Kim et al., 2016; Kayansamruaj et al., 2017), probiotics (B. subtilis, Lb. acidophilus, L. lactic, E. casseliflavus, S. cerevisiae, and Aspergillus oryzae) and prebiotics (GrobioticTMAE) (Li and Gatlin, 2004; Aly et al., 2008; Kim et al., 2013; Iwashita et al., 2015; Safari et al., 2016), medicinal plants (inositol, essential oil, Aloe vera, and Spirulina platensis) (Peres et al., 2004; Soltani et al., 2014; Gabriel et al., 2015; Adel et al., 2016), nucleotides (Li et al., 2004; Tahmasebi-Kohyani et al., 2011), and vitamins(A and E) (Lim et al., 2009; Guimarães et al., 2014). Besides these two common pathogens, several species within genus Streptococcus such as Streptococcus dysgalactiae (Yang and Li, 2009), S. parauberis (Haines et al., 2013), and Streptococcus uberis (Luo et al., 2017) have been reported to be pathogenic in aquaculture. However, to our knowledge, there is no treatment against these species.
enterococcus
Enterococcus sp. is an important pathogen in aquaculture, with severely impacts in commercial aquaculture practices worldwide (Martins et al., 2008; Rahman et al., 2017). The first report on the occurrence of pathogenic Enterococcus sp. in fish was revealed in yellow tail (Seriola quinqueradiata) in Japan (Kusuda and Salati, 1993). Later, Enterococcus was revealed in turbot (S. maximus) (Nieto et al., 1995), and tilapia (O. niloticus) (Plumb and Hanson, 2010). E. faecalis has been reported as causative agent of streptococcal infection in tilapia in lakes of Egypt, Thailand, and Bangladesh (Petersen and Dalsgaard, 2003; Abou El-Geit et al., 2013; Rahman et al., 2017). To our knowledge, limited information regarding prevention and treatment methods against E. faecalis has been reported. However, recently, Rahman et al. (2017) demonstrated that extraction of some medicinal plants, such as tamarind (Tamarinds indica), Indian gooseberry (Phyllanthus emblica), garlic (Allium sativum), and clove (Syzygium aromaticum) significantly protected fish against E. faecalis infection.
lactococcus garvieae
The pathogenicity of L. garvieae is well-known (Vendrell et al., 2006; Michel et al., 2007; Fukushima et al., 2017; Meyburgh et al., 2017) and the bacterium is the causative agent of lactococcosis, a hyperacute haemorrhagic septicaemia of fish. Huge economic loss in several economical freshwater - and marine fish species has been reported as a result of lactococcosis infection in Red sea wrasse (Coris aygula) (Colorni et al., 2003), Nile tilapia and pintado (Pseudoplathystoma corruscans) (Evans et al., 2009), rainbow trout (Aguado-Urda et al., 2011; Reimundo et al., 2011), gray mullet (Mugil cephalus) (Chen et al., 2002), catfish (Silurus glanis) (Ravelo et al., 2003), and freshwater prawn (Macrobrachium rosenbergii) (Shih-Chu et al., 2001). The common way to deal with this bacterium was the use of antibiotic, such as lincomycin, oxytetracycline and macrolides (Aoki et al., 1990; Kawanishi et al., 2005), and erythromycin, oxytetracycline, amoxicillin, and doxycycline have been widely used to control outbreaks of lactococcosis through rainbow trout (Vendrell et al., 2006). It is known that antibiotics were highly effective against L. garvieae in in vitro studies, but not in field conditions because of anorexia of infected fish (Bercovier et al., 1997) and possibly by ineffective metabolism of antibiotics in fish (Meyburgh et al., 2017). Due to this limitation of antibiotics and their side-effects in aquaculture practice, vaccination was considered as most effective to control lactococcosis (Meyburgh et al., 2017). Several types of vaccine have been developed such as autogenous formalin-inactivated vaccines (Bercovier et al., 1997), inactivated vaccine Ichtiovac-Lg (Vendrell et al., 2007), bivalent vaccine (Bastardo et al., 2012), and subunit vaccines (Ra et al., 2009). In addition to vaccines, several functional feed additives have been demonstrated to protect the fish against this bacterium which include essential oil (Soltani et al., 2015), mushroom extracts (Baba et al., 2015), stinging nettle (Saeidi Asl et al., 2017), extract of noni leaves (Marisa Halim et al., 2017), and Huanglian Jiedu decoction (Choi et al., 2014).
carnobacterium
C. maltaromaticum was reported as an important species and is reported in numerous fish species and meat products (Leisner et al., 2012). This bacterium has been demonstrated as a promising probiotic for aquaculture (Ringø et al., 2005; Kim and Austin, 2008; Pikuta and Hoover, 2014). However, some strains of this bacterium has been reported as fish pathogens with low virulence and stressed fish are especially susceptible, particularly post spawning (Michel et al., 1986; Starliper et al., 1992). Several fish species has been infected with C. maltaromaticum such as rainbow trout (Hiu et al., 1984; Baya et al., 1991; Starliper et al., 1992; Toranzo et al., 1993b), striped bass and channel catfish (Baya et al., 1991; Toranzo et al., 1993a), salmon (Hiu et al., 1984; Michel et al., 1986), and lake whitefish (Loch et al., 2008). However, to our knowledge, there is no information available regarding prevention and treatment approaches of this bacterium in aquaculture.
Practical uses of LAB as an immunostimulant in finfish aquaculture
Finfish share many common structures and functions with warm-blooded animals in innate immunity (Whyte, 2007), adaptive immunity (Laing and Hansen, 2011), and mucosal immunity (Gomez et al., 2013), although apparent differences exist. The finfish immune systems are regulated in the same or very similar manners to those of other vertebrates. Since antibiotics have significant limitations in finfish aquaculture, the field has sought safer and more effective antibiotic alternatives. Natually, LAB became a candidate for a substitute for antibiotics because the immunostimulant effects of LAB have been well established in other animals including human.
Various strains of LAB have been studied in their immune modulatory effects on many different finfish species; summarized in Tables 7, 8. Genus Lactobacillus is most studied (Salinas et al., 2006; Balcázar et al., 2007b; Picchietti et al., 2009; Harikrishnan et al., 2011; Biswas et al., 2013; Giri et al., 2013; Liu et al., 2013; Gioacchini et al., 2014; Van Doan et al., 2014, 2016a,b; Beck et al., 2015, 2016; Mohammadian et al., 2016; Lee et al., 2017; Zhang Z. et al., 2017). The second most investigated genus is Lactococcus (Balcázar et al., 2007b; Kim et al., 2013; Beck et al., 2015, 2016; Nguyen et al., 2017). Genera of Enterococcus, Pediococcus, and Leuconostoc have also been studied at a significant level; Enterococcus (Wang et al., 2008; Kim et al., 2012; Rodriguez-Estrada et al., 2013; Matsuura et al., 2017), Pediococcus (Neissi et al., 2013; Dawood et al., 2016a; Kaew-on et al., 2016), Leuconostoc (Balcázar et al., 2007b). Although the majority of the studies used a specific strain of live LAB (Table 7), some studies were performed with their inactivated form of LAB (Table 8). The immunostimulant effects of a mixture of two different LAB were also investigated. These studies revealed that the mixture LAB were superior to a single homogenous LAB in the probiotic effects (Beck et al., 2015; Maji et al., 2017). Not only various strains of LAB, but diverse species of subject fish were investigated as well; olive flounder, Nile tilapia, shirbot, Huanghe common carp (C. carpio Huanghe var.), European sea bass; basa fish (P. bocourti), Japanese eel (Anguilla japonica), rohu, zebrafish, striped beakfish (Oplegnathus fasciatus), rainbow trout, green terror (A. rivulatus), goldfish (Carassius auratus), gilthead sea bream, tiger puffer (Takifugu rubripes) and red sea bream.
Table 7.
Immunological changes of finfish resulted by live LAB treatment.
| LAB | Fish model (weight) | Administration route and dose | Administration length | Immunological changes | References |
|---|---|---|---|---|---|
| E. faecium (strain not mentioned) | Olive flounder (Paralichthys olivaceus) (33.4 ± 10 g) | Intraperitoneal injection 109 CFU/fish | 15 days | Alternative complement activity ↑, Serum lysozyme activity ↑, Spleen: IL-1β ↑, Kidney: IL-1β ↑, TNF-α ↑ |
Kim et al., 2012 |
| E. faecium ZJ4 | Nile tilapia (Oreochromis niloticus) (6.834 ± 0.18 g) | Immersion 107 CFU/mL supplemented in aquaria for every 4 days | 40 days | Complement C3 ↑, Myeloperoxidase activity ↑, NBT reaction (respiratory burst) ↑, Serum lysozyme activity ↑, |
Wang et al., 2008 |
| Lb. acidophilus JCM 1132 | Nile tilapia (Oreochromis niloticus ♀ × Oreochromis aureus ♂) (0.9 g) | Diet 105, 107, 109 CFU/g feed | 10, 20, 35 days (consecutive) | Spleen: IL-1β ↑, TGF-β ↑,TNF-α ↑ at day 20, TNF-α ↓ at day 35 Kidney: IL-1β ↑ at day 20, IL-1β ↓ at day 35, TGF-β ↑,TGF-β ↓ at day 35 in 105 CFU/g feed, TNF-α ↑ Protection against A. hydrophila ↑ * Increased or decreased gene expressions were varied by dose and sample collection time mark. |
Liu et al., 2013 |
| Lb. brevis JCM 1170 | Nile tilapia (0.9 g) | Diet 105, 107, 109 CFU/g feed | 10, 20, 35 days (consecutive) | Spleen: IL-1β ↑, TGF-β ↑ at day 20, TGF-β ↓ at day 35, TNF-α ↑ at day 20, TNF-α ↓ at day 35 Kidney: IL-1β ↑ at day 20, IL-1β ↓ at day 35, TGF-β ↑ at day 20, TNF-α ↑ at day 35 Protection against A. hydrophila ↑ * Increased or decreased gene expressions were varied by dose and sample collection time mark. |
Liu et al., 2013 |
| Lb. casei PTCC1608 | Shirbot (Barbus grypus) (50 g) | Diet 5 × 107 CFU/g feed | 6 weeks | Alternative complement activity ↑, NBT reaction (respiratory burst) ↑, Protection against A. hydrophila ↑ |
Mohammadian et al., 2016 |
| Lb. delbrueckii (Angel Company, Wuhan, China) | Huanghe common carp (Cyprinus carpio Huanghe var.) (1.05 ± 0.03 g) | Diet 105, 106, 107, 108 CFU/g feed | 8 weeks | Intestine: IL-1β ↓, IL-8 ↓, TNF-α ↓, NF-κB P65 ↓, IL-10 ↑, TGF-β ↑ IgM concentration ↑, Myeloperoxidase activity ↑, Serum lysozyme activity ↑, Protection against A. hydrophila ↑ |
Zhang C.-N. et al., 2017 |
| Lb. delbrueckii ssp. delbrueckii AS13B | European sea bass (Dicentrarchus labrax (L.)) (not available, 1 day post hatch) | Diet 105 CFU/cm3 via enriched in Brachionus plicatilis or Artemia nauplii | 72 days | Acidophilic granulocytes ↑, T cells ↑, IL-1 β ↓ |
Picchietti et al., 2009 |
| Lb. delbrueckii ssp. bulgaricus (original isolate by authors) | Shirbot (50 g) | Diet 5 × 107 CFU/g feed | 6 weeks | Alternative complement activity ↑, NBT reaction (respiratory burst) ↑, Protection against A. hydrophila ↑ |
Mohammadian et al., 2016 |
| Lb. plantarum (original isolate by authors) | Shirbot (50 g) | Diet 5 × 107 CFU/g feed | 6 weeks | Alternative complement activity ↑, NBT reaction (respiratory burst) ↑, Serum lysozyme activity (only at day 60) ↑, Protection against A. hydrophila ↑ |
Mohammadian et al., 2016 |
| Lb. plantarum CR1T5 | Basa fish (Pangasius bocourti) (82.01 g) | Diet 108 CFU/g feed | 4 weeks | Alternative complement activity ↑, Protection against A. hydrophila ↑ |
Van Doan et al., 2014 |
| Lb. plantarum CR1T5 | Basa fish (3.57 g) | Diet 108 CFU/g feed | 3, 6, 9, 12 weeks (consecutive) | Alternative complement activity ↑, Phagocytic activity ↑, Serum lysozyme activity ↑, Protection against A. hydrophila ↑ |
Van Doan et al., 2016a |
| Lb. plantarum CR1T5 | Nile tilapia (15.56 ± 0.02 g) | Diet 108 CFU/g feed | 30 and 60 days (consecutive) | Alternative complement activity ↑, NBT reaction (respiratory burst) ↑, Phagocytic activity ↑, Serum lysozyme activity ↑, Protection against S. agalactiae ↑ |
Van Doan et al., 2016b |
| Lb. plantarum FGL0001 | Olive flounder (37.5 ± 1.26 g) | Diet 107 CFU/g feed | 4 weeks | NBT reaction (respiratory burst) ↑, Phagocytic activity ↑, Skin mucus lysozyme activity ↑, Intestine: IL-6 ↑, IL-8 ↑, TNF-α ↑, Protection from S. iniae ↑ |
Beck et al., 2015 |
| Lb. plantarum FGL0001 | Olive flounder (42.7 ± 1.61 g) | Diet 107 CFU/g feed | 4 weeks | Intestine: CD4-1 ↑, T-bet ↑, GATA3 ↑, IL-1β ↑, IFN-γ ↑, IL-17A/F ↓, Gut permeability ↓, Protection from E. tarda ↑ |
Beck et al., 2016 |
| Lb. plantarum KCTC3928 | Japanese eel (Anguilla japonica) (8.29 ± 00.6 g) | Diet 106, 107, 108 CFU/g feed | 8 weeks | Myeloperoxidase activity (108 CFU/g feed only) ↑, Serum lysozyme activity ↑, Superoxide dismutase ↑, Intestine: IgM ↑, Protection from V. anguilarum (108 CFU/g feed only) ↑ |
Lee et al., 2017 |
| Lb. plantarum VSG3 | Rohu (60 g) | Diet 106, 108, 1010 CFU/g feed | 30 and 60 days (consecutive) | Alternative complement activity ↑, IgM concentration at 30th day (108 and 1010 CFU/g feed) ↑ NBT reaction (respiratory burst) ↑, Phagocytic activity ↑, Serum lysozyme activity ↑, Protection from A. hydrophila ↑ |
Giri et al., 2013 |
| Lb. rhamnosus IMC 501 | Zebrafish (Danio rerio) (adult, weight is not mentioned) | Diet 106 CFU/g feed | 10 days | Liver: IL-1β ↑, TNF-α ↑ |
Gioacchini et al., 2014 |
| Lb. sakei BK19 | Striped beakfish (Oplegnathus fasciatus) (32 ± 3 g) | Diet 2.2 × 107 CFU/g feed | 1, 3, 6 weeks (consecutive) | Alternative complement activity ↑, Eosinophils ↑, Monocytes ↑, NBT reaction (respiratory burst) ↑, Neutrophils ↑, Reactive nitrogen species ↑, Serum lysozyme activity ↑ |
Harikrishnan et al., 2011 |
| Lb. sakei CLFP 202 | Rainbow trout (40 g) | Diet 106 CFU/g feed | 2 weeks | Alternative complement activity ↑, Phagocytic activity ↑, Serum lysozyme activity ↑, Protection from A. salmonicida ↑ |
Balcázar et al., 2007b |
| L. lactis BFE920 | Olive flounder (37.5 ± 1.26 g, 40 ± 3 g, 55 ± 5 g) | Diet 107 CFU/g feed | 4 weeks | Myeloperoxidase activity ↑, NBT reaction (respiratory burst) ↑, Phagocytic activity ↑, Skin mucus lysozyme activity ↑, Spleen: IL-12p40 ↑, IFN-γ ↑, Intestine: IL-6 ↑, IL-8 ↑, Protection from S. iniae ↑ |
Kim et al., 2013; Beck et al., 2015 |
| L. lactis BFE920 | Olive flounder (42.7 ± 1.61 g) | Diet 107 CFU/g feed | 4 weeks | Intestine: CD4-1 ↑, FOXP3 ↑, IL-10 ↑, TGF-β1 ↑, IFN-γ ↑, RORγ ↓, IL-17A/F ↓, Gut permeability ↓, Protection from E. tarda ↑ |
Beck et al., 2016 |
| L. lactis ssp. lactis CLFP 100 | Rainbow trout (40 g) | Diet 106 CFU/g feed | 2 weeks | Alternative complement activity ↑, NBT reaction (respiratory burst) ↑, Phagocytic activity ↑, Serum lysozyme activity ↑, Protection from A. salmonicida ↑ |
Balcázar et al., 2007b |
| L. lactis WFLU12 | Olive flounder (80.84 ± 9.37 g) | Diet 109 CFU/g feed | 2, 4, 8 weeks (consecutive) | Intestine: IL-6 (at week 4) ↑ Kidney: IL-6 (at week 2) ↑, IL-8 (at week 4) ↑, IFN-γ (at week 4) ↑, g-lysozyme (at week 4) ↑ Phagocytic activity (at week 2) ↑, NBT reaction (respiratory burst, at week 4) ↑, Natural infection of S. parauberis ↓ |
Nguyen et al., 2017 |
| Lc. mesenteroides CLFP 196 | Rainbow trout (40 g) | Diet 106 CFU/g feed | 2 weeks | Alternative complement activity ↑, Phagocytic activity ↑, NBT reaction (respiratory burst) ↑, Serum lysozyme activity ↑, Protection from A. salmonicida ↑ |
Balcázar et al., 2007b |
| P. acidilactici MA 18/5 M | Green terror (Aequidens rivulatus) (0.388 ± 0.0021 g) | Diet 0.9 × 107 CFU/g feed | 56 days | Alternative complement activity ↑, Serum lysozyme activity ↑, Total immunoglobulin counts ↑ |
Neissi et al., 2013 |
| P. pentosaceus PKWA-1 | Nile tilapia (0.68 ± 0.02 g, 36.89 ± 3.34 g) | Diet 107 CFU/g feed | 1, 14, 28, 42 days (consecutive) | Alternative complement activity ↑, Phagocytic activity ↑, Serum lysozyme activity ↑, Total leukocyte counts ↑, Protection from A. hydrophila ↑ |
Kaew-on et al., 2016 |
| Mixed LAB (Lb. plantarum FGL0001, L. lactis BFE920) | Olive flounder (37.5 ± 1.26 g) | Diet 107 CFU/g feed of each strain | 4 weeks | NBT reaction (respiratory burst) ↑, Phagocytic activity ↑, Skin mucus lysozyme activity ↑, Intestine: IL-6 ↑, IL-8 ↑, TNF-α ↑, Protection from S. iniae ↑ |
Beck et al., 2015 |
| Mixed LAB (Lb. plantarum SM16, Lb. plantarum SM33, Lb. fermentum SM51, Lb. brevis SM56, P. pentosaceus SM65) | Rohu (19.72 ± 0.18 g) | Diet 2 × 108 CFU/g feed of each strain | 30 days | NBT reaction (respiratory burst) ↑, Intestine and liver: TNF-α ↑, IL-10 ↑ Protection from A. hydrophila ↑ |
Maji et al., 2017 |
Table 8.
Immunological changes of finfish resulted by inactivated LAB treatment.
| LAB | Fish model (weight) | Administration route and dose | Administration length | Immunological changes | References |
|---|---|---|---|---|---|
| E. faecalis (Nichinichi Pharmaceutical, Japan) | Rainbow trout (Oncorhynchus mykiss) (36.3 ± 0.42 g) | Diet 0.25, 0.5% w/w inclusion to feeds | 12 weeks | Mucus secretion ↑, Phagocytic activity ↑, Protection from A. salmonicida ↑ Systemic invasion of A. salmonicida ↓ |
Rodriguez-Estrada et al., 2013 |
| E. faecalis KH2 | Goldfish (Carassius auratus)(15–20 g) In vitro, kidney leukocytes | In vivo, intraperitoneal injection 500 μg/fish In vitro treatment 50 μg/well | In vivo, 7 days; In vitro, 12 h | In vivo: CD4-1+ cells ↑, CD8α+ cells ↑, Myeloid cells ↑, Macrophages ↑, IL-12p35 ↑, IL-12p40 ↑, IFN-γ1 ↑ In vitro: IL4/13a ↑, IL-12p35 ↑, IL-12p40 ↑, IFN-γ1 ↑, IFN-γ2 ↑, infgrel1 ↑, infgrel2 ↑ |
Matsuura et al., 2017 |
| Lb. delbrueckii ssp. lactis CECT287 | Gilthead sea bream (Sparus aurata L.)(65 g) In vitro, head kidney cells | In vitro treatment 5 × 105, 5 × 106 5 × 107 CFU/mL | 30 min | Respiratory burst ↑, Natural cytotoxic activity ↑, | Salinas et al., 2006 |
| Lb. paracasei spp. paracasei 06TCa22 | Tiger puffer (Takifugu rubripes)(205 ± 8 g) In vitro, head kidney cells | In vitro treatment 20 μg/mL | 1, 4, 8, 12, 24, 48 h | IL-1β ↑, IL-2 ↑, IL-6 ↑, IL-7 ↑, IL-12p40 ↑, IL-17AF-3 ↑, IL-18 ↑, TNF-α ↑, TNF-N ↑, I-IFN-1 ↑, IFN-γ ↑ | Biswas et al., 2013 |
| Lb. plantarum 06CC2 | Tiger puffer (205 ± 8 g) In vitro, head kidney cells | In vitro treatment 20 μg/mL | 1, 4, 8, 12, 24, 48 h | IL-1β ↑, IL-2 ↑, IL-6 ↑, IL-7 ↑, IL-12p40 ↑, IL-10 ↑, IL-15 ↑, IL-18 ↑, IL-21 ↑, TNF-α ↑, TNF-N ↑, I-IFN-1 ↑ | Biswas et al., 2013 |
| P. pentosaceus D3268 | Red sea bream (Pagrus major) (6 ± 0.2 g) | Diet 1.6 × 1010, 1.6 × 1011, 1.6 × 1012, 3.2 × 1012 CFU/g feed | 56 days | Mucus lysozyme activity ↑, Mucus secretion ↑, Serum lysozyme activity ↑ |
Dawood et al., 2016a |
The mode of administration of LAB is an important factor for practical use of LAB in the field. Feeding the LAB adsorbed into regular diets may be the best way for administration because this feeding method reduces labor and stress to fish. As expected, a vast majority of studies employed dietary LAB as the mode of administration. However, some studies treated the fish by intraperitoneal injection (Kim et al., 2012; Matsuura et al., 2017) or immersion in a LAB-containing bath (Wang et al., 2008). Many studies indicated that the feeding administration demonstrated better immunostimulant effects, compared to any other modes of application. The viability of LAB is another important issue to consider. The viability of microbes is a necessity for probiotics by definition. In general, live LAB triggered higher immune stimulation compared to that of the inactivated LAB (Panigrahi et al., 2005; Munoz-Atienza et al., 2015; Tables 7, 8). However, more studies that compare the activities between the live and the inactivated condition of the same LAB need to be done for further confirmation. Nevertheless, only live LAB can produce bioactive products such as exopolysaccharides and maintain the natural state of microbe-associated molecular patterns (MAMP) structures. These unique properties of live LAB may contribute to the superiority in immunostimulant effects over the inactivated form of the LAB. In this context, the establishment of proper techniques for storing and applying live LAB is an important aspect to consider. As summarized in Table 8, the inactive LAB also showed significant immunostimulant effects, but less than those of live LAB. However, in the aspect of manufacturing LAB products, the inactivated condition of LAB may be advantageous because the cost for storage and distribution can be reduced. For the practical utilization of LAB in the finfish aquaculture field, the species, the living status, the mode of administration, and the optimum dosage of the LAB should be carefully considered for the best results.
LAB effects on innate immunity
Innate immunity takes the place of the first line of defense toward a wide range of pathogens. The interaction between MAMP in microbes and pattern-recognition receptors (PRR) on innate immune cells is one of the critical initiators for activation of the innate immune system. Some probiotics that have immunostimulant activity such as LAB can protect the host from various pathogens by stimulating the immune system. The LAB studies of warm-blooded animals seem to influence the similar studies in finfish. However, the finfish studies were heavily biased toward the LAB effects on innate immunity as shown Tables 7, 8. Furthermore, most of the studies simply described the physiological status without exploring the specific immune subsets responsible for disease resistance or the underlying mechanism. The studies of the adaptive immune system are even more limited. Antibody was the only subject studied, and the studies concerning T cell responses were very few, if any. Understanding the regulatory mechanism of the finfish immune system is a big challenge to the field of finfish immunology. This understanding is essential for developing safe and potent immunological means for the protection and cure of fish diseases.
Immune parameters for studying finfish immunity
The immune parameters that have frequently been used for studying finfish immunity are listed and briefly explained in Table 9. The innate immune parameters include complement activity, lysozyme, phagocytosis, and respiratory burst. The level of antigen-specific antibodies is mostly used for representing adaptive immune responses. The types and the levels of cytokines are important indicators of both innate and adaptive immune status of fish.
Table 9.
Frequently measured immune parameters in finfish studies.
| Immune parameters | Functions | References |
|---|---|---|
| Antibody | Produced by B cells Recognizes and binds to specific antigens of pathogens Neutralization of pathogens Opsonization of antibody bound pathogens Activation of the complement system Activation of antibody-dependent cellular cytotoxicity |
Abbas et al., 2014 |
| Cytokine | Signal proteins of host cells Activation of inflammation through proinflammatory cytokines (eg., IL-1β, INF-γ, TNF-α) Regulation of immune activities/anti-inflammation through regulatory cytokines (eg., IL-10, TGF-β) |
Wang and Secombes, 2013; Abbas et al., 2014; Turner et al., 2014 |
| Complement activity | Non-cellular immune response which is activated by antigen-specific antibodies or lectin Formation of membrane attack complexes (MAC) of the surface of pathogens Induction of inflammation at local infection sites Opsonization of pathogens by antibody binding or complement subunits to the surface of pathogens |
Alexander and Ingram, 1992; Abbas et al., 2014 |
| Lysozyme | Non-cellular immune response toward bacterial pathogens Hydrolysis β-(1, 4) glycosidic linkages in N-acetylmuramic acid and N-acetylglucosamine of bacterial cell wall peptidoglycan |
Alexander and Ingram, 1992 |
| Phagocytosis | Engulfing activity of phagocytic cells such as dendritic cells, macrophages, and monocytes Direct killing of pathogen by intracellular lysosome of phagocytic cells Antigen presentation of phagocytosed antigens to T cell by dendritic cells and macrophages | Abbas et al., 2014 |
| Respiratory burst | Oxidative potential of innate cells Pathogen killing effect by reactive oxygen species including hydrogen peroxide, superoxide anions, and hydroxyl radicals | Abbas et al., 2014 |
Cytokines as important immune modulators of finfish immunity
Cytokines are small proteins (~5–20 KDa) that are important in cell signaling. They act through receptors and are particularly important in the immune system because cytokines modulate the balance between humoral and cellular immune responses. Cytokines regulate the maturation, growth, and responsiveness of particular cell populations (Abbas et al., 2014; Turner et al., 2014). Many studies demonstrated the cytokine induction effects of LAB in various finfish models (Picchietti et al., 2009; Kim et al., 2012, 2013; Biswas et al., 2013; Liu et al., 2013; Beck et al., 2015, 2016; Matsuura et al., 2017; Nguyen et al., 2017; Zhang Z. et al., 2017). The cytokine profiles modified by LAB administration are summarized in Tables 7, 8. Increased expression of proinflammatory cytokines (e.g., IL-1β, IL-6, IL-8, or TNF-α) directly correlates to disease protection against challenged pathogens. This protective activity of inflammatory cytokines may be because of the potentiation of the host immune system, resulting in rapid and efficient responses to the invading pathogens (Wang and Secombes, 2013; Turner et al., 2014). However, excessive inflammation can cause acute inflammatory symptoms leading to the death of the host. Therefore, maintaining a balanced inflammation status is critical. IL-10, an anti-inflammatory cytokine, is a well-known immune regulator. Some strains of dietary LAB induced IL-10 expression in finfish; Biswas et al. (2013) (Lb. plantarum 06CC2 treated T. rubripes), Beck et al. (2016) (L. lactis BFE920 treated P. olivaceus), and Maji et al. (2017) (a mixture of Lb. plantarum SM16, Lb. plantarum SM33, Lb. fermentum SM51, Lb. brevis SM56, P. pentosaceus SM65 treated Labeo rohita). Beck and co-authors demonstrated that LAB plays an important role in the establishment of the “immune tone” in the finfish gut. The immune tone is a higher status of immunological-readiness to combat against pathogens. LAB established the proinflammatory or anti-inflammatory immune tone in a strain-specific manner. The finfish in proinflammatory immune tone was able to protect the challenged pathogen better compared to those with an anti-inflammatory immune tone. However, the fish in anti-inflammatory immune tone gained more weight (Beck et al., 2016). Therefore, monitoring the types of cytokines expressed after LAB treatment may be important to maximize the beneficial effects of the LAB. The underlying mechanisms involved in the establishment of the two different types of immune tones and their relationships to the adaptive immune system need to be further investigated.
LAB effects on adaptive immunity
In addition to innate immunity, LAB treatment also influenced the adaptive immunity of finfish. The fish fed with LAB increased total T cell numbers (Picchietti et al., 2009). The LAB also activated the subtype-specific factors of CD4+ T helper cells (Th1, Th2, Th17, and Treg cell) (Beck et al., 2016) and CD8+ cytotoxic T cells (Beck et al., 2016; Matsuura et al., 2017). The modification of T cell composition may be due to the cytokines released from various subsets of immune cells that are induced by the treated LAB. IL-12, IL-18, and IFN-γ act on Th1 cell differentiation and activation. IL-4, IL-13, IL-5 are involved in Th2 cells, and IL-17, IL-22, IL-21 promote Th17 cell differentiation. Treg cell differentiation is controlled by IL-10 and TGF-β (Abbas et al., 2014). The relationships between cytokines and immune cells are mutually regulated; cytokines secreted from stimulated immune cells control the same or other immune cells through signaling pathways. The responding immune cells then release cytokines accordingly (Knosp and Johnston, 2012). The cytokine networks are closely linked between the innate and the adaptive immune system as well. IL-10 released from activated M2 macrophages (Martinez and Gordon, 2014) influences Treg cell differentiation. Also, IL-12 released by activated DCs and macrophages stimulate Th1 cells and NK cells to release IFN-γ. This IFN-γ then activates DCs and macrophages. The LAB's roles involved in this kind of immune modulation have been well-demonstrated in warm-blooded animals (Delcenserie et al., 2008; Bron et al., 2012). Although it appears that LAB play similar roles in the finfish immune system, further studies are required.
Conclusions
Numerous reports exist in finfish regarding the microbiota modulating effects of dietary modifications and the presence of LAB in the GI tract. However, when investigating the GI tract microbiota, one major concern occur; most studies evaluating the fish gut microbiota have focus to characterize the communities in the GI lumen (the allochthonous microbiota), while those bacteria that adhere to the mucosal surface (the autochthonous microbiota); which may be important in specialized physiological functions, remain uncharacterized. We therefore recommend more focus on the autochthonous gut microbiota in future studies.
Previous studies were based on culture-based approaches, but this may be question. Although there is a discussion over the value and need of using culture-based techniques vs. culture-independent approaches, it is apparent that viable cells are valuable to culture collections, in vaccine production, and as probiotics and synbiotics. During the last decades, 16S rRNA gene fingerprinting methods such as denaturing gradient gel electrophoresis (DGGE) have been widely used, but the DGGE method only detect 1–2% of the microbial diversity. Next-Generation Sequencing (NGS) has been used in recent years to examine the gut microbiome of humans, terrestrial and marine vertebrate including some finfish species. However, as NGS has only been used in few finfish species such as rainbow trout, Atlantic salmon, Siberian sturgeon, zebrafish and gilthead sea bream, we recommend that this technique is used to explore the gut bacterial community of finfish.
LAB and their bacteriocins are alternatives to chemicals and antibiotics as antimicrobial activities toward pathogens have been revealed. In some cases LAB and their bacteriocins may be used in combination with low dosages of antibiotics. As novel applications of LAB and bacteriocins are increasing; within prospects of anti-quorum sensing strategies and site-specific drug delivery, this topic merits further investigations.
As the specific bio-active compounds and mechanism behind the antagonism of LAB bacteriocins have rarely investigated, this merits further investigations to validate the health claims. Furthermore, as there may be risk of possible horizontal transfer of antibiotic resistance genes through LAB, the use of promising LAB must follow strict guidelines in addition to antimicrobial actions. As the efficacy of the bacteriocins is dictated by environmental factors, there is also a need to determine the effective conditions for application of each LAB bacteriocin (Balciunas et al., 2013).
Recent studies regarding probiotic administration as revealed beneficial effects on growth performance, immune responses and disease resistance. However, still there is limited information available about the exact mode of action on physiology of host organism. Although, there are some assumptions and speculations, this should be clarified in future through in depth studies. Also, different studies revealed varied results on different species. Considering the species-specific effects, there should be studies to determined optimum probiotic and inclusion level for each cultured species. During the recent years, there has been increased attention toward probiotics effects on mucosal parameters and expression of immune, and antioxidant related genes expression. The possible mode of action on gene expression profile merit further researches.
In addition to the numerous beneficial LAB, there are several pathogenic species within genera Streptococcus, Enterococcus, Lactobacillus, Carnobacterium, and Lactococcus. They have caused considerable losses in aquaculture practice. Huge effort been contributed to deal with these pathogens such as vaccines, dietary supplements; medicinal plants, prebiotics, probiotics and other immunostimulants. Such treatments needs to be developed in the future for sustainable aquaculture.
It is quite clear that LAB administration results in beneficial effects such as disease resistance and weight gain in finfish aquaculture. However, the underlying mechanism is poorly understood; the microbe-associated molecular patterns (MAMPs) of the LAB, their pattern recognition receptors (PRRs) on immune cells, and byproducts released from the LAB that are responsible for immunomodulation. The immunomodulatory effects of the LAB are strain-specific, and therefore, the information of the studies performed with various strains of LAB need to be further accumulated and actively shared for finfish aquaculture industries.
Author contributions
ER: introduction, GI tract, editorial. KG: antibacterial effects of LAB. SH: LAB as probiotic. HD: pathogenic LAB. BB and SS: immunology of LAB.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. National Research Foundation of Korea (NRF-2015R1D1A1A01056959) and the Korea GyeongSangbuk-Do Fisheries Technology Center support SS. The publication charges for this article have been funded by a grant from the publication fund of UiT The Arctic University of Norway.
References
- Abbas A. K., Lichtman A. H., Pillai S. (2014). Cellular and Molecular Immunology, 8th Edn. Philadelphia, PA: Saunders Elsevier. [Google Scholar]
- Abou El-Geit E., Saad T., Abdo M., Mona S. Z. (2013). Microbial infections among some fishes and crustacean species during blooming phenomenon in Qaroun Lake-Egypt. Life Sci. J. 10, 1217–1224. Available online at: http://www.lifesciencesite.com [Google Scholar]
- Adel M., Yeganeh S., Dadar M., Sakai M., Dawood M. A. O. (2016). Effects of dietary Spirulina platensis on growth performance, humoral and mucosal immune responses and disease resistance in juvenile great sturgeon (Huso huso Linnaeus, 1754). Fish Shellfish Immunol. 56, 436–444. 10.1016/j.fsi.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Adel M., Safari R., Yeganeh S., Binaii M., Ghiasi M., Ahmadvad S. (2017). Effects of dietary GroBiotic® – A supplementation as a probiotic on the intestinal microflora, growth performance, haemato-serological parameters, survival rate and body composition in juvenile beluga (Huso huso Linnaeus, 1754). Aquacult. Nutr. 23, 492–499. 10.1111/anu.12417 [DOI] [Google Scholar]
- Agnew W., Barnes A. C. (2007). Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet. Microbiol. 122, 1–15. 10.1016/j.vetmic.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Aguado-Urda M., López-Campos G. H., Gibello A., Cutuli M. T., López-Alonso V., Fernández-Garayzábal J. F., et al. (2011). Genome sequence of Lactococcus garvieae 8831, isolated from rainbow trout Lactococcosis outbreaks in Spain. J. Bacteriol. 193, 4263–4264. 10.1128/JB.05326-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakomi H. L., Skyttä E., Saarela M., Mattila-Sandholm T., Latva-Kala K., Helander I. M. (2000). Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66, 2001–2005. 10.1128/AEM.66.5.2001-2005.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. B., Ingram G. A. (1992). Noncellular nonspecific defense mechanisms of fish. Ann. Rev. Fish Dis. 2, 249–280. 10.1016/0959-8030(92)90066-7 [DOI] [Google Scholar]
- Al-Harbi A. H. (2011). Molecular characterization of Streptococcus iniae isolated from hybrid tilapia (Oreochromis niloticus×Oreochromis aureus). Aquaculture 312, 15–18. 10.1016/j.aquaculture.2010.12.014 [DOI] [Google Scholar]
- Al-Harbi A. H. (2016). Phenotypic and genotypic characterization of Streptococcus agalactiae isolated from hybrid tilapia (Oreochromis niloticus×O. aureus). Aquaculture 464, 515–520. 10.1016/j.aquaculture.2016.07.036 [DOI] [Google Scholar]
- Allameh S., Ringø E., Yusoff F., Daud H., Ideris A. (2015). Dietary supplement of Enterococcus faecalis on digestive enzyme activities, short-chain fatty acid production, immune system response and disease resistance of Javanese carp (Puntius gonionotus, Bleeker 1850). Aquacult. Nutr. 23, 331–338. 10.1111/anu.12397 [DOI] [Google Scholar]
- Allameh S. K., Yusoff F. M., Ringø E., Daud H. M., Saad C. R., Ideris A. (2016). Effects of dietary mono- and multiprobiotic strains on growth performance, gut bacteria and body composition of Javanese carp (Puntius gonionotus, Bleeker 1850). Aquacult. Nutr. 22, 367–373. 10.1111/anu.12265 [DOI] [Google Scholar]
- Almeida T., Brandao A., Muñoz-Atienza E., Gonçalves A., Torres C., Igrejas G., et al. (2011). Identification of bacteriocin genes in enterococci isolated from game animals and saltwater fish. J. Food Protect. 74, 1252–1260. 10.4315/0362-028X.JFP-11-016 [DOI] [PubMed] [Google Scholar]
- Aly S. M., Abdel-Galil Ahmed Y., Abdel-Aziz Ghareeb A., Mohamed M. F. (2008). Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 25, 128–136. 10.1016/j.fsi.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Amal M. N. A., Zamri-Saad M., Iftikhar A. R., Siti-Zahrah A., Aziel S., Fahmi S. (2012). An outbreak of Streptococcus agalactiae infection in cage-cultured golden pompano, Trachinotus blochii (Lacépède), in Malaysia. J. Fish Dis. 35, 849–852. 10.1111/j.1365-2761.2012.01443.x [DOI] [PubMed] [Google Scholar]
- Annamalai N., Manivasagan P., Balasubramanian T., Vijayalakshmi S. (2009). Enterocin from Enterococcus faecium isolated from mangrove environment. Afr. J. Biotechnol. 8, 6311–6316. 10.5897/AJB2009.000-9478 [DOI] [Google Scholar]
- Aoki T., Takami K., Kitao T. (1990). Drug resistance in a non-hemolytic Streptococcus sp. isolated from cultured yellowtail Seriola quinqueradiata. Dis. Aquat. Org. 8, 171–177. 10.3354/dao008171 [DOI] [Google Scholar]
- Arlindo S., Calo P., Franco C., Prado M., Cepeda A., Barros-Velázquez J. (2006). Single nucleotide polymorphism analysis of the enterocin P structural gene of Enterococcus faecium strains isolated from nonfermented animal foods. Mol. Nutr. Food Res. 50, 1229–1238. 10.1002/mnfr.200600178 [DOI] [PubMed] [Google Scholar]
- Austreng E. (1978). Digestibility determination in fish using chromic oxide marking and analysis of contents from different segments if the gastrointestinal tract. Aquaculture 13, 265–272. 10.1016/0044-8486(78)90008-X [DOI] [Google Scholar]
- Baba E., Uluköy G., Öntaş C. (2015). Effects of feed supplemented with Lentinula edodes mushroom extract on the immune response of rainbow trout, Oncorhynchus mykiss, and disease resistance against Lactococcus garvieae. Aquaculture 448, 476–482. 10.1016/j.aquaculture.2015.04.031 [DOI] [Google Scholar]
- Bahramian S., Parsa A. (2017). A survey of growth performance, intestinal micro-flora and meat shelf-life in rainbow trout fed with Pistacia atlantica kurdica essential oil. Iran. J. Fish. Sci. 16, 619–624. [Google Scholar]
- Bakkal S., Robinson S. M., Riley M. A. (2012). Bacteriocins of aquatic microorganisms and their potential applications in the seafood industry in Health and Environment in Aquaculture, ed Carvalho E. (Croatia: InTech; ), 303–328. [Google Scholar]
- Balcázar J. L., Vendrell D., de Blas I., Ruiz-Zarzuela I., Gironés O., Múzquiz J. L. (2007a). In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet. Microbiol. 122, 373–380. 10.1016/j.vetmic.2007.01.023 [DOI] [PubMed] [Google Scholar]
- Balcázar J. L., De Blas I., Ruiz-Zarzuela I., Vendrell D., Girones O., Muzquiz J. L. (2007b). Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunol. Med. Microbiol. 51, 185–193. 10.1111/j.1574-695X.2007.00294.x [DOI] [PubMed] [Google Scholar]
- Balciunas E. M., Martinez F. A. C., Todorov S. D., de Melo G., Franco B. D., Converti A., et al. (2013). Novel biotechnological applications of bacteriocins: a review. Food Control 32, 134–142. 10.1016/j.foodcont.2012.11.025 [DOI] [Google Scholar]
- Banerjee G., Ray A. K. (2017). The advancement of probiotic research and its application in fish farming industries. Res. Vet. Sci. 115, 66–77. 10.1016/j.rvsc.2017.01.016 [DOI] [PubMed] [Google Scholar]
- Banerjee S. P., Dora K. C., Chowdhury S. (2013). Detection, partial purification and characterization of bacteriocin produced by Lactobacillus brevis FPTLB3 isolated from freshwater fish. J. Food Sci. Technol. 50, 17–25. 10.1007/s13197-011-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo A., Ravelo C., Castro N., Calheiros J., Romalde J. L. (2012). Effectiveness of bivalent vaccines against Aeromonas hydrophila and Lactococcus garvieae infections in rainbow trout Oncorhynchus mykiss (Walbaum). Fish Shellfish Immunol. 32, 756–761. 10.1016/j.fsi.2012.01.028 [DOI] [PubMed] [Google Scholar]
- Baya A. M., Toranzo A. E., Lupiani B., Li T., Roberson B. S., Hetrick F. M. (1991). Biochemical and serological characterization of Carnobacterium spp. isolated from farmed and natural populations of striped bass and catfish. Appl. Environ. Microbiol. 57, 3114–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley S. S., Saris P. E. J. (2004). Nisin-producing Lactococcus lactis strains isolated from human milk. Appl. Environ. Microbiol. 70, 5051–5053. 10.1128/AEM.70.8.5051-5053.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. R., Kim D., Jeon J., Lee S.-M., Kim H. K., Kim O.-J., et al. (2015). The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 42, 177–183. 10.1016/j.fsi.2014.10.035 [DOI] [PubMed] [Google Scholar]
- Beck B. R., Song J. H., Park B. S., Kim D., Kwak J.-H., Do H. K., et al. (2016). Distinct immune tones are established by Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 in the gut of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 55, 434–443. 10.1016/j.fsi.2016.06.022 [DOI] [PubMed] [Google Scholar]
- Belicova A., Mikulasova M., Dusinsky R. (2013). Probiotic potential and safety properties of Lactobacillus plantarum from Slovak bryndza cheese. BioMed Res Int. 2013:760298. 10.1155/2013/760298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovier H., Ghittino C., Eldar A. (1997). Immunization with bacterial antigens: infections with streptococci and related organisms. Dev. Biol. 90, 153–160. [PubMed] [Google Scholar]
- Bhugaloo-Vial P., Dousset X., Metivier A., Sorokine O., Anglade P., Boyaval P., et al. (1996). Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significantly different levels of specific inhibitory activity. Appl. Environ. Microbiol. 62, 4410–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindiya E. S., Tina K. J., Raghul S. S., Bhat S. G. (2015). Characterization of deep sea fish gut bacteria with antagonistic potential from Centroscyllium fabricii (deep sea shark). Prob. Antimicrob. Prot. 7, 157–163. 10.1007/s12602-015-9190-x [DOI] [PubMed] [Google Scholar]
- Biswas G., Korenaga H., Nagamine R., Takayama H., Kawahara S., Takeda S., et al. (2013). Cytokine responses in the Japanese pufferfish (Takifugu rubripes) head kidney cells induced with heat-killed probiotics isolated from the Mongolian dairy products. Fish Shellfish Immunol. 34, 1170–1177. 10.1016/j.fsi.2013.01.024 [DOI] [PubMed] [Google Scholar]
- Boonanuntanasarn S., Tiengtam N., Pitaksong T., Piromyou P., Teaumroong N. (2017). Effects of dietary inulin and Jerusalem artichoke (Helianthus tuberosus) on intestinal microbiota community and morphology of Nile tilapia (Oreochromis niloticus) fingerlings. Aquacult. Nutr. 24, 712–722. 10.1111/anu.12600 [DOI] [Google Scholar]
- Bromage E. S., Thomas A., Owens L. (1999). Streptococcus iniae, a bacterial infection in barramundi Lates calcarifer. Dis. Aquat. Org. 36, 177–181. 10.3354/dao036177 [DOI] [PubMed] [Google Scholar]
- Bron P. A., van Baarlen P., Kleerebezem M. (2012). Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 10, 66–78. 10.1038/nrmicro2690 [DOI] [PubMed] [Google Scholar]
- Brum A., Pereira S. A., Owatari M. S., Chagas E. C., Chaves F. C. M., Mouriño J. L. P., et al. (2017). Effect of dietary essential oils of clove basil and ginger on Nile tilapia (Oreochromis niloticus) following challenge with Streptococcus agalactiae. Aquaculture 468, 235–243. 10.1016/j.aquaculture.2016.10.020 [DOI] [Google Scholar]
- Bruni L., Pastorelli R., Viti C., Gasco L., Parisi G. (2018). Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Acuaculture 487, 56–63. 10.1016/j.aquaculture.2018.01.006 [DOI] [Google Scholar]
- Burridge L., Weis J. S., Cabello F., Pizarro J., Bostick K. (2010). Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306, 7–23. 10.1016/j.aquaculture.2010.05.020 [DOI] [Google Scholar]
- Cabello F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8, 1137–1144. 10.1111/j.1462-2920.2006.01054.x [DOI] [PubMed] [Google Scholar]
- Cai X., Wang B., Peng Y., Li Y., Lu Y., Huang Y., et al. (2017). Construction of a Streptococcus agalactiae phoB mutant and evaluation of its potential as an attenuated modified live vaccine in golden pompano, Trachinotus ovatus. Fish Shellfish Immunol. 63, 405–416. 10.1016/j.fsi.2016.11.050 [DOI] [PubMed] [Google Scholar]
- Cai Y., Suyanandana P., Saman P., Benno Y. (1999). Classification and characterization of lactic acid bacteria isolated from the intestines of common carp and freshwater prawns. J. Gen. Appl. Microbiol. 45, 177–184. 10.2323/jgam.45.177 [DOI] [PubMed] [Google Scholar]
- Calo-Mata P., Arlindo S., Boehme K., de Miguel T., Pascoal A., Barros-Velazquez J. (2007). Current applications and future trends of lactic acid bacteria and their bacteriocins for the biopreservation of aquatic food products. Food Biopro. Technol. 1, 43–63. 10.1007/s11947-007-0021-2 [DOI] [Google Scholar]
- Carnevali O., Sun Y.-Z., Merrifield D. L., Zhou Z., Picchietti S. (2014). Probiotic applications in temperate and warm water fish species in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, eds Merrifield D., Ringø E. (Oxford, UK: Wiley-Blackwell Publishing; ), 253–289. [Google Scholar]
- Carr F. J., Chill D., Maida N. (2002). The lactic acid bacteria: a literature survey. Crit. Rev. Microbiol. 28, 281–370. 10.1080/1040-840291046759 [DOI] [PubMed] [Google Scholar]
- Castex M., Daniels C., Chim L. (2014). Probiotic applications in crustaceans in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, eds Merrifield D., Ringø E. (Oxford, UK: Wiley-Blackwell Publishing; ), 290–327. [Google Scholar]
- Chahad O. B., El Bour M., Calo-Mata P., Boudabous A., Barros-Vela‘zquez J. (2012). Discovery of novel biopreservation agents with inhibitory effects on growth of food-borne pathogens and their application to seafood products. Res. Microbiol. 163, 44–54. 10.1016/j.resmic.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Chen S. C., Liaw L. L., Su H. Y., Ko S. C., Wu C. Y., Chaung H. C., et al. (2002). Lactococcus garvieae, a cause of disease in grey mullet, Mugil cephalus L., in Taiwan. J. Fish Dis. 25, 727–732. 10.1046/j.1365-2761.2002.00415.x [DOI] [Google Scholar]
- Choi W. M., Lam C. L., Mo W. Y., Cheng Z., Mak N. K., Bian Z. X., et al. (2014). Effects of the modified Huanglian Jiedu decoction on the disease resistance in grey mullet (Mugil cephalus) to Lactococcus garvieae. Mar. Poll. Bull. 85, 816–823. 10.1016/j.marpolbul.2014.04.043 [DOI] [PubMed] [Google Scholar]
- Collins M. D., Ash C., Farrow J. A. E., Wallbanks S., Williams A. M. (1989). 16S ribosomal ribonucleic acid sequence analyses of lactococci and related taxa. Description of Vagococcus fluvialis gen. nov., sp. nov. J. Appl. Bacteriol. 67, 453 – 460. 10.1111/j.1365-2672.1989.tb02516.x [DOI] [PubMed] [Google Scholar]
- Colorni A., Diamant A., Eldar A., Kvitt H., Zlotkin A. (2002). Streptococcus iniae infections in Red sea cage-cultured and wild fishes. Dis. Aquatic Org. 49, 165–170. 10.3354/dao049165 [DOI] [PubMed] [Google Scholar]
- Colorni A., Ravelo C., Romalde J. L., Toranzo A. E., Diamant A. (2003). Lactococcus garvieae in wild Red sea wrasse Coris aygula (Labridae). Dis. Aquat. Org. 56, 275–278. 10.3354/dao056275 [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Hill C., Ross R. P. (2005). Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3, 777–788. 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Ross R. P., Hill C. (2013). Bacteriocins - a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105. 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- Czaran T. L., Hoekstra R. F., Pagie L. (2002). Chemical warfare between microbes promotes biodiversity. Proc. Nat. Acad. Sci. U.S.A. 99, 786–790. 10.1073/pnas.012399899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood M. A. O., Koshio S. (2016). Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture 454, 243–251. 10.1016/j.aquaculture.2015.12.033 [DOI] [Google Scholar]
- Dawood M. A. O., Koshio S., Ishikawa M., El-Sabagh M., Yokoyama S., Wang W.-L., et al. (2017). Physiological response, blood chemistry profile and mucus secretion of red sea bream (Pagrus major) fed diets supplemented with Lactobacillus rhamnosus under low salinity stress. Fish Physiol. Biochem. 43, 179–192. 10.1007/s10695-016-0277-4 [DOI] [PubMed] [Google Scholar]
- Dawood M. A. O., Koshio S., Ishikawa M., Yokoyama S. (2015). Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 442, 29–36. 10.1016/j.aquaculture.2015.02.005 [DOI] [Google Scholar]
- Dawood M. A. O., Koshio S., Ishikawa M., Yokoyama S. (2016a). Effects of dietary inactivated Pediococcus pentosaceus on growth performance, feed utilization and blood characteristics of red sea bream, Pagrus major juvenile. Aquacult. Nutr. 22, 923–932. 10.1111/anu.12314 [DOI] [Google Scholar]
- Dawood M. A. O., Koshio S., Ishikawa M., Yokoyama S., El Basuini M. F., Hossain M. S., et al. (2016b). Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol. 49, 275–285. 10.1016/j.fsi.2015.12.047 [DOI] [PubMed] [Google Scholar]
- De B. C., Meena D., Behera B., Das P., Mohapatra P. D., Sharma A. (2014). Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol. Biochem. 40, 921–971. 10.1007/s10695-13-9897-0. [DOI] [PubMed] [Google Scholar]
- De Kwaadsteniet M., ten Doeschate K., Dicks L. M. T. (2008). Characterization of the structural gene encoding nisin f, a new lantibiotic produced by a Lactococcus lactis subsp. lactis isolate from freshwater catfish (Clarias gariepinus). Appl. Environ. Microbiol. 74, 547–549. 10.1128/AEM.01862-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst L., Leroy F. (2007). Bacteriocins from lactic acid bacteria: Production, purification and food applications. J. Mol. Microbiol. Biotechnol. 13, 194–199. 10.1159/000104752 [DOI] [PubMed] [Google Scholar]
- Dehler C. E., Secombes C. J., Martin S. A. M. (2017a). Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.). Aquaculture 467, 149–157. 10.1016/j.aquaculture.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehler C. E., Secombes C. J., Martin S. A. M. (2017b). Seawater transfer alters the intestinal microbiota profiles of Atlantic salmon parr (Salmo salar L.). Sci Rep. 7:13877 10.1038/s41598-017-13249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del‘Duca A., Cesar D. E., Albreu P. C. (2015). Bacterial community of pond‘s water, sediment and in the guts of tilapia (Oreochromis niloticus) juveniles characterized by fluorescent in situ hybridization technique. Aquacult Res. 46, 707–715. 10.1111/are.12218 [DOI] [Google Scholar]
- Delcenserie V., Martel D., Lamoureux M., Amiot J., Boutin Y., Roy D. (2008). Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 10, 37–54. 10.21775/cimb.010.037 [DOI] [PubMed] [Google Scholar]
- Desai A. R., Links M. G., Collins S. A., Mansfield G. S., Drew M. D., Van Kessel A. G., et al. (2012). Effects of plant based diets on the distal gut microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 350–353, 134–142. 10.1016/j.aquaculture.2012.04.005 [DOI] [Google Scholar]
- Desriac F., Defer D., Bourgougnon N., Brillet B., Le Chevalier P., Fleury Y. (2010). Bacteriocin as weapons in the marine animal-associated bacteria warfare: inventory and potential applications as an aquaculture probiotic. Mar. Drugs 8, 1153–1177. 10.3390/md8041153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didinen B. I., Onuk E. E., Metin S., Cayli O. (2018). Identification and characterization of lactic acid bacteria isolated from rainbow trout (Oncorhynchus mykiss, Walbaum 1792), with inhibitory activity against Vagococcus salmoninarum and Lactococcus garvieae. Aquacult. Nutr. 24, 400–407. 10.1111/anu.12571 [DOI] [Google Scholar]
- Diop M. B., Dubois-Dauphin R., Destain J., Tine E., Thonart P. (2009). Use of a nisin-producing starter culture of Lactococcus lactis subsp, lactis to improve traditional fish fermentation in Senegal. J. Food Protect. 72, 1930–1934. 10.4315/0362-028X-72.9.1930 [DOI] [PubMed] [Google Scholar]
- do Vale Pereira G., da Cunha D. G., Mourino J. L. P., Rodiles A., Jaramillo-Torres A., Merrifield D. L. (2017). Characterization of microbiota in Arapaima gigas intestine and isolation of potential probiotic bacteria. J. Appl. Microbiol. 123, 1298–1311. 10.1111/jam.13572 [DOI] [PubMed] [Google Scholar]
- Doan H. V., Hoseinifar S. H., Tapingkae W., Chitmanat C., Mekchay S. (2017). Effects of Cordyceps militaris spent mushroom substrate on mucosal and serum immune parameters, disease resistance and growth performance of Nile tilapia, (Oreochromis niloticus). Fish Shellfish Immunol. 67, 78–85. 10.1016/j.fsi.2017.05.062 [DOI] [PubMed] [Google Scholar]
- Dong B., Yi Y., Liang L., Shi Q. (2017). High throughput identification of antimicrobial peptides from fish gastrointestinal microbiota. Toxins 9:266. 10.3390/toxins9090266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drider D., Fimland G., Hechard Y., McMullen L. M., Prevost H. (2006). The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70, 564–582. 10.1128/MMBR.00016-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont A. W., DuPont H. L. (2011). The intestinal microbiota and chronic disorders of the gut. Nat. Rev. Gastroenterol. Hepatol. 8, 523–531. 10.1038/nrgastro.2011.133 [DOI] [PubMed] [Google Scholar]
- Duremdez R., Al-Marzouk A., Qasem J. A., Al-Harbi A., Gharabally H. (2004). Isolation of Streptococcus agalactiae from cultured silver pomfret, Pampus argenteus (Euphrasen), in Kuwait. J. Fish Dis. 27, 307–310. 10.1111/j.1365-2761.2004.00538.x [DOI] [PubMed] [Google Scholar]
- Eijsink V. G. H., Axelsson L., Diep D. B., Havarstein L. S., Holo H., Nes I. F. (2002). Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Van Leeuwenhoek 81, 639–654. 10.1023/A:1020582211262 [DOI] [PubMed] [Google Scholar]
- Elayaraja S., Annamalai N., Mayavu P., Balasubramanian T. (2014). Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 4, S305–S311. 10.12980/APJTB.4.2014C537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. J., Klesius P. H., Shoemaker C. A. (2004). Efficacy of Streptococcus agalactiae (group B) vaccine in tilapia (Oreochromis niloticus) by intraperitoneal and bath immersion administration. Vaccine 22, 3769–3773. 10.1016/j.vaccine.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Evans J. J., Klesius P. H., Shoemaker C. A. (2009). First isolation and characterization of Lactococcus garvieae from Brazilian Nile tilapia, Oreochromis niloticus (L.), and pintado, Pseudoplathystoma corruscans (Spix & Agassiz). J. Fish Dis. 32, 943–951. 10.1111/j.1365-2761.2009.01075.x [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2002). Guidelines for the Evaluation of Probiotics in Food. Report of a joint FAO/WHO working group, London, ON. [Google Scholar]
- Faikoh E. N., Hong Y.-H., Hu S.-Y. (2014). Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol. 38, 15–24. 10.1016/j.fsi.2014.02.024 [DOI] [PubMed] [Google Scholar]
- Fečkaninová A., Koščová J., Mudronová D., Popelka P., Toropilová J. (2017). The use of probiotic bacteria against Aeromonas infections in salmonid aquaculture. Aquaculture 469, 1–8. 10.1016/j.aquaculture.2016.11.042 [DOI] [Google Scholar]
- Ford A. C., Quigely E. M. M., Lacy B. E., Lembo A. J., Saito Y. A., Schiller L. P., et al. (2014). Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am. J. Gastroenterol. 109, 1547–1561. 10.1038/ajg.2014.202 [DOI] [PubMed] [Google Scholar]
- Fukushima H. C. S., Leal C. A. G., Cavalcante R. B., Figueiredo H. C. P., Arijo S., Morinigo M. A., et al. (2017). Lactococcus garvieae outbreak in Brazilian farms lactococcosis in Pseudoplatystoma sp. – development of an autogenous vaccine as a control strategy. J. Fish Dis. 40, 263–272. 10.1111/jfd.12509 [DOI] [PubMed] [Google Scholar]
- Fusco V., Quero G. M., Cho G.-S., Kabisch J., Meske D., Neve H., et al. (2015). The genus Weissella: taxonomy, ecology and biotechnological potential. Front. Microbiol. 6:155. 10.3389/fmicb.2015.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel N. N., Qiang J., He J., Ma X. Y., Kpundeh M. D., Xu P. (2015). Dietary aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT). Fish Shellfish Immunol. 44, 504–514. 10.1016/j.fsi.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Gajardo K., Jaramillo-Torres A., Kortner T. M., Merrifield D. L., Tinsley J., Bakke A. M., et al. (2017). Alternative protein sources in the diet modulate microbiota and functionality in the distal intestine of Atlantic salmon (Salmo salar). Appl. Environ. Microbiol. 83:e02615. 10.1128/AEM.02615-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Dora K. C., Sarkar S., Chowdhury S. (2012). Supplementation of prebiotics in fish feed: a review. Rev. Fish Biol. Fish. 23, 195–199. 10.1007/s11160-012-9291-5 [DOI] [Google Scholar]
- Gao Y., He J., He Z., Li Z., Zhao B., Mu Y., et al. (2017). Effects of fulvic acid on growth performance and intestinal health of juvenile loach Paramisgurnus dabryanus (Sauvage). Fish Shellfish Immunol. 62, 47–56. 10.1016/j.fsi.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Gatesoupe F. J. (2008). Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J. Mol. Microbiol. Biotechnol. 14, 107–114. 10.1159/000106089 [DOI] [PubMed] [Google Scholar]
- German D. P. (2009). Inside the gut of wood-eating catfishes: can they digest wood? J. Comp. Physiol. B 179, 1011–1023. 10.1007/s00360-009-0381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari M., Jami M., Kneifel W., Domig K. J. (2013). Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from sturgeon fish. Food Control 32, 379–385. 10.1016/j.foodcont.2012.12.024 [DOI] [Google Scholar]
- Ghosh S., Ringø E., Selvam A. D. G., Rahiman K. M. M., Sathyan N., John N., et al. (2014). Gut associated lactic acid bacteria isolated from the estuarine fish Mugil cephalus: Molecular diversity and antibacterial activities against pathogens. Int. J. Aquacult. 4, 1–11. 10.5376/ija.2014.04.0001 [DOI] [Google Scholar]
- Gibson G. R., Roberfroid M. B. (1995). Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412. 10.1093/jn/125.6.1401 [DOI] [PubMed] [Google Scholar]
- Gillor O., Etzion A., Riley M. A. (2008). The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 81, 591–606. 10.1007/s00253-008-1726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioacchini G., Giorgini E., Olivotto I., Maradonna F., Merrifield D. L., Carnevali O. (2014). The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress. Zebrafish 11, 98–106. 10.1089/zeb.2013.0932 [DOI] [PubMed] [Google Scholar]
- Giri S. S., Sukumaran V., Oviya M. (2013). Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 34, 660–666. 10.1016/j.fsi.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Giri S. S., Sukumaran V., Sen S. S., Jena P. K. (2014). Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeo rohita. Aquacult. Nutr. 20, 163–171. 10.1111/anu.12062 [DOI] [Google Scholar]
- Gobbetti M., De Angelis M., Di Cagno R., Minervini F., Limitone A. (2007). Cell–cell communication in food related bacteria. Int. J. Food Microbiol. 120, 34–45. 10.1016/j.ijfoodmicro.2007.06.012 [DOI] [PubMed] [Google Scholar]
- Gomez D., Sunyer J. O., Salinas I. (2013). The mucosal immune system of fish: the evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 35, 1729–1739. 10.1016/j.fsi.2013.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez G. D., Balcázar J. L. (2008). A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 52, 145–154. 10.1111/j.1574-695X.2007.00343.x [DOI] [PubMed] [Google Scholar]
- Gómez-Sala B., Munoz-Atienza E., Sánchez J., Basanta A., Herranz C., Hernández P. E., et al. (2015). Bacteriocin production by lactic acid bacteria isolated from fish, seafood and fish products. Eur. Food Res. Technol. 241, 341–356. 10.1007/s00217-015-2465-3 [DOI] [Google Scholar]
- González C. J., Encinas J. P., García-López M. L., Otero A. (2000). Characterization and identification of lactic acid bacteria from freshwater fishes. Food Microbiol. 17, 383 – 391. 10.1006/fmic.1999.0330 [DOI] [Google Scholar]
- Gudding R., Van-Muiswinkel W. B. (2013). A history of fish vaccination science-based disease prevention in aquaculture. Fish Shellfish Immunol. 35, 1683–1688. 10.1016/j.fsi.2013.09.031 [DOI] [PubMed] [Google Scholar]
- Guerreiro I., Serra C. R., Pousão-Ferreira P., Oliva-Teles A., Enes P. (2018a). Prebiotics effect on growth performance, hepatic intermediary metabolism, gut microbiota and digestive enzymes of white sea bream (Diplodus sargus). Aquacult. Nutr. 24, 153–163. 10.1111/anu.12543 [DOI] [Google Scholar]
- Guerreiro I., Serra C. R., Oliva-Teles A., Enes P. (2018b). Short communication: gut microbiota of European sea bass (Dicentrarchus labrax) is modulated by short-chain fructooligosaccharides and xylooligosaccharides. Aquacult. Int. 26, 279–288. 10.1007/s10499-017-0220-4 [DOI] [Google Scholar]
- Guimarães I. G., Lim C., Yildirim-Aksoy M., Li M. H., Klesius P. H. (2014). Effects of dietary levels of vitamin A on growth, hematology, immune response and resistance of Nile tilapia (Oreochromis niloticus) to Streptococcus iniae. Anim. Feed Sci. Technol. 188, 126–136. 10.1016/j.anifeedsci.2013.12.003 [DOI] [Google Scholar]
- Gutowska M. A., Drazen J. C., Robison B. H. (2004). Digestive chitinolytic activity in marine fishes of Monterey bay, California. Comp. Biochem. Physiol. Part A 13, 351–358. 10.1016/j.cbpb.2004.09.020 [DOI] [PubMed] [Google Scholar]
- Haines A. N., Gauthier D. T., Nebergall E. E., Cole S. D., Nguyen K. M., Rhodes M. W., et al. (2013). First report of Streptococcus parauberis in wild finfish from North America. Vet Microbiol. 166, 270–275. 10.1016/j.vetmic.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Halami P. M., Chandrashekar A., Joseph R. (1999). Characterization of bacteriocinogenic strains of lactic acid bacteria in fowl and fish intestine and mushroom. Food Biotechnol. 13, 121–136. 10.1080/08905439909549966 [DOI] [Google Scholar]
- Hammami R., Zouhir A., Le Lay C., Ben Hamida J., Fliss I. (2010). BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol. 10:22. 10.1186/1471-2180-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikrishnan R., Kim M.-C., Kim J.-S., Balasundaram C., Heo M.-S. (2011). Protective effect of herbal and probiotics enriched diet on haematological and immunity status of Oplegnathus fasciatus (Temminck & Schlegel) against Edwardsiella tarda. Fish Shellfish Immunol. 30, 886–893. 10.1016/j.fsi.2011.01.013 [DOI] [PubMed] [Google Scholar]
- He R.-P., Feng J., Tian X.-L., Dong S.-L., Wen B. (2017). Effects of dietary supplementation of probiotics on the growth, activities of digestive and non-specific immune enzymes in hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Aquacult. Res. 48, 5782–5790. 10.1111/are.13401 [DOI] [Google Scholar]
- Heo W. S., Kim E. Y., Kim Y. R., Hossain M. T., Kong I. S. (2012). Salt effect of nisin Z isolated from a marine fish on the growth inhibition of Streptococcus iniae, a pathogen of streptococcosis. Biotechnol. Lett. 34, 315–320. 10.1007/s10529-011-0766-6 [DOI] [PubMed] [Google Scholar]
- Hiu S. F., Holt R. A., Sriranganathan N. (1984). Lactobacillus piscicola, a new species from salmonid fish. Int. J. Syst. Bacteriol. 34, 393–400. 10.1099/00207713-34-4-393 [DOI] [Google Scholar]
- Hoseinifar S. H., Mirvaghefi A., Amoozegar M. A., Merrifield D., Ringø E. (2015a). In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquacult. Nutr. 23, 111–118. 10.1111/anu.12373. [DOI] [Google Scholar]
- Hoseinifar S. H., Mirvaghefi A., Amoozegar M. A., Sharifian M., Esteban M. Á. (2015b). Modulation of innate immune response, mucosal parameters and disease resistance in rainbow trout (Oncorhynchus mykiss) upon synbiotic feeding. Fish Shellfish Immunol. 45, 27–32. 10.1016/j.fsi.2015.03.029 [DOI] [PubMed] [Google Scholar]
- Hoseinifar S. H., Roosta Z., Hajimoradloo A., Vakili F. (2015c). The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish Shellfish Immunol. 42, 533–538. 10.1016/j.fsi.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Hoseinifar S. H., Khalili M., Sun Y.-Z. (2016a). Intestinal histomorphology, autochthonus microbiota and growth performance of the oscar (Astronotus ocellatus Agassiz, 1831) following dietary administration of xylooligosaccharide. J. Ichthyol. 32, 1137–1141. 10.1111/jai.13118 [DOI] [Google Scholar]
- Hoseinifar S. H., Hoseini S. M., Bagheri D. (2016b). Effects of galactooligosaccharide and Pediococcus acidilactici on antioxidant defence and disease resistance of rainbow trout, Oncorhynchus mykiss. Ann. Anim. Sci. 17, 217–227. 10.1515/aoas-2016-0024 [DOI] [Google Scholar]
- Hoseinifar S. H., Ringø E., Shenavar Masouleh A., Esteban M. Á. (2016c). Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: a review. Rev. Aquacult. 8, 89–102, 10.1111/raq.12082 [DOI] [Google Scholar]
- Hoseinifar S. H., Safari R., Dadar M. (2017). Dietary sodium propionate affects mucosal immune parameters, growth and appetite related genes expression: Insights from zebrafish model. Gen. Comp. Endocrinol. 243, 78–83. 10.1016/j.ygcen.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Hosseini M., Miandare H. K., Hoseinifar S. H., Yarahmadi P. (2016). Dietary Lactobacillus acidophilus modulated skin mucus protein profile, immune and appetite genes expression in gold fish (Carassius auratus gibelio). Fish Shellfish Immunol. 59, 149–154. 10.1016/j.fsi.2016.10.026 [DOI] [PubMed] [Google Scholar]
- Huang J.-B., Wu Y.-C., Chi S.-C. (2014). Dietary supplementation of Pediococcus pentosaceus enhances innate immunity, physiological health and resistance to Vibrio anguillarum in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 39, 196–205. 10.1016/j.fsi.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Huang L. Y., Wang K. Y., Xiao D., Chen D. F., Geng Y., Wang J., et al. (2014). Safety and immunogenicity of an oral DNA vaccine encoding Sip of Streptococcus agalactiae from Nile tilapia Oreochromis niloticus delivered by live attenuated Salmonella typhimurium. Fish Shellfish Immunol. 38, 34–41. 10.1016/j.fsi.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Huyben D., Nyman A., Vidakovi'c A., Passoth V., Moccia R., Kiessling A., et al. (2017). Effects of dietary inclusion of the yeast Saccharomyces cerevisiae and Wickerhamomyces anomalus on gut microbiota of rainbow trout. Aquaculture 473, 528–537, 10.1016/j.aquaculture.2017.03.024 [DOI] [Google Scholar]
- Huyben D., Sun L., Moccia R., Kiessling A., Dicksved J., Lundh T. (2018). Dietary live yeast and increased water temperature influence the gut microbiota of rainbow trout. J. Appl. Microbiol. 124, 1377–1392. 10.1111/jam.13738 [DOI] [PubMed] [Google Scholar]
- Indira K., Jayalakshmi S., Gopalakrishnan A., Srinivasan M. (2011). Biopreservative potential of marine Lactobacillus spp. Afr. J. Microbiol. Res. 5, 2287–2296. 10.5897/AJMR11.613 [DOI] [Google Scholar]
- Irianto A., Austin B. (2002). Probiotics in aquaculture. J. Fish Dis. 25, 63–642. 10.1046/j.1365-2761.2002.00422.x [DOI] [Google Scholar]
- Issazadeh K., Pahlaviani M., Massiha A. (2012). Isolation of Lactobacillus species from sediments of Caspian sea for bacteriocin production in 2nd Int Con. Biomed. Eng. Tech IPCBEE (Singapore: ), 79–84. [Google Scholar]
- Iwashita M. K., Nakandakare I. B., Terhune J. S., Wood T., Ranzani-Paiva M. J. (2015). Dietary supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae enhance immunity and disease resistance against Aeromonas hydrophila and Streptococcus iniae infection in juvenile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 43, 60–66. 10.1016/j.fsi.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Jesus G., Vieira F., Silva B., Junior M., Ushizima T., Schmidt E., et al. (2017). Probiotic bacteria may prevent haemorrhagic septicaemia by maturing intestinal host defences in Brazilian native surubins. Aquacult. Nutr. 23, 484–491. 10.1111/anu.12416 [DOI] [Google Scholar]
- Kaew-on S., Areechon N., Wanchaitanawong P. (2016). Effects of Pediococcus pentosaceus PKWA-1 and Bacillus subtilis BA04 on growth performances, immune responses and disease resistance against Aeromonas hydrophila in Nile Tilapia (Oreochromis niloticus Linn.). Chiang Mai J. Sci. 43, 997–1006. Available online at: http://it.science.cmu.ac.th/ejournal/journalDetail.php?journal_id=7358 [Google Scholar]
- Kane A. M., Soltani M., Ebrahimzahe-Mousavi H. A., Pakzad K. (2016). Influence of probiotic, Lactobacillus plantarum on serum biochemical and immune parameters in vaccinated rainbow trout (Oncorhynchus mykiss) against streptococcosis/lactococosis. Int J. Aquat. Biol. 4, 285–294. 10.22034/ijab.v4i4.226 [DOI] [Google Scholar]
- Karthikeyan V., Santhosh S. W. (2009). Study of bacteriocin as a food preservative and the L. acidophilus strain as probiotic. Pakistan J. Nutr. 8, 335–340. 10.3923/pjn.2009.335.340 [DOI] [Google Scholar]
- Kawanishi M., Kojima A., Ishihara K., Esaki H., Kijima M., Takahashi T., et al. (2005). Drug resistance and pulsed-field gel electrophoresis patterns of Lactococcus garvieae isolates from cultured seriola (yellowtail, amberjack and kingfish) in Japan. Lett. Appl. Microbiol. 40, 322–328. 10.1111/j.1472-765X.2005.01690.x [DOI] [PubMed] [Google Scholar]
- Kayansamruaj P., Dong H. T., Pirarat N., Nilubol D., Rodkhum C. (2017). Efficacy of α-enolase-based DNA vaccine against pathogenic Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Aquaculture 468, 102–106. 10.1016/j.aquaculture.2016.10.001 [DOI] [Google Scholar]
- Ke X., Chen X., Liu Z., Lu M., Gao F., Cao J. (2017). Immunogenicity of the LrrG protein encapsulated in PLGA microparticles in Nile tilapia (Oreochromis niloticus) vaccinated against Streptococcus agalactiae. Aquaculture 480, 51–57. 10.1016/j.aquaculture.2017.08.003 [DOI] [Google Scholar]
- Kesarcodi-Watson A., Kaspar H., Lategan M. J., Gibson L. (2008). Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274, 1–14. 10.1016/j.aquaculture.2007.11.019 [DOI] [Google Scholar]
- Kim D., Beck B. R., Heo S.-B., Kim J., Kim H. D., Lee S.-M., et al. (2013). Lactococcus lactis BFE920 activates the innate immune system of olive flounder (Paralichthys olivaceus), resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large-scale field studies. Fish Shellfish Immunol. 35, 1585–1590. 10.1016/j.fsi.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Kim D., Beck B. R., Lee S. M., Jeon J., Lee D. W., Lee J. I., et al. (2016). Pellet feed adsorbed with the recombinant Lactococcus lactis BFE920 expressing SiMA antigen induced strong recall vaccine effects against Streptococcus iniae infection in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 55, 374–383. 10.1016/j.fsi.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Kim D. H., Austin B. (2008). Characterization of probiotic carnobacteria isolated from rainbow trout (Oncorhynchus mykiss) intestine. Lett. Appl. Microbiol. 47, 141–147. 10.1111/j.1472-765X.2008.02401.x [DOI] [PubMed] [Google Scholar]
- Kim Y.-R., Kim E.-Y., Choi S.-Y., Hossain M. T., Oh R., Heo W.-S., et al. (2012). Effect of a probiotic strain, Enterococcus faecium, on the immune responses of olive flounder (Paralichthys olivaceus). J. Microbiol. Biotechnol. 22, 526–529. 10.4014/jmb.1108.08047 [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R. (1993). Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Lett. 12, 224–227. 10.1111/j.1574-6976.1993.tb00012.x [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Shoemaker C. A., Evans J. J. (2000). Efficacy of single and combined Streptococcus iniae isolate vaccine administered by intraperitoneal and intramuscular routes in tilapia (Oreochromis niloticus). Aquaculture 188, 237–246. 10.1016/S0044-8486(00)00345-8 [DOI] [Google Scholar]
- Knosp C. A., Johnston J. A. (2012). Regulation of CD4+ T-cell polarization by suppressor of cytokine signaling proteins. Immunology 135, 101–111. 10.1111/j.1365-2567.2011.03520.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolndadacha O. D., Adikwu I. A., Okaeme A. N., Atiribom R. Y., Mohammed A., Musa Y. M. (2011). The role of probiotics in aquaculture in Nigeria – a review. Cont. J. Fish Aquat. Sci. 5, 8–15. Available online at: http://www.wiloludjournal.com [Google Scholar]
- Kraus H. (1961). Kurze mitteilung über das vorkommen von Lactobazillien auf fischen heringen. Arch. Lebensmittelhyg. 12, 101–102. [Google Scholar]
- Kumar S. R., Arul V. (2009). Purification and characterization of phocaecin PI80: an anti-listerial bacteriocin produced by Streptococcus phocae PI80 Isolated from the gut of Peneaus indicus (Indian white shrimp). J. Microbiol. Biotechnol. 19, 1393–1400. 10.4014/jmb.0901.0003 [DOI] [PubMed] [Google Scholar]
- Kusuda R., Salati F. (1993). Major bacterial diseases affecting mariculture in Japan. Ann. Rev. Fish Dis. 3, 69–85. 10.1016/0959-8030(93)90029-B [DOI] [Google Scholar]
- Lagha A. B., Haas B., Gottschalk M., Grenier D. (2017). Antimicrobial potential of bacteriocins in poultry and swine production. Vet Res. 48:22. 10.1186/s13567-017-0425-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing K. J., Hansen J. D. (2011). Fish T cells: recent advances through genomics. Dev. Comp. Immunol. 35, 1282–1295. 10.1016/j.dci.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Laith A. A., Mazlan A. G., Effendy A. W., Ambak M. A., Nurhafizah W. W. I., Alia A. S., et al. (2017). Effect of Excoecaria agallocha on non-specific immune responses and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Res. Vet. Sci. 112, 192–200. 10.1016/j.rvsc.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Lauzon H. L., Ringø E. (2011). Prevalence and application of lactic acid bacteria in aquatic environments in Lactic Acid Bacteria: Microbiological and Functional Aspects, 4th Edn, eds Lahtinen S., Ouwehand A., Salminen S., von Wright A. (New York, NY: CRC Press, Taylor and Francis Group; ), 593–631. [Google Scholar]
- Lauzon H. L., Dimitroglou A., Merrifield D. L., Ringø E., Davies S. J. (2014). Probiotics and prebiotics: concepts, definitions and history in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, eds Merrifield D., Ringø E. (Oxford, UK: Wiley-Blackwell Publishing; ), 169–184. [Google Scholar]
- Lavoie C., Courcelle M., Redivo B., Derome N. (2018). Structural and compositional mismatch between captive and wild Atlantic salmon (Salmo salar) parrs gut microbiota highlights the relevance of integrating molecular ecology for management and conservation methods. Evolution. Appl. 10.1111/eva.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Katya K., Park Y., Won S., Seong M., Hamidoghli A., et al. (2017). Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 61, 201–210. 10.1016/j.fsi.2016.12.035 [DOI] [PubMed] [Google Scholar]
- Leisner J. J., Groth Laursen B. G., Prévost H., Drider D., Dalgaard P. (2007). Carnobacterium: positive and negative effects in the environment and in foods. FEMS Microbiol Rev. 31, 592–613. 10.1111/j.1574-6976.2007.00080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisner J. J., Hansen M. A., Larsen M. H., Hansen L., Ingmer H., Sørensen S. J. (2012). The genome sequence of the lactic acid bacterium, Carnobacterium maltaromaticum ATCC 35586 encodes potential virulence factors. Int. J. Food Microbiol. 152, 107–115. 10.1016/j.ijfoodmicro.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Lescak E. A., Milligan K. C. (2017). Teleost as model organisms to understand host-microbe interactions. J. Bacteriol. 199, e00868–16. 10.1128/JB.00868-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhong Q., Wirth S., Wang W., Hao Y., Zou H., et al. (2015). Diversity of autochthonous bacterial communities in the intestinal mucosa of grass carp (Ctenopharyngodon idellus) (Valenciennes) by culture-dependent and culture-independent techniques. Aquacult. Res. 46, 2344–2359. 10.1111/are.12391 [DOI] [Google Scholar]
- Li P., Gatlin D. M., III. (2004). Dietary brewers yeast and the prebiotic Grobiotic™AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops×M. saxatilis) to Streptococcus iniae infection. Aquaculture 231, 445–456. 10.1016/j.aquaculture.2003.08.021 [DOI] [PubMed] [Google Scholar]
- Li P., Lewis D. H., Gatlin D. M. (2004). Dietary oligonucleotides from yeast RNA influence immune responses and resistance of hybrid striped bass (Morone chrysops × Morone saxatilis) to Streptococcus iniae infection. Fish Shellfish Immunol. 16, 561–569. 10.1016/j.fsi.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Li X., Ringø E., Hoseinifar S. H., Lauzon H., Birkbeck H., Yang D. (2018). Adherence and colonisation of microorganisms in the fish gastrointestinal tract. Rev Aquacult. 10.1111/raq.12248 [DOI] [Google Scholar]
- Lim C., Yildirim-Aksoy M., Li M. H., Welker T. L., Klesius P. H. (2009). Influence of dietary levels of lipid and vitamin E on growth and resistance of Nile tilapia to Streptococcus iniae challenge. Aquaculture 298, 76–82. 10.1016/j.aquaculture.2009.09.025 [DOI] [Google Scholar]
- Lin Y. H., Chen Y. S., Wu H. C., Pan S. F., Yu B., Chiang C. M., et al. (2012). Screening and characterization of LAB-produced bacteriocin-like substances from the intestine of grey mullet (Mugil cephalus L.) as potential biocontrol agents in aquaculture. J. Appl. Microbiol. 114, 299–307. 10.1111/jam.12041 [DOI] [PubMed] [Google Scholar]
- Lisboa M. P., Bonatto D., Bizani D., Henriques J. A. P., Brandelli A. (2006). Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic Forest. Int. Microbiol. 9, 111–118. Available online at: www.im.microbios.org [PubMed] [Google Scholar]
- Liu H., Wang S., Cai Y., Guo X., Cao Z., Zhang Y., et al. (2017). Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 60, 326–333. 10.1016/j.fsi.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Liu L., Li Y. W., He R. Z., Xiao X. X., Zhang X., Su Y. L., et al. (2014). Outbreak of Streptococcus agalactiae infection in barcoo grunter, Scortum barcoo (McCulloch & Waite), in an intensive fish farm in China. J. Fish Dis. 37, 1067–1072. 10.1111/jfd.12187 [DOI] [PubMed] [Google Scholar]
- Liu W., Ren P., He S., Xu L., Yang Y., Gu Z., et al. (2013). Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish Shellfish Immunol. 35, 54–62. 10.1016/j.fsi.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Liu W., Wang W., Ran C., He S., Yang Y., Zhou Z. (2017). Effects of dietary scFOS and lactobacilli on survival, growth, and disease resistance of hybrid tilapia. Aquaculture 470, 50–55. 10.1016/j.aquaculture.2016.12.013 [DOI] [Google Scholar]
- Loch T. P., Xu W., Fitzgerald S. M., Faisal M. (2008). Isolation of a Carnobacterium maltaromaticum-like bacterium from systemically infected lake whitefish (Coregonus clupeaformis). FEMS Microbiol. Lett. 288, 76–84. 10.1111/j.1574-6968.2008.01338.x [DOI] [PubMed] [Google Scholar]
- Lopez Cazorla A., Sica M. G., Brugnoni L. I., Marucci P. L., Cubitto M. A. (2015). Evaluation of Lactobacillus paracasei subsp. tolerans isolated from Jenyn's sprat (Ramnogaster arcuata) as probiotic for juvenile rainbow trout Oncorhynchus mykiss (Walbaum, 1792). J. Appl. Ichthyol. 31, 88–94. 10.1111/jai.12496 [DOI] [Google Scholar]
- Luo X., Fu X., Liao G., Chang O., Huang Z., Li N. (2017). Isolation, pathogenicity and characterization of a novel bacterial pathogen Streptococcus uberis from diseased mandarin fish Siniperca chuatsi. Microb. Pathogen. 107, 380–389. 10.1016/j.micpath.2017.03.049 [DOI] [PubMed] [Google Scholar]
- Lyons P. P., Turnbull J. F., Dawson K. A., Crumlish M. (2016). Phylogenetic and functional characterization of the distal intestine microbiome of rainbow trout Oncorhynchus mykiss from both farm and aquarium settings. J. Appl. Microbiol. 122, 347–363. 10.1111/jam.13347 [DOI] [PubMed] [Google Scholar]
- Lyons P. P., Turnbull J. F., Dawson K. A., Crumlish M. (2017a). Exploring the microbial diversity of the distal lumen and mucosa of farmed rainbow trout Oncorhynchus mykiss (Walbaum) using next generation sequencing (NGS). Aquacult Res. 48, 77–91. 10.1111/are.12863 [DOI] [Google Scholar]
- Lyons P. P., Turnbull J. F., Dawson K. A., Crumlish M. (2017b). Effects of low-level dietary microalgae supplementation on the distal intestine microbiome of farmed rainbow trout Oncorhynchus mykiss (Walbaum). Aquacult. Res. 48, 2438–2452. 10.1111/are.13080 [DOI] [Google Scholar]
- Ma Y.-P., Ke H., Liang Z.-L., Ma J.-Y., Hao L., Liu Z.-X. (2017). Protective efficacy of cationic-PLGA microspheres loaded with DNA vaccine encoding the sip gene of Streptococcus agalactiae in tilapia. Fish Shellfish Immunol. 66, 345–353. 10.1016/j.fsi.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Maiuta N. D., Schwarzentruber P., Schenker M., Schoelkopf J. (2013). Microbial population dynamics in the faeces of wood-eating loricariid catfishes. Lett. Appl. Microbiol. 56, 401–407, 10.1111/lam.12061 [DOI] [PubMed] [Google Scholar]
- Maji U. J., Mohanty S., Pradhan A., Maiti N. K. (2017). Immune modulation, disease resistance and growth performance of Indian farmed carp, Labeo rohita (Hamilton), in response to a dietary consortium of putative lactic acid bacteria. Aquacult. Int. 25, 1391–1407. 10.1007/s10499-017-0122-5 [DOI] [Google Scholar]
- Marisa Halim A., Lee P.-P., Chang Z.-W., Chang C.-C. (2017). The hot-water extract of leaves of noni, Morinda citrifolia, promotes the immunocompetence of giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 64, 457–468. 10.1016/j.fsi.2017.03.045 [DOI] [PubMed] [Google Scholar]
- Martinez F. O., Gordon S. (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 6:13. 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M., Mouriño J., Amaral G., Vieira F., Dotta G., Jatobá A., et al. (2008). Haematological changes in Nile tilapia experimentally infected with Enterococcus sp. Braz. J. Biol. 68, 657–661. 10.1590/S1519-69842008000300025 [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Takasaki M., Miyazawa R., Nakanishi T. (2017). Stimulatory effects of heat-killed Enterococcus faecalis on cell-mediated immunity in fish. Dev. Comp. Immunol. 74, 1–9. 10.1016/j.dci.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Merrifield D. L., Balcázar J. L., Daniels C., Zhou Z., Carnevali O., Sun Y.-Z., et al. (2014). Indigenous lactic acid bacteria in fish and crustaceans in Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics, eds D. Merrifield and E. Ringø (Oxford, UK: Wiley-Blackwell Publishing; ), 128–168. [Google Scholar]
- Meyburgh C. M., Bragg R. R., Boucher C. E. (2017). Lactococcus garvieae: an emerging bacterial pathogen of fish. Dis. Aquatic. Org. 123, 67–79. 10.3354/dao03083 [DOI] [PubMed] [Google Scholar]
- Miao S., Zhao C., Zhu J., Hu J., Dong X., Sun L. (2018). Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 8:113. 10.1038/s41598-017-18430-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C., Faivre B., Kerouault B. (1986). Biochemical identification of Lactobacillus piscicola strains from France and Belgium. Dis. Aquatic. Org. 2, 27–30. 10.3354/dao002027 [DOI] [Google Scholar]
- Michel C., Pelletier C., Boussaha M., Douet D. G., Lautraite A., Tailliez P. (2007). Diversity of lactic acid bacteria associated with fish and the farm environment, established by amplified rRNA gene restriction analysis. Appl. Environ. Microbiol. 73, 2947–2955. 10.1128/AEM.01852-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzapour-Rezaee S., Farhangi M., Rafiee G. (2017). Combined effects of dietary mannan- and fructo-oligosaccharide on growth indices, body composition, intestinal bacterial flora and digestive enzymes activity of regal peacock (Aulonocara stuartgranti). Aquacult. Nutr. 23, 629–636. 10.1111/anu.12430 [DOI] [Google Scholar]
- Mukherjee A., Dutta D., Banerjee S., Ringø E., Breines E. M., Hareide E., et al. (2016). Potential probiotics from Indian major carp, Cirrhinus mrigala. Characterization, pathogen inhibitory activity, partial characterization of bacteriocin and production of exoenzymes. Res. Vet. Sci. 108, 76–84. 10.1016/j.rvsc.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Modanloo M., Soltanian S., Akhlaghi M., Hoseinifar S. H. (2017). The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish Immunol. 70, 391–397. 10.1016/j.fsi.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Mohammadian T., Alishahi M., Tabandeh M. R., Ghorbanpoor M., Gharibi D., Tollabi M., et al. (2016). Probiotic effects of Lactobacillus plantarum and L. delbrueckii ssp. bulguricus on some immune-related parameters in Barbus grypus. Aquacult. Int. 24, 225–242. 10.1007/s10499-015-9921-8 [DOI] [Google Scholar]
- Mohammadian T., Alishahi M., Tabande M., Ali Z., Nejad A. (2017). Effect of different levels of Lactobacillus casei on growth performance and digestive enzymes activity of shirbot (Barbus gryprus). J. Vet. Res. 72, 43–52. [Google Scholar]
- Mokoena M. P. (2017). Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22, 1255. 10.3390/molecules22081255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslehi F., Sattari M., Masouleh A. S. (2016). Effects of Pediococcus pentosaceus as a probiotic on intestinal microbiota and body composition of Siberian sturgeon, Acipenser baerii Brandt, 1869. Int. J. Aquatic. Biol. 4, 11–16. 10.22034/ijab.v4i1.117 [DOI] [Google Scholar]
- Munoz-Atienza E., Araújo C., Lluch N., Hernández P. E., Herranz C., Cintas L. M., et al. (2015). Different impact of heat-inactivated and viable lactic acid bacteria of aquatic origin on turbot (Scophthalmus maximus L.) head-kidney leucocytes. Fish Shellfish Immunol. 44, 214–223. 10.1016/j.fsi.2015.02.021 [DOI] [PubMed] [Google Scholar]
- Muñoz-Atienza E., Gómez-Sala B., Araújo C., Campanero C., del Campo R., Hernández P. E., et al. (2013). Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 13:15. 10.1186/1471-2180-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa N., Wei L. S., Musa N., Hamdan R. H., Leong L. K., Wee W., et al. (2009). Streptococcosis in red hybrid tilapia (Oreochromis niloticus) commercial farms in Malaysia. Aquacult. Res. 40, 630–632. 10.1111/j.1365-2109.2008.02142.x [DOI] [Google Scholar]
- Nayak S. K. (2010). Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 29, 2–14. 10.1016/j.fsi.2010.02.017 [DOI] [PubMed] [Google Scholar]
- Neissi A., Rafiee G., Nematollahi M., Safari O. (2013). The effect of Pediococcus acidilactici bacteria used as probiotic supplement on the growth and non-specific immune responses of green terror, Aequidens rivulatus. Fish Shellfish Immunol. 35, 1976–1980, 10.1016/j.fsi.2013.09.036 [DOI] [PubMed] [Google Scholar]
- Neuman C., Hatje E., Zarkasi K., Smullen R., Bowman J. P., Katouli M. (2015). The effect of diet and environmental temperature on faecal microbiota of farmed Tasmanian Atlantic salmon (Salmo salar L.). Aquacult Res. 47, 660–672. 10.1111/are.12522 [DOI] [Google Scholar]
- Newaj-Fyzul A., Austin B. (2014). Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. J. Fish Dis. 38, 937–955. 10.1111/jfd.12313 [DOI] [PubMed] [Google Scholar]
- Ng W. K., Kim Y. C., Romano N., Koh C. B., Yang S. Y. (2014). Effects of dietary probiotics on the growth and feeding efficiency of red hybrid tilapia, Oreochromis sp., and subsequent resistance to Streptococcus agalactiae. J. Appl. Aquacult. 26, 22–31. 10.1080/10454438.2013.874961 [DOI] [Google Scholar]
- Nguyen H. T., Kanai K., Yoshikoshi K. (2002). Ecological investigation of Streptococcus iniae in cultured Japanese flounder (Paralichthys olivaceus) using selective isolation procedures. Aquaculture 205, 7–17. 10.1016/S0044-8486(01)00667-6 [DOI] [Google Scholar]
- Nguyen T. L., Park C.-I., Kim D.-H. (2017). Improved growth rate and disease resistance in olive flounder, Paralichthys olivaceus, by probiotic Lactococcus lactis WFLU12 isolated from wild marine fish. Aquaculture 471, 113–120. 10.1016/j.aquaculture.2017.01.008 [DOI] [Google Scholar]
- Nieto J. M., Devesa S., Quiroga I., Toranzo A. E. (1995). Pathology of Enterococcus sp. infection in farmed turbot, Scophthalmus maximus L. J. Fish Dis. 18, 21–30. 10.1111/j.1365-2761.1995.tb01262.x [DOI] [Google Scholar]
- Nikoskelainen S., Salminen S., Bylund G., Ouwehand A. C. (2001). Characterization of the properties of human- and dairy-derived probiotics for prevention of infectious diseases in fish. Appl. Environ. Microbiol. 67, 2430–2435, 10.1128/aem.67.6.2430-2435.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur-Nazifah M., Sabri M. Y., Siti-Zahrah A. (2014). Development and efficacy of feed-based recombinant vaccine encoding the cell wall surface anchor family protein of Streptococcus agalactiae against streptococcosis in Oreochromis sp. Fish Shellfish Immunol. 37, 193–200. 10.1016/j.fsi.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Nyman A., Huyben D., Lundh T., Dicksved J. (2017). Effects of microbe- and mussel-based diets on the gut microbiota in Arctic charr (Salvelinus alpinus). Aquacult Rep. 5, 34–40. 10.1016/j.aqrep.2016.12.003 [DOI] [Google Scholar]
- Ovissipour M., Abedian Kenari A., Nazari R., Motamedzadegan A., Rasco B. (2014). Tuna viscera protein hydrolysate: nutritive and disease resistance properties for Persian sturgeon (Acipenser persicus L.) larvae. Aquacult. Res. 45, 591–601. 10.1111/j.1365-2109.212.03257x [DOI] [Google Scholar]
- Pandiyan P., Balaraman D., Thirunavukkarasu R., George E. G. T., Subaramaniyan K., Manikkam S., et al. (2013). Probiotics in aquaculture. Drug Inven. Today 5, 55–59. 10.1016/j.dit.2013.03.003 [DOI] [Google Scholar]
- Panigrahi A., Kiron V., Puangkaew J., Kobayashi T., Satoh S., Sugita H. (2005). The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243, 241–254. 10.1016/j/aquaculture.2004.09.032 [DOI] [Google Scholar]
- Parada J. L., Caron C. R., Medeiros A. B. P., Soccol C. R. (2007). Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch Biol. Technol. 50, 521–542. 10.1590/S1516-89132007000300018 [DOI] [Google Scholar]
- Patil R., Jeyasekaran G., Shanmugam S. A., Shakila J. R. (2001). Control of bacterial pathogens, associated with fish diseases, by antagonistic marine actinomycetes isolated from marine sediments. Indian J. Mar. Sci. 30, 264–267. Available online: http://nopr.niscair.res.in/handle/123456789/4632 [Google Scholar]
- Penberthy W. T., Shafizadeh E., Lin S. (2002). The zebrafish as a model for human disease. Front. Biosci. 7, D1439–D1453. 10.2741/penber [DOI] [PubMed] [Google Scholar]
- Peres H., Lim C., Klesius P. H. (2004). Growth, chemical composition and resistance to Streptococcus iniae challenge of juvenile Nile tilapia (Oreochromis niloticus) fed graded levels of dietary inositol. Aquaculture 235, 423–432. 10.1016/j.aquaculture.2003.09.021 [DOI] [Google Scholar]
- Perez R. H., Zendo T., Sonomoto K. (2014). Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Fact. 13:S3. 10.1186/1475-2859-13-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Dalsgaard A. (2003). Antimicrobial resistance of intestinal Aeromonas spp. and Enterococcus spp. in fish cultured in integrated broiler-fish farms in Thailand. Aquaculture 219, 71–82. 10.1016/S0044-8486(03)00018-8 [DOI] [Google Scholar]
- Piazzon M. C., Calduch-Giner J. A., Fouz B., Estensoro I., Simó-Mirabet P., Puyalto M., et al. (2017). Under control: how a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. Microbiome 5:164. 10.1186/s40168-017-0390-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchietti S., Fausto A. M., Randelli E., Carnevali O., Taddei A. R., Buonocore F., et al. (2009). Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal T-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol. 26, 368–376. 10.1016/j.fsi.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Piccolo G., Bovera F., Lombardi P., Mastellone V., Nizza S., Di Meo C., et al. (2015). Effect of Lactobacillus plantarum on growth performance and hematological traits of European sea bass (Dicentrarchus labrax). Aquacult. Inter. 23, 1025–1032. 10.1007/s10499-014-9861-8 [DOI] [Google Scholar]
- Pikuta E. V., Hoover R. B. (2014). The genus Carnobacterium in Lactic Acid Bacteria: Biodiversity and Taxonomy, eds Pikuta E. V., Hoover R. B. (Chichester: John Wiley & Sons, Ltd.) 109–123. 10.1002/9781118655252.ch10 [DOI] [Google Scholar]
- Pilarski F., Ferreira De Oliveira C. A., Darpossolo De Souza F. P. B., Zanuzzo F. S. (2017). Different β-glucans improve the growth performance and bacterial resistance in Nile tilapia. Fish Shellfish Immunol. 70, 25–29. 10.1016/j.fsi.2017.06.059 [DOI] [PubMed] [Google Scholar]
- Pinpimai K., Rodkhum C., Chansue N., Katagiri T., Maita M., Pirarat N. (2015). The study on the candidate probiotic properties of encapsulated yeast, Saccharomyces cerevisiae JCM 7255, in Nile Tilapia (Oreochromis niloticus). Res. Vet. Sci. 102, 103–111. 10.1016/j.rvsc.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Fernandes M., Pinto C., Albano H., Castilho F., Teixeira P., et al. (2009). Characterization of anti Listeria bacteriocins isolated from shellfish: potential antimicrobials to control non-fermented seafood. Int. J. Food Microbiol. 129, 50–58, 10.1016/j.ijfoodmicro.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Pirarat N., Pinpimai K., Rodkhum C., Chansue N., Ooi E. L., Katagiri T., et al. (2015). Viability and morphological evaluation of alginate-encapsulated Lactobacillus rhamnosus GG under simulated tilapia gastrointestinal conditions and its effect on growth performance, intestinal morphology and protection against Streptococcus agalactiae. Anim. Feed Sci. Technol. 207, 93–103. 10.1016/j.anifeedsci.2015.03.002 [DOI] [Google Scholar]
- Plumb J. A., Hanson L. A. (2010). Tilapia bacterial diseases in Health Maintenance and Principal Microbial Diseases of Cultured Fishes, eds Plumb J. A., Hanson L. A. (Wiley-Blackwell; ), 445–463. [Google Scholar]
- Popovic N. T., Strunjak-Perovic I., Sauerborn-Klobucar R., Barisic J., Jadan M., Kazazic S., et al. (2017). The effects of diet supplemented with Lactobacillus rhamnosus on tissue parameters of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquacult. Res. 48, 2388–2401. 10.1111/are.13074 [DOI] [Google Scholar]
- Pourgholam M. A., Khara H., Safari R., Sadati M. A. Y., Aramli M. S. (2016). Dietary administration of Lactobacillus plantarum enhanced growth performance and innate immune response of Siberian sturgeon, Acipenser baerii. Prob. Antimicrob. Prot. 8, 1–7. 10.1007/s12602-015-9205-7 [DOI] [PubMed] [Google Scholar]
- Prasad S., Morris P. C., Hansen R., Meaden P. G., Austin B. (2005). A novel bacteriocin-like substance (BLIS) from a pathogenic strain of Vibrio harveyi. Microbiology 151, 3051–3058. 10.1099/mic.0.28011-0 [DOI] [PubMed] [Google Scholar]
- Qin C., Xu L., Yang Y., He S., Dai Y., Zhao H., et al. (2014). Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reproduction 147, 53–64. 10.1530/REP-13-0141 [DOI] [PubMed] [Google Scholar]
- Ra C. H., Kim Y. J., Park S. J., Jeong C. W., Nam Y. K., Kim K. H., et al. (2009). Evaluation of optimal culture conditions for recombinant ghost bacteria vaccine production with the antigen of Streptococcus iniae GAPDH. J. Microbiol. Biotechnol. 19, 982–986. 10.4014/jmb.0901.007 [DOI] [PubMed] [Google Scholar]
- Rahman M., Rahman M. M., Deb S. C., Alam M. S., Alam M. J., Islam M. T. (2017). Molecular identification of multiple antibiotic resistant fish pathogenic Enterococcus faecalis and their control by medicinal herbs. Sci. Rep. 7:3747. 10.1038/s41598-017-03673-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram G., Manivasagan P., Thilagavathi B., Saravanakumar A. (2010). Purification and characterization of a bacteriocin produced by Lactobacillus lactis isolated from marine environment. Adv. J. Food Sci. Technol. 2, 138–144. [Google Scholar]
- Rather I. A., Galope R., Bajpai V. K., Lim J., Paek W. K., Park Y. (2017). Diversity of marine bacteria and their bacteriocins: applications in aquaculture. Rev. Fish Sci. Aquacult. 25, 257–269. 10.1080/23308249.2017.1282417 [DOI] [Google Scholar]
- Ravelo C., Magariños B., López-Romalde S., Toranzo A. E., Romalde J. L. (2003). Molecular fingerprinting of fish-pathogenic Lactococcus garvieae strains by random amplified polymorphic DNA Analysis. J. Clin. Microbiol. 41, 751–756. 10.1128/JCM.41.2.751-756.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. F., Samuel B. S., Gordon J. I. (2004). Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Nat. Acad. Sci. U.S.A. 101, 4596–4601. 10.1073/pnas.0400706101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. F., Mahowald M. A., Ley R. E., Gordon J. I. (2006). Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433. 10.1016/j.cell.2006.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A. K., Ghosh K., Ringø E. (2012). Enzyme-producing bacteria isolated from fish gut: a review. Aquacult. Nutr. 18, 465–492. 10.1111/j.1365-2095.2012.00943.x [DOI] [Google Scholar]
- Reimundo P., Pignatelli M., Alcaraz L. D., D'auria G., Moya A., Guijarro J. A. (2011). Genome sequence of Lactococcus garvieae UNIUD074, isolated in Italy from a Lactococcosis outbreak. J. Bacteriol. 193, 3684–3685. 10.1128/JB.05210-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengpipat S., Rueangruklikhit T., Piyatiratitivorakul S. (2014). Evaluations of lactic acid bacteria as probiotics for juvenile seabass Lates calcarifer. Aquacult. Res. 39, 134–143. 10.1111/j.1365-2109.2007.01864.x [DOI] [Google Scholar]
- Resende J. A., Silva V. L., Cesar D. E., Del‘Duca A., Fontes C. O., Diniz C. G. (2015). Seasonal dynamics of microbial community in an aquaculture system of Nile tilapia (Oreochromis niloticus). Aquacult. Res. 46, 1233–1244. 10.1111/are.12281. [DOI] [Google Scholar]
- Rimoldi S., Terova G., Ascione C., Giannico R., Brambilla F. (2018). Next generation sequencing for gut microbiome characterization in rainbow trout (Oncorhynchus mykiss) fed animal by-product meals as an alternative to fish meal protein sources. PLoS ONE 13:e0193652 10.1371/journal.pone.0193652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringø E. (1993a). Does chromic oxide (Cr2O3) affect faecal lipid and intestinal bacterial flora in Arctic charr, Salvelinus alpinus (L.)? Aquacult. Fish. Manage. 24, 767–776. 10.1111/j.1365-2109.1993.tb00656.x [DOI] [Google Scholar]
- Ringø E. (1993b). The effect of chromic oxide (Cr2O3) on aerobic bacterial populations associated with the epithelial mucosa of Arctic charr (Salvelinus alpinus L.). Can. J. Microbiol. 39, 1169–1173. 10.1139/m93-177 [DOI] [Google Scholar]
- Ringø E. (1994). The effect of chromic oxide (Cr2O3) on faecal lipid and intestinal bacterial flora of sea water reared Arctic charr, Salvelinus alpinus (L.)? Aquacult. Fish. Manage. 25, 341–344. 10.1111/j.1365-2109.1994.tb00697.x [DOI] [Google Scholar]
- Ringø E. (2004). Lactic acid bacteria in fish and fish farming in Lactic Acid Bacteria, eds Salminen S., Ouwehand A., von Wrigth A. (New York, NY: Marcel Dekker Inc; ), 581–610. [Google Scholar]
- Ringø E. (2008). The ability of carnobacteria isolated from fish intestine to inhibit growth of fish pathogenic bacteria: a screening study. Aquacult Res. 39, 171–180. 10.1111/j.1365-2109.2007.01876.x [DOI] [Google Scholar]
- Ringø E., Olsen R. E. (1994). Lipid nutrition in Arctic charr, Salvelinus alpinus (L.): a mini review. Aquacult. Fish. Manag. 25, 823–838. 10.1111/j.1365-2109.1994.tb00746.x [DOI] [Google Scholar]
- Ringø E., Gatesoupe F.-J. (1998). Lactic acid bacteria in fish: a review. Aquaculture 160, 177–203. 10.1016/S0044-8486(97)00299-8 [DOI] [Google Scholar]
- Ringø E., Song S. K. (2016). Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture: Aquacult. Nutr. 22, 4–24. 10.1111/anu12349. [DOI] [Google Scholar]
- Ringø E., Birkbeck T. H., Munro P. D., Vadstein O., Hjelmeland K. (1996). The effect of early exposure to Vibrio pelagius on the aerobic bacterial flora of turbot, Scophthalmus maximus (L.) larvae. J. Appl. Bacteriol. 81, 207–211. 10.1111/j.1365-2672.1996.tb04502.x [DOI] [Google Scholar]
- Ringø E., Olsen R. E., Mayhew T. M., Myklebust R. (2003). Electron microscopy of the intestinal microflora of fish. Aquaculture 227, 395–415. 10.1016/j.aquaculture.2003.05.001 [DOI] [Google Scholar]
- Ringø E., Schillinger U., Holzapfel W. (2005). Antimicrobial activity of lactic acid bacteria isolated from aquatic animals and the use of lactic acid bacteria in aquaculture in Biology of Growing Animals, eds Holzapfel W. H., Naughton P. J., Pierzynowski S. G. (Edinburgh, UK: Elsevier; ), 418–453. [Google Scholar]
- Ringø E., Olsen R. E., Gonzales Vecino J. L., Wadsworth S., Song S. K. (2012a). Use of immunostimulants and nucleotides in aquaculture: a review. J. Mar. Sci. Res. Develop. 2:104 10.4172/2155-9910.1000104 [DOI] [Google Scholar]
- Ringø E., Zhou Z., Olsen R. E., Song S. K. (2012b). Use of chitin and krill in aquaculture – effect on gut microbiota and the immune system: a review. Aquacult. Nutr. 18, 117–131. 10.1111/j.1365-2095.2011.00919.x [DOI] [Google Scholar]
- Ringø E., Olsen R. E., Jensen I., Romero J., Lauzon H. L. (2014). Application of vaccines and dietary supplements in aquaculture: possibilities and challenges. Rev. Fish Biol. Fish. 24, 1005–1032. 10.1007/s11160-014-9361-y [DOI] [Google Scholar]
- Ringø E., Zhou Z., Gonzalez Vecino J. L., Wadsworth S., Romero J., Krogdahl Å., et al. (2016). Effects of dietary components on the gut microbiota of aquatic animals: a never-ending story? Aquacult. Nutr. 22, 219–282. 10.1111/anu12346 [DOI] [Google Scholar]
- Ringø E, Wesmajervi M. S., Bendiksen H. R., Berg A., Olsen R. E., Johnsen T., et al. (2001). Identification and characterization of carnobacteria isolated from fish intestine. Syst. Appl. Microbiol. 24, 183–191. 10.1078/0723-2020-00020 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Estrada U., Satoh S., Haga Y., Fushimi H., Sweetman J. (2013). Effects of inactivated Enterococcus faecalis and mannan oligosaccharide and their combination of growth, immunity, and disease protection in rainbow trout. N. Am. J. Aquacult. 75, 416–428. 10.1080/15222055.2013.799620 [DOI] [Google Scholar]
- Román L., Padilla D., Acosta F., Sorroza L., Fátima E., Déniz S., et al. (2015). The effect of probiotic Enterococcus gallinarum L-1 on the innate immune parameters of outstanding species to marine aquaculture. J. Appl. Anim. Res. 43, 177–183. 10.1080/09712119.2014.928635 [DOI] [Google Scholar]
- Romero J., Feijoo C. G., Navarrete P. (2012). Antibiotics in aquaculture – use, abuse and alternatives in Health and Environment in Aquaculture, ed Carvalho E. (In-Tech), 159–198. [Google Scholar]
- Round J. L., Mazmanian S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323. 10.1038/nri2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudi K., Angell I. L., Pope P. B., Vik J. O., Sandve S. R., Snipen L.-G. (2018). Stable core gut microbiota across the freshwater-tosaltwater transition for farmed Atlantic salmon. Appl. Environ. Microbiol. 84, e01974–17. 10.1128/AEM.01974-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi Asl M. R., Adel M., Caipang C. M. A., Dawood M. A. O. (2017). Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish Shellfish Immunol. 71, 230–238. 10.1016/j.fsi.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Safari R., Adel M., Lazado C. C., Caipang C. M. A., Dadar M. (2016). Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss) via immunomodulation. Fish Shellfish Immunol. 52, 198–205. 10.1016/j.fsi.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Safari R., Hoseinifar S. H., Nejadmoghadam S., Khalili M. (2017). Apple cider vinegar boosted immunomodulatory and health promoting effects of Lactobacillus casei in common carp (Cyprinus carpio). Fish Shellfish Immunol. 67, 441–448. 10.1016/j.fsi.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Salinas I., Díaz-Rosales P., Cuesta A., Meseguer J., Chabrillón M., Morinigo M. A., et al. (2006). Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet. Immunol. Immunopatol. 111, 279–286. 10.1016/j.vetimm.2006.01.020 [DOI] [PubMed] [Google Scholar]
- Sahoo T. K., Jena P. K., Patel A. K., Seshadri S. (2016). Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: a review. Aquacult. Res. 47, 1013–1027. 10.1111/are.12556 [DOI] [Google Scholar]
- Salas-Leiva J., Opazo R., Remond C., Uribe E., Velez A., Romero J. (2017). Characterization of the intestinal microbiota of wild-caught and farmed fine flounder (Paralichthys adspersus). Lat. Am. J. Aquat. Res. 42, 370–378. 10.3856/vol45-issue2-fulltext-12 [DOI] [Google Scholar]
- Salma W., Zhou Z., Wang W., Askarian F., Kousha A., Ebrahimi M. T., et al. (2011). Histological and bacteriological changes in intestine of beluga (Huso huso) following ex vivo exposure to bacterial strains. Aquaculture 314, 24–33. 10.1016/j.aquaculture.2011.01.047 [DOI] [Google Scholar]
- Sarika A. R., Lipton A. P., Aishwarya M. S., Dhivya R. S. (2012). Isolation of a bacteriocin-producing Lactococcus lactis and application of its bacteriocin to manage spoilage bacteria in high-value marine fish under different storage temperatures. Appl. Biochem. Biotechnol. 167, 1280–1289. 10.1007/s12010-012-9701-0 [DOI] [PubMed] [Google Scholar]
- Sarika A. R., Lipton A. P., Aishwarya M. S., Rachana mol R. S. (2017). Lactic acid bacteria from marine fish: antimicrobial resistance and production of bacteriocin effective against L. monocytogenes in situ. J. Food: Microbiol. Safety Hyg. 3:1 10.4172/2476-2059.1000128 [DOI] [Google Scholar]
- Satish Kumar R., Kanmani P., Yuvaraj N., Paari K. A., Pattukumar V., Arul V. (2011). Purification and characterization of enterocin MC13 produced by a potential aquaculture probiont Enterococcus faecium MC13 isolated from the gut of Mugil cephalus. Can. J. Microbiol. 57, 993–1001. 10.1139/w11-092 [DOI] [PubMed] [Google Scholar]
- Schillinger U., Lücke F. K. (1989). Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 55, 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kilpper-Bälz R. (1984). Transfer of Streptococcus faecalis and Streptococcus faecium to the Genus Enterococcus norn. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Syst. Bacteriol. 34, 31–34. 10.1099/00207713-34-1-31 [DOI] [Google Scholar]
- Schleifer K. H., Kraus J., Dvorak C., Kilpper-Balz R., Collins M. D., Fischer W. (1985). Transfer of Streptococcus lactis and related streptococci to the genus Lactococcus gen nov. Syst. Appl. Microbiol. 6, 183–195. 10.1016/S0723-2020(85)80052-7 [DOI] [Google Scholar]
- Serra C. R., Magalháes Júnior F., Couto A. M., Oliva-Teles A., Enes P. (2018). Gut microbiota and gut morphology of gilthead sea bream (Sparus aurata) juveniles are not affected by chromic oxide as digestibility marker. Aquacult. Res. 49, 1347–1356. 10.1111/are.13596 [DOI] [Google Scholar]
- Shahid M., Hussain B., Riaz D., Khurshid M., Ismail M., Tariq M. (2017). Identification and partial characterization of potential probiotic lactic acid bacteria in freshwater Labeo rohita and Cirrhinus mrigala. Aquacult. Res. 48, 1688–1698. 10.1111/are.13006 [DOI] [Google Scholar]
- Shih-Chu C., Yu-De L., Li-Ling L., Pei-Chi W. (2001). Lactococcus garvieae infection in the giant freshwater prawn Macrobranchium rosenbergii confirmed by polymerase chain reaction and 16S rDNA sequencing. Dis. Aquat. Org. 45, 45–52. 10.3354/dao045045 [DOI] [PubMed] [Google Scholar]
- Shoemaker C. A., Klesius P. H., Evans J. J. (2001). Prevalence of Streptococcus iniae in tilapia, hybrid striped bass, and channel catfish on commercial fish farms in the United States. Am. J. Vet. Res. 62, 174–177. 10.2460/ajvr.2001.62.174 [DOI] [PubMed] [Google Scholar]
- Silva C. C. G., Silva S. P. M., Riberio S. C. (2018). Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 9:594. 10.3389/fmicb.2018.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Sivasubramani K., Jayalakshmi S., Satheesh Kumar S., Selvi C. (2013). Isolation and production of bacteriocin by marine Lactobacillus fermentum SBS001. Int. J. Curr. Microbiol. Appl. Sci. 2, 67–73. [Google Scholar]
- Soltani M., Ghodratnama M., Ebrahimzadeh-Mosavi H. A., Nikbakht-Brujeni G., Mohamadian S., Ghasemian M. (2014). Shirazi thyme (Zataria multiflora Boiss) and Rosemary (Rosmarinus officinalis) essential oils repress expression of sagA, a streptolysin S-related gene in Streptococcus iniae. Aquaculture 430, 248–252. 10.1016/j.aquaculture.2014.04.012 [DOI] [Google Scholar]
- Soltani M., Mohamadian S., Rouholahi S., Soltani E., Rezvani S. (2015). Shirazi thyme (Zataria multiflora) essential oil suppresses the expression of PavA and Hly genes in Lactococcus garvieae, the causative agent of lactococcosis in farmed fish. Aquaculture 442, 74–77. 10.1016/j.aquaculture.2015.03.001 [DOI] [Google Scholar]
- Soltani M., Pakzad K., Taheri-Mirghaed A., Mirzargar S., Shekarabi S. P. H., Yosefi P., et al. (2017a). Dietary application of the probiotic Lactobacillus plantarum 426951 enhances immune status and growth of rainbow trout (Oncorhynchus mykiss) vaccinated against Yersinia ruckeri. Probiotics Antimicrob. Proteins. 10.1007/s12602-017-9376-5 [DOI] [PubMed] [Google Scholar]
- Soltani M., Abdy E., Alishahi M., Mirghaed A. T., Hosseini-Shekarabi P. (2017b). Growth performance, immune-physiological variables and disease resistance of common carp (Cyprinus carpio) orally subjected to different concentrations of Lactobacillus plantarum. Aquacult. Inter. 25, 1913–1933. 10.1007/s10499-017-0164-8 [DOI] [Google Scholar]
- Srionnual S., Yanagida F., Lin L. H., Hsiao K. N., Chen Y. S. (2007). Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl. Environ. Microbiol. 73, 2247–2250. 10.1128/AEM.02484-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisapoome P., Areechon N. (2017). Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): laboratory and on-farm trials. Fish Shellfish Immunol. 67, 199–210. 10.1016/j.fsi.2017.06.018 [DOI] [PubMed] [Google Scholar]
- Standen B. T., Rodiles A., Peggs D. L., Davies S. J., Santos G. A., Merrifield D. L. (2015). Modulation of the intestinal microbiota and morphology of tilapia, Ocerchromis niloticus, following the application of a multi-species probiotic. Appl. Microbiol. Biotechnol. 99, 84013–88417. 10.1007/s00253-015-6702-2 [DOI] [PubMed] [Google Scholar]
- Standen B. T., Peggs D. L., Rawling M. D., Foey A., Davies S. J., Santos G. A., et al. (2016). Dietary administration of a commercial mixed-species probiotic improves growyh performance and modulates the intestinal immunity of tilapia, Ocerchromis niloticus. Fish Shellfish Immuniol. 49, 427–435. 10.1016/j.fsi.2015.11.037 [DOI] [PubMed] [Google Scholar]
- Starliper C. E., Shotts E. B., Brown J. (1992). Isolation of Carnobacterium piscicola and an unidentified Gram-positive bacillus from sexually mature and post-spawning rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 13, 181–187. 10.3354/dao013181 [DOI] [Google Scholar]
- Stoffregen D. A., Backman S. C., Perham R. E., Bowser P. R., Babish J. G. (1996). Initial disease report of Streptococcus iniae infection in hybrid striped (Sunshine) bass and successful therapeutic intervention with the fluoroquinolone antibacterial enrofloxacin. J. World Aquacult. Soc. 27, 420–434. 10.1111/j.1749-7345.1996.tb00626.x [DOI] [Google Scholar]
- Strøm E. (1988). Melkesyrebakterier i Fisketarm: Isolasjon, Karakterisering og Egenskaper [in Norwegian]. MSc thesis, The Norwegian College of Fishery Science, University of Tromsø, Norway. [Google Scholar]
- Sugita H., Ito Y. (2006). Identification of intestinal bacteria from Japanese flounder (Paralichthys olivaceus) and their ability to digest chitin. Lett. Appl. Microbiol. 43, 336–342. 10.1111/j.1472-765X.2006.01943.x [DOI] [PubMed] [Google Scholar]
- Sugita H., Ohta K., Kuruma A., Sagesaka T. (2007). An antibacterial effect of Lactococcus lactis isolated from the intestinal tract of the Amur catfish, Silurus asotus Linnaeus. Aquacult. Res. 38, 1002–1004. 10.1111/j.1365-2109.2007.01765.x [DOI] [Google Scholar]
- Šušković J., Kos B., Beganović J., Leboš Pavunc A., Habjanič K., Matošić S. (2010). Antimicrobial activity - the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 48, 296–307. [Google Scholar]
- Tahmasebi-Kohyani A., Keyvanshokooh S., Nematollahi A., Mahmoudi N., Pasha-Zanoosi H. (2011). Dietary administration of nucleotides to enhance growth, humoral immune responses, and disease resistance of the rainbow trout (Oncorhynchus mykiss) fingerlings. Fish Shellfish Immunol. 30, 189–193. 10.1016/j.fsi.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Tarnecki A. M., Rhody N. R. (2017). Microbiota of common snook Centropomus undecimalis larvae exhibiting high mortality. Aquacult. Res. 48, 5693–5698. 10.1111/are.13377 [DOI] [Google Scholar]
- Tarnecki A. M., Burgos F. A., Ray C. L., Arias C. R. (2017). Fish intestinal microbiome: diversity and symbiosis unraveled by metagenomics. J. Appl. Microbiol. 123, 2–17. 10.1111/jam.13415 [DOI] [PubMed] [Google Scholar]
- Todorov S. D. (2009). Bacteriocins from Lactobacillus plantarum- production, genetic organization and mode of action. A review. Braz. J. Microbiol. 40, 209–221. 10.1590/S1517-83822009000200001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Novoa B., Baya A. M., Hetrick F. M., Barja J. L., Figueras A. (1993a). Histopathology of rainbow trout, Oncorhynchus mykiss (Walbaum), and striped bass, Morone saxatilis (Walbaum), experimentally infected with Carnobacterium piscicola. J. Fish Dis. 16, 261–267. 10.1111/j.1365-2761.1993.tb01256.x [DOI] [Google Scholar]
- Toranzo A. E., Romalde J. L., Nunez S., Figueras A., Barja J. L. (1993b). An epizootic in farmed, market-size rainbow trout in Spain caused by a strain of Carnobacterium piscicola of unusual virulence. Dis. Aquat. Org. 17, 87–99. 10.3354/dao017087 [DOI] [Google Scholar]
- Torrecillas S., Mompel D., Caballero M. J., Monteri D., Merrifield D., Rodiles A., et al. (2017). Effect of fishmeal and fish oil replacement by vegetable meals and oils on gut health of European sea bass (Dicentrarchus labrax). Aquaculture 468, 386–398. 10.1016/j.aquaculture.2016.11.005 [DOI] [Google Scholar]
- Tran N. T., Wang G.-T., Wu S.-G. (2017). A review of intestinal microbes in grass carp Ctenopharyngodon idellua (Valenciennes). Aquacult Res. 48, 3287–3397. 10.111/are.13367 [DOI] [Google Scholar]
- Turner M. D., Nedjai B., Hurst T., Pennington D. J. (2014). Cytokines and chemokines: at the crossroads of cell signaling and inflammatory disease. Biochim. Biophys. Acta 1843, 2563–2582. 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- Tytgat H. L. P., Douillard F. P., Reuanen J., Rasinkangas P., Hendrickx A. P. A., Laine P. K., et al. (2016). Lactobacillus rhamnosus GG outcompete Enterococcus faecium via mucus-binding pili: Evidence for a novel and heterospecific probiotic mechanism. Appl. Environ. Microbiol. 82, 5756–5762. 10.1128/AEM.01243-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadanasundari V., Rangabhashiyam S., Sankaran K., Hemavathy R. V. (2013). Production, purification and characterization of bacteriocins by Lactobacillus lactis from marine environment. Int. J. Adv. Innov. Res. 1, 340–346. [Google Scholar]
- Valcarce D. G., Pardo M. Á., Riesco M. F., Cruz Z., Robles V. (2015). Effect of diet supplementation with a commercial probiotic containing Pediococcus acidilactici (Lindner, 1887) on the expression of five quality markers in zebrafish (Danio rerio (Hamilton, 1822)) testis. J. Appl. Ichthyol. 31, 18–21. 10.1111/jai.12731 [DOI] [Google Scholar]
- Valenzuela A. S., Benomar N., Abriouel H., Canamero M. M., Galvez A. (2010). Isolation and identification of Enterococcus faecium from seafoods: antimicrobial resistance and production of bacteriocin-like substances. Food Microbiol. 27, 955–961. 10.1016/j.fm.2010.05.033 [DOI] [PubMed] [Google Scholar]
- Van Doan H., Doolgindachbapron S., Suksri A. (2014). Effects of low molecular weight agar and Lactobacillus plantarum on growth performance, immunity, and disease resistance of basa fish (Pangasius bocourti, Sauvage 1880). Fish Shellfish Immunol. 41, 340–345. 10.1016/j.fsi.2014.09.015 [DOI] [PubMed] [Google Scholar]
- Van Doan H., Hoseinifar S. H., Tapingkae W., Tongsiri S., Khamtavee P. (2016a). Combined administration of low molecular weight sodium alginate boosted immunomodulatory, disease resistance and growth enhancing effects of Lactobacillus plantarum in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 58, 678–685. 10.1016/j.fsi.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Van Doan H., Doolgindachbaporn S., Suksri A. (2016b). Effect of Lactobacillus plantarum and Jerusalem artichoke (Helianthus tuberosus) on growth performance, immunity and disease resistance of Pangasius catfish (Pangasius bocourti, Sauvage 1880). Aquacult. Nutr. 22, 444–456. 10.1111/anu.12263 [DOI] [Google Scholar]
- Van Doan H., Doolgindachbaporn S., Suksri A. (2016c). Effects of Eryngii mushroom (Pleurotus eryngii) and Lactobacillus plantarum on growth performance, immunity and disease resistance of Pangasius catfish (Pangasius bocourti, Sauvage 1880). Fish Physiol. Biochem. 45, 1427–1440. 10.1007/s10695-016-0230-6 [DOI] [PubMed] [Google Scholar]
- Van Doan H., Hoseinifar S. H., Dawood M. A. O., Chitmanat C., Tayyamath K. (2017a). Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 70, 87–94. 10.1016/j.fsi.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Van Doan H., Hoseinifar S. H., Tapingkae W., Khamtavee P. (2017b). The effects of dietary kefir and low molecular weight sodium alginate on serum immune parameters, resistance against Streptococcus agalactiae and growth performance in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 62, 139–146. 10.1016/j.fsi.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Vendrell D., Balcázar J. L., Ruiz-Zarzuela I., de Blas I., Gironès O., Mùzquiz J. L. (2006). Lactococcus garvieae in fish: a review. Comp. Immunol. Microbiol. Inf. Dis. 29, 177–198. 10.1016/j.cimid.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Vendrell D., Balcázar J. L., Ruiz-Zarzuela I., De Blas I., Gironés O., Múzquiz J. L. (2007). Safety and efficacy of an inactivated vaccine against Lactococcus garvieae in rainbow trout (Oncorhynchus mykiss). Prevent Vet. Med. 80, 222–229. 10.1016/j.prevetmed.2007.02.008 [DOI] [PubMed] [Google Scholar]
- Ventura M., O'Flaherty S., Claesson M. J., Turroni F., Klaenhammer T. R., van Sinderen D., et al. (2009). Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat. Rev. Microbiol. 7, 61–77. 10.1038/nrmicro2047 [DOI] [PubMed] [Google Scholar]
- Verschuere L., Rombaut G., Sorgeloos P., Verstraete W. (2000). Probiotic bacteria as biocontrol agents in aquaculture. Microbiol. Mol. Biol. Rev. 64, 655–671. 10.1128/MMBR.64.4.655-671.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vílchez M. C., Santangeli S., Maradonna F., Gioacchini G., Verdenelli C., Gallego V., et al. (2015). Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 84, 1321–1331. 10.1016/j.theriogenology.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Villamil L., Reyes C., Martínez-Silva M. A. (2014). In vivo and in vitro assessment of Lactobacillus acidophilus as probiotic for tilapia (Oreochromis niloticus, Perciformes: Cichlidae) culture improvement. Aquacult. Res. 45, 1116–1125. 10.1111/are.12051 [DOI] [Google Scholar]
- Walter J. (2008). Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 74, 4985–4996. 10.1128/AEM.00753-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.n R., Ran C., Ringø E., Zhou Z. (2018). Progress in fish gastrointestinal microbiota research. Rev. Aquacult. 10.1111/raq.12191 [DOI] [Google Scholar]
- Wang J., Zou L. L., Li A. X. (2014). Construction of a Streptococcus iniae sortase A mutant and evaluation of its potential as an attenuated modified live vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 40, 392–398. 10.1016/j.fsi.2014.07.028 [DOI] [PubMed] [Google Scholar]
- Wang T., Secombes C. J. (2013). The cytokine networks of adaptive immunity in fish. Fish Shellfish Immunol. 35, 1703–1718. 10.1016/j.fsi.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Wang Y. B., Tian Z. Q., Yao J. T., Li W. F. (2008). Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 277, 203–207. 10.1016/j.aquaculture.2008.03.007 [DOI] [Google Scholar]
- Whyte S. K. (2007). The innate immune response of finfish – a review of current knowledge. Fish Shellfish Immunol. 23, 1127–1151. 10.1016/j.fsi.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Wu Y.-R., Gong Q.-F., Fang H., Liang W.-W., Chen M., He R.-J. (2013). Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 34, 220–227. 10.1016/j.fsi.2012.10.020 [DOI] [PubMed] [Google Scholar]
- Wu Z. B., Gatesoupe F. J., Li T. T., Wang X. H., Zhang Q. Q., Feng D. Y., et al. (2018). Significant improvement of intestinal microbiota of gibel carp (Carassius auratus gebelio) after traditional Chinese medicine feeding. J. Appl. Microbiol. 124, 829–841. 10.1111/jam.13674 [DOI] [PubMed] [Google Scholar]
- Yamashita M., Pereira S., Cardoso L., Araujo A., Oda C., Schmidt É., et al. (2017). Probiotic dietary supplementation in Nile tilapia as prophylaxis against streptococcosis. Aquacult. Nutr. 23, 1235–1243. 10.1111/anu.12498 [DOI] [Google Scholar]
- Yan Y. Y., Xia H. Q., Yang H. L., Hoseinifar S. H., Sun Y. Z. (2016). Effects of dietary live or heat-inactivated autochthonous Bacillus pumilus SE5 on growth performance, immune responses and immune gene expression in grouper Epinephelus coioides. Aquacult. Nutr. 22, 698–707. 10.1111/anu.12297 [DOI] [Google Scholar]
- Yang H.-T., Zou S.-S., Zhai L.-J., Wang Y., Zhang F.-M., An L.-G., et al. (2017). Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol. 71, 35–42. 10.1016/j.fsi.2017.09.075 [DOI] [PubMed] [Google Scholar]
- Yang P., Hu H., Liu Y., Li Y., Ai Q., Xu W., et al. (2018). Dietary stachyose altered the intestinal microbiota profile and improved the intestinal mucosal barrier function of juvenile turbot, Scophthalmus maximus L. Aquaculture 486, 98–106. 10.1016/j.aquaculture.2017.12.014 [DOI] [Google Scholar]
- Yang W., Li A. (2009). Isolation and characterization of Streptococcus dysgalactiae from diseased Acipenser schrenckii. Aquaculture 294, 14–17. 10.1016/j.aquaculture.2009.05.018 [DOI] [Google Scholar]
- Yi T., Li Y.-W., Liu L., Xiao X.-X., Li A.-X. (2014). Protection of Nile tilapia (Oreochromis niloticus L.) against Streptococcus agalactiae following immunization with recombinant FbsA and α-enolase. Aquaculture 428–429, 35–40. 10.1016/j.aquaculture.2014.02.027 [DOI] [Google Scholar]
- Yin L. J., Wu C. W., Jiang S. T. (2007). Biopreservative effect of pediocin ACCEL on refrigerated seafood. Fish Sci. 73, 907–912. 10.1111/j.1444-2906.2007.01413.x [DOI] [Google Scholar]
- Yu L., Zhai Q., Zhu J., Zhang C., Li T., Liu X., et al. (2017). Dietary Lactobacillus plantarum supplementation enhances growth performance and alleviates aluminum toxicity in tilapia. Ecotoxicol Environ. Saf. 143, 307–314. 10.1016/j.ecoenv.2017.05.023 [DOI] [PubMed] [Google Scholar]
- Yuasa K., Kitancharoen N., Kataoka Y., Al-Murbaty F. A. (1999). Streptococcus iniae, the causative agent of mass mortality in rabbitfish Siganus canaliculatus in Bahrain. J. Aquat. Anim. Health 11, 87–93. [DOI] [Google Scholar]
- Zacharof M. P., Lovitt R. W. (2012). Bacteriocins produced by lactic acid bacteria: a review article. APCBEE Proc. 2, 50–56. 10.1016/j.apcbee.2012.06.010 [DOI] [Google Scholar]
- Zai A. S., Ahmad S., Rasool S. A. (2009). Bacteriocin production by indigenous marine catfish associated Vibrio spp. Pakistan J. Pharma. Sci. 22, 162–167. 10.1007/s11274-015-1903-5 [DOI] [PubMed] [Google Scholar]
- Zarkasi K. Z., Taylor R., Abell G. C. J., Tamplin M. L., Glencross B. D., Bowman J. P. (2016). Atlantic salmon (Salmo salar L.) gastrointestinal microbial community dynamics in relation to digesta properties and diet. Microb. Ecol. 71, 589–603. 10.1007/s00248-015-0728-y [DOI] [PubMed] [Google Scholar]
- Zhai Q. X., Yu L., Li T., Zhu J., Zhang C., Zhao J., et al. (2017). Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie Van Leeuwenhoek 110, 501–513. 10.1007/s10482-016-0819-x [DOI] [PubMed] [Google Scholar]
- Zhang C.-N., Zhang J.-L., Guan W.-C., Zhang X.-F., Guan S.-H., Zeng Q.-H., et al. (2017). Effects of Lactobacillus delbrueckii on immune response, disease resistance against Aeromonas hydrophila, antioxidant capability and growth performance of Cyprinus carpio var Huanghe. Fish Shellfish Immunol. 68, 84–91. 10.1016/j.fsi.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yu A., Lan J., Zhang H., Hu M., Cheng J., et al. (2017). GapA, a potential vaccine candidate antigen against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 63, 255–260. 10.1016/j.fsi.2017.02.019 [DOI] [PubMed] [Google Scholar]
- Zhou L., Limbu S. M., Qiao F., Du F.-Q., Zhang M. (2018). Influence of long-term feeding antibiotics on the gut health of zebrafish. Zebrafish. 10.1089/zeb.2017.1526. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zhou M., Liang R., Mo J., Yang S., Gu N., Wu Z., et al. (2018). Effects of brewer‘s yeast hydrolysate on the growth performance and the intestinal bacterial diversity of largemouth bass (Micropterus salmoides). Aquaculture 484, 139–144. 10.1016/j.aquaculture.2017.11.006 [DOI] [Google Scholar]
- Zhou Z., Ringø E., Olsen R. E., Song S. K. (2018). Dietary effects of soybean products on gut microbiota and immunity of aquatic animals: a review. Aquacult. Nutr. 24, 644–665. 10.1111/anu12532 [DOI] [Google Scholar]
- Zhu L., Yang Q., Huang L., Wang K., Wang X., Chen D., et al. (2017). Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia. Dev. Comp. Immunol. 77, 77–87. 10.1016/j.dci.2017.07.024 [DOI] [PubMed] [Google Scholar]