Abstract
Objective
This study retrospectively evaluated fungal dissemination due to hospital reconstruction and explored effective methods of predicting an outbreak.
Methods
Patients suspected of having invasive aspergillosis were tested for Aspergillus galactomannan antigen before and after reconstruction, and the mean values of three months of testing for positive patients were determined. The characteristics of patients with aspergillosis during this period were also assessed.
Results
Forty-five patients were positive for Aspergillus antigen (>0.5 cut-off index) from January 2013 to December 2014. Mean Aspergillus antigen values significantly increased following reconstruction (p<0.05). Three patients developed pneumonia due to Aspergillus and were diagnosed with “probable” invasive aspergillosis according to the European Organization for Research and Treatment of Cancer and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria. We also discovered that the anteroom to contain dust was not prefabricated and a negative pressure system to remove dust was not used. After construction of the unit, no new cases of aspergillosis were diagnosed.
Conclusion
Many Aspergillus spores may be transiently floating during hospital reconstruction. Therefore, monthly surveillance with frequent serum galactomannan antigen testing to predict outbreaks is necessary. Surveillance of all patients in the hematology ward is especially important. Reconsideration of prophylactic antifungals may also be necessary during hospital reconstruction.
Keywords: hospital reconstruction, aspergillosis, galactomannan antigen
Introduction
Aspergillus comprises a large genus of ubiquitous filamentous fungi, accounting for up to 40% of cases of hospital and home fungal contamination. Aspergillus spores are dispersed in great amounts during construction and renovation work, and many work-related aspergillosis outbreaks have been described (1, 2). Hematologists who have treated immunocompromised patients are acutely aware of the extremely high mortality of progressive fungal disease. Many studies have attempted to evaluate the acquisition of invasive fungal infection by medically compromised patients (2-8). In particular, patients with hematological malignancy are at risk of invasive aspergillosis during hospital reconstruction. However, several reports have demonstrated a decreased risk of aspergillosis during hospital reconstruction when protective measures were taken (2, 9).
Therefore, this study was performed retrospectively to evaluate fungal dissemination due to construction and determine methods to predict Aspergillus outbreak at a single hospital in which three patients had been diagnosed with invasive aspergillosis despite protective measures having been taken. We also discuss the usefulness of Aspergillus galactomannan antigen testing and surveillance during hospital reconstruction.
Materials and Methods
Reconstruction
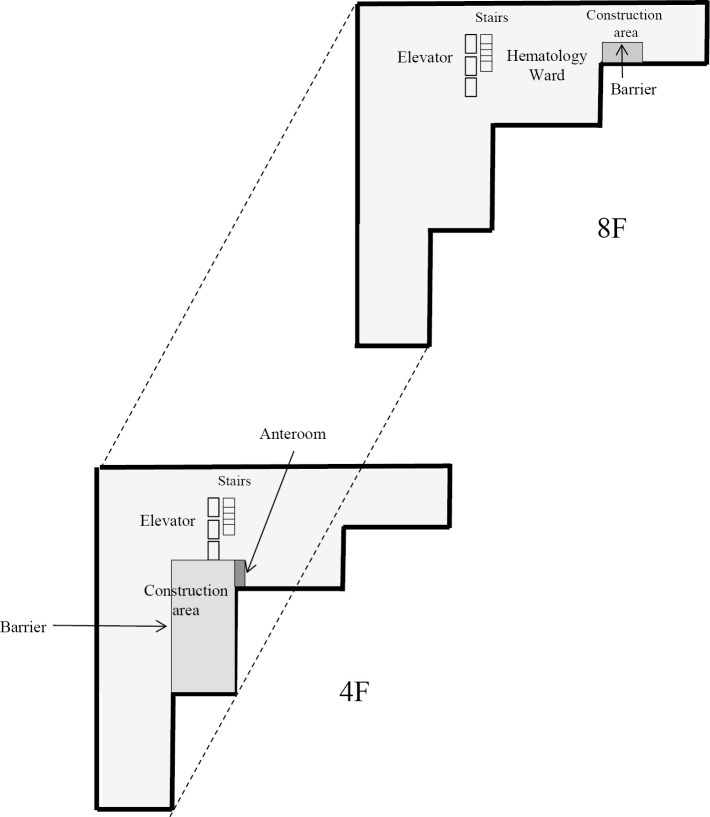
In our hospital, the 4th floor intensive-care unit was reconstructed from September 2013 to May 2014. Nurse call speakers were also repaired in the 8th floor hematology ward in December 2013. In addition, two large clean rooms for four patients in the hematology ward were constructed internally from January to February 2014. A partition was installed between the patient area and the construction site. An anteroom was to be constructed, and workers were required to pass through the anteroom so that they could be cleaned using a high-efficiency particulate air (HEPA) vacuum cleaner. The source of the outbreak was suspected to be the construction area on the 4th floor, which did not have a sufficient negative pressure system to remove dust from the construction workers. A unit with a negative pressure system was subsequently constructed beside the construction area in January 2014 (Fig. 1).
Figure 1.
Diagram of construction. In our hospital, the 4th floor intensive-care unit was reconstructed from September 2013 to May 2014. Nurse call speakers were also repaired in the 8th floor hematology ward in December 2013. In addition, two large clean rooms for four patients in the hematology ward were constructed internally from January 2014 to February 2014. A partition was installed between the patient area and construction site. The source of the outbreak was suspected to be the construction area on the 4th floor, which did not have a sufficient negative pressure system to remove dust from construction workers. A unit with a negative pressure system was subsequently constructed beside the construction area in January 2014.
Environmental assessment
The infection control team (ICT) performed an environmental assessment once a week during construction according to the infection control risk assessment (ICRA). During this assessment, the barrier between the patient area and work area, the anteroom and cleaning around the work area or workwear was checked. The ICT also conducted a daily environmental assessment to determine the outbreak source during a period without epidemiologic evidence of transmission after an outbreak of invasive aspergillosis (Category IB) in December 2013 (10).
Patients
We examined 351 patients with hematologic disease from January 2013 to December 2014. Forty-five patients were positive for Aspergillus galactomannan antigen [>0.5 cut-off index (COI)]. We further examined only Aspergillus galactomannan antigen-positive patients, as the immune state of patients with hematologic disease varies, and the infectivity of Aspergillus spores also varies in such patients. Three patients were diagnosed with invasive aspergillosis according to the European Organization for Research and Treatment of Cancer and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria in this period (11).
Aspergillus antigen testing
Aspergillus galactomannan antigen testing was performed as usual before, during and after reconstruction. For patients with hematologic disease, Aspergillus galactomannan antigen was examined by an enzyme-linked immunosorbent assay (SRL, Tokyo, Japan) at most once a week when fungal infection was suspected because of lung nodules or a fever of unknown origin. We defined a result as positive if the Aspergillus galactomannan antigen value was >0.5 COI and determined the maximum value from a series of data during the course of infection as the final value for the patient.
The data are shown as the positive rates and the mean±standard deviation (SD) of Aspergillus antigen. For positive patients, the values were calculated as the mean of testing of all hematology wards for 3 months. Statistical analyses were performed using the EZR software program (12). Intergroup comparisons were assessed by the chi-squared test or the unpaired t-test. p values <0.05 were considered statistically significant. This study was approved by the Toyama Prefectural Central Hospital ethics committee (Approval No. 5126).
Results
After we confirmed an outbreak of invasive aspergillosis had occurred, the ICT conducted a daily environmental assessment to determine the source of infection during a time without epidemiologic evidence of transmission. The source was suspected to be the construction area on the 4th floor that did not have a sufficient negative pressure system to remove dust from construction workers. Based on the assessment, the ICT recommended the construction of an anteroom with a negative pressure system. No new cases of invasive aspergillosis were diagnosed after the construction of this unit in the intensive-care unit on the 4th floor or during reconstruction of two large clean rooms on the 8th floor.
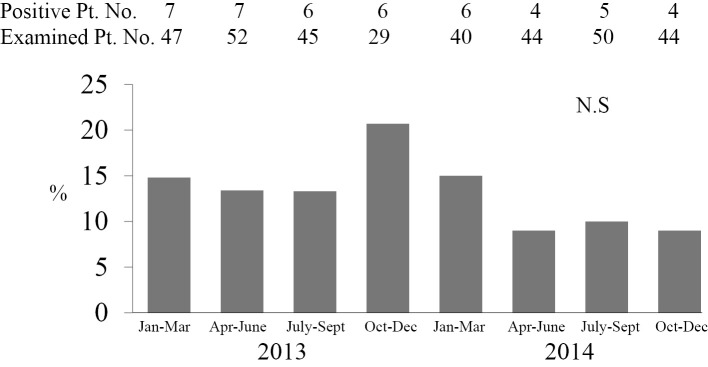
Fig. 2 shows the rate of Aspergillus antigen-positive patients every 3 months among 351 examined patients. The positive rate was 13% from July to September 2013 (before reconstruction) but increased to 21% from October to December 2013 (during reconstruction). The positive rate was the highest after reconstruction started and before the unit with a negative pressure system was constructed beside the construction area in January 2014. From January to December 2014, the positive rates remained at about 10%. There were no significant differences in the positive rates before, during or after reconstruction.
Figure 2.
Rate of Aspergillus antigen-positive patients every three months. We calculated the positive rate of patients with Aspergillus antigen (>0.5 cut-off index) from 3 months of testing. In our hospital, the 4th floor intensive-care unit was reconstructed starting in October 2013. A unit with a negative pressure system to remove dust from construction workers was constructed after an invasive aspergillosis outbreak occurred between December 2013 and January 2014. The positive rate was the highest from October to December 2013.
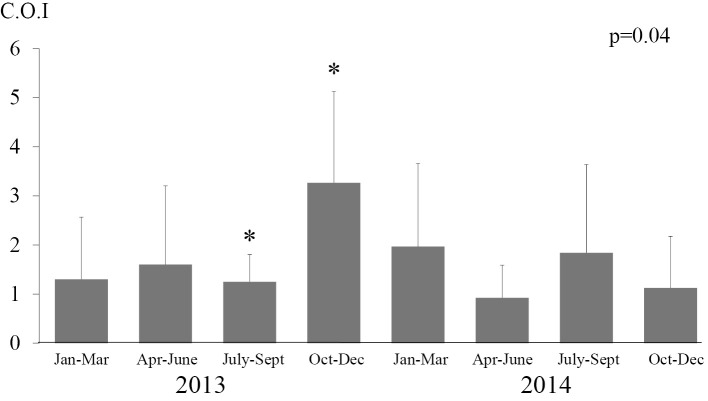
Fig. 3 shows the mean Aspergillus antigen values every 3 months in positive patients in the hematology ward. The mean Aspergillus antigen values of hematologic patients from July to September 2013, October to December 2013 and January to March 2014 were 1.3±0.55, 3.6±1.62 and 2.2±1.72 COI, respectively. These results show that the mean Aspergillus antigen values significantly increased after the start of reconstruction (p<0.05). Although the mean Aspergillus antigen values decreased after January 2014, this difference was not significant.
Figure 3.
Testing of mean Aspergillus antigen values every three months. The mean Aspergillus antigen values in patients in the hematology ward were calculated from three months of testing. In our hospital, the 4th floor intensive-care unit was reconstructed starting in October 2013. A unit with a negative pressure system to remove dust from construction workers was constructed after an invasive aspergillosis outbreak occurred between December 2013 and January 2014. The mean values significantly increased following reconstruction (p<0.05).
Fig. 4 shows the mean Aspergillus antigen values every 3 months in positive patients without aspergillosis in the hematology ward only from July 2013 to March 2014. The mean Aspergillus antigen values of hematologic patients from July to September 2013, October to December 2013 and January to March 2014 were 1.3±0.55, 4.9±0.14 and 3.5±1.5 COI, respectively. These results show the mean Aspergillus antigen values also significantly increased after the start of reconstruction in patients without aspergillosis (p<0.05).
Figure 4.
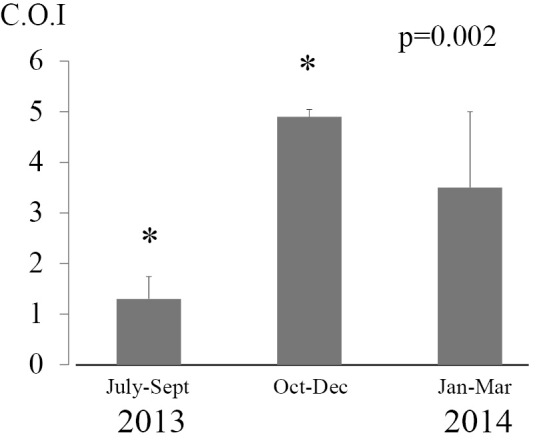
Testing of mean Aspergillus antigen values in patients without aspergillosis every three months. The mean Aspergillusantigen values in patients without aspergillosis in the hematology ward were calculated from three months of testing. In our hospital, the 4th floor intensive-care unit was reconstructed starting in October 2013. A unit with a negative pressure system to remove dust from construction workers was constructed after an invasive aspergillosis outbreak occurred between December 2013 and January 2014. The mean values significantly increased following reconstruction (p<0.05).
Table shows the characteristics of patients with aspergillosis. All three patients developed pneumonia due to Aspergillus and were diagnosed with “probable” invasive aspergillosis according to the EORTC/MSG criteria. Two patients had acute myeloid leukemia and one had multiple myeloma. Two patients were diagnosed at the end of December 2013, and one patient was diagnosed in the middle of January 2014. The maximum Aspergillus antigen values ranged from 1 to 2.5 COI. Two patients had been treated with micafungin (150 mg/body, 5 days or 23 days) previously but were treated with liposomal amphotericin B (2.5 mg/kg, 6 days or 8 days) after the diagnosis. One patient had been treated with voriconazole (400 mg/body, per os, 23 days) previously but was treated with liposomal amphotericin B (2.5 mg/kg, 24 days) after the diagnosis. Two patients ultimately died due to aspergillosis, and the other patient died due to transplantation-related lymphoma. All patients had received allogeneic stem cell transplantation.
Table.
Characteristics of Three Patients Diagnosed with Invasive Aspergillosis according to the EORTC/MSG Criteria.
| Pt. | disease | age | max value of antigen | Infection focus | EORTC/MSG criteria | treatment | outcome | cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | AML | 63 | 1 | pneumonia | probable | MCFG (150 mg/body 23 days)→ LAMB (2.5 mg/kg 8 days) | dead | aspergillosis |
| 2 | MM | 65 | 2.5 | pneumonia | probable | MCFG (150 mg/body 5 days)→ LAMB (2.5 mg/kg 6 days) | dead | aspergillosis |
| 3 | AML | 30 | 1 | pneumonia | probable | VRCZ (400 mg/body po 5 months)→ LAMB (2.5 mg/kg 24 days) | dead | lymphoma |
LAMB: liposomal amphotericin, MCFG: micafungin, VRCZ: voriconazole
Discussion
An outbreak of invasive aspergillosis in a hematology ward was confirmed by molecular methods in 1996 (13). The Centers for Disease Control (CDC) reports that immunocompromised patients are at risk of aspergillosis during hospital reconstruction; therefore, infection control for Aspergillus is needed (10). For infection control in hospitals, no standard recommendations are available regarding routine microbiological air sampling before, during or after construction or before or during occupancy of areas housing immunocompromised patients. However, the CDC recommended airborne-particle sampling as a tool to evaluate barrier integrity (Category II) in 2003 (10). HEPA filtration is known to reduce airborne Aspergillus contamination (14, 15). Mobile air treatment decontamination units have been developed as an alternative to laminar air filtration. In our hospital, approximately 10 of the patients were treated in mobile air treatment decontamination units or using laminar air filtration. The outbreak was difficult to resolve completely because standard air sampling techniques and methods to predict Aspergillus outbreak have not been reported. Therefore, we investigated infection control measures suitable for our hospital before and during hospital reconstruction in this study.
The first point to be discussed is serum galactomannan testing. Yoshida et al. reported about recent advances in the serodiagnosis of systemic fungal infections (16). Fujiuchi et al. reported that serum galactomannan is useful as a prognostic value in chronic pulmonary aspergillosis (17). In addition, Maezaki et al. evaluated serum (1, 3)-β-D-glucan and Aspergillus galactomannan before and after construction in a ward for patients with hematologic diseases (18). They showed that the mean serum Aspergillus galactomannan level was 0.3 COI before and 0.4 COI after construction in 60 patients (18). However, no patients had aspergillosis. Furthermore, Kawakami et al. reported that there was a correlation between increased fungal contamination from 4 AM to 8 AM and bronchial asthma attacks (19). This finding suggests that the concentration of Aspergillus spores in the air may change throughout the day. Many Aspergillus spores may float transiently during situations such as peeling ceilings, and patients with hematological malignancy may be at a greater risk of inhaling spores at any moment than others. Hamaki et al. reported that because patients who received allogeneic stem cell transplantation had dysfunction of the intestinal or bronchial mucosal barrier, fecal or bronchial galactomannan antigens might reach the circulation, thus leading to false-positive antigenemia (20). Kami et al. speculated that Aspergillus might enter the bloodstream from the upper respiratory tract or catheter insertion sites and subsequently be cleared without the development of invasive aspergillosis in some patients (21). In our patients who receive chemotherapy or hematopoietic stem cell transplantation, galactomannan antigens might reach the circulation due to the dysfunction of the intestinal or bronchial mucosal barrier. Based on our finding of a high serum Aspergillus galactomannan level in patients without aspergillosis in Fig. 4, most Aspergillus spores might be cleared without the development of invasive aspergillosis, and only a few patients might develop of invasive aspergillosis during insufficient infection control for construction. We therefore considered the possibility of false-positive responses as being a marker of airborne Aspergillus spore contamination in our patients. Finally, we found that the mean serum Aspergillus galactomannan level of patients every 3 months was 1.3 COI before and 3.6 COI after construction. In addition, there was a significant association between the mean serum Aspergillus galactomannan level and construction. We speculate that spores disseminated from the construction area and were inhaled by the patients, although we used portable HEPA filter units in some parts of the hematology ward, sealed windows in the work area, and constructed barriers to prevent dust from the construction areas spreading. This finding suggests that monthly surveillance with frequent serum Aspergillus galactomannan antigen and (1, 3)-β-D-glucan testing in the hematological ward may be useful for predicting an invasive aspergillosis outbreak. Furthermore, serum Aspergillus galactomannan antigen should be examined every few days if health insurance coverage is not limited.
The second point to be discussed is the rigorous surveillance of patients with invasive aspergillosis. Although patients in our hospital had received antifungal therapy, an outbreak of invasive aspergillosis still occurred. Several factors are involved in aspergillosis, including the patient's immune state, the history of antifungal therapy and the quantity of Aspergillus spores. Therefore, an aggressive study using N95 masks, computed tomography, biopsies and serum galactomannan testing should be performed to diagnose invasive aspergillosis, as even patients who receive antifungal therapy might still develop aspergillosis. Furthermore, a report by Chabrol et al. suggested that prophylaxis with voriconazole or caspofungin is useful in limited situations (22). Our patients who developed aspergillosis had been treated with micafungin or voriconazole, so the antifungals were changed to liposomal amphotericin B. Thus, the dosage or antifungal class must be reconsidered in order to control Aspergillus infection completely during hospital reconstruction.
The third point to be discussed is the environmental assessment and detailed communication with construction workers. After the outbreak of invasive aspergillosis occurred between December 2013 and January 2014, we conducted an environmental assessment to determine the source of transmission. We suspected the source of the outbreak was the construction area on the 4th floor, which did not have a unit with a negative pressure system to remove dust from the construction workers, as no issues had been observed with the air ventilation system on all floors. After such a unit was constructed, no new cases of invasive aspergillosis were diagnosed. Therefore, frequent environmental assessments and strong communication with construction workers, especially during ceiling demolition, are critical for preventing an Aspergillus outbreak.
We did not examine other hospital environments, such as the bathrooms or patient rooms, outside of the corridor. Further investigation is necessary to evaluate other potential sources of Aspergillus contamination to predict outbreaks. Furthermore, airborne-particle sampling should be investigated as a tool to evaluate barrier integrity, although the CDC recommends airborne-particle sampling as Category II.
In summary, many Aspergillus spores are transiently floating during hospital reconstruction. Therefore, monthly surveillance with frequent serum galactomannan antigen testing to predict outbreaks and an environmental assessment to determine the source of the outbreak are necessary. The surveillance of all patients in the hematology ward is especially important. Reconsideration of prophylactic antifungals may also be required during hospital reconstruction.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Masahiko Nakamura, Mariko Sugita and Naomi Shimizu from Toyama Prefectural Central Hospital Infection Control Team for their assistance with this study.
References
- 1.Manuel RJ, Kibbler CC. The epidemiology and prevention of invasive aspergillosis. J Hosp Infect 39: 95-109, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Fournel I, Sautour M, Lafon I, et al. Airborne Aspergillus contamination during hospital construction works: efficacy of protective measures. Am J Infect Control 38: 189-194, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Goodley JM, Clayton YM, Hay RJ. Environmental sampling for aspergilla during building construction on a hospital site. J Hosp Infect 26: 27-35, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Hospenthal DR, Kwon-Chung KJ, Bennet JE. Concentrations of airborne Aspergillus compared to the incidence of invasive aspergillosis: lack of correlation. Med Mycol 36: 165-168, 1998. [PubMed] [Google Scholar]
- 5.Morris G, Kokki MH, Anderson K, Richardson MD. Sampling of Aspergillus spores in air. J Hosp Infect 44: 81-92, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Barnes RA, Rogers TR. Control of an outbreak of nosocomial aspergillosis by laminar air-flow isolation. J Hospital Infect 14: 89-94, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Alberti C, Bouakline A, Ribaud P, et al. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J Hop Infect 48: 198-206, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Reyboux G, Gbaguidi-Haore H, Bellanger AP, et al. A 10-year survey of fungal aerocontamination in hospital corridors: a reliable sentinel to predict fungal exposure risk? J Hosp Infect 87: 34-40, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Consensus conference. . Preventing the risk of Aspergillus infection in immunocompromised patients. Bull Cancer 88: 589-600, 2001. [PubMed] [Google Scholar]
- 10.Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 52: 1-42, 2003. [PubMed] [Google Scholar]
- 11.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46: 1813-1821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leenders A, van Belkum A, Janssen S, et al. Molecular epidemiology of apparent outbreak of invasive aspergillosis in a hematology ward. J Clin Micobiol 34: 345-351, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornet M, Levy V, Fleury L, et al. Efficacy of prevention by high-efficiency particulate air filtration or laminar airflow against Aspergillus airbone contamination during hospital renovation. Infect control Hosp Epidemiol 20: 508-513, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction:before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 66: 257-262, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida K. Reccent advances of serodiagnosis for systemic fungal infections. Jpn J Med Mycol 47: 135-142, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Fujiuchi S, Yamamoto Y, Takeda A, et al. Evaluation of galactomannan antigen and β-D glucan value for diagnosis of chronic necrotizing pulmonary aspergillosis. Annals of Japanese Respiratory Society 47: 7-11, 2009(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 18.Maezaki S, Tarumoto N, Abe Y, Yamaguchi T, Matsumoto C. Evaluation of serum (1,3)-B-d-glucan and Asperillus galactomannan before and after construction in the ward for patients with hematological disease. Jpn J Infect Prevent Control 24: 233-235, 2009. [Google Scholar]
- 19.Kawakami Y, Hashimoto K. A survey on the diurnal variation of airbone fungi in rooms of common houses. Indoor Environment 17: 11-17, 2014. [Google Scholar]
- 20.Hamaki T, Kami M, Kanda Y, et al. False-positive results of Aspergillus enzyme-linked immunosorbent assay in a patient with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant 28: 633-634, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kami M, Kanda Y, Ogawa S, et al. Frequent false-positive results of Aspergillus latex agglutination test. transient Aspergillus antigenemia during neutropenia. Cancer 86: 274-281, 1999. [PubMed] [Google Scholar]
- 22.Chabrol A, Cuzin L, Huguet F, et al. Prophylaxis of invasive aspergillosis with voriconazol or caspofungin in patients with acute leukemia during building works. Haematologica 95: 996-1003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]