Abstract
We herein report a case of recurrent Campylobacter coli bacteremia in a 37-year-old Japanese man with X-linked agammaglobulinemia (XLA). The patient experienced seven episodes of C. coli bacteremia over one year, with an erythematous rash intermittently emerged on the lower limbs. Although hospitalization for intravenous treatment was repeatedly recommended, he obstinately declined it. Following long-term oral antibiotic treatment with tebipenem and faropenem for the persistent infection, C. coli showed elevated minimum inhibitory concentrations to meropenem, a key drug for severe campylobacteriosis. Physicians should note that the overuse of antibiotics can lead to the emergence of carbapenem-non-susceptible Campylobacter strains.
Keywords: bacteremia, Campylobacter coli, carbapenem, faropenem, soft tissue infection, tebipenem
Introduction
Among Campylobacter spp., Campylobacter coli is the second-most frequent pathogen of community-onset Campylobacter infection, followed by Campylobacter jejuni (1). Based on previous reports, usually less than 1% of C. coli infections are complicated with bacteremia (2-7). However, the incidence of systemic infection with C. coli might be underestimated due to its slow growth and the infrequency of blood culture examinations for outpatients with enterocolitis (8, 9). Thus, the clinical characteristics of C. coli bacteremia have not been fully uncovered. Compared with C. fetus, which has a specific affinity for vascular tissues, C. coli rarely evokes bacteremia and extra-intestinal complications. Immunocompromised patients, however, may fail to survive without appropriate treatment (10).
We herein report an adult patient with X-linked agammaglobulinemia (XLA) who was suffering from repetitive C. coli bacteremia without any gastrointestinal symptoms. Through a prolonged treatment course, the pathogenic organism gradually lost its susceptibility to carbapenem.
Case Report
A 37-year-old man (body weight, 53 kg) with genetically-diagnosed XLA due to a mutation in the Bruton tyrosine kinase gene had undergone intravenous immunoglobulin (Ig)-replacement therapy every 3 weeks since he was 3 years of age. Six months earlier, Ig-replacement therapy had been switched from intravenous administration to subcutaneous injection. The serum IgG levels had been maintained approximately between 870 and 1,000 mg/dL. The patient was not receiving any prophylactic antibiotics.
At the end of May 2015, he suddenly suffered from a swelling of his left leg with an accompanying high fever. The patient was diagnosed with cellulitis and was treated with cefazolin at a neighborhood clinic for one week. However, the high fever and swollen leg persisted, and the patient visited our outpatient department. On arrival, his left lower leg appeared reddish and swollen. He denied any other symptoms, including any gastrointestinal complaints. Serum C-reactive protein was elevated (up to 6.19 mg/dL); otherwise, no remarkable findings were obtained. For a further examination and treatment, hospitalization was proposed to the patient, but he refused. After blood was drawn for a bacterial culture, he returned home. One week later, the patient visited us again, since the results of the blood culture examination were positive for Gram-negative spiral rods, with a 2.33-day incubation period (BacT/ALERT system; bioMérieux, Marcy l'Etoile, France). The patient received a daily administration of cefazolin at the clinic in the meantime; however, the swelling and claudication still persisted, and he was ultimately admitted to our hospital.
On admission, he was afebrile, and his vital signs were stable. There were still no gastrointestinal symptoms, and blood cultures remained positive for the Gram-negative spiral rods. The organism was identified as C. coli based on the matrix-assisted laser desorption/ionization-time of flight mass spectrometry using a MALDI Biotyper (Bruker Daltonics, Bremen, Germany) with a score of 2.231 and a positive result for glyA gene by polymerase chain reaction test (11). A stool culture was also positive for C. coli. The results of antimicrobial susceptibility testing are shown in Table. A whole-body investigation by computed tomography revealed no remarkable findings of visceral infection, and the results of transthoracic echocardiography were not suggestive of infective endocarditis. Magnetic resonance imaging of his lower legs revealed high intensity at the subcutaneous tissues on short TI inversion recovery, but there was no evidence indicating the formation of an abscess or osteomyelitis. After treatment with meropenem (1 g every 8 hours), the swelling of his lower left leg gradually improved, and the bacteremia disappeared. Two weeks later, the patient was discharged with continued treatment by oral tebipenem-pivoxil (200 mg, twice daily) for 3 weeks.
Table.
Minimum Inhibitory Concentrations (MIC) of Campylobacter coli
| June 2015 | February 2016 | May 2016 | |
|---|---|---|---|
| Meropenema | ≤0.125 | 1 | 4 |
| Azithromycina | >4 | >4 (>256b) | >4 |
| Minocyclinea | ≤0.5 | ≤0.5 | ≤0.5 |
| Levofloxacina | >2 | >8 | >8 |
| Metronidazoleb | n.p. | 0.06 | 0.06 |
| Chloramphenicolb | 2 | 2 | 2 |
aMicroScan Walkaway 96 plus System (Beckman Coulter)
bE-test (SYSMEX bioMerieux).
MIC of meropenem continued elevating throughout the course.
n.p.: not performed
Two months after discharge, the patient experienced a recurrence of the swollen legs, and tebipenem-pivoxil was again orally administered for three weeks. Nevertheless, three months later, the patient experienced a high fever with leg swelling and continuing recurrent C. coli bacteremia. For the subsequent five months, even though the symptoms remained, the patient adamantly refused to be re-admitted to the hospital. During this time, oral administration of tebipenem-pivoxil and faropenem (200 mg, twice daily) was continued, and the minimum inhibitory concentration (MIC) for meropenem was found to be elevated (Table). Approximately one year after the onset of the initial episode of C. coli bacteremia, he complained of abdominal discomfort. A gastroscopic examination revealed an elevated gastric mucosal lesion that was pathologically diagnosed as gastric cancer. He eventually agreed to be admitted for endoscopic mucosal resection of the gastric malignancy as well as antibiotic treatment for the recurrent bacteremia.
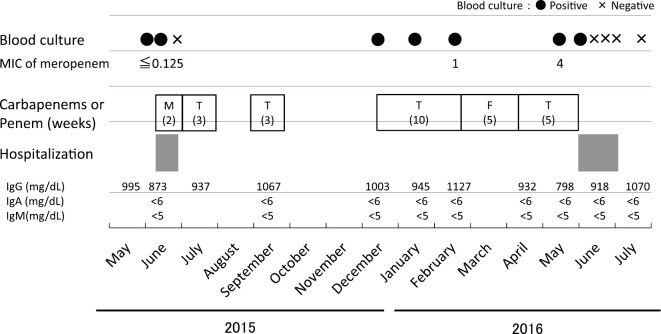
The MIC level for meropenem of the pathogen was further increased on admission (Table). The patient underwent antibiotic therapy with intravenous biapenem (600 mg, every 12 hours) and oral minocycline (100 mg, twice daily) for 2 weeks. The persistent bacteremia resolved, and the antibiotics were switched to oral metronidazole (250 mg, 4 times daily) for an additional 4 weeks. After discharge, the antibiotic treatment was continued with oral minocycline for 10 weeks in total. Subsequently, no recurrent episodes have been reported in more than one year. The clinical course of the patient is shown in Figure.
Figure.
Clinical course of the patient. The patient was admitted to the hospital for two weeks in June 2015 and four weeks in June 2016. Over one year, various kinds of carbapenems (M: meropenem, T: tebipenem-pivoxil) and penems (F: faropenem) were administered for the treatment of recurrent Campylobacter coli bacteremia. MIC: minimum inhibitory concentration (μg/mL)
Discussion
We presented a case of multiple episodes of C. coli bacteremia in an adult patient with XLA. Over one year, the patient had at least seven episodes of C. coli bacteremia, with an erythematous rash that intermittently emerged on his lower limbs. Due to his refusal for hospitalization therapy, we were unable to provide the appropriate antibiotic treatment. As a result, the meropenem MIC of the pathogen apparently increased in association with the long-term antibiotic treatment mainly by oral antimicrobials.
The emergence of multidrug-resistant Campylobacter strains has become a global health problem (12, 13). Most Campylobacter spp. remain susceptible to a range of antibiotics (6), but strains resistant to third-generation cephalosporins, macrolides, and fluoroquinolones have been increasingly reported (14-16). Carbapenem is recommended as an empiric agent for Campylobacter bacteremia (7, 10, 17, 18); however, the causative organism in the present case demonstrated an increasing MIC level for meropenem after the prolonged antibiotic therapy. In Japan, the recommended dose of tebipenem is 4 to 6 mg/kg twice daily for children, while the optimum dose for adults is not described on the drug package insert. Faropenem is administered at 150 to 300 mg three times a day. In this case, both of the drugs were given at 200 mg twice daily, which may have been an under-dose for the infection. Although carbapenem-resistance in Campylobacter species has yet to be well defined by authorities, our case suggested possible resistance subsequent to drug exposure in a clinical situation. In case of refractory Campylobacter infections, combination therapy may be preferred. In addition, aminoglycosides may be a favorable choice for the treatment of multidrug-resistant Campylobacter infections. The rate of aminoglycosides-resistant organisms is approximately 3% (19, 20); therefore, treatment with aminoglycosides is recommended, particularly in the case of severe infections (1, 21).
XLA predisposes patients to severe hypogammaglobulinemia due to the inadequate maturity of B lymphocytes (22). Interestingly, previous studies have revealed a peculiar association with XLA patients and Campylobacter systemic infection (8, 23). In a large cohort study, all of the recurrent cases were those with humoral immunodeficiency; as such, a relationship between systemic campylobacteriosis and XLA may exist (14). Worldwide, more than 20 cases of C. coli or C. jejuni bacteremia in patients with XLA or hypogammaglobulinemia were reported before 2010 (8). In Japan, three cases of C. coli bacteremia in XLA patients (24-26) and one case of C. jejuni bacteremia in a patient with hypogammaglobulinemia have been reported (27). Patients with XLA are regularly given Ig-replacement therapy to maintain the serum level of IgG for infection prevention. However, replacement therapy is not sufficient to prevent infection with enteropathogenic organisms, such as Campylobacter spp. or Helicobacter spp. (28), particularly because IgA, which plays an important role in the elimination of gut bacteria, is not provided with such therapy (29). Indeed, C. coli bacteremia occurred even in the presence of high serum IgG levels in our case, similar to the findings in an Italian patient with XLA (23). Thus, gut immunity via IgA is likely a key factor in the prevention of this rare infection (30).
The clinical manifestations of C. coli bacteremia have only rarely been reported. Previous studies have shown that patients with immunocompromising factors, like our patient, tend to suffer from recurrent C. coli bacteremia without gastrointestinal symptoms (7, 31). Cutaneous involvement is less recognized and may often be overlooked as a presentation of Campylobacter bacteremia (32). The incidence of cellulitis ranges from 3% to 20% and is particularly frequent in the lower extremities with recurrent episodes of bacteremia (18), as was also seen in this case.
In conclusion, this case highlighted several important issues. First, a carbapenem-non-susceptible C. coli strain appeared subsequent to prolonged antibiotic treatment for recurrent infection. Second, patients with XLA are at risk for systemic campylobacteriosis. Finally, Ig-replacement therapy alone is inadequate for the prevention of Campylobacter bacteremia, and another method that can enhance gut immunity may be required.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28: 687-720, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain MA, Kabir I, Albert MJ, Kibriya AK, Alam K, Alam AN. Campylobacter jejuni bacteraemia in children with diarrhoea in Bangladesh: report of six cases. J Diarrhoeal Dis Res 10: 101-104, 1992. [PubMed] [Google Scholar]
- 3.Skirrow MB, Jones DM, Sutcliffe E, Benjamin J. Campylobacter bacteraemia in England and Wales, 1981-91. Epidemiol Infect 110: 567-573, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel MC, Vugia DJ, Shallow S, et al. . Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996-1999. Clin Infect Dis 38 (Suppl 3): S165-S174, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen H, Hansen KK, Gradel KO, et al. . Bacteraemia as a result of Campylobacter species: A population-based study of epidemiology and clinical risk factors. Clin Microbiol Infect 16: 57-61, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Feodoroff B, Lauhio A, Ellstrom P, Rautelin H. A nationwide study of Campylobacter jejuni and Campylobacter coli bacteremia in Finland over a 10-Year Period, 1998-2007, with special reference to clinical characteristics and antimicrobial susceptibility. Clin Infect Dis 53: e99-e106, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasaka K, Matsubara K, Nigami H, Iwata A, Isome K, Yamamoto G. [Invasive Campylobacter jejuni/coli infections: 9 case reports at a single center between 2000 and 2015, and a review of literature describing Japanese patients]. Kansenshogaku Zasshi 90: 297-304, 2016(in Japanese). [DOI] [PubMed] [Google Scholar]
- 8.van den Bruele T, Mourad-Baars PE, Claas EC, van der Plas RN, Kuijper EJ, Bredius RG. Campylobacter jejuni bacteremia and Helicobacter pylori in a patient with X-linked agammaglobulinemia. Eur J Clin Microbiol Infect Dis 29: 1315-1319, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louwen R, van Baarlen P, van Vliet AH, van Belkum A, Hays JP, Endtz HP. Campylobacter bacteremia: a rare and under-reported event? Eur J Microbiol Immunol (Bp) 2: 76-87, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacanowski J, Lalande V, Lacombe K, et al. . Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin Infect Dis 47: 790-796, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, Clark CG, Taylor TM, et al. . Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J Clin Microbiol 40: 4744-4747, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakanen AJ, Lehtopolku M, Siitonen A, Huovinen P, Kotilainen P. Multidrug resistance in Campylobacter jejuni strains collected from Finnish patients during 1995-2000. J Antimicrob Chemother 52: 1035-1039, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Qin SS, Wu CM, Wang Y, et al. . Antimicrobial resistance in Campylobacter coli isolated from pigs in two provinces of China. Int J Food Microbiol 146: 94-98, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Cruz A, Munoz P, Mohedano R, et al. . Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore) 89: 319-330, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Liao CH, Chuang CY, Huang YT, Lee PI, Hsueh PR. Bacteremia caused by antimicrobial resistant Campylobacter species at a medical center in Taiwan, 1998-2008. J Infect 65: 392-399, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Lehtopolku M, Nakari U-M, Kotilainen P, Huovinen P, Siitonen A, Hakanen AJ. Antimicrobial susceptibilities of multidrug-resistant Campylobacter jejuni and C. coli strains: in vitro activities of 20 antimicrobial agents. Antimicrob Agents Chemother 54: 1232-1236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira L, Sampaio S, Tavares I, Bustorff M, Pestana M. Bacteremia due to Campylobacter in renal transplantation: a case report and review of literature. Transpl Infect Dis 16: 1007-1011, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Monselise A, Blickstein D, Ostfeld I, Segal R, Weinberger M. A case of cellulitis complicating Campylobacter jejuni subspecies jejuni bacteremia and review of the literature. Eur J Clin Microbiol Infect Dis 23: 718-721, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hakanen AJ, Lehtopolku M, Siitonen A, Huovinen P, Kotilainen P. Multidrug resistance in Campylobacter jejuni strains collected from Finnish patients during 1995-2000. J Antimicrob Chemother 52: 1035-1039, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Moore JE, Barton MD, Blair IS, et al. . The epidemiology of antibiotic resistance in Campylobacter. Microbes Infect 8: 1955-1966, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Qin S, Wang Y, Zhang Q, et al. . Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli. Antimicrob Agents Chemother 56: 5332-5339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conley ME, Broides A, Hernandez-Trujillo V, et al. . Genetic analysis of patients with defects in early B-cell development. Immunol Rev 203: 216-234, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Ariganello P, Angelino G, Scarselli A, et al. . Relapsing Campylobacter jejuni systemic infections in a child with X-linked agammaglobulinemia. Case Rep Pediatr 2013: 735108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokuda K, Nishi J, Miyanohara H, et al. . Relapsing cellulitis associated with Campylobacter coli bacteremia in an agammaglobulinemic patient. Pediatr Infect Dis J 23: 577-579, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Arai A, Kitano A, Sawabe E, Kanegane H, Miyawaki T, Miura O. Relapsing Campylobacter coli bacteremia with reactive arthritis in a patient with X-linked agammaglobulinemia. Intern Med 46: 605-609, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Okada H, Kitazawa T, Harada S, et al. . Combined treatment with oral kanamycin and parenteral antibiotics for a case of persistent bacteremia and intestinal carriage with Campylobacter coli. Intern Med 47: 1363-1366, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Mori T, Hasegawa N, Sugita K, et al. . Clinical features of bacteremia due to Campylobacter jejuni. 53: 1941-1944, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Desar IM, van Deuren M, Sprong T, et al. . Serum bactericidal activity against Helicobacter pylori in patients with hypogammaglobulinaemia. Clin Exp Immunol 156: 434-439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Hilst JC, Smits BW, van der Meer JW. Hypogammaglobulinaemia: cumulative experience in 49 patients in a tertiary care institution. Neth J Med 60: 140-147, 2002. [PubMed] [Google Scholar]
- 30.Wooldridge KG, Ketley JM. Campylobacter-host cell interactions. Trends Microbiol 5: 96-102, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Shimol S, Carmi A, Greenberg D. Demographic and clinical characteristics of Campylobacter bacteremia in children with and without predisposing factors. Pediatr Infect Dis J 32: e414-e418, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Schønheyder HC, Søgaard P, Frederiksen W. A survey of Campylobacter bacteremia in three Danish counties, 1989 to 1994. Scand J Infect Dis 27: 145-148, 1995. [DOI] [PubMed] [Google Scholar]