Abstract
A 69-year-old man was admitted to a hospital with complaints of abdominal pain. Computed tomography showed hepatic portal venous gas and pneumatosis cystoides intestinalis. Conservative treatment was effective; however, after discharge, he developed complaints of vomiting. Fluoroscopic enteroclysis revealed a stricture in the jejunum necessitating admission to our hospital. Transoral balloon-assisted enteroscopy showed a circumferential ulcer with a stricture. The stricture was surgically resected, and a histopathological examination was consistent with ischemic enteritis. Stenotic ischemic enteritis should be considered among the differential diagnoses in a patient presenting with hepatic portal venous gas and pneumatosis cystoides intestinalis showing small intestinal obstruction.
Keywords: ischemic enteritis, hepatic portal venous gas, pneumatosis cystoides intestinalis, balloon-assisted enteroscopy, intestinal stricture
Introduction
Ischemic enteritis is a rare disease compared to ischemic colitis because of the rich collateral blood supply to the small intestine, although both conditions are associated with a decreased arterial inflow (1). An accurate and early diagnosis of ischemic enteritis is difficult because of the anatomy of the small intestine. However, capsule endoscopy and balloon-assisted enteroscopy successfully demonstrate the endoscopic findings of ischemic enteritis (1-4).
The presence of hepatic portal venous gas (HPVG) was previously considered a sign of intestinal necrosis warranting urgent surgery. However, it has been recently shown that HPVG can be treated conservatively in some patients (5-7).
We herein report a patient who presented with stenotic ischemic enteritis with concomitant HPVG and pneumatosis cystoides intestinalis (PCI).
Case Report
A 69-year-old man who had been traveling was admitted to a local hospital with complaints of abdominal pain in October 2013. Plain abdominal computed tomography revealed an insignificant degree of HPVG (Fig. 1A, white arrow) and PCI (Fig. 1B). Abdominal computed tomography showed that a small amount of HPVG existed in the liver, and PCI appeared on only one side of the lumen. However, there was no stenosis observed in the small intestine. He was diagnosed with regional enteritis without peritoneal irritation and was treated conservatively using broad-spectrum antibiotics for HPVG and PCI. The following day, computed tomography showed the resolution of both the HPVG and PCI (Fig. 2). He was discharged on day 16 of hospitalization. However, two months later, he was admitted to another hospital with complaints of vomiting and weight loss. He was discharged following the improvement in his condition after receiving conservative treatment; however, he later presented with worsening vomiting. Fluoroscopic enteroclysis performed at the previous hospital suggested a stricture in the jejunum, and he was admitted to our hospital in July 2014.
Figure 1.
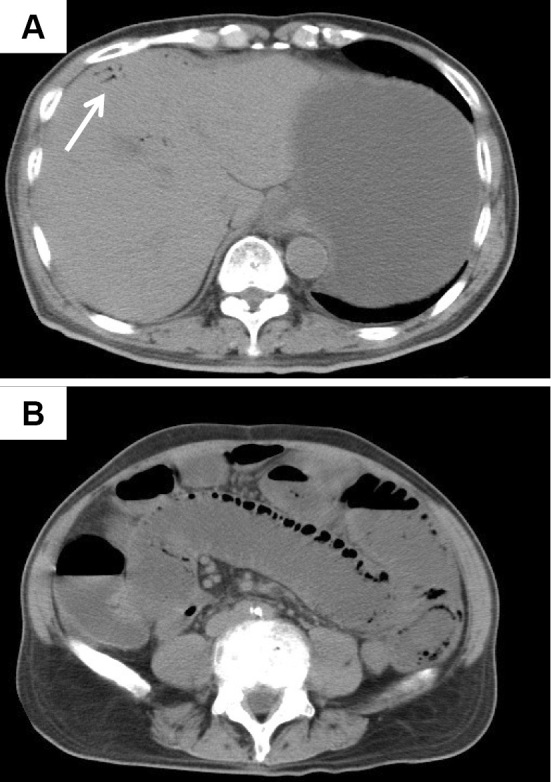
Computed tomography findings on admission. Plain abdominal computed tomography revealed an insignificant degree of hepatic portal venous gas (HPVG) (A: white arrow). Plain abdominal computed tomography revealed pneumatosis cystoides intestinalis (PCI) (B).
Figure 2.
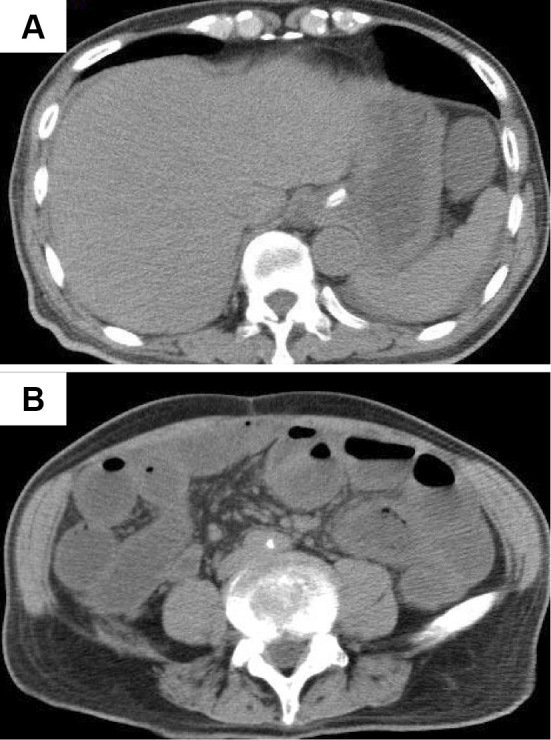
Computed tomography findings on post-admission day 1. Plain abdominal computed tomography revealed the resolution of the hepatic portal venous gas (HPVG) and pneumatosis cystoides intestinalis (PCI).
A physical examination on admission revealed the patient to be 163 cm tall and having lost 13 kg over the 10 months prior to presentation, now weighing 51 kg. His body temperature was 36.5℃, blood pressure 99/66 mmHg, and pulse rate 57 beats/min. A systemic examination revealed abdominal distension with diffuse tenderness but no signs of peritoneal irritation. He had a history of hypertension and had undergone laparotomy to treat rectal carcinoma. Laboratory investigations showed the following: hemoglobin 11.7 g/dL, white blood cells 3,500/mm3, and platelets 297,000/mm3. Serum biochemistry showed the following: total protein 6.1 g/L, albumin 3.8 g/L, blood urea nitrogen 16.9 mg/dL, creatinine 0.78 mg/dL, total bilirubin 0.50 mg/dL, aspartate aminotransferase 29 IU/L, alanine aminotransferasae 33 IU/L, C-reactive protein 0.08 mg/dL, carcinoembryonic antigen 2.6 ng/mL, and carbohydrate antigen 19-9 3.5 U/mL.
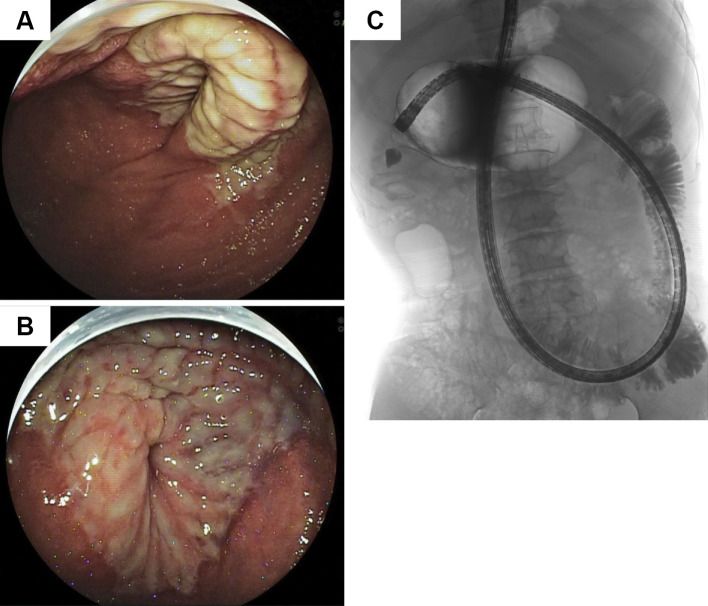
Enhanced abdominal computed tomography showed ascites (Fig. 3A) and a stricture in a segment of the jejunum (Fig. 3B, white arrow), although no stenosis of the major abdominal arteries was noted. Transoral double-balloon enteroscopy performed using an EN-450T5 double-balloon enteroscope (Fujifilm, Tokyo, Japan) revealed a circumferential ulcer in a segment of the jejunum (Fig. 4A and B). Because the circumferential ulcer caused a stricture across which we could not pass our scope, we performed Gastrografin fluoroscopy through the scope. We noted a change in the caliber and a 6-cm stricture, which was too extensive to perform balloon dilation therapy (Fig. 4C). Endoscopic biopsy specimens obtained from the ulcer showed no malignancy. Because conservative treatment could not effectively treat the symptoms related to the stricture, he eventually underwent laparotomy in October 2014.
Figure 3.
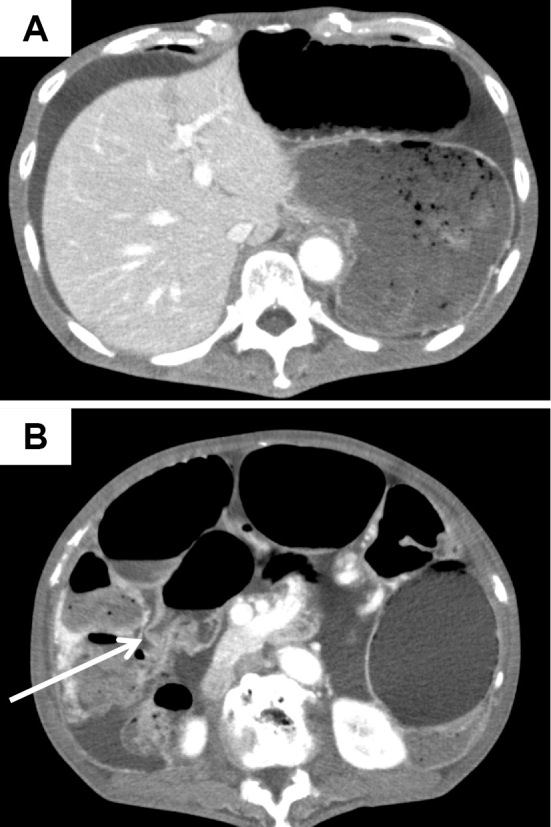
Enhanced computed tomography findings 10 months after the onset of the disease. Abdominal computed tomography showed ascites (A) and a stricture in a segment of the jejunum (B: white arrow).
Figure 4.
Findings of double-balloon endoscopy and Gastrografin fluoroscopy. Transoral double-balloon endoscopy revealed a circumferential ulcer in a segment of the jejunum (A and B). Gastrografin fluoroscopy showed a change in the caliber (C).
Intraoperatively, a stricture was identified in the jejunum 70 cm distal to the ligament of Treitz. We performed intestinal resection (approximately 10 cm in length) followed by side-to-side anastomosis. Postoperatively, his clinical course was uneventful.
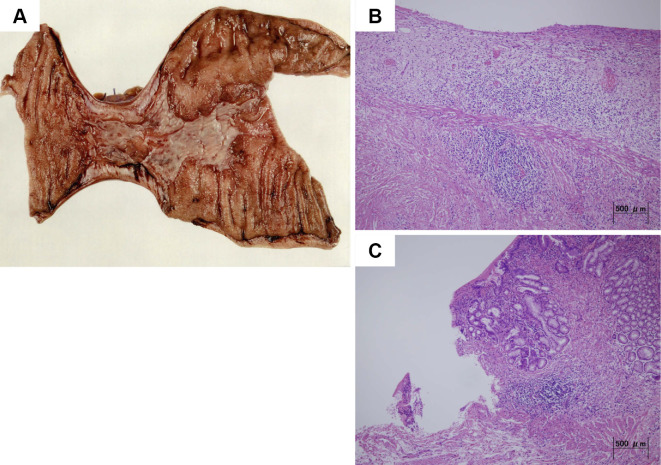
Macroscopic examinations of the resected specimen revealed a 60-mm circumferential ulcer of grade Ul-IIIs showing a clear region and a partial stricture with no signs of thrombi (Fig. 5A). A microscopic examination revealed the loss of the mucosal layer, an edematous submucosa, and chronic inflammation with lymphocytic and plasma cell infiltration. Furthermore, the peri-ulcer area showed significant edema and fibrosis in the submucosal layer. However, there was no evidence of granuloma or sideroferous cells (Fig. 5B and C). The findings of macro- and microscopic examinations were consistent with ischemic enteritis.
Figure 5.
Histological findings. Macroscopic examinations of the resected specimen revealed a 60-mm circumferential ulcer of grade Ul-IIIs with a partial stricture (A). Microscopic examinations of the resected specimen revealed chronic inflammation with lymphocytic and plasma cell infiltration and an edematous and congested submucosa. However, there was no evidence of sideroferous cells (B and C).
Discussion
Ischemic colitis, first reported by Boley et al. (8) and Marston et al. (9),is one of the most common vascular diseases of the colon and is caused by an insufficient blood supply through the mesenteric arteries. It is hypothesized that ischemic enteritis is caused by the same pathomechanism that leads to ischemic colitis (1). In our patient, the degree/extent of ischemia at the time of his first admission was so severe that the ulcer extended to the submucosal layer, and gas in the bowel lumen traveled through the injured mucosal layer into the portal venous system, resulting in HPVG and PCI. While his initial symptoms did improve, the stenotic symptoms gradually worsened secondary to chronic inflammation. Ischemic enteritis is classified as transient or stenotic in type (1, 3, 10, 11). Retrospectively, our patient's clinical course could be classified as stenotic ischemic enteritis.
HPVG was initially reported as a radiographic sign associated with necrotizing enterocolitis (12). HPVG was previously considered to be associated with a high mortality and an indication for urgent surgery (13). However, with the development of diagnostic techniques such as multi-detector computed tomography, some patients diagnosed with HPVG can now be treated using conservative therapy. Indeed, it was recently reported that HPVG is not a predictor of urgent surgery and high mortality per se (5-7).
PCI usually has diffuse lesions in the small bowel and/or colon. However, in the present patient, plain abdominal computed tomography revealed an insignificant degree of PCI. Therefore, we deemed the degree of PCI not severe and selected conservative therapy. As with HPVG, PCI is not an indicator for surgical resection per se (14), and no reports have found that the degree of PCI is correlated with the severity of the intestinal ischemia or timing of computed tomography. More case reports are needed to clarify whether the degree of PCI is correlated with the severity of the intestinal ischemia or timing of computed tomography. In our patient, while ischemia may have led to HPVG and PCI, he was still able to be treated conservatively, as there was no peritoneal irritation. Necrosis of the entire bowel wall and the consequent need for urgent surgery were prevented by the initiation of prompt therapy in this patient.
Previously, the diagnosis of ischemic enteritis was difficult, as a radiographic evaluation was the only modality available for the investigation of the small intestine. In recent years, however, capsule endoscopy and balloon-assisted enteroscopy have come to be widely used as established diagnostic methods for investigating small intestinal diseases (15). A few reports have described the diagnosis of ischemic enteritis by capsule endoscopy (2, 4, 16, 17). Previous reports reveal that the characteristic endoscopic findings of ischemic enteritis are circumferential ulcers and afferent stenosis (1-3). However, an accurate diagnosis of small intestinal diseases remains difficult. Thus, an endoscopic examination and biopsies are recommended for distinguishing between ischemic enteritis and other small intestinal diseases. Balloon-assisted enteroscopy is therefore a very useful examination for diagnosing ischemic enteritis. In our patient, we performed double-balloon enteroscopy, and the biopsy specimen findings were consistent with those typical of ischemic enteritis.
Stenotic ischemic enteritis often requires surgical management. Balloon dilation using balloon-assisted enteroscopy is reportedly effective for treating stenotic ischemic enteritis (18, 19), particularly for strictures ≤3 cm in length (18). However, in most patients, the strictures associated with stenotic ischemic enteritis tend to be longer than 3 cm (3). Indeed, in our patient, the stricture with an open ulcer measured approximately 6 cm in length. A long stricture associated with an open ulcer increases the risk of adverse events, such as perforation and bleeding. Therefore, the adaptation of balloon dilation for stenotic ischemic enteritis needs to be considered carefully. Stenotic ischemic enteritis is a rare disease entity; as such, more data need to be obtained through studies in a greater number of patients to conclusively establish the clinical features of this pathophysiology.
In conclusion, stenotic ischemic enteritis should be considered among the differential diagnoses in patients presenting with PCI and HPVG showing small intestinal obstruction.
Author's disclosure of potential Conflicts of Interest (COI).
Yoshito Itoh: Research funding, Fujifilm Medical.
References
- 1.Takeuchi N, Naba K. Small intestinal obstruction resulting from ischemic enteritis: a case report. Clin J Gastroenterol 6: 281-286, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong WS, Song HJ, Na SY, et al. Acute extensive ischemic enteritis in a young man diagnosed with wireless capsule endoscopy: a case report. Korean J Gastroenterol 61: 160-165, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Koshikawa Y, Nakase H, Matsuura M, et al. Ischemic enteritis with intestinal stenosis. Intest Res 14: 89-95, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami K, Ishida K, Inoue T, Higuchi K. Endoscopic findings of ischemic enteritis. Intern Med 54: 1943-1944, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Faberman RS, Mayo-Smith WW. Outcome of 17 patients with portal venous gas detected by CT. AJR Am J Roentgenol 169: 1535-1538, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita H, Shinozaki M, Tanimura H, et al. Clinical features and management of hepatic portal venous gas: four case reports and cumulative review of the literature. Arch Surg 136: 1410-1414, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Koami H, Isa T, Ishimine T, et al. Risk factors for bowel necrosis in patients with hepatic portal venous gas. Surg Today 45: 156-161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boley SJ, Schwartz S, Lash J, Sternhill V. Reversible vascular occlusion of the colon. Surg Gynecol Obstet 116: 53-60, 1963. [PubMed] [Google Scholar]
- 9.Marston A, Pheils MT, Thomas ML, Morson BC. Ischaemic colitis. Gut 7: 1-15, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Kim B, Chung S, Park CW, Chang YS. Ischaemic enteritis in a patient with chronic renal failure: diagnosis and management decisions. BMJ Case Rep 2010, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iida M, Matsui T, Yao T, et al. Radiographic features in ischemic jejunoileitis: serial changes and comparison with pathologic findings. Gastrointest Radiol 17: 327-332, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med 74: 486-488, 1955. [PubMed] [Google Scholar]
- 13.Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB. Hepatic-portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg 187: 281-287, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umapathi BA, Friel CM, Stukenborg GJ, Hedrick TL. Estimating the risk of bowel ischemia requiring surgery in patients with tomographic evidence of pneumatosis intestinalis. Am J Surg 212: 762-768, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Handa O, Naito Y, Okayama T, et al. Endoscopic diagnosis of small intestinal diseases. Clin J Gastroenterol 6: 94-98, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Liatsos C, Goulas S, Karagiannis S, Patelaros E, Sabaziotis D, Mavrogiannis C. Diagnosis of small-bowel ischemic necrosis by capsule endoscopy. Gastrointest Endosc 62: 439-440; discussion 440, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Yang XY, Chen CX, Zhang BL, et al. Diagnostic effect of capsule endoscopy in 31 cases of subacute small bowel obstruction. World J Gastroenterol 15: 2401-2405, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura N, Yamamoto H, Yano T, et al. Balloon dilation when using double-balloon enteroscopy for small-bowel strictures associated with ischemic enteritis. Gastrointest Endosc 74: 1157-1161, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Ohmiya N, Arakawa D, Nakamura M, et al. Small-bowel obstruction: diagnostic comparison between double-balloon endoscopy and fluoroscopic enteroclysis, and the outcome of enteroscopic treatment. Gastrointest Endosc 69: 84-93, 2009. [DOI] [PubMed] [Google Scholar]