Abstract
Lupus nephritis (LN) occurs in up to 60% of systemic lupus erythematosus patients. Combination therapy involving a corticosteroid and cyclophosphamide or mycophenolate mofetil (MMF) has been a standard therapy for LN. However, clinicians generally prefer to minimize steroid use in LN treatment. We herein report the case of a Japanese man with LN whose severe chronic heart failure prevented us from using steroid therapy. Instead, his LN was successfully treated with MMF monotherapy. Based on our experience with this case, we suggest that MMF monotherapy may represent a feasible LN treatment option in patients who cannot tolerate steroid therapy.
Keywords: lupus nephritis, monotherapy, mycophenolate mofetil, steroid-free
Introduction
Systemic lupus erythematosus (SLE) is a type of autoimmune disease that can cause inflammation and tissue damage throughout the body. Lupus nephritis (LN) is a serious complication of SLE, occurring in up to 60% of patients with SLE (1). While the 5-year survival rate of LN patients was 44% in 1953-1969, the availability of immunosuppression therapy has substantially improved the 10-year survival, which has been reported to be 88% in recent decades (1). LN is pathologically staged according to the classification put forth by the World Health Organization and the International Society of Nephrology/Renal Pathology Society (ISN/RPS) (2). Class IV LN is defined as diffuse LN involving 50% or more of glomeruli, which indicates severe LN requiring more-intensive therapy (2, 3). At present, combination therapy of a corticosteroid with cyclophosphamide or mycophenolate mofetil (MMF) is recommended as induction therapy for class III and IV LN (1, 3). However, the effectiveness and safety of MMF monotherapy (i.e., without a corticosteroid) for LN remain unclear.
We herein report the case of a Japanese man with LN whose severe chronic heart failure prevented us from using steroid therapy. Instead, his LN was successfully treated with MMF monotherapy.
Case Report
A 43-year-old Japanese man presented to our hospital with a 1-month history of a fever and productive cough. Fifteen years before his admission, he had been diagnosed with idiopathic thrombocytopenic purpura (ITP) and prescribed prednisolone, cyclosporine, and rituximab. The patient had also undergone splenectomy for the treatment of idiopathic thrombocytopenic purpura. Five years later, he developed acute myocardial infarction, which led to severe chronic heart failure (CHF). At that time, there was little evidence suggesting SLE. However, one year before the current admission, laboratory investigations revealed the presence of anti-dsDNA and anti-Sm antibodies, low complement levels, and leukopenia. Based on these findings, the patient was diagnosed with SLE. There were no other findings to suggest the activity of the disease. The patient was not on any specific medications for SLE; hydroxychloroquine had been withdrawn for causing ventricular tachycardia.
On admission, the patient had a fever, but his other vital signs were normal. A physical examination revealed facial erythema, enlargement of the bilateral neck lymph nodes, and mild bilateral leg swelling. Laboratory investigations showed elevated serum C-reactive protein levels and erythrocyte sedimentation rate (2.28 mg/dL and 27 mm at 1 hour, respectively). A complete blood cell count showed mild anemia (hemoglobin, 11.8 g/dL vs. 13.7-16.8 g/dL normal range; leucocytes, 34.2×108/L vs. 33.0-86.0×108/L normal range; platelets, 19.5×1010/L vs. 15.8-34.8×1010/L normal range). Anti-dsDNA antibody levels were elevated (398.6 U/mL vs. ≤12.0 U/mL normal range), with hypocomplementemia (total hemolytic complement CH50, 13.1 U/mL vs. 30.0-40.0 U/mL normal range; complement C3, 29.3 mg/dL vs. 73.0-138.0 mg/dL normal range; complement C4, 4.5 mg/dL vs. 11.0-31.0 mg/dL normal range). The lupus anticoagulant test was also positive. Although the serum creatinine levels were within the normal limits (0.95 mg/dL), the serum albumin levels were low (2.9 g/dL vs. 4.1-5.2 g/dL normal range). The protein-to-creatinine ratio in a random urine sample was 2.40, suggesting severe proteinuria. The presence of abnormal urinary casts was also noted, including red blood cell casts, white blood cell casts, and fatty casts. A renal biopsy showed LN ISN/RPS class IV (A/C) (Fig. 1). Based on these findings, the patient was diagnosed as having active SLE with LN. In addition, the elevation of serum brain natriuretic peptide levels (518.3 pg/nL vs. ≤18.4 pg/nL normal range) and abnormal findings on an echocardiographic examination revealed an additional diagnosis of acute exacerbation of CHF (Fig. 2A).
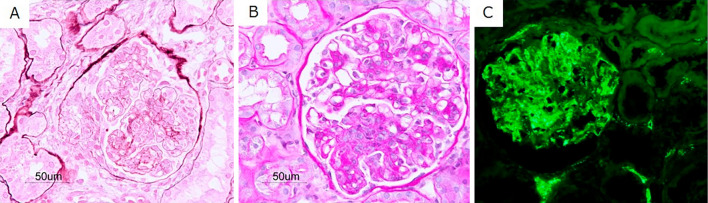
Figure 1.
Histopathology of renal biopsy samples. (A) Light-microscopy periodic acid-methenamine silver staining showing segmental endocapillary proliferation and a fibrocellular crescent (original magnification ×400). (B) Light-microscopy periodic acid-Schiff staining showing moderate mesangial proliferation with segmental endocapillary proliferation (original magnification ×400). (C) Immunofluorescence staining for C1q showing global peripheral and segmental mesangial deposits (original magnification ×400).
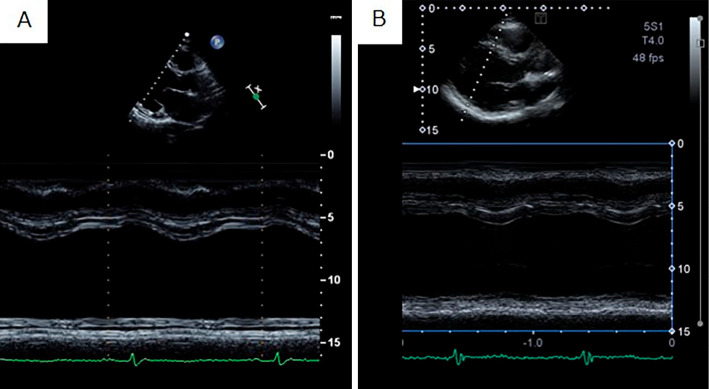
Figure 2.
Echocardiogram before and after treatment for acute heart failure. (A) Before treatment, severe heart failure was noted (left ventricular end-systolic diameter and left ventricular ejection fraction of 74 mm and 30%, respectively). (B) After treatment, the heart failure improved, but the cardiac function remained poor (left ventricular end-systolic diameter and left ventricular ejection fraction of 73 mm and 40%, respectively).
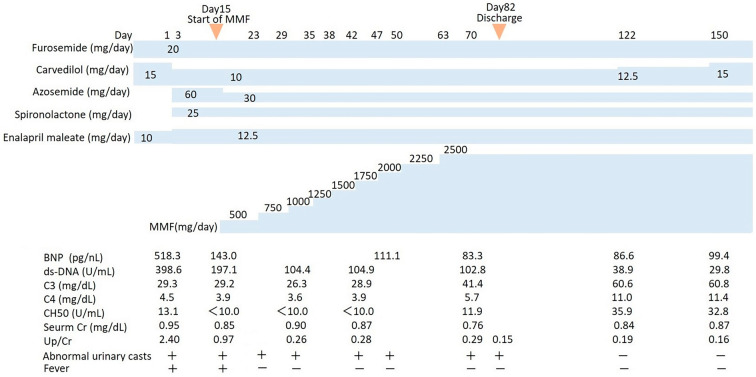
The patient was started on acute heart failure treatment involving a loop diuretic, an angiotensin-converting enzyme inhibitor, a beta-blocker, and spironolactone. Following this treatment, his heart failure improved, but the cardiac function remained poor (Fig. 2B). Therefore, we started the patient on MMF monotherapy (250 mg twice daily) as treatment for LN. We did not include a corticosteroid to prevent the progression of heart failure and remodeling of the cardiac muscle. One week after the initiation of MMF therapy, his fever resolved and the uric protein excretion decreased. After 2 months of gradual loading of MMF (up to 1,250 mg twice daily, with a 12-h post-MMF intake concentration of 2.3 μg/mL), the protein-to-creatinine ratio in a random urine sample decreased to 0.15, suggesting remission of LN. After four months of MMF monotherapy, no abnormal urinary casts were noted, and the complement levels recovered to nearly normal values (Fig. 3).
Figure 3.
Clinical course of lupus nephritis, including medications, changes in serum findings and urinary findings. BNP: brain natriuretic peptide, C3: complement C3, C4: complement C4, CH50: total hemolytic complement, Cr: creatinine, MMF: mycophenolate mofetil, Up/Cr: protein-to-creatinine ratio in a random urine sample
Discussion
To our knowledge, this is the first case report documenting the successful treatment of class IV LN with MMF monotherapy. Although the mechanism underlying SLE pathogenesis has yet to be fully elucidated, it has been hypothesized that B-cell-to-T-cell interactions may have an important role (4). Combination therapy with cyclophosphamide and MMF represents the standard immunosuppression therapy for LN at present (1, 3).
Cyclophosphamide is an alkylating agent that functions by introducing alkyl radicals to DNA strands. Cyclophosphamide has been used for chemotherapy and immunosuppression therapy (5), with low doses exerting a selective effect on B-cell populations (6). In contrast, MMF is an immunosuppressive prodrug of mycophenolic acid that reversibly inhibits inosine monophosphate dehydrogenase, resulting in decreased B- and T-cell proliferation and decreased antibody production (7). Fassbinder et al. reported that, although both MMF and cyclophosphamide showed a significant effect on circulating B-cell subpopulations and disease activity in SLE patients, the mechanisms of action of these drugs were different (8). However, the clinical efficacies of MMF and cyclophosphamide appear to be equivalent (9, 10). Furthermore, MMF has been reported to exhibit fewer and less-severe adverse effects than cyclophosphamide (9).
Interestingly, the effectiveness of MMF may exhibit race-specific differences (10). Although MMF and cyclophosphamide play important roles in the treatment of LN, steroid therapy has long been considered the key aspect of LN treatment (3). However, long-term steroid therapy can induce several complications, such as an increased susceptibility to infection as well as an increased risk of osteoporosis and cardiovascular diseases (11, 12). It was recently shown that the pathological activation of the mineralocorticoid receptor promotes cardiovascular remodeling and inflammation (13). Therefore, minimizing steroid use is desirable (14).
Condon et al. previously described an oral steroid-free regimen for LN. In this regimen, patients with LN took MMF daily and 2 doses of rituximab (1 g) and methylprednisolone (0.5 g) on days 1 and 15. This treatment regimen resulted in 86% complete biochemical remission or partial remission by 54 weeks (15). With regard to completely steroid-free therapy for LN, Tanaka et al. reported a case of LN treated with tacrolimus monotherapy (16). As already mentioned, the paradigm of LN treatment is shifting, and such treatments may become steroid-free in the near future. Until then, MMF may be a key drug for the treatment of LN.
However, the safety and long-term effectiveness of MMF monotherapy for LN have yet to be established. In the present case, we may have to consider the role of race (Asian/Japanese) and the history of splenectomy in the outcome. In addition, we should also consider the possibility that the reduction in uric protein excretion in the early phase of treatment might reflect not only the action of MMF but also the improvement of heart failure (17). However, our experience with this case suggests that MMF monotherapy may serve as a feasible treatment option in LN patients who cannot tolerate steroid therapy.
Further studies are warranted regarding the indications and dosing requirements of MMF monotherapy as a type of steroid-free therapy, as well as the effectiveness of steroid-free therapy for LN.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Chen Y, Sun J, Zou K, Yang Y, Liu G. Treatment for lupus nephritis: an overview of systematic reviews and meta-analyses. Rheumatol Int 37: 1089-1099, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Weening JJ, D'Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241-250, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Hahn BH, McMahon MA, Wilkinson A, et al. American college of rheumatology guidelines for screening, case definition, treatment and management of lupus nephritis. Arthritis Care Res (Hoboken) 64: 797-808, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dörner T, Lipsky PE. Beyond pan-B-cell-directed therapy - new avenues and insights into the pathogenesis of SLE. Nat Rev Rheumatol 12: 645-657, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Hall AG, Tilby MJ. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev 6: 163-173, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 128: 2453-2457, 1982. [PubMed] [Google Scholar]
- 7.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47: 85-118, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Fassbinder T, Saunders U, Mickholz E, et al. Differential effects of cyclophosphamide and mycophenolate mofetil on cellular and serological parameters in patients with systemic lupus erythematosus. Arthritis Res Ther 17: 92, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group.. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 16: 1076-1084, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 20: 1103-1112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96: 23-43, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 141: 764-770, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Shen JZ, Young MJ. Corticosteroids, heart failure, and hypertension: a role for immune cells? Endocrinology 153: 5692-5700, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-Irastorza G, Danza A, Perales I, et al. Prednisone in lupus nephritis: how much is enough? Autoimmun Rev 13: 206-214, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 72: 1280-1286, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Tsuruga K, Watanabe S, Aizawa-Yashiro T, Chiba-Fukada N, Ito E. Tacrolimus monotherapy in a patient with lupus flare using once-daily administration protocol. NDT Plus 4: 363-365, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albright R, Brensilver J, Cortell S. Proteinuria in congestive heart failure. Am J Nephrol 3: 272-275, 1983. [DOI] [PubMed] [Google Scholar]