Abstract
Developmental dyslexia is one of the most prevalent learning disabilities, thought to be associated with dysfunction in the neural systems underlying typical reading acquisition. Neuroimaging research has shown that readers with dyslexia exhibit regional hypoactivation in left hemisphere reading nodes, relative to control counterparts. This evidence, however, comes from studies that have focused only on isolated aspects of reading. The present study aims to characterize left hemisphere regional hypoactivation in readers with dyslexia for the main processes involved in successful reading: phonological, orthographic and semantic. Forty-one participants performed a demanding reading task during MRI scanning. Results showed that readers with dyslexia exhibited hypoactivation associated with phonological processing in parietal regions; with orthographic processing in parietal regions, Broca's area, ventral occipitotemporal cortex and thalamus; and with semantic processing in angular gyrus and hippocampus. Stronger functional connectivity was observed for readers with dyslexia than for control readers 1) between the thalamus and the inferior parietal cortex/ventral occipitotemporal cortex during pseudoword reading; and, 2) between the hippocampus and the pars opercularis during word reading. These findings constitute the strongest evidence to date for the interplay between regional hypoactivation and functional connectivity in the main processes supporting reading in dyslexia.
Keywords: Dyslexia, Reading, Hypoactivation, Functional connectivity, Thalamus, Hippocampus
Highlights
-
•
Regional hypoactivation in dyslexic readers specifically associated with phonological, orthographic and semantic processes
-
•
Stronger functional connectivity for dyslexic versus control readers among regional hypoactivated nodes
-
•
Increased subcortico-cortical connectivity among regions showing regional reading deficits
1. Introduction
Over the last two decades there has been a substantial increase in the efforts devoted to understanding the neural bases of developmental dyslexia, which is defined as a disability in reading despite normal intelligence, adequate education and lack of obvious sensory or neurological damage (American Psychiatric Association, 2013; World Health Organization, 2008), and which is the most prevalent reading disability in the population (i.e., 3–7% depending on definitional criteria and language orthography; e.g., Barbiero et al., 2012; Peterson and Pennington, 2012). These efforts have led to a small number of systematic findings and also to a considerable accumulation of mixed results and theoretical views in regard to the causes of this developmental disorder.
Proficient reading involves mapping visual features, such as letters (orthography) to sound (input phonology) and to meaning (semantics) as well as, for reading aloud, to articulatory codes (output phonology) that generate the corresponding speech sounds (phonetics). These reading operations are typically accomplished by means of two processing streams that run through left hemisphere regions: a lexical ventral stream, including the ventral occipitotemporal (vOT) and anterior inferior frontal gyrus (IFG) regions, that supports mapping of orthographic-lexical stimuli to whole words (i.e., the direct route); and a non-lexical dorsal stream, encompassing the parietal lobe, superior temporal gyrus (STG) and posterior IFG, that is thought to subserve orthographic-to-phonological conversion (i.e., the indirect route; Oliver et al., 2017; Pugh et al., 2001; Sandak et al., 2004; Schlaggar and McCandliss, 2007). Neuroimaging research using reading tasks has consistently shown regional hypoactivation for readers with dyslexia as opposed to control readers in all of these left hemisphere ventral and dorsal areas, including IFG, temporal and parietal regions, and vOT (see Maisog et al., 2008, Paulesu et al., 2014; Richlan et al., 2009, Richlan et al., 2011, for metanalytical reviews). Similarly, functional and structural MRI studies have revealed hypoactivation and structural differences in thalamic nuclei, including the lateral geniculate nucleus (LGN), medial geniculate nucleus (MGN), pulvinar and posterior thalamic nuclei in readers with dyslexia and samples of atypically-developing readers/talkers relative to their control counterparts (Díaz et al., 2012; Giraldo-Chica et al., 2015; Jednorog et al., 2015; Maisog et al., 2008; Preston et al., 2010; Pugh et al., 2008, Pugh et al., 2013). Although some studies have also reported regional hyperactivation for readers with dyslexia relative to control readers, especially in the left IFG, such hyperactivation may reflect processes related to the level of current reading ability independent of dyslexia, while hypoactivation instead reflects functional atypicalities related to dyslexia itself independent of current reading ability (Hoeft et al., 2007).
Recent research has also underscored that individuals with dyslexia, as opposed to control readers, exhibit difficulties in performing sequential procedural tasks and learning from feedback (e.g., Gabay et al., 2015; Pavlidou et al., 2010), skills which rely on hippocampal function and its interactions with other cortical and subcortical regions (e.g., Opitz and Friederici, 2003; Plante et al., 2014). Deficits in these implicit learning processes are likely to impact aspects of language that require learning complex probabilistic and sequential rules (Krishnan et al., 2016). This evidence, together with recent neuroimaging findings showing hippocampal involvement in reading processes (Binder et al., 2009; Duff and Brown-Schmidt, 2012; Piai et al., 2016), points to the importance of examining hippocampal differences in activation and connectivity between readers with dyslexia and controls.
Despite the extensive neuroimaging evidence showing regional hypoactivation for readers with dyslexia versus control readers, most of the previous functional MRI (fMRI) and PET studies have focused on one specific reading aspect, especially in phonologically-related reading processing deficits. However, much less is known about basic orthographic and semantic deficits in dyslexia and, importantly, no studies so far have specifically examined regional hypoactivation in left hemisphere regions for phonological, orthographic and semantic aspects of reading within the same sample of both readers with and without dyslexia, and to what extent these reduced activations are associated with between-group differences in task functional connectivity. This is critical for a unified understanding of differential regional activations between readers with and without dyslexia in the main reading systems and to further inform accounts that highlight differences in connectivity between these groups of readers (Boets et al., 2013; Boets, 2014; Klingberg et al., 2000; Paulesu et al., 1996; Silani et al., 2005; Vandermosten et al., 2012). Examining phonological, orthographic and semantic constituents of reading within the same study for readers with and without dyslexia is also relevant for theoretical accounts that consider these constituents represent the most important levels of lexical processing (i.e., the lexical quality hypothesis; Perfetti and Hart, 2001, Perfetti and Hart, 2002) and those that, based on cross-linguistic evidence, emphasize that understanding visual word recognition necessarily entails simultaneously considering covariations between orthographic, phonological, and semantic sublinguistic units, as well as morphological aspects (Frost, 2012).
Here, we used functional fMRI to investigate neural modulations at regional and connectivity levels of left hemisphere reading-related regions in individuals with dyslexia and matched controls by means of a reading aloud (naming), a task widely used to measure reading abilities that posed strong cognitive demands, together with an experimental design that allowed us to examine reading-related processes specifically associated with phonological, orthographic and semantic processing. Several authors have emphasized that the deficits showed by readers with dyslexia are apparent only when tasks impose strong cognitive demands, such as fast responses (e.g., Ramus and Szenkovits, 2008). Thus, using a naming task allowed us to tax the performance of readers with dyslexia, as well as guarantee that participants were actually doing (reading) the task, and subsequently classify fMRI trials as correct and incorrect.
To examine reading-related processes specifically associated with phonological, orthographic and semantic processing, our fMRI experimental design included four different types of stimuli: 1) consistent words; 2) inconsistent words or words with specific pronunciation rules; 3) pseudowords; and, 4) pseudohomophones derived from misspells in real words. Based on previous neuroimaging reading research with normal readers, contrasts between these conditions allow examination of phonological effects (i.e., Pseudowords > Consistent Words; e.g., Carreiras et al., 2007; Fiebach et al., 2002), orthographic effects (i.e., Pseudohomophones > Inconsistent Words; e.g., Woollams et al., 2011; van der Mark et al., 2011), and pseudohomophone or semantic effects (i.e., Pseudohomophones > Pseudowords; e.g., Braun et al., 2015).
More specifically, first, phonological effects can be observed based on the functional contrast Pseudowords > Consistent Words because pseudowords rely more on non-semantic orthography-to-phonology mapping, while consistent words benefit more from semantic processing. Previous research using this functional contrast to examine phonological effects has consistently revealed the involvement of regions along the nonlexical stream, including left parietal cortex, STG and pars opercularis (e.g., Carreiras et al., 2007; Fiebach et al., 2002; Fiez et al., 1999; Hagoort et al., 1999; Mechelli et al., 2003, Mechelli et al., 2005). Second, another major deficit in individuals with dyslexia is the impaired automaticity of visual word processing, which prevents skilled, fluent (automatic) reading (e.g., van der Mark et al., 2009). Orthographic effects can be examined using the functional contrast Pseudohomophones > Inconsistent Words, which capitalizes on detecting an orthographic misspell, since the pseudohomophones are pseudowords derived from a one-letter error in actual words with specific pronunciation rules (e.g., ajencia) and inconsistent words are correctly written words that are subject to the same specific pronunciation rules (e.g., agencia). Although orthographic aspects of reading have been less explored in dyslexia, neuroimaging studies using visuo-orthographic letter strings and word processing tasks with minimal phonological involvement have revealed underactivations in occipital cortex in readers with dyslexia versus controls (e.g., Boros et al., 2016; Brunswick et al., 1999), vOT (e.g., Cao et al., 2006; Paulesu et al., 2001; Shaywitz et al., 2003, Shaywitz et al., 2007; van der Mark et al., 2009; Wimmer et al., 2010) and parietal cortex (e.g., Lobier et al., 2014; Peyrin et al., 2012; Reilhac et al., 2013). Finally, pseudohomophone or semantic effects have been examined in typically developing readers as a marker of reading development (e.g., Goswami et al., 2001). This effect has typically been examined using the contrast Pseudohomophones > Pseudowords, which capitalizes on the fact that a non-word that shares phonology but not orthography with a word, (e.g., in Spanish, the pseudohomophone ajencia and the real word agencia), leads to differential processing compared to that seen for other non-words (e.g., pseudoword: alencia). These effects are typically explained by the fact that a given pseudohomophone triggers the lexical representation of its phonologically identical baseword in the mental lexicon, which in turn activates the semantic information associated with that representation (e.g., Braun et al., 2015). Previous neuroimaging research on the pseudohomophone effect in normal readers has shown the engagement of regions involved in lexico-semantic processing, including the angular gyrus (AG), middle temporal gyrus (MTG), and anterior IFG regions (e.g., Binder et al., 2009; Bitan et al., 2005; Gold et al., 2005; Lau et al., 2008).
Based on previous evidence (e.g., Maisog et al., 2008; Richlan et al., 2009, Richlan et al., 2011), in the present study we expected to observe regional hypoactivation in readers with dyslexia as opposed to control readers in left parietal cortex, STG and pars opercularis for the phonological effects; left vOT and parietal cortex for orthographic effects; and in AG, MTG and anterior IFG for the semantic (pseudohomophone) effect.
Importantly, we will also examine between-group differences in task-related functional connectivity for each of the four experimental conditions (i.e., Consistent Words, Inconsistent Words, Pseudowords, Pseudohomophones) among the left hemisphere regions showing hypoactivation for readers with dyslexia versus control readers. Since functional connectivity is thought to reflect the prior history of coactivations, in line with a Hebbian-like learning rule (Harmelech et al., 2013; Hebb, 1949; Oliver et al., 2017), the present study constitutes an important attempt to further examine if the areas showing regional hypoactivations in dyslexia also show differences in functional connectivity for readers with and without dyslexia in terms of phonological, orthographic and semantic-related processes. If hypoactivation in readers with dyslexia reflects deficits in adequately resolving reading processes at the regional level, it could be possible to predict increases in functional connectivity among these regions in individuals with dyslexia to compensate for regional reading deficits. However, based on previous findings suggesting that dyslexia can be determined to some extent to altered connectivity (e.g., Boets et al., 2013; Boets, 2014; Paulesu et al., 1996, Paulesu et al., 2014), it might be possible that reading deficits in dyslexia are associated with underactivations at the regional level as well as with a reduced coupling among critical nodes within the reading network. Based on the limited number of studies that have examined differential task-based functional connectivity among readers with and without dyslexia, and the mixed findings reported in these studies, it would be reasonable to expect differences in left vOT-parietal cortex (Horwitz et al., 1998; van der Mark et al., 2011) and left vOT-IFG coactivation (Finn et al., 2013; Schurz et al., 2014; van der Mark et al., 2011).
Finally, in line with evidence highlighting the role of the thalamus in reading processes and dyslexia (e.g., Díaz et al., 2012), as well as recent findings suggesting that the deficits observed in dyslexic readers on sequential procedural learning may be associated with hippocampal function (Krishnan et al., 2016) and evidence showing that the hippocampus is involved in semantic reading processes (Binder et al., 2009; Duff and Brown-Schmidt, 2012; Piai et al., 2016), we will explore to what extent these regions show hypoactivation and a differential task-related functional connectivity pattern in readers with dyslexia compared to control readers.
2. Methods
2.1. Participants
The final study sample included 41 participants, 20 readers with dyslexia (Mean Age = 21.71 years; 8 Female) and 21 matched controls (Mean Age = 21.40 years; 9 Female). All participants were right-handed monolinguals with Spanish as their native language (see Table 1). All participants had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. Data from 6 additional participants (4 readers with dyslexia and 2 control readers) were excluded from analysis due to excessive head motion during imaging (see fMRI data analyses section below). Prior to taking part in the experiment, all participants gave written informed consent in compliance with the ethical regulations established by the BCBL Ethics Committee and the guidelines of the Helsinki Declaration.
Table 1.
Participant demographics and behavioral scores by group. Standard deviations in parentheses.
| Dyslexic group (n = 20) | Control group (n = 21) | p values | |
|---|---|---|---|
| Age (years) | 21.71 (12.55) | 21.40 (11.84) | 0.93 |
| Gender (% female) | 40 | 42.8 | 0.42 |
| Fluid Reasoning (ss) | 117.06 (13.93) | 114.33 (16.31) | 0.58 |
| Working Memory Span | 4.17 (1.15) | 4.45 (0.94) | 0.41 |
| Word Reading | |||
| Accuracy (/40) | 36.50 (4.95) | 39.71 (0.56) | 0.005 |
| Time (sec) | 46.55 (18.60) | 25.00 (4.24) | <0.001 |
| Pseudoword Reading | |||
| Accuracy (/40) | 31.94 (5.22) | 38.38 (1.12) | <0.001 |
| Time (sec) | 71.67 (28.95) | 43.42 (7.56) | <0.001 |
| Pseudoword Repetition | |||
| Accuracy (%) | 0.80 (0.09) | 0.90 (0.07) | <0.001 |
| Num phonemic errors | 6.60 (3.52) | 2.89 (2.83) | 0.002 |
| Phonemic Deletion | |||
| Accuracy (%) | 0.81 (0.21) | 0.92 (0.11) | 0.04 |
| Num. deletion errors | 3.56 (4.22) | 1.63 (2.43) | 0.10 |
| Num. misplacing errors | 2.56 (3.22) | 0.58 (1.02) | 0.01 |
ss = standard scores.
The inclusion criteria for selecting individuals with dyslexia were (1) self-reported childhood and/or reading difficulties at the time of testing, (2) intelligence quotient above 80, (3) below-normal reading performance (−1.5 standard deviation below average) on reading time and accuracy and (4) previous formal diagnosis of dyslexia. The exclusion criteria for the selection of all participants were (1) presence of language-related disorders, other than dyslexia, or learning disabilities (Specific Language Impairment, dyscalculia, dyspraxia, ADHD), (2) a long absence from school for personal reasons, and (3) history of vision and/or audition problems.
Readers with dyslexia and control counterparts were matched in individual variables (i.e., age and gender) as well as in high-order cognitive functions, including fluid reasoning abilities and working memory span. Fluid reasoning was measured with the corresponding performance subtests of the Wechsler Intelligence Scales (Wechsler, 2001, Wechsler, 2008). Group differences in reading performance were assessed with the word and pseudoword reading lists of the Evaluation of Processes of Reading-Revised (PROLEC-R) battery (Cuetos et al., 2009) using accuracy and total time for list reading. Also, group differences in phonological processing skills were evaluated with pseudoword repetition (i.e., phonological short-term memory), phonemic deletion (i.e., phonological awareness) and phonological fluency tasks.
In the pseudoword repetition task, participants listened to 24 pseudowords, one at a time, and were instructed to repeat them as accurately as possible. Items varied from 2 to 4 syllables (eight of 2, 3 and 4 syllables) and their structure followed Spanish phonotactic rules. They did not include the repetition of any phoneme within each pseudoword. The number of correctly-repeated pseudowords was recorded and converted into percentage. Phonemic errors were analyzed and assigned into the following categories: phonemic addition (/taØforbegun/→/tasforbegun/), phonemic substitution (/talsomen/ → /kalsomen/), phonemic permutation (/musbolife/ → /muslobife/) and phonemic omission (/taforbegun/ → /taforbeguØ/).
In the phonemic deletion task, participants listened to pseudowords, one at a time, and were instructed to remove the first sound of the pseudoword and produce what remained. Twenty-four items 2 syllables long, respecting Spanish phonotactic rules were presented. Half of the items started with a consonantal cluster (e.g., /tr/) and the remaining half with a simple consonant-vowel syllable (e.g., /pa/). The number of correct answers was measured and converted into percentage. Errors were classified into the following categories: phoneme deletions errors (e.g., /pladi/ → /adi/) and phonemic errors occurring outside of the deletion site (e.g., /pladi/ → /lati/).
2.2. Materials and experimental procedure
At the scanner, participants performed a single-word naming task with four different experimental conditions of interest: a) consistent words (e.g., portería); b) inconsistent words or words with specific pronunciation rules (e.g., ingeniero); c) pseudowords (e.g., cinguda) and d) pseudohomophones (e.g., ajencia), which are phonologically correct but orthographically misspelled, in contrast to the correct spelling: agencia. All pseudowords and pseudohomophones were created as a function of the selected real words. For the pseudowords, a single letter from the original word was changed. In contrast, for pseudohomophones, only the phoneme b was changed to v, and the phoneme j to g. In Spanish, the phonemes b-v have the same pronunciation. The same applies to the phonemes g-j when followed by vowels e and i. There were a total of 240 stimuli (60 items per condition). Stimuli were visually presented at the center of the screen. All stimuli were matched on frequency, number of orthographic neighbors and length (i.e., 5 to 9 letters; 2 to 5 syllables).
2.3. fMRI data acquisition
Whole-brain fMRI data acquisition was conducted on a 3-T Siemens TRIO whole-body MRI scanner (Siemens Medical Solutions, Erlangen, Germany) at the Basque Center on Cognition, Brain and Language (BCBL), using a 32-channel head coil. Snugly-fitting headphones (MR Confon, Magdeburg, Germany) were used to dampen background scanner noise and to enable communication with experimenters while in the scanner. Participants viewed stimuli back-projected onto a screen with a mirror mounted on the head coil. To limit head movement, the area between participants' heads and the coil was padded with foam and participants were asked to remain as still as possible.
Functional images were acquired in two separated runs using a gradient-echo echo-planar pulse sequence with the following acquisition parameters: TR = 2000 ms, TE = 25 ms, 35 contiguous 3 cubic mm axial slices, no inter-slice gap, flip angle = 90°, Field of view = 192 mm, 64 × 64 matrix, 230 volumes per run. Prior to each scan, four volumes were discarded to allow T1-equilibration effects. High-resolution T1-weighted MPRAGE sequence was also acquired with the following acquisition parameters: TR = 2300 ms, TE = 2.97 ms, flip angle = 9°, Field of view = 256 mm, 176 volumes per run, voxel size = 1 cubic mm.
The order of the experimental conditions (consistent words, inconsistent words, pseudowords and pseudohomophones) and the inter-trial intervals (jitter fixation) within each functional run were determined with an algorithm designed to maximize the efficiency of the estimation of the blood oxygen level-dependent (BOLD) response (Optseq II; Dale, 1999). Participants' in-scanner responses were recorded with a 40 dB noise-reducing microphone system (FOMRI-II, Optoacoustics Ltd.) allowing for an on-line naming synchronization. A dual adaptive filter system subtracted the reference input (MRI noise) from the source input (naming) and filtered the production instantly while recording the output. The optic fiber microphone was mounted on the head coil and wired to the sound filter box, of which the output port was directly wired to the audio in-line plug of the computer sound card. The audio files were saved and analyzed to obtain participants' in-scanner accuracy via transcription and reaction times using Chronset (Roux et al., 2017).
2.4. fMRI data analysis
Standard SPM8 (Wellcome Department of Cognitive Neurology, London) preprocessing routines and analysis methods were employed. Images were corrected for differences in timing of slice acquisition and were realigned to the first volume by means of rigid-body motion transformation. Motion parameters were extracted from this process and were used, after a partial spatial smoothing of 4-mm full width at half-maximum (FWHM) isotropic Gaussian kernel, to inform additional motion correction algorithms implemented by the Artifact Repair toolbox (ArtRepair; Stanford Psychiatric Neuroimaging Laboratory) intended to repair outlier volumes with sudden scan-to-scan motion exceeding 1 mm and volumes whose global intensity was >1 SD away from the mean, and that corrects these outlier volumes via linear interpolation between the nearest non-outliers time points (Mazaika et al., 2009). Participants with >20% to-be-corrected outlier volumes across or within each of the functional runs were excluded. Before applying this additional motion correction procedure we also excluded participants who showed a drift over 3-mm/degrees in any of the translation (x, y, z) and rotation (yaw, pitch, roll) directions. As a result of applying these motion correction criteria, we excluded a total of 6 participants from further data analyses. Importantly, the amount of corrected outlier volumes did not differ statistically between readers with dyslexia (Mean = 13.56; SD = 25.31) and controls (Mean = 8.67; SD = 16.40) groups (p = .49).
After volume repair, structural and functional volumes were co-registered and spatially normalized to T1 and echo-planar imaging templates, respectively. The normalization algorithm used a 12-parameter affine transformation, together with a non-linear transformation involving cosine basis functions. During normalization, the volumes were sampled to 3-mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997), an approximation of Talairach space (Talairach and Tournoux, 1988). Functional volumes were then spatially smoothed with a 7-mm FWHM isotropic Gaussian kernel. Finally, time series were temporally filtered to eliminate contamination from slow frequency drift (high-pass filter with cut-off period: 128 s).
Statistical analyses were performed on individual participant data using the general linear model (GLM). fMRI time series data were modeled by a series of impulses convolved with a canonical hemodynamic response function (HRF). The motion parameters for translation (i.e., x, y, z) and rotation (i.e., yaw, pitch, roll) were included as covariates of noninterest in the GLM. Each trial was modeled as an event, time-locked to the onset of the presentation of each character string. The resulting functions were used as covariates in a GLM, along with a basic set of cosine functions that high-pass filtered the data. Least-squares parameter estimates of the effect for each study condition were used in pairwise contrasts. Contrast images, computed on a participant-by-participant basis, were submitted to group analyses.
At the group level, whole-brain contrasts between conditions were computed by performing one-sample t-tests on these images, treating participants as a random effect. We performed a general All Correct > Null (fixation baseline) contrast to identify brain regions involved in our fMRI experimental design across conditions and groups for all the in-scanner correct responses. This contrast was thresholded at a voxel-wise corrected false discovery rate (FDR) of q < 0.001 and was subsequently used to identify regions that were non-biased by the experimental manipulations in the region-of-interest (ROI) analyses described below. We also performed specific whole-brain contrast across all participants for the main comparisons of interest: phonological effect (i.e., Pseudowords – Consistent words), orthographic effect (i.e., Pseudohomophone – Inconsistent words), and the semantic pseudohomophone effect (i.e., Pseudohomophone – Pseudowords). These specific contrasts were voxel-wise thresholded at a FDR q < 0.05 (see Supplementary Materials and Supplementary Fig. 1). Brain coordinates throughout the text, as well as in tables and figures, are reported in MNI atlas space (Cocosco et al., 1997).
ROI analyses were performed with the MARSBAR toolbox for use with SPM8 (Brett et al., 2002). To gain further sensitivity to examine the effects of interest in our sample and to specifically control that the voxels going into these analyses were activated for correct responses in our fMRI experimental design, we functionally selected left-lateralized regions of a priori interest based on previous neuroimaging evidence and meta-analytical reviews (Jednorog et al., 2015; Paulesu et al., 2014; Richlan et al., 2009, Richlan et al., 2011), including areas within the parietal cortex: AG (BA 39; center of mass = −44 -71 35; volume = 664 mm3), inferior parietal cortex (IPC; BA 40; center of mass = −37 -49 48; volume = 4112 mm3), and superior parietal cortex (SPC; BA 7; center of mass = −26 -65 51; volume = 3984 mm3); areas within the temporal cortex: vOT (BA 37; center of mass = −34 -69 -15; volume = 3184 mm3), posterior MTG (BA 21; center of mass = −55 -38 2; volume = 8128 mm3); prefrontal cortex (PFC): pars opercularis (BA 44; center of mass = −50 10 17; volume = 5240 mm3), pars triangularis (BA 45; center of mass = −47 25 12; volume = 5536 mm3), and pars orbitalis (BA 47; center of mass = −45 26–7; volume = 2248 mm3); and the left thalamus (center of mass = −11 -20 7; volume = 7504 mm3). We also include the left hippocampus (center of mass = −26 -26 -7; volume = 2208 mm3) due to recent evidence suggesting its role in normal reading (e.g., Piai et al., 2016) as well as deficits in sequential procedural learning observed in dyslexic readers (e.g., Krishnan et al., 2016). All these regions consisted of functional active voxels identified from the whole-brain contrast All Correct > Null across all participants (q < 0.001 false discovery rate (FDR) voxel-wise corrected threshold) and anatomically masked to the areas of interest.
Functional connectivity analyses were conducted via the beta correlation method (Rissman et al., 2004), implemented in SPM8 with custom Matlab scripts. The canonical HRF in SPM was fit to each trial from each experimental condition and the resulting parameter estimates (i.e., beta values) were sorted according to the study conditions to produce a condition-specific beta series for each voxel. Two different functional connectivity analyses were performed: 1) pairwise connectivity between each pair of the left-lateralized ROIs that showed hypoactivation in readers with dyslexia relative to control readers (i.e., IPC, SPC, pars triangularis, pars opercularis, vOT, thalamus, hippocampus and AG); and 2) whole-brain functional connectivity using left thalamus and hippocampus as seed regions.
Pairwise functional connectivity analyses were used to examine 1) the overall coupling strength across study conditions, separately for controls and readers with dyslexia, between each pair of the left-lateralized ROIs that showed hypoactivation in readers with dyslexia relative controls (i.e., IPC, SPC, pars triangularis, pars opercularis, vOT, thalamus, hippocampus and AG), and 2) the differential coupling strength in the connectivity among these ROIs for controls versus readers with dyslexia. Importantly, to control that differences in functional connectivity were not determined by differences in the cluster size of the functionally defined ROIs, we repeated the same pairwise functional connectivity analyses using 5-mm radius spheres centered at the highest local maxima for all the ROIs (except for the hippocampal ROI due to the size and shape of this region).
First, to examine significant functional coactivations between these nodes of interest for readers with and without dyslexia, we calculated beta-series correlation values for each pair of ROIs. To do so, an arc-hyperbolic tangent transform (Fisher, 1921) was applied at the subject level to the beta-series correlation values (r values) of each pair of ROIs and each study condition. These values were then averaged across conditions for participants in the control and dyslexic groups. Since the correlation coefficient is inherently restricted to range from −1 to +1, this transformation served to make its null hypothesis sampling distribution approach that of the normal distribution. To test for significant differences in the coupling strength of the pairwise connectivity between controls and readers with dyslexia for each condition in our experimental fMRI design, Fisher's Z normally distributed values for each pair of ROIs for each participant and condition were submitted to two-sample t-tests, which were thesholded for significance using a FDR q < 0.05 to correct for multiple comparisons.
Second, to confirm and further examine results obtained with pairwise functional connectivity analyses, we also performed two separate whole-brain functional connectivity analyses using the left thalamus and hippocampus as seed regions. In these analyses, the beta series associated with these seeds were correlated with voxels across the entire left hemisphere of the brain to produce beta correlation images for each subject. Group-level two sample t-tests were performed on the resulting subject All Correct > Null contrast images for each of the selected seeds (i.e., left hippocampus and left thalamus) for the comparisons Dyslexic > Control groups and Control > Dyslexic groups (q < 0.05, FDR corrected). All these contrasts were also subjected to an arc-hyperbolic tangent transform to allow for statistical inference based on the correlation magnitudes. We selected the All Correct-Null contrast for whole-brain functional connectivity analyses to gain further sensitivity due to the higher number of observations going into this contrast.
3. Results
3.1. In-scanner behavioral results
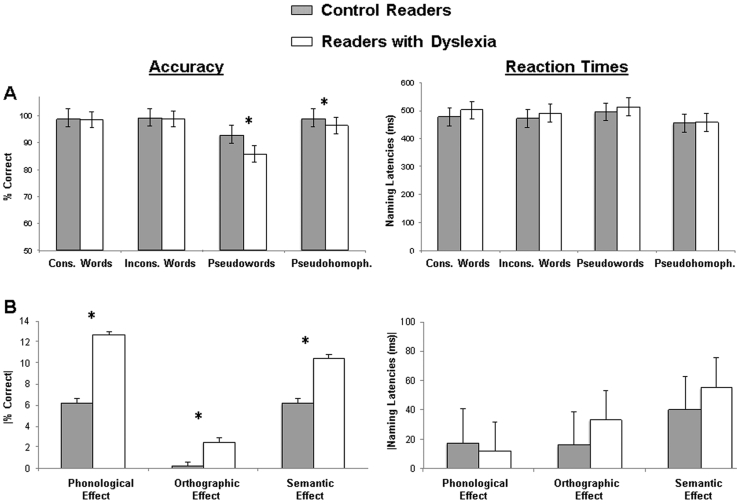
To examine participants' in-scanner performance on naming accuracy and naming latencies we carried out two separate 2 (Group: control, dyslexic) X 4 (Condition: consistent words, inconsistent words, pseudowords, pseudohomophones) mixed-model analyses of variance (ANOVAs), with Condition as the only within-subjects factor, with percent of correct responses and reaction times as dependent measures. Analysis for the percent of correct responses revealed that the main effects of Group, (F(1, 31) = 26.80; p < .001, ηp2 = 0.46), and Condition, (F(3, 93) = 46.19; p < .001, ηp2 = 0.60), were subsumed by a Group X Condition interaction, (F(3, 93) = 14.23; p < .001, ηp2 = 0.31). Simple-effects analyses revealed that control readers exhibited a higher percent of correct responses for pseudowords and pseudohomophones relative to readers with dyslexia (ps < 0.001; see Fig. 1A). Moreover, whereas both controls and readers with dyslexia showed a higher percent of correct responses to all the conditions relative to pseudowords (ps < 0.001), readers with dyslexia also exhibited a higher percent of correct responses to consistent and inconsistent words relative to pseudohomophones (ps < 0.01). Analyses for naming latencies did not reveal Group main or interactive effects (Fs < 1; p ≥ .01, ηp2 ≤ 0.02).
Fig. 1.
In-scanner behavioral results. (A) Percent correct naming responses and naming latencies as a function of Group (control, dyslexic) and Condition (consistent words, inconsistent words, pseudowords, pseudohomophones). (B) Differential percent correct naming responses and differential naming latencies in absolute values as a function of group and contrasts of interest: Phonological effect (i.e., Pseudowords-Consistent Words), Orthographic effect (i.e., Pseudohomophone-Inconsistent Words), and Semantic (Pseudohomophone) effect (i.e., Pseudohomophone-Pseudowords). Error bars show the standard error with a 0.95 confidence interval. Asterisks denote statistically significant (p < .05) group effects within single conditions/contrasts of interest. Cons. = Consistent; Incons. = Inconsistent; Pseudohomop. = Pseudohomophones.
Given the specific interest in examining fMRI contrasts focused on reading-related phonological, orthography and semantic effects, we conducted separate one-way ANOVAs with the factor Group and the absolute values of the subtraction between conditions for the Phonological Effect (i.e., |Pseudoword – Consistent Words|), Orthographic Effect (i.e., |Pseudohomophone – Inconsistent Words|), and the Semantic (Pseudohomophone) Effect (i.e., |Pseudohomophone – Pseudowords|) with naming accuracy and naming latencies as dependent measures. These analyses revealed statistically significant effects of Group in naming accuracy across all the reading-related effects of interest (Fs(1, 36) ≥ 11.35; ps < 0.01, ηp2 ≥ 0.26), with readers with dyslexia exhibiting stronger differences between the conditions in these comparisons relative to control readers (see Fig. 1B). No Group effects emerged in the naming latencies analyses (Fs < 1; ps ≥ 0.34, ηp2 ≤ 0.03).
3.2. fMRI results
3.2.1. Whole-brain contrasts
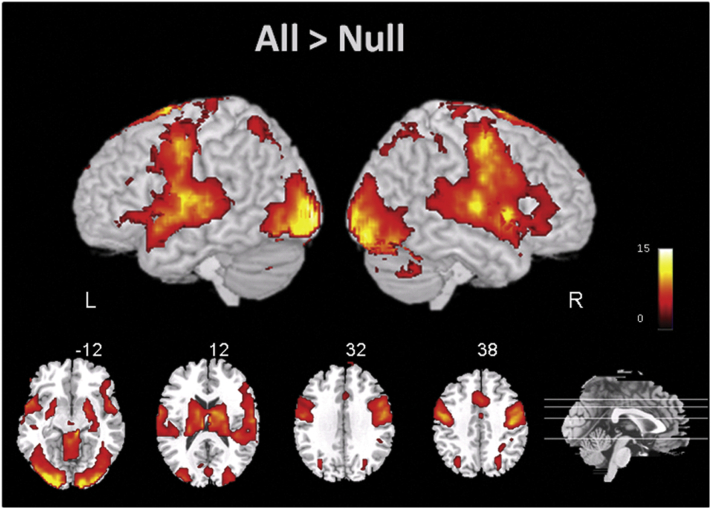
Regions involved across conditions and participants. To identify brain regions associated with reading processes across all participants and factors in the experimental fMRI design, we computed a whole-brain contrast for All Correct trials > Null or fixation baseline (Fig. 2). Importantly, to obtain common voxels contributing to reading processes in both control and readers with dyslexia and not bias subsequent fMRI analyses based on the activation in one of the groups, this whole-brain analysis was conducted across all participants. Consistent with prior neuroimaging evidence (e.g., Lau et al., 2008), this contrast revealed the involvement of left hemisphere regions typically involved in reading-related processes including pars triangularis (BA 45), pars opercularis (BA 44) and pars orbitalis (BA 47) in the PFC; MTG (BA 21), superior temporal gyrus (STG; BA 22), and fusiform gyrus/vOT (BA 37), in the temporal lobe; IPC (BA 40) and SPC (BA 7) in the parietal lobe. This contrast also showed homologues in right hemisphere regions, as typically shown in previous naming neuroimaging studies (Binder et al., 2005; Carreiras et al., 2007; Mechelli et al., 2007), as well as left hippocampal and bilateral thalamic activation.
Fig. 2.
Brain rendering and axial slice sections showing activations for the All Correct > Null whole-brain contrast across all subjects at a statistical threshold of q < 0.001 FDR voxel-wise corrected.
3.2.2. ROI analyses
We conducted ROI analyses to characterize Group effects in the activation profile of regions of a priori interest based on previous studies and meta-analysis in naming- and reading-related processes in dyslexia, including IFG, lateral temporal cortex, parietal lobe, thalamus and vOT. As indicated, to avoid potential biases in the patterns of activation observed in these ROI analyses and to make sure that these regional analyses included relevant voxels involved in processes engaged by readers with and without dyslexia, these regions were selected across all subjects from the general whole-brain All > Null contrast. This contrast yielded activations in most of the left-lateralized key regions involved in reading processes: pars triangularis, opercularis, orbitalis, MTG, STG, IPC, SPC, thalamus and vOT (Fig. 2). Also, given that we observed left hippocampal activation in this general contrast (i.e., All Correct > Null) and in specific contrasts of interest related to semantic processing (i.e., pseudohomophone effect: Pseudohomophones > Consistent Words), and that there is evidence suggesting that the hippocampus is at the base of the sequential procedural learning deficits exhibited by dyslexic readers (e.g., Krishnan et al., 2016) and it is involvement in reading-related processes (e.g., Binder et al., 2009; Duff and Brown-Schmidt, 2012; Jaimes-Bautista et al., 2015; Piai et al., 2016), we also included in this analysis the left hippocampus region that was activated in the All Correct > Null general contrast.
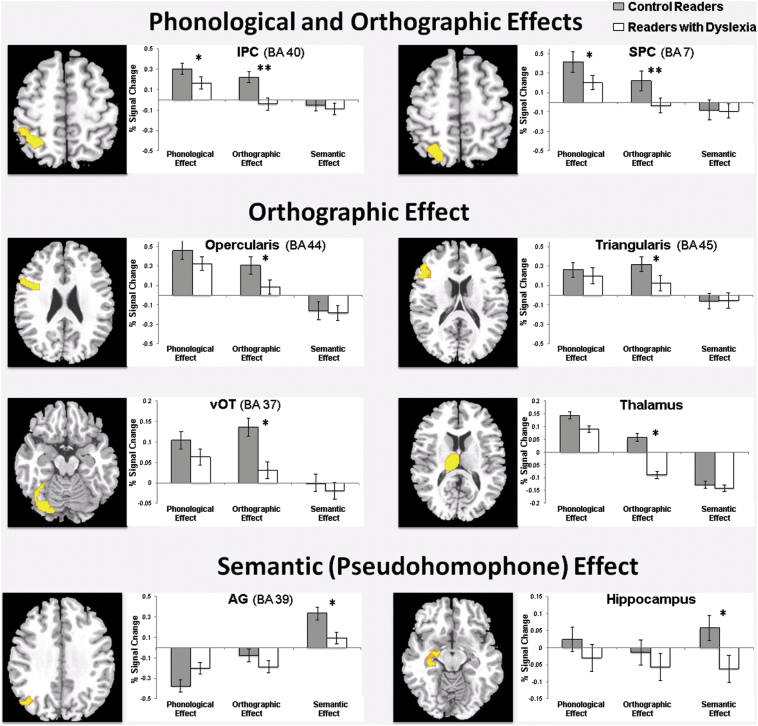
Because of our specific interest in examining Group effects for the phonological, orthographic and semantic (pseudohomophone) effects, we extracted fMRI parameter estimates for these three specific contrasts (i.e., Pseudowords > Consistent Words; Pseudohomophones > Inconsistent Words; Pseudohomophones > Pseudowords) from these ROIs and submitted then to one-way ANOVAs with Group (control, dyslexic) as the between-subjects factor. As shown in Fig. 3 and Table 2, these analyses revealed group differences in common and specific regions: a) phonological effects were found in IPC and SPC; b) orthographic effects were found also in IPC and SPC, but also in pars opercularis and triangularis, as well as vOT and thalamus; and, c) semantically related pseudohomophone effects were found in AG and hippocampus. Importantly, all these regions showed statistically significant hypoactivation for readers with dyslexia relative to control readers across the effects of interest. ROI analyses for posterior MTG and pars orbitalis did not show Group effects across any of these three contrasts of interest. No regions showed hyperactivation for readers with dyslexia versus controls, consistent with findings from previous meta-analytical reviews (e.g., Maisog et al., 2008; Paulesu et al., 2014; Richlan et al., 2009).
Fig. 3.
ROI analyses showing group differences in % signal change as a function of the effects of interest. (A) Regions that showed group differences for both phonological and orthographic effects: IPC and SPC. (B) Regions that revealed group differences only for the orthographic effect: pars opercularis, pars triangularis, vOT, and thalamus. (C) Regions that showed group differences for the semantic (pseudohomophone) effect: AG and hippocampus. Asterisks indicate group comparisons that showed statistically significant effects: *p < .05, **p < .01. Brain coordinates correspond to the MNI coordinates for the center of mass of each ROI. IPC = inferior parietal cortex; SPC = superior parietal cortex; vOT = ventral occipitotemporal cortex; AG = angular gyrus.
Table 2.
Summary of ROIs results (group effects).
| Effects/ROIs | F-values | FDR q-values |
|---|---|---|
| Phonological Effects | ||
| L. IPC (BA 40) | F(1, 39) = 4.56 | 0.043 |
| L. SPC (BA 7) | F(1, 39) = 5.79 | 0.033 |
| Orthographic Effects | ||
| L. IPC (BA 40) | F(1, 40) = 16.22 | 0.001 |
| L. SPC (BA 7) | F(1, 40) = 8.84 | 0.025 |
| L. opercularis (BA 44) | F(1, 40) = 5.84 | 0.033 |
| L. triangularis (BA 45) | F(1, 39) = 5.12 | 0.036 |
| L. vOT (BA 37) | F(1, 38) = 4.12 | 0.050 |
| L. Thalamus | F(1, 40) = 5.60 | 0.033 |
| Semantic Effects | ||
| L. AG (BA 39) | F(1, 40) = 6.33 | 0.033 |
| L. Hippocampus | F(1, 39) = 7.16 | 0.033 |
3.2.3. Functional connectivity analyses
Previous studies and theoretical models have suggested potential ways in which left hemisphere regions interact during naming and reading tasks (e.g., Lau et al., 2008). As previously indicated, although there is extensive evidence showing regional hypoactivation in readers with dyslexia compared to controls (e.g., Lobier et al., 2014; Maisog et al., 2008; McCrory et al., 2005; Richlan et al., 2009; Peyrin et al., 2011) and mixed evidence in terms of differential connectivity between controls and readers with dyslexia (e.g., Boets et al., 2013; Richards and Berninger, 2008; van der Mark et al., 2011), previous research has not examined differences in the functional connectivity of the reading network between readers with dyslexia and controls specifically for nodes showing regional hypoactivation in readers with dyslexia. To this end, and to further understand to what extent regional hypoactivation relates to concomitant differences in functional connectivity between readers with dyslexia and control readers, we used pairwise and whole-brain functional connectivity analyses (Rissman et al., 2004).
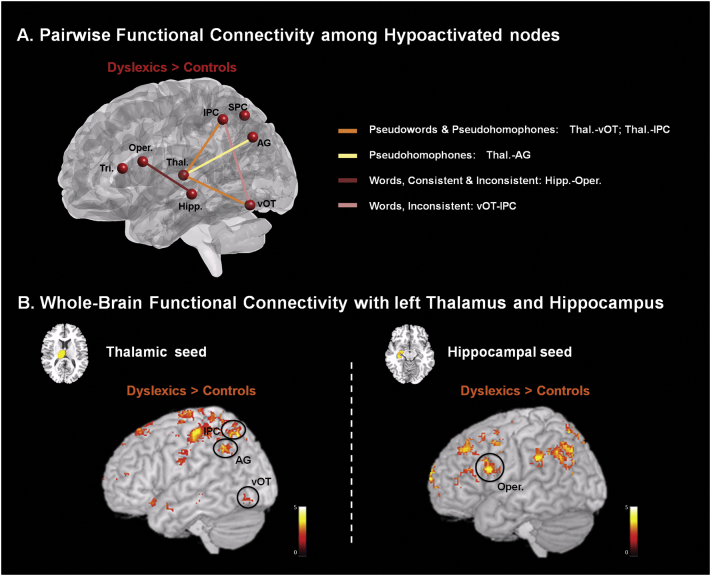
First, analysis examining overall coupling strength across study conditions separately for controls and readers with dyslexia between each pair of the left-lateralized ROIs that showed hypoactivation in readers with dyslexia relative to control readers (i.e., IPC, SPC, pars triangularis, pars opercularis, vOT, thalamus, hippocampus and AG) revealed a strikingly similar pattern of overall significant connections in both groups. Nevertheless, when comparing the differential coupling strength between these groups, results showed that readers with dyslexia exhibit tighter functional connectivity than control readers for several pairs of nodes (Fig. 4A). More specifically, for consistent pseudowords and inconsistent pseudowords (i.e., pseudohomophones) readers with dyslexia exhibited strong thalamic-vOT and thalamic-IPC functional coactivation. Also, only for pseudohomophones, which allow access to the lexical representation of phonologically identical base words in a mental lexicon, activating semantic information associated with that representation, we additionally observed stronger thalamic-AG functional connectivity. Moreover, for consistent and inconsistent words, readers with dyslexia exhibited tighter hippocampal-pars opercularis functional coactivation relative to control readers. Also, in the case of words with specific pronunciation rules (i.e., inconsistent words), readers with dyslexia exhibited stronger vOT-IPC connectivity than control readers. Importantly, none of the pairwise functional connectivity analyses for the different conditions of the present experimental design revealed statistically stronger functional connectivity for controls versus readers with dyslexia. The same pattern of results emerged when 5-mm radius spheres, centered at the region local maxima were used in these pairwise connectivity analyses.
Fig. 4.
Functional connectivity analyses. (A) Left sagittal rendering showing hypoactivated regions in readers with dyslexia and the pairwise connections among them showing differential coupling for Dyslexic > Control groups (q < 0.05 FDR-corrected). (B) Left-hemisphere brain renderings showing whole-brain functional connectivity maps with left thalamus (left panel) and left hippocampus (right panel) for Dyslexic > Control groups two-sample t-tests (q < 0.05 FDR-corrected). Tri. = pars triangularis; Oper. = pars opercularis; Thal. = thalamus; Hipp. = hippocampus; IPC = inferior parietal cortex; SPC = superior parietal cortex; AG = angular gyrus; vOT = ventral occipitotemporal cortex.
Second, to further examine the possibility that our functional connectivity approach of using regions showing hypoactivation for readers with dyslexia relative to controls in the main reading-related contrasts of interest might miss statistically significant functional connectivity differences for controls relative to readers with dyslexia, we conducted two separate whole-brain functional connectivity two-sample t-test analyses seeding the central nodes showing between-group differences in the pairwise connectivity analysis: left thalamus and left hippocampus (Fig. 4B). These analyses examined voxels in the left hemisphere that were tightly coactivated with these seeds across all the main experimental conditions (i.e., All > Null). The analysis using the left thalamic seed revealed stronger coupling for Dyslexic > Control groups with vOT, IPC and AG, confirming our pairwise functional connectivity results; as well as with clusters in the postcentral, SPC, pars orbitalis and insula. Aside from the vOT, IPC and AG, none of the other significantly coactivated clusters with the thalamic seed fully overlap with the ROIs of interest used in our previous pairwise functional connectivity analyses. Also, there were no clusters showing stronger coactivation in the two-sample t-test for Control > Dyslexic groups. Finally, the analysis using the left hippocampal seed revealed tighter coupling with pars opercularis for Dyslexic > Control groups, also confirming the results from the pairwise connectivity analyses; as well as with clusters in IPC, precuneus, postcentral, precentral, MFG and SFG. Aside from the pars opercularis, none of the other clusters significantly coactivated with the hippocampal seed fully overlap with the ROIs of interest used in our previous pairwise functional connectivity analyses. The Control > Dyslexic groups two-sample t-tests for the left hippocampus whole-brain functional connectivity only revealed a statistically significant coactivated cluster in the posterior parahippocampal gyrus.
4. Discussion
The present study aimed at investigating regional hypoactivation and functional coupling among hypoactivated nodes in readers with dyslexia versus control readers, using a demanding reading task and an experimental design that allowed us to examine the between-groups functional dynamics related to phonological, orthographic and semantic reading operations. Reduced regional activation in dyslexia has been extensively documented in studies examining specific reading-related components. Here, within the same sample and study, we sought to examine the relation between the specific operations involved in reading (phonological, orthographic and semantic) and regional hypoactivations in readers with dyslexia across classical reading areas, including also the thalamus and the hippocampus. Furthermore, examined the previously-unexplored question of whether these regional reduced activations are associated with concomitant differences in the functional coupling among these hypoactivated regions, which can shed further light on how the brain with dyslexia deals with difficulties in resolving reading operations at the regional level. Our findings showed that regional hypoactivation in readers with dyslexia relative to control readers were associated with specific phonological, orthographic and semantic reading processes. Importantly, functional connectivity was stronger for readers with dyslexia versus control readers between the thalamus and regions showing hypoactivation for phonological (IPC), orthographic (IPC, vOT) and semantic processing (AG) for pseudoword reading, and between the hippocampus and a region typically involved in accessing representations (pars opercularis) for word reading. These main findings are discussed below.
4.1. Regional hypoactivation in readers with dyslexia specifically associated with phonological, orthographic and semantic reading processes
Despite extensive neuroimaging research, evidence of regional hypoactivations in readers with dyslexia versus control readers has been mixed. Here, we show underactivations in several brain regions specifically associated with phonological, orthographic and semantic processes. The main reasons for the differences in regional hypoactivation found between previous studies can be attributed to 1) limitations in analytical approaches and statistical power and 2) differences in the experimental paradigms employed and selected contrasts. Most of the previous neuroimaging studies published to date have searched for underactivations by conducting whole-brain analysis to identify the activity underlying reading or reading-related skills, and then reporting on between-group differences. Also, many of these studies did not correct for multiple comparisons at the voxel-wise level and/or did not use adequate cluster-level corrections, increasing the risk of Type I errors or false positives (e.g., Bennett et al., 2009; Eklund et al., 2016; Poldrack, 2012). Heterogeneity in the type of experimental reading tasks (e.g., word rhyming, overt naming, covert naming, letter-speech sound integration, sentence processing) and contrasts selected to examine brain activation differences between groups (e.g., Pseudowords > Fixation, Words & Pseudowords > Fixation, Words > False Fonts, Sentence Reading > Fixation) has also been the norm (see for instance Table 1 in Richlan et al.'s, 2011 meta-analytical review for a description of the statistical thresholds, experimental tasks and selected contrasts used).
The relevance of previous neuroimaging reading studies in elucidating the reading deficits in individuals with dyslexia is unquestionable. Nevertheless, the study of the neurobiology of dyslexia can strongly benefit from integrative approaches that can shed further light on our understanding of these deficits in the functioning of the main aspects supporting successful reading. Specifically, the lexical quality hypothesis (Perfetti and Hart, 2001, Perfetti and Hart, 2002) proposes that one important difference between skilled and less-skilled readers involves the degree to which there is efficient integration across phonological, orthographic, and semantic levels of linguistic representations. In this regard, understanding regional deficits in readers with dyslexia for each of these major constituents of lexical processing is a critical initial step towards better understanding to what extent these constituents are compromised and to establishing their neural correlates in readers with dyslexia.
4.1.1. Phonological effect
Our results for the phonological effect revealed underactivation for readers with dyslexia relative to control readers in the IPC and SPC. Previous studies using tasks emphasizing phonological aspects have consistently shown hypoactivations in posterior parietal cortex along the dorsal reading pathway believed to mediate assembled phonology (e.g., Brunswick et al., 1999; Cao et al., 2006; Eden et al., 2004; Hoeft et al., 2006; Meyler et al., 2008; Richlan et al., 2010; Rumsey et al., 1997; van der Mark et al., 2009; Wimmer et al., 2010; but see, for instance, Brambati et al., 2006, McCrory et al., 2005, Paulesu et al., 2001, for between-group null effects in parietal lobe regions). Hypoactivations in left parietal cortex for readers with dyslexia versus control readers were observed for specifically-contrasting pseudowords versus fixation in phonological lexical decision tasks (e.g., Richlan et al., 2010; van der Mark et al., 2009; Wimmer et al., 2010), implicit and explicit reading tasks (e.g., Brunswick et al., 1999), and overt reading in phonological and orthographic tasks (e.g., Rumsey et al., 1997).
Contrary to our hypothesis, results from the present study did not reveal hypoactivation in the left MTG associated with phonological effects in readers with dyslexia relative to control readers. In fact, the left MTG and pars orbitalis were the only ROIs not showing differential group activations for any of the effects of interest. Multiple reasons may determine this null effect. One potential reason for not observing hypoactivations in these regions is the type of task (naming) used in the present study, which was chosen to increase reading demands but it differed from the tasks used in other studies showing hypoactivation effects in these regions for dyslexic readers relative to controls (see Maisog et al., 2008; Richlan et al., 2009, Richlan et al., 2011).
4.1.2. Orthographic effect
In the present study, the left IPC and SPC also showed hypoactivation for readers with dyslexia compared to controls for orthographic effects. The IPC is implicated in the mapping between orthographic and phonological representations and it is sensitive to conflict between orthographic and phonological information (Bitan et al., 2007). Previous studies have shown that the IPC is hypoactivated in readers with dyslexia relative to control readers for orthographic tasks and conflicting stimuli to examine orthography-to-phonology mapping (e.g., Cao et al., 2006; Rumsey et al., 1997). Consistent with these results, in the present study, using a demanding reading task and a contrast (Pseudohomophones > Inconsistent Words) that enhances orthography-to-phonology mapping demands (Pugh et al., 2000; Shaywitz, 1998), we also observed IPC underactivation in readers with dyslexia. Further evidence showing the involvement of the left IPC and SPC in orthographic processes has come from recent studies showing that this region is involved in letter identity and, critically, in letter position coding in normal readers (Carreiras et al., 2015). Specifically with regard to the SPC, this region has consistently been found to be involved in visual attention span deficits in readers with dyslexia (e.g., Lobier et al., 2014; Peyrin et al., 2011), with visual attention span strongly linked to orthographic processing (e.g., Lobier et al., 2012).
In typical readers, the vOT is specialized for print processing and sensitive to the orthographic familiarity of letter strings. Our results showed vOT underactivation for readers with dyslexia versus controls for orthographic effects, which is consistent with previous reports showing hypoactivation in this region in individuals with dyslexia for a variety of word processing tasks (Brunswick et al., 1999; Cao et al., 2006; McCrory et al., 2005; Maurer et al., 2007; Olulade et al., 2015; Paulesu et al., 2001; Rumsey et al., 1997; Salmelin et al., 1996; Shaywitz et al., 2002; van der Mark et al., 2009; Wimmer et al., 2010) and with meta-analytic results from children and adults contrasting readers with dyslexia with normal readers (Richlan et al., 2011). However, although the relation of vOT with dyslexia is a relatively well-documented finding, not all studies examining differences between readers with dyslexia and controls have reported hypoactivity in this region (e.g., Ingvar et al., 2002; Georgiewa et al., 1999, Georgiewa et al., 2002; Hoeft et al., 2006; Meyler et al., 2007; Schulz et al., 2008), which could be due to differences in the experimental paradigms employed and contrasts selected.
With respect to the IFG, this region has traditionally been considered a contributor to phonological assembly and articulatory planning (e.g., Pugh et al., 2001). Studies comparing individuals with dyslexia with proficient-reading counterparts have generally observed hypoactivation in readers with dyslexia in the IFG (Booth et al., 2007; Brambati et al., 2006; Cao et al., 2006; Schulz et al., 2008; but see for instance Georgiewa et al., 2002 for null effects; and Brunswick et al., 1999, Grünling et al., 2004, Shaywitz, 1998 for studies showing hypearctivation in readers with dyslexia relative to control readers). Our results showing hypoactivation in pars opercularis and triangularis in relation to the orthographic effects are in line with recent findings with normal readers highlighting the role of the IFG in orthographic processing. Vinckier et al.'s (2007) study found concomitant gradients of increasing word selectivity along the anterior-posterior y-axis of vOT and along the medial-lateral x-axis of Broca's area, indicating that the duplication of the word-selectivity gradient pattern in these two left hemisphere regions may be a result of the neural connections between them, a suggestion posteriorly confirmed by Olulade et al. (2015). Also, recent views of reading propose a more direct role of the IFG in orthographic processing via grapheme-to-phoneme conversion (Richlan, 2014). Furthermore, the presence of functional connections between the IFG and vOT regions (Richlan, 2012, Richlan, 2014) suggests that some level of top-down modulation of vOT function by the IFG may be occurring. Phonological remapping, as required for articulation, is subserved by the IFG (Owen et al., 2004; Pugh et al., 2001) and likely involves constant access to orthographic representations established within the OTC. Such continuous communication may lead to similar neuronal tuning in both of these left hemisphere regions (Olulade et al., 2015). Our vOT-IFG functional connectivity results did not reveal differences between controls and readers with dyslexia, which suggests that although readers with dyslexia may have deficits in solving orthographic processes at the regional level in vOT and IFG, these deficits do not appear to be modulated by a differential strength in connectivity among them. This null finding is also consistent with studies in dyslexia demonstrating no differences between readers with dyslexia and controls in ventral structural connectivity via the inferior fronto-occipital fasciculus (Vandermosten et al., 2012).
Finally, the left thalamus was also underactivated in readers with dyslexia relative to control readers for orthographic effects in the present study. As indicated, the thalamus is a key structure for language processing and has typically been found associated with dyslexia in functional and structural MRI studies (Díaz et al., 2012; Giraldo-Chica et al., 2015; Jednorog et al., 2015; Maisog et al., 2008), as well as in histological studies showing abnormal cellular organization in first-order thalamic visual and auditory sensory nuclei (LGN, MGN) in the brains of individuals with dyslexia (e.g., Galaburda et al., 1994; Livingstone et al., 1991; see also Stein and Walsh, 1997). As well as in first-order sensory nuclei, neuroimaging research has also evinced hypoactivation in the pulvinar nucleus in readers with dyslexia and in atypically-developing participants (e.g., late talkers), relative to control counterparts (Brunswick et al., 1999; Georgiewa et al., 1999; Maisog et al., 2008; Preston et al., 2010; Pugh et al., 2008, Pugh et al., 2013). The left thalamus ROI functionally identified in the present study included mostly the pulvinar, which is thought to play a coordinating role in cortical-cortical communication, allowing cortical areas to communicate indirectly via non-reciprocal cortical-pulvinar circuitries (Shipp, 2003), and it has been associated with gating of perceptual information and the regulation of visual attention (Posner and Raichle, 1995; Saalmann et al., 2012). The pulvinar appears to mediate selective attention to features that shape orthographic learning with the input from regions sensitive to linguistic forms, and thus when disrupted results in impaired reading abilities (e.g., Crosson, 1999).
4.1.3. Semantic (pseudohomophone) effect
Our results showed that the AG and the hippocampus were underactivated for readers with dyslexia relative to control readers for the pseudohomophone semantic-related effect. On the one hand, the AG has been typically associated to processing word meaning across multiple neuroimaging studies (e.g., Binder and Desai, 2011; Graves et al., 2010), large-scale meta-analyses (Binder et al., 2009) and neuroanatomical models of reading (e.g., Lau et al., 2008). Previous neuroimaging studies on dyslexia have reported AG hypoactivation in readers with dyslexia relative to controls (e.g., Gross-Glenn et al., 1991; Grünling et al., 2004; Shaywitz, 1998). However, meta-analytical reviews have generally failed to find evidence for consistent AG underactivation effects in dyslexia (e.g., Maisog et al., 2008; Paulesu et al., 2014; Richlan et al., 2009, Richlan et al., 2011), which might be due to the fact that most of the studies included in these meta-analytical reviews were not focused on fMRI tasks emphasizing semantic processing. On the other hand, the hippocampus has been largely ignored as a region of interest in reading-related tasks and in studies examining differences among readers with dyslexia and controls (but see Krishnan et al., 2016). However, results from single-word reading and sentence processing MRI studies conducted in our lab (Molinaro et al., 2015; Oliver et al., 2017; Rueckl et al., 2015) and recent findings confirm the relevance of the hippocampus in semantic processes during reading (Binder et al., 2009; Duff and Brown-Schmidt, 2012; Piai et al., 2016; see Jaimes-Bautista et al., 2015 for a neuropsychological review). Additionally, recent evidence has suggested that hippocampal function may be associated with sequential procedural learning deficits observed in readers with dyslexia (Krishnan et al., 2016). Consistently, in the present study, reduced hippocampal activation for readers with dyslexia relative to control readers was observed, in particular for the semantic (pseudohomophone) effect.
4.2. Stronger functional connectivity for readers with dyslexia versus controls among regional hipoactivated nodes
There are a limited number of fMRI studies that have examined activation-based functional connectivity during reading tasks comparing readers with dyslexia and control counterparts. Among them, overall, increased left hemisphere vOT-IFG, vOT-IPC, vOT-AG, visual regions-IPC and STG-IFG functional coupling were typically reported for controls versus readers with dyslexia (Boets et al., 2013; Cao et al., 2008; Horwitz et al., 1998; Olulade et al., 2015; Schurz et al., 2014; van Der Mark et al., 2011).
Compared to controls, readers with dyslexia have typically shown increased functional connectivity between left hemisphere regions and default-mode network regions, and also between left hemisphere regions and right homologous language regions (e.g., Finn et al., 2013; Koyama et al., 2013). Other studies have also reported tighter coupling for readers with dyslexia as opposed to control readers between left IFG and other PFC regions (Finn et al., 2013; Richards and Berninger, 2008). These increases in functional coupling in readers with dyslexia relative to control readers have usually been explained as the result of compensatory strategies intended to overcome reading processing deficits (Finn et al., 2013; Koyama et al., 2013). In this vein, a longitudinal study examining functional connectivity before and after the implementation of an instructional intervention showed that increased functional connectivity for readers with dyslexia relative to controls disappeared after remediation (Richards and Berninger, 2008; see also Koyama et al., 2013, for a cross-sectional study with groups of participants exposed to different types of remediation).
Nevertheless, it is important to note that most of the results with regard to group differences in functional coupling mentioned above have not been consistently replicated and most of these studies examining task-based connectivity did not disentangle these effects as a function of the principal components of the reading system and/or just focused on one specific aspect of reading, using tasks such as rhyming (Cao et al., 2008), naming words and pseudowords (Horwitz et al., 1998), phonological lexical decision (Schurz et al., 2014; van der Mark et al., 2011) and phoneme mapping (Richards and Berninger, 2008; Schurz et al., 2014). Therefore, the different patterns in functional connectivity typically explained as dysfunctional or compensatory have strongly depended so far on the nature of the task used (Paulesu et al., 2014).
In the present study, group differences in functional connectivity were examined for relevant left hemisphere nodes showing specific regional hypoactivation for phonological, orthographic and semantic reading processes. Functional connectivity analyses revealed that individuals with dyslexia exhibited tighter functional coactivation relative to controls between the thalamus and posterior occipito-temporal and parietal cortical regions (vOT, IPC, AG) during pseudoword reading; and between the hippocampus and the IFG pars opercularis during word reading. In line with previous functional and structural evidence with readers with dyslexia highlighting the involvement of the thalamus in general, and of the pulvinar complex in particular, our results underline the critical role of this region by showing hypoactivation for orthographic effects as well as group differences in connectivity with posterior cortical regions. It has been argued that the pulvinar mediates interactions between visual language and attentional regions. When dealing with orthographic features, the connectivity between the pulvinar and ventral visual regions may allow for the selection (or attentional enhancement) of those visual features that will come to shape the functional organization of the ventral visual pathway for orthographic forms (e.g., Serences and Yantis, 2006). However, orthography does not only involve visual recognition but is fundamentally relational and constrained by phonological and possibly semantic knowledge. Therefore, it is reasonable to expect that this thalamic region, which is hypoactivated in readers with dyslexia relative to controls for orthographic effects, is also more strongly coactivated in these individuals with regions that have shown regional hypoactivation for orthographic (IPC, vOT), phonological (IPC), and semantic processing (AG). Clearly, neuroimaging studies focused on examining the role of the thalamus in reading are needed to shed further light on the functional interactions of this region with classical nodes within the reading network.
On the other hand, our data show stronger left hippocampus coupling with left pars opercularis during word reading for readers with dyslexia relative to controls. Recent evidence has highlighted the involvement of the hippocampus in semantic reading processes (Binder et al., 2009; Duff and Brown-Schmidt, 2012; Jaimes-Bautista et al., 2015; Piai et al., 2016). In this vein, a meta-analysis of fMRI activations associated with the term “reading” in a total of 427 studies (Neurosynth.org, Yarkoni et al., 2011) showed significant participation of left hippocampus (see Paz-Alonso et al., 2018). Also, the hypoactivation observed in the present study in the left hippocampus for semantic-related effects is also consistent with this evidence, highlighting its involvement in reading and semantic operations. Tighter functional coupling among left hippocampus and left pars opercularis during word reading for readers with dyslexia relative to controls may reflect individuals with dyslexia greater need to integrate and maintain the memory representations required for online reading (Duff and Brown-Schmidt, 2012), in interaction with the regions involved in accessing representations (e.g., Ramus and Szenkovits, 2008).
Thus, our functional connectivity results focusing on the hypoactivated nodes in readers with dyslexia highlight the critical role of regions that, despite not being usually considered as part of the reading network, present strong connections with cortical regions of the reading network for pseudoword and word reading. Importantly, these results were confirmed across pairwise and whole-brain functional connectivity analyses, indicating that subcortical structures, such as the thalamus and hippocampus, might enable functional dynamics for orthographic and semantic reading processes, to compensate regional reading deficits in dyslexia.
4.3. Increased subcortico-cortical connectivity among regions showing regional reading deficits
Different hypotheses with regard to the underlying causes of dyslexia, including among others the phonological deficit hypothesis (e.g., Goswami, 2002; Ramus, 2003; Shaywitz, 1998) and the magnocellular theory (Galaburda et al., 1994; Stein, 2001; Stein and Walsh, 1997), consider that the major reading deficits observed in this developmental disorder are related to the function of cortical and subcortical regions. Results from the present study highlight the importance of cortical regions within the reading network in dyslexia and provide valuable information to further understand regional activation deficits as a function of phonological, orthographic and semantic reading processes. Including the thalamus and hippocampus in these regional analyses revealed that these regions also showed hypoactivations in readers with dyslexia relative to control readers for orthographic and semantic effects respectively, emphasizing that regional deficits in dyslexia are also present at the subcortical level. In this vein, the present study advances our knowledge by showing that regional deficits are common across cortical and subcortical structures. Further, our results reveal for the first time that these cortical and subcortical regional deficits in readers with dyslexia occur concomitantly with increases in functional coactivation between subcortical and cortical nodes exhibiting hypoactivation for phonological, orthographic and semantic reading processes. Although in the past, some theoretical accounts have highlighted the role of cortical as opposed to subcortical reading deficits, both of them are useful in understanding the neurobiology of dyslexia. Our results clearly point out the need to investigate more precisely the interactions between subcortical and cortical regions for the main reading systems to further understand the patterns of regional deficits and brain connectivity related to reading processes in individuals with dyslexia.
4.4. Conclusion
By means of a reading aloud (naming) task that poses strong cognitive demands and an experimental design that allowed examination of the reading-related processes specifically associated with phonological, orthographic and semantic processing, the present fMRI study investigated regional deficits in activation and functional connectivity among hypoactivated nodes in a group of readers with dyslexia and control counterparts. Readers with dyslexia exhibited hypoactivation associated with phonological processing in parietal regions (IPC, SPC); with orthographic processing in parietal regions (IPC, SPC), Broca's area (pars opercularis and triangularis), vOT and thalamus; and with semantic processing in the AG and hippocampus. Furthermore, task pairwise and whole-brain functional connectivity revealed tighter functional coactivation in readers with dyslexia versus control readers between the thalamus and regions showing hypoactivation for phonological (IPC), orthographic (IPC, vOT) and semantic processing (AG) for pseudoword reading, and between the hippocampus and regions exhibiting hypoactivation for orthographic processing (pars opercularis), for word reading.
The fact that regional deficits and differences in functional coactivation were associated with these three different reading components underscores the importance of further developing integrative views that advance our understanding of the neurobiology of dyslexia, in line with theoretical accounts that have highlighted that reading necessarily involves simultaneous covariations between phonological, orthographic and semantic processes (Frost, 2012; Perfetti and Hart, 2001). Based on this initial evidence, it is important for future research (ideally with larger sample sizes that would allow regression-based analytical approaches) to investigate within a single study the percentage of reading behavior variance explained by each of these reading constituents in readers with and without dyslexia. It will be especially important to examine whether or not dyslexic readers use semantic and/or orthographic skills to compensate for other reading difficulties (e.g., Cavalli et al., 2017) and to what extent they do so. Another important goal for future research would be to investigate regional hypoactivations and functional connectivity in right-lateralized regions that may play a specific role in some of the major components of reading examined here, in line with accounts that highlight the role of right-hemisphere compensatory mechanisms in dyslexia.
Importantly, the present study was conducted with Spanish monolinguals. Previous evidence has suggested that clinical difficulties associated with dyslexia are less common in transparent languages with consistent orthographies (e.g., Italian) than in languages with inconsistent orthographies (e.g., English; Barbiero et al., 2012; Peterson and Pennington, 2012). Nevertheless, cross-cultural comparisons have shown that poor readers in English, French and Italian showed similar deficits in neural activation relative to poor readers in Italian, despite the fact that Italian participants exhibited higher overall reading accuracy (Paulesu et al., 2001). Although in the present study we did not include any manipulation related to language orthography, consistent with previous neuroimaging results mostly from studies conducted with English monolinguals, our findings clearly suggest the presence of regional deficits across the major reading networks nodes.
In sum, findings from the present study constitute the first and strongest evidence so far of the interplay between regional hypoactivation and functional connectivity among the underactivated nodes in dyslexia, as a function of critical subcortical and cortical brain structures and the main systems supporting reading.
Acknowledgements and funding
Supported by grants (RYC-2014-15440, PSI2015-65696) from the Spanish Ministry of Economy and Competitiveness (MINECO), a grant (PI2016-12) from the Basque Government and a grant (Exp. 65/15) from the Programa Red guipuzcoana de Ciencia, Tecnología e Innovación from the Diputación Foral de Gipuzkoa (P.M.P-A.); a predoctoral grant from the Department of Education, Universities and Research from the Basque Government (M.O.); grant (PSI2015-64174P) from the MINECO (F.C.); grants (PSI2015-67353-R) from the MINECO and (ERC-2011-ADG-295362) from the European Research Council (M.C.). BCBL acknowledges funding from Ayuda Centro de Excelencia Severo OchoaSEV-2015-0490 from the MINECO. We thank Larraitz Lopez and Oihana Vadillo for their assistance with data collection and Margaret Gillon-Dowens and Magda Altman for proofreading the manuscript.
Conflict-of-interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Declaration of interests
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.08.018.
Appendix A. Supplementary data
Supplementary material
References
- American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Barbiero C., Lonciari I., Montico M., Monasta L., Penge R., Vio C., Tressoldi P.E., Ferluga V., Bigoni A., Tullio A., Carrozzi M. The submerged dyslexia iceberg: how many school children are not diagnosed? Results from an Italian study. PLoS One. 2012;7(10):e48082. doi: 10.1371/journal.pone.0048082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.M., Wolford G.L., Miller M.B. The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 2009;4:417–422. doi: 10.1093/scan/nsp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H. The neurobiology of semantic memory. Trends Cogn. Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Medler D.A., Desai R., Conant L.L., Liebenthal E. Some neurophysiological constraints on models of word naming. NeuroImage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Booth J.R., Choy J., Burman D.D., Gitelman D.R., Mesulam M.M. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Cheon J., Lu D., Burman D.D., Gitelman D.R., Mesulam M.-M., Booth J.R. Developmental changes in activation and effective connectivity in phonological processing. NeuroImage. 2007;38:564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B. Dyslexia: reconciling controversies within an integrative developmental perspective. Trends Cogn. Sci. 2014;18:501–503. doi: 10.1016/j.tics.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Boets B., Op de Beeck H.P., Vandermosten M., Scottm S.K., Gillebert C.R., Mantini D., Bulthe J., Sunaert S., Wouters J., Ghesquiere P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342:1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Bebko G., Burman D.D., Bitan T. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 2007;45:775–783. doi: 10.1016/j.neuropsychologia.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros M., Anton J.L., Pech-Georgel C., Grainger J., Szwed M., Ziegler J.C. Orthographic processing deficits in developmental dyslexia: beyond the ventral visual stream. NeuroImage. 2016;128:316–327. doi: 10.1016/j.neuroimage.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Brambati S.M., Termine C., Ruffino M., Danna M., Lanzi G., Stella G., Cappa S.F., Perani D. Neuropsychological deficits and neural dysfunction in familial dyslexia. Brain Res. 2006;1113:174–185. doi: 10.1016/j.brainres.2006.06.099. [DOI] [PubMed] [Google Scholar]
- Braun M., Hutzler F., Münte T.F., Rotte M., Dambacher M., Richlan F., Jacobs A.M. The neural bases of the pseudohomophone effect: phonological constraints on lexico-semantic access in reading. Neuroscience. 2015;295:151–163. doi: 10.1016/j.neuroscience.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Conference on Functional Mapping of the Human Brain, June 2–6. Japan; Sendai: 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Brunswick N., McCrory E., Price C.J., Frith C.D., Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Cao F., Bitan T., Chou T.L., Burman D.D., Booth J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J. Child Psychol. Psychiatry. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Booth J.R. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008;107:91–101. doi: 10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M., Mechelli A., Estevez A., Price C.J. Brain activation for lexical decision and reading aloud: two sides of the same coin? J. Cogn. Neurosci. 2007;19:433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- Carreiras M., Quiñones I., Hernández-Cabrera J.A., Duñabeitia J.A. Orthographic coding: brain activation for letters, symbols, and digits. Cereb. Cortex. 2015;25:4748–4760. doi: 10.1093/cercor/bhu163. [DOI] [PubMed] [Google Scholar]
- Cavalli E., Colé P., Pattamadilok C., Badier J.M., Zielinski C., Chanoine V., Ziegler J.C. Spatiotemporal reorganization of the reading network in adult dyslexia. Cortex. 2017;92:204–221. doi: 10.1016/j.cortex.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Cocosco C.A., Kollokian V., Kwan R.K.S., Evans A.C. BrainWeb: online interface to a 3D MRI simulated brain database. NeuroImage. 1997;5:S425. [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus. Brain Cogn. 1999;40:414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Cuetos F., Rodriguez B., Ruano E., Arribas D. TEA Ediciones; Madrid (Spain): 2009. Bateria de evaluacion de los procesos lectores, revisada. [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S., Bucher K., Maurer U., Schulz E., Brem S., Buckelmüller J., Kronbichler M., Loenneker T., Klaver P., Martin E., Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Mark S., Klaver P., Bucher K., Maurer U., Schulz E., Brem S., Martin E., Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. NeuroImage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Díaz B., Hintz F., Kiebel S.J., von Kriegstein K. Dysfunction of the auditory thalamus in developmental dyslexia. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13841–13846. doi: 10.1073/pnas.1119828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff M.C., Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front. Hum. Neurosci. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden G.F., Jones K.M., Cappell K., Gareau L., Wood F.B., Zeffiro T.A., Dietz N.A., Agnew J.A., Flowers D.L. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach C.J., Friederici A.D., Muller K., von Cramon D.Y. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez J.A., Balota D.A., Raichle M.E., Petersen S.E. Effects of lexicality, frequency, and spelling-to-sound consistency on the functionalanatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Holahan J.M., Papademetris X., Scheinost D., Lacadie C., Shaywitz S.E., Shaywitz B.A., Constable R.T. Disruption of functional networks in dyslexia: a whole-brain, data-driven approach to fMRI connectivity analysis. Biol. Psychiatry. 2013;76:397–404. doi: 10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. On the probable error of a coefficient of correlation deduced from a small sample. Metro. 1921;1:3–32. [Google Scholar]
- Frost R. A universal approach to modeling visual word recognition and reading: not only possible, but also inevitable. Behav. Brain Sci. 2012;35:310–329. doi: 10.1017/s0140525x12000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay Y., Thiessen E.D., Holt L.L. Impaired statistical learning in develop-mental dyslexia. J. Speech Lang. Hear. Res. 2015;58:934–945. doi: 10.1044/2015_JSLHR-L-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A.M., Menard M.T., Rosen G.D. Evidence for aberrant auditory anatomy in developmental dyslexia. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiewa P., Rzanny R., Hopf J.M., Knab R., Glauche V., Kaiser W.A., Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Georgiewa P., Rzanny R., Gaser C., Gerhard U.J., Vieweg U., Freesmeyer D., Mentzel H.J., Kaiser W.A., Blanz B. Phonological processing in dyslexic children: a study combining functional imaging and event related potentials. Neurosci. Lett. 2002;318:5–8. doi: 10.1016/s0304-3940(01)02236-4. [DOI] [PubMed] [Google Scholar]
- Giraldo-Chica M., Hegarty J.P., Schneider K.A. Morphological differences in the lateral geniculate nucleus associated with dyslexia. NeuroImage Clin. 2015;7:830–836. doi: 10.1016/j.nicl.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B.T., Balota D.A., Kirchhoff B.A., Buckner R.L. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from fMRI adaptation. Cereb. Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Goswami U. Phonology, reading development, and dyslexia: a cross-linguistic perspective. Ann. Dyslexia. 2002;52:139–163. [Google Scholar]
- Goswami U., Ziegler J.C., Dalton L., Schneider W. Pseudohomophone effects and phonological recoding procedures in reading development in English and German. J. Mem. Lang. 2001;45:648–664. [Google Scholar]
- Graves W.W., Desai R., Humphries C., Seidenberg M.S., Binder J.R. Neural systems for reading aloud: a multiparametric approach. Cereb. Cortex. 2010;20:1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Glenn K., Duara R., Barker W.W., Loewenstein D., Chang J.Y., Yoshii F., Apicella A.M., Pascal S., Boothe T., Sevush S. Positron emission tomographic studies during serial word-reading by normal and dyslexic adults. J. Clin. Exp. Neuropsychol. 1991;13:531–544. doi: 10.1080/01688639108401069. [DOI] [PubMed] [Google Scholar]
- Grünling C., Ligges M., Huonker R., Klingert M., Mentzel H.-J., Rzanny R., Kaiser W.A., Witte H., Blanz B. Dyslexia: the possible benefit of multimodal integration of fMRI- and EEG-data. J. Neural Transm. 2004;111:951–969. doi: 10.1007/s00702-004-0117-z. [DOI] [PubMed] [Google Scholar]
- Hagoort P., Indefrey P., Brown C., Herzog H., Steinmetz H., Seitz R.J. The neural circuitry involved in the reading of German words and pseudowords: a PET study. J. Cogn. Neurosci. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Harmelech T., Preminger S., Wertman E., Malach R. The day-after effect: long-term, hebbian-like restructuring of resting-state fMRI patterns induced by a single epoch of cortical activation. J. Neurosci. 2013;33:9488–9497. doi: 10.1523/JNEUROSCI.5911-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O. Psychology Press; New York: 1949. The Organization of Behavior: A Neuropsychological Theory. [Google Scholar]
- Hoeft F., Hernandez A., McMillon G., Taylor-Hill H., Martindale J.L., Meylerm A., Keller T.A., Siok W.T., Deutsch G.K., Just M.A., Whitfield-Gabrieli S., Gabrieli J.D. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J. Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Meyler A., Hernandez A., Juel C., Taylor-Hill H., Martindale J.L., McMillon G., Kolchugina G., Black J.M., Faizi A., Deutsch G.K., Siok W.T., Reiss A.L., Whitfield-Gabrieli S., Gabrieli J.D.E. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz P., Rumsey J.M., Donohue B.C. Functional connectivity of the angular in normal reading and dyslexia: neurobiology. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar M., Af Trampe P., Greitz T., Eriksson L., Stone-Elander S., von Euler C. Residual differences in language processing in compensated dyslexics revealed in simple word reading tasks. Brain Lang. 2002;83:249–267. doi: 10.1016/s0093-934x(02)00055-x. [DOI] [PubMed] [Google Scholar]
- Jaimes-Bautista A.G., Rodríguez-Camacho M., Martínez-Juárez I.E., Rodríguez-Agudelo Y. Semantic processing impairment in patients with temporal lobe epilepsy. Epilepsy Res. Treat. 2015;2015:746745. doi: 10.1155/2015/746745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednorog K., Marchewka A., Altarelli I., Monzalvo Lopez A.K., van Ermingen-Marbach M., Grande M., Grabowska A., Heim S., Ramus F. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? Insights from a large-scale voxel-based morphometry study. Hum. Brain Mapp. 2015;36:1741–1754. doi: 10.1002/hbm.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T., Hedehus M., Temple E., Salz T., Gabrieli J.D., Moseley M.E., Poldrack R.A. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Koyama M.S., Di Martino A., Kelly C., Jutagir D.R., Sunshine J., Schwartz S.J., Castellanos F.X., Milham M.P. Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S., Watkins K.E., Bishop D.V. Neurobiological basis of language learning difficulties. Trends Cogn. Sci. 2016;20:701–714. doi: 10.1016/j.tics.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E.F., Phillips C., Poeppel D. A cortical network for semantics:(de) constructing the N400. Nat. Rev. Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Livingstone M.S., Rosen G.D., Drislane F.W., Galaburda A.M. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobier M., Peyrin C., Le Bas J.F., Valdois S. Pre-orthographic character string processing and parietal cortex: a role for visual attention in reading? Neuropsychologia. 2012;50:2195–2204. doi: 10.1016/j.neuropsychologia.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Lobier M.A., Peyrin C., Pichat C., Le Bas J.F., Valdois S. Visual processing of multiple elements in the dyslexic brain: evidence for a superior parietal dysfunction. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog J.M., Einbinder E.R., Flowers D.L., Turkeltaub P.E., Eden G.F. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Maurer U., Brem S., Bucher K., Kranz F., Benz R., Steinhausen H.C., Brandeis D. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130:3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- Mazaika P., Hoeft F., Glover G.H., Reiss A.L. Human Brain Mapping Conference. June 11–15; Florence, Italy. 2009. Methods and software for fMRI analysis for clinical subjects. [Google Scholar]
- McCrory E.J., Mechelli A., Frith U., Price C.J. More than words: a common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Gorno-Tempini M.L., Price C.J. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J. Cogn. Neurosci. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Crinion J.T., Long S., Friston K.J., Lambon Ralph M.A., Patterson K., McClelland J.L., Price C.J. Dissociating reading processes on the basis of neuronal interactions. J. Cogn. Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Josephs O., Ralph L., Matthew A., McClelland J.L., Price C.J. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Hum. Brain Mapp. 2007;28:205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler A., Keller T.A., Cherkassky V.L., Lee D., Hoeft F., Whitfield-Gabrieli S., Gabrieli J.D., Just M.A. Brain activation during sentence comprehension among good and poor readers. Cereb. Cortex. 2007;17:2780–2787. doi: 10.1093/cercor/bhm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler A., Keller T.A., Cherkassky V.L., Gabrieli J.D., Just M.A. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro N., Paz-Alonso P.M., Duñabeitia J.A., Carreiras M. Combinatorial semantics strengthens angular-anterior temporal coupling. Cortex. 2015;65:113–127. doi: 10.1016/j.cortex.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Oliver M., Carreiras M., Paz-Alonso P.M. Functional dynamics of dorsal and ventral reading networks in bilinguals. Cereb. Cortex. 2017;27:5431–5443. doi: 10.1093/cercor/bhw310. [DOI] [PubMed] [Google Scholar]
- Olulade O.A., Flowers D.L., Napoliello E.M., Eden G.F. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. NeuroImage Clin. 2015;7:742–754. doi: 10.1016/j.nicl.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B., Friederici A.D. Interactions of the hippo-campal system and the prefrontal cortex in learning language-like rules. NeuroImage. 2003;19:1730–1737. doi: 10.1016/s1053-8119(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Owen W.J., Borowsky R., Sarty G.E. FMRI of two measures of phonological processing in visual word recognition: ecological validity matters. Brain Lang. 2004;90:40–46. doi: 10.1016/S0093-934X(03)00418-8. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Frith U., Snowling M., Gallagher A., Morton J., Frackowiak R.S., Frith C.D. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Démonet J.F., Fazio F., McCrory E., Chanoine V., Brunswick N., Cappa S.F., Cossu G., Habib M., Frith C.D., Frith U. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Danelli L., Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidou E.V., Louise Kelly M., Williams J.M. Do children with developmental dyslexia have impairments in implicit learning? Dyslexia. 2010;16:143–161. doi: 10.1002/dys.400. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Oliver M., Quiñones I., Carrerias M. Neural basis of monolingual and bilingual reading. In: de Zubicaray G., Schiller N.O., Miozzo M., editors. The Oxford Handbook of Neurolinguistics. Oxford University Press; New York, NY: 2018. [Google Scholar]
- Perfetti C.A., Hart L. The lexical basis of comprehension skill. In: Gorfien D.S., editor. On the Consequences of Meaning Selection: Perspectives on Resolving Lexical Ambiguity. Washington; DC, American Psychological Association: 2001. [Google Scholar]
- Perfetti C.A., Hart L. The lexical quality hypothesis. In: Verhoeven L., Elbro C., Reitsma P., editors. Precursors of Functional Literacy. John Benjamins; Amsterdam: 2002. [Google Scholar]
- Peterson R.L., Pennington B.F. Developmental dyslexia. Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin C., Démonet J.F., N'Guyen-Morel M.A., Le Bas J.F., Valdois S. Superior parietal lobule dysfunction in a homogeneous group of dyslexic children with a visual attention span disorder. Brain Lang. 2011;118:128–138. doi: 10.1016/j.bandl.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Peyrin C., Lallier M., Démonet J.F., Pernet C., Baciu M., Le Bas J.F., Valdois S. Neural dissociation of phonological and visual attention span disorders in developmental dyslexia: FMRI evidence from two case reports. Brain Lang. 2012;120:381–394. doi: 10.1016/j.bandl.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Piai V., Anderson K.L., Lin J.J., Dewar C., Parvizi J., Dronkers N.F., Knight R.T. Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci. U. S. A. 2016;113:11366–11371. doi: 10.1073/pnas.1603312113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante E., Patterson D., Dailey N.S., Kyle R.A., Fridriksson J. Dynamic changes in network activations characterize early learning of a natural language. Neuropsychologia. 2014;62:77–86. doi: 10.1016/j.neuropsychologia.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. The future of fMRI in cognitive neuroscience. NeuroImage. 2012;62:1216–1220. doi: 10.1016/j.neuroimage.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Raichle M.E. Precis of images of mind. Behav. Brain Sci. 1995;18:327–339. [Google Scholar]
- Preston J.L., Frost S.J., Mencl W.E., Fulbright R.K., Landi N., Grigorenko E., Jacobsen L., Pugh K.R. Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain. 2010;133:2185–2195. doi: 10.1093/brain/awq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R., Shaywitz S.E., Shaywitz B.A. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment. Retard. Dev. Disabil. Res. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R., Shaywitz S.E., Shaywitz B.A. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Frost S.J., Sandak R., Landi N., Rueckl J.G., Constable R.T., Seidenberg M.S., Fulbright R.K., Katz L., Mencl W.E. Effects of stimulus difficulty and repetition on printed word identification: an fMRI comparison of nonimpaired and reading-disabled adolescent cohorts. J. Cogn. Neurosci. 2008;20:1146–1160. doi: 10.1162/jocn.2008.20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.R., Landi N., Preston J.L., Mencl W.E., Austin A.C., Sibley D., Fulbright R.K., Seidenberg M.S., Grigorenko E.L., Constable R.T., Molfese P., Frost S.J. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013;125:173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Curr. Opin. Neurobiol. 2003;13:212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ramus F., Szenkovits G. What phonological deficit? Q. J. Exp. Psychol. 2008;61:129–141. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- Reilhac C., Peyrin C., Démonet J.-F., Valdois S. Role of the superior parietal lobules in letter-identity processing within strings: FMRI evidence from skilled and dyslexic readers. Neuropsychologia. 2013;51:601–612. doi: 10.1016/j.neuropsychologia.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Richards T.L., Berninger V.W. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: before but not after treatment. J. Neurolinguistics. 2008;21:294–304. doi: 10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Front. Hum. Neurosci. 2012;6:120. doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Functional neuroanatomy of developmental dyslexia: the role of orthographic depth. Front. Hum. Neurosci. 2014;8:347. doi: 10.3389/fnhum.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Sturm D., Schurz M., Kronbichler M., Ladurner G., Wimmer H. A common left occipito-temporal dysfunction in developmental dyslexia and letter-by-letter reading? PLoS One. 2010;5 doi: 10.1371/journal.pone.0012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Rissman J., Gazzaley A., D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Roux F., Armstrong B.C., Carreiras M. Chronset: an automated tool for detecting speech onset. Behav. Res. Methods. 2017;49:1864–1881. doi: 10.3758/s13428-016-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckl J.G., Paz-Alonso P.M., Molfese P.J., Kuo W.J., Bick A., Frost S.J., Hancock R., Wu D.H., Mencl W.E., Duñabeitia J.A., Lee J.R., Oliver M., Zevin J.D., Hoeft F., Carreiras M., Tzeng O.J.L., Pugh K.R., Frost R. Universal brain signature of proficient reading: evidence from four contrasting languages. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15510–15515. doi: 10.1073/pnas.1509321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey J.M., Nace K., Donohue B., Wise D., Maisog J.M., Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch. Neurol. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Saalmann Y.B., Pinsk M.A., Wang L., Li X., Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R., Service E., Kiesilä P., Uutela K., Salonen O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann. Neurol. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- Sandak R., Mencl W.E., Frost S.J., Pugh K.R. The neurobiological basis of skilled and impaired reading: recent findings and new directions. Sci. Stud. Read. 2004;8:273–292. [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schulz E., Maurer U., van der Mark S., Bucher K., Brem S., Martin E., Brandeis D. Impaired semantic processing during sentence reading in children with dyslexia: combined fMRI and ERP evidence. NeuroImage. 2008;41:153–168. doi: 10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Schurz M., Wimmer H., Richlan F., Ludersdorfer P., Klackl J., Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb. Cortex. 2014;25:3502–3514. doi: 10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences J.T., Yantis S. Selective visual attention and perceptual coherence. Trends Cogn. Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E. Dyslexia. N. Engl. J. Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Mencl W.E., Fulbright R.K., Skudlarski P., Constable R.T., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Fulbright R.K., Skudlarski P., Mencl W.E., Constable R.T., Pugh K.R., Holahan J.M., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol. Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Skudlarski P., Holahan J.M., Marchione K.E., Constable R.T., Fulbright R.K., Zelterman D., Lacadie C., Shaywitz S.E. Age-related changes in reading systems of dyslexic children. Ann. Neurol. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico–pulvinar connections. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G., Frith U., Demonet J.F., Fazio F., Perani D., Price C.J. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Stein J., Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Germany, Thieme Verlag; Stuttgart: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F., Dehaene S., Jobert A., Dubus J.P., Sigman M., Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; New York, NY: 2001. Intelligence Scale for Children-Revised. [Google Scholar]
- Wechsler D. San Antonio, TX; NCS Pearson: 2008. Wechsler Adult Intelligence Scale–4th Edition (WAIS–IV) [Google Scholar]
- Wimmer H., Schurz M., Sturm D., Richlan F., Klackl J., Kronbichler M., Ladurner G. A dual-route perspective on poor reading in a regular orthography: an fMRI study. Cortex. 2010;46:1284–1298. doi: 10.1016/j.cortex.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams A.M., Silani G., Okada K., Patterson K., Price C.J. Word or word-like? Dissociating orthographic typicality from lexicality in the left occipito-temporal cortex. J. Cogn. Neurosci. 2011;23:992–1002. doi: 10.1162/jocn.2010.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2nd ed. World Health Organization; Geneva, Switzerland: 2008. International Statistical Classification of Diseases and Related Health Problems—Tenth Revision. [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material