Abstract
Salidroside has a wide range of pharmacological activities, including antitumor, anti-inflammatory, analgesic, antibacterial, antiviral and anti-fertility abilities. In the present study, the effects of salidroside on the viability and apoptosis of bladder cancer cells, and the potential underlying mechanisms, were examined. In the present study, treatment with salidroside reduced cell viability, and induced apoptosis and caspase-9/3 activation in the T24 human bladder carcinoma cell line. Salidroside induced autophagy, promoted the protein expression of nucleoporin p62 and the microtubule-associated proteins 1A/1B light chain 3B, suppressed phosphoinositide 3-kinase (PI3K) and phosphorylated protein kinase B (p-Akt) expression, inhibited matrix metalloproteinase-9 (MMP-9) expression and increased that of Bcl-2-associated X protein, which functions as an apoptosis regulator in T24 cells. In the present study, it was demonstrated that the effect of salidroside reduced the viability and induced the apoptosis of bladder cancer cells through the autophagy/PI3K/Akt and MMP-9 signaling pathways.
Keywords: salidroside, bladder cancer, autophagy, phosphoinositide 3-kinase, matrix metalloproteinase-9
Introduction
In 2008, the incidence of bladder cancer was 7.49/100,000 according to the National Cancer Registry, and the standardized global incidence was 4.53/100,000 (1). The incidence of bladder cancer was ranked first among all genitourinary cancer types in the Chinese male population and eighth of all malignant tumor types in China, and accounted for 2.50% of all malignant tumors diagnosed in China in 2008 (2,3). For Chinese individuals between 0 and 74 years old, the cumulative incidence of bladder cancer was 0.52% in 2008 (4).
Autophagy refers to the process in which damaged, degenerated or aging proteins or organelles in cells are transported to lysosomes for digestion and degradation (5). The occurrence of autophagy requires the multiple stages. First, under the regulation of initial autophagic signals, the precursors of autophagy with a cup-shaped double layer coating are formed in the cytoplasm (6). Then, the precursors of autophagy are gradually lengthened, assisted by autophagic proteins. The lengthened precursors of autophagy cover degradative substrates and finally form completely closed autophagic vacuoles (7). Finally, the autophagic vacuoles reach the lysosomes through the intracellular transport system. The adventitia of the autophagic vacuoles integrates with lysosomal membranes and degrades their wrapping via the lysosomes hydrolase activity (7).
Under normal physiological conditions, autophagy is beneficial in maintaining a self-stabilized state (8). When there is stress, autophagy may prevent the accumulation of toxic or carcinogenic proteins and organelles and inhibit cellular cancerization (9). However, a number of previous studies have indicated that, following neoplasia, autophagy may provide more abundant nutrition for cancer cells and promote tumor growth (8,9). Therefore, in the process of tumorigenesis and tumor development, autophagy serves an important function.
The suppressed phosphoinositide 3-kinase (PI3K)/phosphorylated protein kinase B (p-Akt) signaling pathway exerts anti-apoptosis activity by influencing multiple downstream effector molecules (10). At present, by knocking out or inhibiting P13K/Akt and other relevant genes using gene intervention techniques or micromolecule drugs, the activation of multiple downstream anti-apoptotic effector molecules may be suppressed, in order to promote apoptosis (11). This topic has already become a central focus of cancer research.
Matrix metalloproteinases (MMPs) are a group of zinc ion-containing endopeptidases, able to degrade the extracellular matrix, and to promote tumor invasion and metastasis; the most notable MMPs include MMP-2, MMP-3 and MMP-9 (12–14). MMP-2 and MMP-9 are two subtypes of type IV collagenase highly expressed in various tumor types, including breast cancer, ovarian cancer and nasopharyngeal carcinoma, and associated with the degree of invasion and malignancy (12–14). Quantitative detection revealed that MMP-2 and MMP-9 are significantly high in bladder cancer tissues, as compared with in the corresponding control tissues; and invasive tumor types have higher expression levels of these proteins compared with superficial tumor types (15,16).
Of the genes involved in the regulation of apoptosis, the B-cell lymphoma 2 (Bcl-2) family is the most important when it comes to regulating apoptosis. Bcl-2 and Bcl-2-associated X protein (Bax) are, respectively, two of the main anti-apoptotic and pro-apoptotic regulators, which mediate apoptosis via influencing mitochondrial function (17). The BCL2 gene was originally cloned from the local breaking point of chromosome 18 of follicular B-cell lymphoma (Bcl), which is an apoptosis-suppressing gene (18). The Bcl-2 protein is located in the mitochondria, at the nuclear membrane and in the endoplasmic reticulum (18). As increased intracellular calcium concentration is an early indicator of apoptosis, Bcl-2 inhibits apoptosis by preventing this elevation in plasma calcium. In previous years, it was revealed that Bcl-2 expression is the most notable feature in human malignant tumor types, is widely recognized as an anti-apoptotic factor, and is involved in the tumorigenesis through the inhibition of apoptosis and by prolonging cell survival (19). In the extrinsic apoptosis pathway, caspase-9 and −3 are key signaling transduction pathway proteins (20). Caspase-9 and −3 form the death-inducing signaling complex for human bladder cancer via the activation of caspase-8 in apoptotic cell death (20).
Salidroside has anti-inflammatory, antibacterial, antiviral, anti-fertility effects amongst other activities; it is currently used for the treatment of rheumatoid arthritis, blood diseases and skin diseases, and is additionally used as an agricultural insecticide (21,22). However, the anticancer effect of salidroside in suppressing the viability of human bladder cancer cells has not yet been fully clarified. In the present study, the effects of salidroside on the viability and apoptosis of bladder cancer cells, and its potential underlying mechanisms, were examined.
Materials and methods
Chemicals and reagents
RPMI-1640 medium was purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Fetal bovine serum (FBS) and MTT bromide were purchased from Invitrogen (Thermo Fisher Scientific, Inc.). Caspase-9 Activity Assay kit and Caspase-3 Activity Assay kit were purchased from Nanjing KeyGen Biotech Co., Ltd (Nanjing, China) and an Enhanced BCA Protein Assay kit was purchased from Beyotime Institute of Biotechnology (Haimen, China). Salidroside was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell culture
Human bladder cancer T24 cells were obtained from Guangzhou Medical University (Guangzhou, Guangdong) and were maintained in RPMI-1640 medium supplemented with 10% FBS in a humidified atmosphere of 5% CO2 at 37°C.
MTT assays
T24 cells were seeded into 96-well dishes at a density of 1×104 cells/well, and treated with 0, 0.5, 1 and 2 µM salidroside for 24, 48 and 72 h each. Cell viability was determined using an MTT assay. T24 cells were supplemented with 10 µl MTT and incubated at 37°C for 4 h in a humidified atmosphere containing 5% CO2. Once the RPMI-1640 was removed, 150 µl dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. The optical density of each well was read at 490 nm.
Cell apoptosis assay
T24 cells were seeded into 6-well plates at a density of 1×106 cells/well, and then treated with 0.5, 1 and 2 µM salidroside for 48 h. The apoptosis of T24 cells was evaluated using a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). Cells were washed with PBS for 5 min three times and resuspended in 500 µl Annexin V Binding Buffer (BD Biosciences). Then, cells were stained with FITC-Annexin V and propidium iodide (BD Biosciences) in the dark for 15 min at room temperature. Apoptotic cells were analyzed using ImageLab 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Caspase-9/3 activation
T24 cells were seeded into 6-well plates (1×106 cells/well), and then treated with 0.5, 1 and 2 µM salidroside for 48 h. The activation of caspase-9/3 in T24 cells was evaluated using ELISA colorimetric kits (cat nos. C1157 and C1115; Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's protocol. The Caspase-9 inhibitor Z-LEHDFMK (2 mM; Nanjing KeyGen Biotech Co., Ltd.) and the Caspase-3 inhibitor Z-DEVD-FMK (2 mM; Nanjing KeyGen Biotech Co., Ltd.) were added into each well. The optical density of each well was read at 450 nm.
Western blot analysis
T24 cells were seeded in 6-well dishes at 1×106 cells/well, and treated with 0.5, 1 and 2 µM salidroside for 48 h. T24 cells were incubated with ice-cold radioimmunoprecipitation assay buffer (Nanjing KeyGen Biotech Co., Ltd.) for 30–50 min on ice at 4°C. Protein concentration was determined using an Enhanced BCA Protein Assay kit (Nanjing KeyGen Biotech Co., Ltd.). Protein extracts (50 µg per lane) were separated via 10% SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc., Hercules). The blotted membranes were blocked with Tris-buffered saline (TBS) containing 5% non-fat milk to block nonspecific binding sites for 1 h at 37°C. Then, the membranes were incubated with anti-p62 (cat. no. sc-25523; 1:100), anti-PI3K (cat. no. sc-7174; 1:1,000), anti-MMP-9 (cat. no. sc-10737; 1:1,000), and anti-Bax (cat. no. sc-6236; 1:2,000; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-LC3 (cat. no. 3868; 1:1,000), anti-Akt (cat. no. 4685; 1:2,000) and anti-p-Akt (cat. no. 4060; 1:1,000; both Cell Signaling Technology, Inc., Danvers, MA, USA), and anti-GAPDH (cat. no. sc-25778, 1:500; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Subsequent to washing in TBS with 0.1% Tween-20 for 5 min 3 times, the membranes were incubated with a horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G (cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The proteins were detected using enhanced chemiluminescence (Tiangen Biotech Co., Ltd., Beijing, China), and quantified with Image Lab v.3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS v.18.0 software (SPSS, Inc., Chicago, IL, USA), and performed using an unpaired one-way analysis of variance followed by Tukey's post hoc test. Data are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
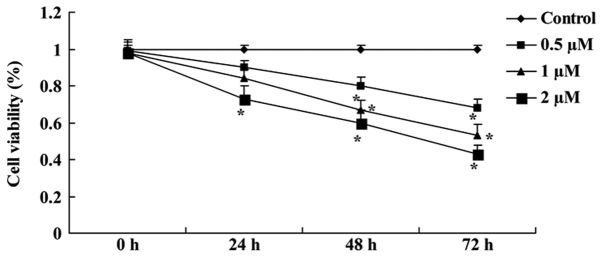
Salidroside suppresses the viability of bladder cancer cells
The chemical structure of salidroside (purity >98%) is illustrated in Fig. 1. To investigate whether salidroside could suppress the viability of T24 cells, the cells were treated with various concentrations of salidroside (0.5, 1 and 2 µM) and then evaluated using an MTT assay. Treatment with salidroside (0.5, 1 and 2 µM) significantly suppressed the viability of T24 cells at 48 and 72 h compared with the control (P<0.05; Fig. 2A). Additionally, the administration of 2 µM salidroside for 24 h significantly suppressed the viability of T24 cells compared with the control (P<0.05; Fig. 2A). The results of the MTT assay revealed that salidroside also inhibited T24 cell viability (Fig. 2B).
Figure 1.
Chemical structure of salidroside.
Figure 2.
Salidroside suppresses the viability of bladder cancer cells. Viability of T24 cells when treated with salidroside (0.5, 1 and 2 µM) was analyzed using MTT assays. *P<0.05 vs. the control group (0 µM salidroside).
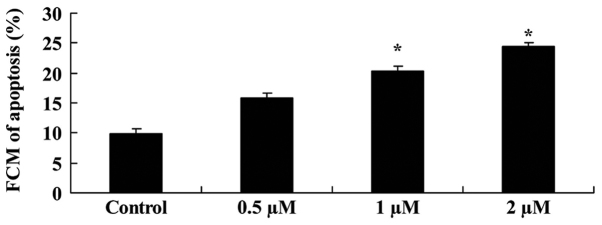
Salidroside induces bladder cancer cell apoptosis
The anticancer effect of salidroside on the cell apoptosis of T24 cells was investigated. T24 cells were treated with different concentrations of salidroside (0.5, 1 and 2 µM) and then evaluated using a flow cytometer. As presented in Fig. 3, 1 and 2 µM of salidroside significantly induced the apoptosis of T24 cells in a dose-dependent manner, compared with the control group (P<0.05).
Figure 3.
Salidroside induces bladder cancer cell apoptosis. Apoptosis of T24 cells treated with various concentrations of salidroside (0.5, 1 and 2 µM) was investigated. *P<0.05 vs. the control group. FCM, flow cytometry.
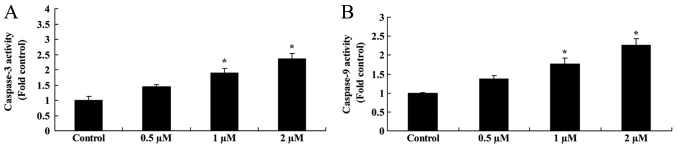
Salidroside induces caspase-9/3 activation in bladder cancer cells
As indicated in Fig. 4, treatment with 0.5 µM salidroside for 48 h non-significantly increased caspase-9/3 activation in T24 cells, compared with in the control group. However, the administration of 1 and 2 µM salidroside for 48 h significantly induced caspase-9/3 activation in T24 cells, compared with in the control group (P<0.05).
Figure 4.
Salidroside induces caspase-9/3 activation in bladder cancer cells. Salidroside was revealed to induce (A) caspase-3 and (B) caspase-9 activation in T24 cells. *P<0.05 vs. the control group.
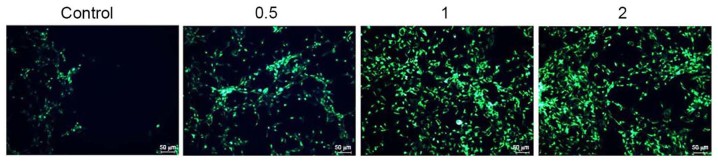
Salidroside induces the autophagy of bladder cancer cells
The anticancer effect of salidroside on the autophagy of T24 cells was investigated. T24 cells were stained with anti-LC3. As presented in Fig. 5, treatment with 0.5, 1 and 2 µM salidroside for 48 h increased the autophagy of T24 cells in a dose-dependent manner, compared with the control.
Figure 5.
Salidroside induces the autophagy of bladder cancer cells. Various concentrations of salidroside (0.5, 1 and 2 µM) were revealed to increase the autophagy of T24 cells in a dose-dependent manner, compared with in the control group.
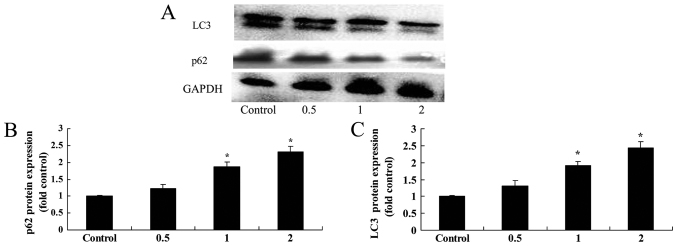
Salidroside induces p62 and LC3 protein expression in bladder cancer cells
Next, the effect of salidroside on the autophagy of T24 cells was further investigated by examining the protein expression of p62 and LC3, which are known biomarkers of autophagy. The results indicated that 1 and 2 µM of salidroside significantly induced p62 and LC3 protein expression in T24 cells in a dose-dependent manner, compared with in the control (P<0.05; Fig. 6).
Figure 6.
Salidroside induces p62 and LC3 protein expression in bladder cancer cells. (A) Salidroside was revealed to induce p62 and LC3 expression in T24 cells, as determined via western blotting. The protein expression levels of (B) p26 and (C) LC3 were quantified. *P<0.05, compared with the control group. p62, nucleoporin p62; LC3, microtubule-associated proteins 1A/1B light chain 3B.
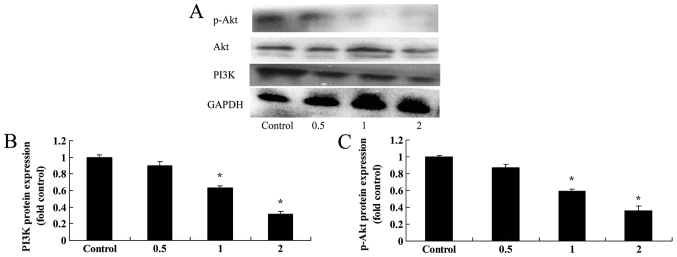
Salidroside suppresses PI3K and Akt protein expression in bladder cancer cells
PI3K and Akt protein expression in bladder cancer cells was assessed in order to elucidate the mechanism by which salidroside induces autophagy in T24 cells. PI3K and p-Akt protein expression levels were significantly suppressed by 1 and 2 µM salidroside in T24 cells in a dose-dependent manner, compared with in the control (P<0.05; Fig. 7).
Figure 7.
Salidroside suppresses PI3K and Akt protein expression in bladder cancer cells. (A) Salidroside suppresses PI3K and Akt protein expression, as revealed via western blotting. The protein expression levels of (B) PI3K and (C) Akt were quantified. *P<0.05, vs. the control group. PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; p-, phosphorylated.
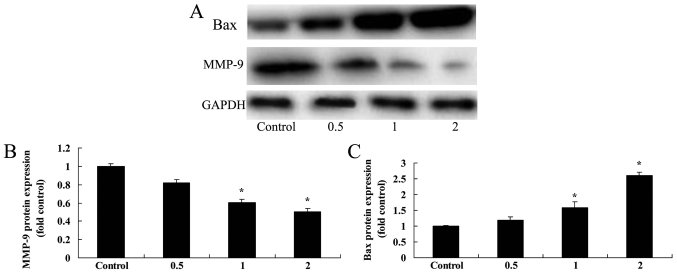
Salidroside suppresses the MMP-9 expression levels of bladder cancer cells
As depicted in Fig. 8A and B, treatment with 0.5 µM salidroside for 48 h non-significantly suppressed MMP-9 protein expression in T24 cells, compared with in the control group. Additionally, the administration of 1 and 2 µM salidroside for 48 h significantly suppressed the MMP-9 protein expression levels in T24 cells, compared with in the control group (P<0.05).
Figure 8.
Salidroside suppressed the MMP-9 protein expression levels and induced Bax protein expression of bladder cancer cells. (A) Salidroside suppressed MMP-9 and Bax expression, as demonstrated using western blotting; (B) MMP-9 and (C) Bax protein levels were quantified. *P<0.05, compared with the control group. MMP-9, matrix metalloproteinase-9.
Salidroside induced Bax expression in bladder cancer cells
As indicated in Fig. 8, treatment with 0.5 µM salidroside for 48 h non-significantly induced Bax protein expression in T24 cells, compared with the control group. However, the administration of 1 and 2 µM salidroside for 48 h significantly increased Bax protein expression in T24 cells, compared with in the control group (P<0.05).
Discussion
Bladder cancer is a common malignant tumor of the urinary system. According to World Health Organization statistics on global cancer epidemic diseases, there were 386,300 new cases globally in 2008, with a 14-fold variance in the incidence of bladder cancer in certain regions (23). The regions with the highest bladder cancer incidence were Europe and North America (with a male incidence rate of ≥20/100,000 and a female incidence rate of up to 5/100,000) (24). The region with the lowest incidence was Central Africa (incidence rate for males of 1.5/100,000 and an incidence rate for females of 0.3/100,000) (25). Previous studies revealed that salidroside significantly suppressed the invasion of and induced the apoptosis of human breast cancer (25) and head and neck cancer cells (26). In the present study, the administration of salidroside significantly suppressed the viability of and significantly increased caspase-9/3 activation in human bladder cancer T24 cells, compared with in the untreated cells (P<0.05).
Autophagy is a normal and ubiquitous physiological process that represents an active defensive strategy for external pessimal stimulation (8); it is also an important form of programmed cell death. Autophagy additionally participates in the pathological development of multiple diseases, including malignant tumor types (27). LC3 is a notable gene that participates in the formation of the autophagosome (9). In the degradation process of autophagic lysosomes, it serves the role of a tumor suppressor; LC3 is an autophagic landmark gene and one of the diagnostic indices of autophagic specificity (9). In the present study, it was revealed that salidroside significantly increased the autophagy of T24 cells in a dose-dependent manner (P<0.05). Fan et al (21) suggested that salidroside induced apoptosis and autophagy in human colorectal cancer cells through inhibition of the PI3K/Akt/mechanistic target of rapamycin (mTOR) pathway.
LC3 is the gene isogeny of yeast and autophagy-related protein 8 (Atg8) in mammalian cells (28). It has ~30% amino acid isogeny with Atg8 (28). LC3 is primarily located in the front autophagic vacuoles and the surface of autophagic vacuoles in the cell interior (28). LC3 that participates in autophagic formation may be divided into type-I and type-II (28). Prior to the presentation of autophagy, LC3 generated in cells is modified and processed to become the regularly expressed, soluble type-I LC3 in the cytoplasm (28). When autophagy occurs, type-I LC3 is modified and processed via ubiquitin-type processing (29). Type-I LC3 forms type-II LC3 by combining with phosphatidyl ethanolamine on the surface of an autophagic membrane; type-II LC3 is always combined on the membrane of intracellular autophagic vacuoles (29). Evaluating the difference in LC3 expression between tumor and normal tissues may provide insight into tumorigenesis and development (9). In the present study, salidroside significantly induced p62 and LC3 protein expression in T24 cells, in a dose-dependent manner, compared with in the control cells (P<0.05).
Under the stimulation of hunger, hormones, chemicals and ultraviolet light, a double-layer coating is independently formed around substances to be degraded (30,31). The key regulatory factor in this stage is TOR, a serine-threonine kinase known as mTOR in mammals (30). It serves as a receptor for various amino acids, ATP and hormones in cells and may lower their levels (31). It also may inhibit the occurrence of autophagy. When there is nutritional sufficiency and the presence of growth factors, it serves as a primary anti-autophagy signal (28). TOR kinase may phosphorylate the associated gene Atg13 (28). The affinity of phosphorylated Atg13 and Atg1 is reduced and blocks the formation of the Atg13-Atg1 complex, reducing the occurrence of autophagy (31). PI3K/Akt signaling may inhibit the activity of mTOR kinase, thus promoting the activity of growth factors and inhibiting autophagy (32). Conversely, type-III PI3K may promote intracellular substances to be degraded by wrapping by the double layer coating structure in order to induce autophagy (33). In the present study, it was demonstrated that salidroside significantly induced PI3K and p-Akt protein expression in T24 cells, in a dose-dependent manner, compared with in the control cells (P<0.05).
The mRNA expression levels of MMP-2 and MMP-9 are increased in bladder cancer cells transfected with the gene encoding fibroblast growth factor (FGF); however, in cells transfected with the FGF-2 antisense gene, MMP-2 and MMP-9 mRNA levels are decreased, and the invasion force is reduced (34). Thus, MMPs are regarded to be associated with bladder cancer cell infiltration (34). A previous study on the expression levels of MMP-2 and tissue inhibitor of metalloproteinases 2 (TIMP-2) in bladder cancer tissues revealed that MMP-2 and TIMP-2 are highly expressed in cancer tissues; the prognosis of patients with high MMP-2 and TIMP-2 expression levels is significantly poorer compared with that of patients with low expression levels (35). In the present study, salidroside significantly decreased MMP-9 protein expression levels in T24 cells. Sun et al (36) revealed that salidroside inhibited the proliferation of, and decreased the migration and invasion of SW1116 cells through the suppression of MMP-2 and MMP-9 expression. The results of the present study corresponded with those of the prior studies discussed here.
Previous molecular biology studies have demonstrated that the occurrence and development of bladder cancer is associated with cell apoptosis in prostate tissue (37,38). Among the genes involved in the regulation of apoptosis, BCL2 is of particular interest. The product of BCL2 is a 26 kDa protein, localized to the mitochondrial and nuclear membranes, and to the endoplasmic reticulum (37). Previously, it was identified that Bcl-2 may inhibit apoptosis in a variety of tissue types, and it is now widely recognized as an anti-apoptotic gene, participating in tumorigenesis by inhibiting apoptosis and prolonging cell survival (38). A previous study demonstrated that for 69% of all patients with bladder cancer, BCL2 is positively expressed, and that the expression level is associated with the cancer; in patients with bladder cancer at a progressive stage, the expression of Bcl-2 is ≤39%, whereas at a less-progressive stage, it is ~24% (39). The results of the present study revealed that the administration of salidroside significantly inhibited the Bax signaling pathway and induced caspase-9/3 activation in T24 cells. Lv et al (22) suggested that salidroside reduces renal cell carcinoma proliferation through the suppression of Bax expression.
In summary, the present study demonstrated for the first time, to the best of our knowledge, that salidroside reduces viability and induces apoptosis in T24 human bladder cancer cells through the autophagy/PI3K/Akt and Bcl-2 signaling pathways. Furthermore, the molecular mechanisms underlying the effects of salidroside in human bladder cancer cells were explored. The results suggested that salidroside may be an effective agent for treating human bladder cancer. However, there were a number of limitations on the present study, including using only T24 cells; further in vitro and in vivo models, in addition to clinical studies, will be required to explore the effects in additional cell lines and other cancer types. Future studies should also be conducted using normal cell lines, in addition to other tumor cell lines.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
TL designed the experiment. KX and YL performed the experiments. TL analyzed the data and wrote the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu L, Zhao X, Zhu X, Zhong Z, Xu R, Wang Z, Cao J, Hou Y. Decreased expression of miR-430 promotes the development of bladder cancer via the upregulation of CXCR7. Mol Med Rep. 2013;8:140–146. doi: 10.3892/mmr.2013.1477. [DOI] [PubMed] [Google Scholar]
- 2.Palmeira C, Oliveira PA, Lameiras C, Amaro T, Silva VM, Lopes C, Santos L. Biological similarities between murine chemical-induced and natural human bladder carcinogenesis. Oncol Lett. 2010;1:373–377. doi: 10.3892/ol_00000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billerey C, Chopin D, Aubriot-Lorton MH, Ricol D, Gil Diez, de Medina S, Van Rhijn B, Bralet MP, Lefrere-Belda MA, Lahaye JB, Abbou CC, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao J, Chen JX, Zhu YP, Zhou LY, Shu QA, Chen LW. Reduced expression of MTUS1 mRNA is correlated with poor prognosis in bladder cancer. Oncol Lett. 2012;4:113–118. doi: 10.3892/ol.2012.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Z, Huangfu X, Liu Z. Effect of autophagy on cisplatin caused bladder cancer cell apoptosis. Panminerva Med. 2016;59:1–8. doi: 10.23736/S0031-0808.16.03182-7. [DOI] [PubMed] [Google Scholar]
- 6.Pinto-Leite R, Arantes-Rodrigues R, Ferreira R, Palmeira C, Colaço A, da Silva Moreira V, Oliveira P, Santos Lara L. Temsirolimus improves cytotoxic efficacy of cisplatin and gemcitabine against urinary bladder cancer cell lines. Urol Oncol. 2014;32(41):e11–e22. doi: 10.1016/j.urolonc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC, Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int J Oncol. 2013;42:993–1000. doi: 10.3892/ijo.2013.1791. [DOI] [PubMed] [Google Scholar]
- 8.Pan XW, Li L, Huang Y, Huang H, Xu DF, Gao Y, Chen L, Ren JZ, Cao JW, Hong Y, Cui XG. Icaritin acts synergistically with epirubicin to suppress bladder cancer growth through inhibition of autophagy. Oncol Rep. 2016;35:334–342. doi: 10.3892/or.2015.4335. [DOI] [PubMed] [Google Scholar]
- 9.Du L, Jiang N, Wang G, Chu Y, Lin W, Qian J, Zhang Y, Zheng J, Chen G. Autophagy inhibition sensitizes bladder cancer cells to the photodynamic effects of the novel photosensitizer chlorophyllin e4. J Photochem Photobiol B. 2014;133:1–10. doi: 10.1016/j.jphotobiol.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Wang H, Peng J. Hispidulin induces apoptosis through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway in HepG2 cancer cells. Cell Biochem Biophys. 2014;69:27–34. doi: 10.1007/s12013-013-9762-x. [DOI] [PubMed] [Google Scholar]
- 11.Wallerand H, Ravaud A, Ferrière JM. Bladder cancer in patients after organ transplantation. Curr Opin Urol. 2010;20:432–436. doi: 10.1097/MOU.0b013e32833cf1ef. [DOI] [PubMed] [Google Scholar]
- 12.Lee KR, Lee JS, Kim YR, Song IG, Hong EK. Polysaccharide from Inonotus obliquus inhibits migration and invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via downregulation of NF-kB signaling pathway. Oncol Rep. 2014;31:2447–2453. doi: 10.3892/or.2014.3103. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Hu HG, Huang J, Zou Q, Wang J, Liu MQ, Zhao Y, Li GZ, Xue S, Wu ZS. Expression and correlation of Twist and gelatinases in breast cancer. Exp Ther Med. 2013;6:97–100. doi: 10.3892/etm.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Ren Y, Wu QC, Lin SX, Liang YJ, Liang HZ. Macrophage migration inhibitory factor enhances neoplastic cell invasion by inducing the expression of matrix metalloproteinase 9 and interleukin-8 in nasopharyngeal carcinoma cell lines. Chin Med J (Engl) 2004;117:107–114. [PubMed] [Google Scholar]
- 15.Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 16.Choi BD, Jeong SJ, Wang G, Park JJ, Lim DS, Kim BH, Cho YI, Kim CS, Jeong MJ. Secretory leukocyte protease inhibitor is associated with MMP-2 and MMP-9 to promote migration and invasion in SNU638 gastric cancer cells. Int J Mol Med. 2011;28:527–534. doi: 10.3892/ijmm.2011.726. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZY, Liang K, Lin Y, Yang F. Study of the UTMD-based delivery system to induce cervical cancer cell apoptosis and inhibit proliferation with shRNA targeting Survivin. Int J Mol Sci. 2013;14:1763–1777. doi: 10.3390/ijms14011763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, He P, Liu F, Shi L, Zhu H, Cheng X, Zhao J, Wang Y, Zhang M. Prognostic significance of B-cell lymphoma 2 expression in acute leukemia: A systematic review and meta-analysis. Mol Clin Oncol. 2014;2:411–414. doi: 10.3892/mco.2014.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, Yu P, Qian DH, Qin ZX, Sun XJ, Yu J, Huang L. Hydrogen-rich medium suppresses the generation of reactive oxygen species, elevates the Bcl-2/Bax ratio and inhibits advanced glycation end product-induced apoptosis. Int J Mol Med. 2013;31:1381–1387. doi: 10.3892/ijmm.2013.1334. [DOI] [PubMed] [Google Scholar]
- 20.Park HY, Kim GY, Moon SK, Kim WJ, Yoo YH, Choi YH. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules. 2014;19:5981–5998. doi: 10.3390/molecules19055981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan XJ, Wang Y, Wang L, Zhu M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36:3559–3567. doi: 10.3892/or.2016.5138. [DOI] [PubMed] [Google Scholar]
- 22.Lv C, Huang Y, Liu ZX, Yu D, Bai ZM. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomark. 2016;17:41–47. doi: 10.3233/CBM-160615. [DOI] [PubMed] [Google Scholar]
- 23.Li HL, Zhang S, Wang Y, Liang RR, Li J, An P, Wang ZM, Yang J, Li ZF. Baicalein induces apoptosis via a mitochondrial-dependent caspase activation pathway in T24 bladder cancer cells. Mol Med Rep. 2013;7:266–270. doi: 10.3892/mmr.2012.1123. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda T, Hori M. Five-year relative survival rate of bladder cancer in the USA, Europe and Japan. Jpn J Clin Oncol. 2014;44:776. doi: 10.1093/jjco/hyu101. [DOI] [PubMed] [Google Scholar]
- 25.Mi C, Shi H, Ma J, Han LZ, Lee JJ, Jin X. Celastrol induces the apoptosis of breast cancer cells and inhibits their invasion via downregulation of MMP-9. Oncol Rep. 2014;32:2527–2532. doi: 10.3892/or.2014.3535. [DOI] [PubMed] [Google Scholar]
- 26.Fribley AM, Miller JR, Brownell AL, Garshott DM, Zeng Q, Reist TE, Narula N, Cai P, Xi Y, Callaghan MU, et al. Celastrol induces unfolded protein response-dependent cell death in head and neck cancer. Exp Cell Res. 2015;330:412–422. doi: 10.1016/j.yexcr.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffen K, Oosting M, Quintin J, Ng A, Kleinnijenhuis J, Kumar V, van de Vosse E, Wijmenga C, van Crevel R, Oosterwijk E, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10:e1004485. doi: 10.1371/journal.ppat.1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz KJ, Ademi C, Bertram S, Schmid KW, Baba HA. Prognostic relevance of autophagy-related markers LC3, p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients with respect to KRAS mutational status. World J Surg Oncol. 2016;14:189. doi: 10.1186/s12957-016-0946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lihuan D, Jingcun Z, Ning J, Guozeng W, Yiwei C, Wei L, Jing Q, Yuanfang Z, Gang C. Photodynamic therapy with the novel photosensitizer chlorophyllin f induces apoptosis and autophagy in human bladder cancer cells. Lasers Surg Med. 2014;46:319–334. doi: 10.1002/lsm.22225. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Sun L, de Carvalho EL, Li X, Lv X, Khan GJ, Semukunzi H, Yuan S, Lin S. DT-13, a saponin monomer of dwarf lilyturf tuber, induces autophagy and potentiates anti-cancer effect of nutrient deprivation. Eur J Pharmacol. 2016;781:164–172. doi: 10.1016/j.ejphar.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Jia X, Wang K, Bao J, Li P, Chen M, Wan JB, Su H, Mei Z, He C. Polyphyllin VII Induces an Autophagic cell death by activation of the JNK pathway and inhibition of PI3K/AKT/mTOR pathway in HepG2 cells. PLoS One. 2016;11:e0147405. doi: 10.1371/journal.pone.0147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CJ, Chen TL, Tseng YY, Wu GJ, Hsieh MH, Lin YW, Chen RM. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol Appl Pharmacol. 2016;304:59–69. doi: 10.1016/j.taap.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Cao ZX, Yang YT, Yu S, Li YZ, Wang WW, Huang J, Xie XF, Xiong L, Lei S, Peng C. Pogostone induces autophagy and apoptosis involving PI3K/Akt/mTOR axis in human colorectal carcinoma HCT116 cells. J Ethnopharmacol. 2017;202:20–27. doi: 10.1016/j.jep.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara Y, Shiraya S, Miyake T, Yamakawa S, Aoki M, Makino H, Nishimura M, Morishita R. Inhibition of experimental abdominal aortic aneurysm in a rat model by the angiotensin receptor blocker valsartan. Int J Mol Med. 2008;22:703–708. [PubMed] [Google Scholar]
- 35.Eissa S, Shabayek MI, Ismail MF, El-Allawy RM, Hamdy MA. Diagnostic evaluation of apoptosis inhibitory gene and tissue inhibitor matrix metalloproteinase-2 in patients with bladder cancer. IUBMB Life. 2010;62:394–399. doi: 10.1002/iub.325. [DOI] [PubMed] [Google Scholar]
- 36.Sun KX, Xia HW, Xia RL. Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway. Int J Clin Exp Pathol. 2015;8:615–621. Perabo FG, Wirger A, Kamp S, Lindner H, Schmidt DH, Müller SC and Kohn EC: Carboxyamido-triazole (CAI), a signal transduction inhibitor induces growth inhibition and apoptosis in bladder cancer cells by modulation of Bcl-2. Anticancer Res 24: 2869–2877, 2004. [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan SS, Chang HL, Chen HW, Kuo FC, Liaw CC, Su JH, Wu YC. Selective cytotoxicity of squamocin on T24 bladder cancer cells at the S-phase via a Bax-, Bad-, and caspase-3-related pathways. Life Sci. 2006;78:869–874. doi: 10.1016/j.lfs.2005.05.068. [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Ding J, Wu Y. Resveratrol induces apoptosis of bladder cancer cells via miR21 regulation of the Akt/Bcl2 signaling pathway. Mol Med Rep. 2014;9:1467–1473. doi: 10.3892/mmr.2014.1950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.