Abstract
Cervical cancer stage-dependent therapies include surgery, chemotherapy, radiotherapy and chemoradiotherapy. Concurrent cisplatin-based chemoradiotherapy (CCRT) is the standard therapy for locally advanced cervical carcinoma (FIGO>IIB), however therapy resistance in a subset of patients is still a major clinical challenge. The present study aimed to analyze the impact of Oncostatin M (OSM) stimulation on CCRT-induced cell death. The present study used cells derived from cervical squamous cell carcinomas (SW756, 808, CaSki and 879) and adenocarcinoma (HeLa). The cervical carcinoma cells were HPV18-positive (HeLa, SW756, 808) or HPV16-positive (CaSki, 879). In addition to the established cell lines HeLa, SW756 and CaSki, the more recently generated cervical cancer cells 808 and 879 were also used. To analyze their radiosensitivity, cells were treated with increasing doses of irradiation (0–8 Gy). To mimic chemotherapy, radiotherapy or CCRT in vitro, the cells were challenged with 0.975 µg/ml cisplatin, irradiated with 6 Gy or a combination. A total of 10 ng/ml OSM was applied for 2 h prior to the respective therapy. The responsiveness toward radiation alone varied among the cervical carcinoma cells. CaSki, 808 and 879 cells were resistant to irradiation up to 8 Gy. OSM pre-treatment sensitized two out of five cell lines (HeLa and 879) to irradiation. Notably, all tested cells were sensitized by OSM for CCRT-treatment, particularly in the less radiosensitive cells. Cell death enhancement was dependent on phosphorylated signal transducer and activator of transcription 3 (STAT3; Tyr705) signaling activation as demonstrated with a dominant-negative version of STAT3 interfering with phosphorylation at Tyr705 (dnSTAT3-Y705F). In conclusion, OSM pre-treatment was able to override resistance to CCRT via the STAT3 signaling pathway.
Keywords: cervical carcinogenesis, human papillomavirus, radiotherapy, chemotherapy, Oncostatin M
Introduction
Cervical cancer is the third most death-related cancer in women worldwide and a consequence of persistent infection with high-risk human papillomaviruses. Neoplastic progression to cancer takes years or decades and develops from low-grade cervical intraepithelial neoplasia (CIN1) through high-grade lesions, CIN2 and CIN3 (carcinoma in situ) (1). Cervical cancer treatment depends on FIGO tumor stages and includes surgery, chemo-, radio- or chemoradiotherapy. For more than 50 years, radiation therapy was the standard treatment for patients with locally advanced cervical carcinoma but patients with advanced stage >IIB disease were cured only in 35–45% of cases with radiation therapy alone (2–4). According to the European clinical guidelines since 1999 locally advanced cervical carcinomas (FIGO>IIB) are treated with simultaneous cisplatin-based chemoradiotherapy (CCRT) (5). CCRT has become the standard treatment for locally advanced cervical carcinoma in North America and Europe (5) and several studies have demonstrated a 40–60% reduction in the relative risk of recurrence and a 30–50% reduction of the risk of death with CCRT (6–8). Nevertheless, resistance to non-surgical therapies is still a major challenge (9). For patients who do not respond to standard therapies, new strategies are needed.
We recently showed that cervical cancer cells can be sensitized for chemotherapeutic drug induced cell death. We found that pre-treatment of cervical carcinoma cells with Oncostatin M (OSM) resulted in enhanced responsiveness of the cells to chemotherapeutic drugs (10). OSM is a member of the IL6-type cytokine family (11) and binds to the OSM receptor-b which then associates with the receptor chain gp130. The recruitment of Janus kinases leads to subsequent signal transducer and activator of transcription 3 (STAT3)-phosphorylation at tyrosine 705 (12). We clarified the molecular mechanism responsible for cell death sensitization, which was dependent on the STAT3/IRF1 signaling pathway. This was unexpected because in cervical cancer patients in situ the STAT3 activation is weak or absent (10). This is in contrast to other malignancies, where STAT3 is constitutively active and is a considered anti-apoptotic factor (13–15).
Because CCRT is more frequently applied than neoadjuvant chemotherapy, we were interested in the impact of OSM pre-treatment on the responsiveness of cervical cancer cell to both irradiation and chemoradiotherapy in this study. We found varying sensitivities or even resistance of different cervical cancer cells toward irradiation alone. Notably, OSM pre-treatment sensitized all tested cervical cancer cells, including the irradiation resistant cells, for CCRT-induced cell death.
Materials and methods
Cells and cell culture
HPV16-positive CaSki [ATCC CRL-1550; (16)] or HPV18-positive cervical carcinoma cell lines SW756 [ATCC CRL-10302; (17)] and HeLa [ATCC CCL-2; (18)] were obtained from M. von Knebel-Doeberitz (Heidelberg, Germany) before 2000. Cells were authenticated by qRT-PCR for HPV16 or HPV18 E6 and E7 expression. Cells were cultured at a density of 1×106 in DMEM supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1 mM sodium pyruvate and 2 mM L-alanyl-L-glutamin (all from PAA, Pasching, Austria). The more recently generated cervical cancer cells 808 (HPV18-positive) and 879 (HPV16-positive) were obtained from P. L. Stern, cultured as previously described (19) and last tested by short tandem repeat profiling in 2014. All cells were tested for mycoplasma infection once per month.
Plasmids and transfections
The vectors pCAGGS and pCAGGS-STAT3F were kindly provided by Dr. K. Nakajima, Osaka City University, Japan and Dr. M. Hibi, Center for Developmental Biology, Kobe, Japan (12). For stable transfections HeLa cells were seeded into 10 cm culture dishes at a density of 8×105 cells/dish and transfected after 24 h with 300 ng linearized (PvuI) pCAGGS or pCAGGS-STAT3F and FuGene 6 (Roche, Mannheim, Germany) according to the manufacturer's guidelines. Clones were selected with 100 µg/ml G418 and analyzed for inhibition of STAT3 activation by western blot analysis.
Protein expression analysis by western blot
HeLa cells stably expressing pCAGGS or pCAGGS-STAT3F were seeded in 6 cm culture dishes at a density of 1.5×106 cells/dish. 24 h later they were incubated with medium or 10 ng/ml OSM (PeproTech, Hamburg, Germany) for 15 min. Stimulated cells were resuspended in sample buffer (62.5 mM Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 100 mM DTT) and equal amounts of protein were analyzed using Abs directed against pTyr705-STAT3 (Cell Signaling Technology, Inc., Danvers, MA, USA), STAT3 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or β-actin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Secondary Abs (Sigma-Aldrich; Merck KGaA) and ECL reagent (Roche) were used for standardized detection with ChemiDoc XRS+ Molecular Imager. Quantification was done with the Quantity One analysis software (both Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Irradiation
Cervical carcinoma cells were seeded in flat-bottom microtiter plates at a density of 1×104 cells/well. 24 h later cells received single-dose of irradiation (2, 4, 6, 8 Gy) using a linear accelerator (Oncor™; Siemens AG, Munich, Germany) as indicated. Separate plates were used for each irradiation dose. The plates were covered by 2 cm thick plexiglass leaf to improve photon dose homogeneity. The radiation characteristics were as follows: Size of the radiation field 30×30 cm; collimator angle 0°; gantry angle 0°; source surface distance 208 cm; beam energy 6 MV photons; dose-rate 2 Gy/min. Computed-tomography-based three-dimensional dose calculations were made with the Pinnacle™ planning system (Philips Radiation Oncology Systems; Philips Medical Systems, Fitchburg, WI, USA) previously.
Stimulation experiments and cytotoxicity assays
Cervical carcinoma cells were seeded in a flat-bottom microtiter plates at a density of 1×104 cells/well and incubated for 24 h. Cervical carcinoma cells were stimulated with 10 ng/ml OSM (PeproTech, Hamburg, Germany) for 2 h or medium as a control. For irradiation experiments cells were subsequently irradiated with increasing irradiation doses (0–8 Gy) as described above. For chemotherapy experiments cells were stimulated with medium or OSM and challenged with a cisplatin concentration of 0.975 µg/ml (Hexal, Holzkirchen, Germany) for 2 h. In chemoradiotherapy experiments cells were stimulated with medium or OSM, challenged with a cisplatin concentration of 0.975 µg/ml (Hexal) for 2 h and subsequent irradiated with a dose of 6 Gy. In all experiments cell viability was assessed 48 h later by the neutral red uptake method as described previously (10).
Statistical analysis
All statistical analyses were performed using the GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) program. To evaluate the statistical differences between multiple groups, one-way analysis of variance with Bonferroni post hoc test was applied. P<0.05 was considered to indicate a statistically significant difference.
Results
Heterogeneous radiosensitivity of cervical carcinoma cells
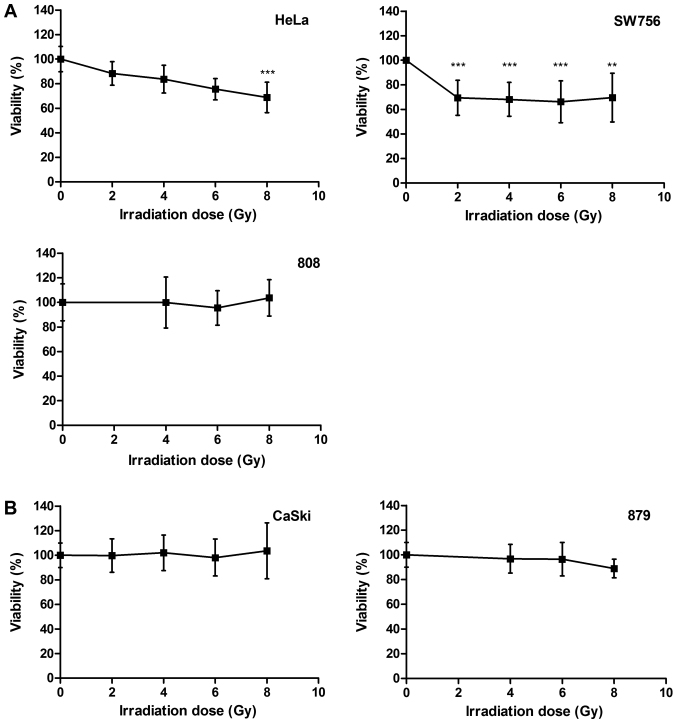
We compared the radiosensitivity of different cervical carcinoma cells. HPV18-positive cell lines HeLa and SW756, HPV16-positive cell line CaSki and the more recently generated cervical cancer cells 808 (HPV18-positive) and 879 (HPV16-positive) were treated with increasing doses of irradiation (0–8 Gy). After irradiation HeLa cells died in a dose dependent manner up to 24.5% at a dose of 6 Gy (P<0.001) and up to 32% at a dose of 8 Gy (P<0.001; Fig. 1A, left panel). In SW756 cells cell death was observed in 31% for 2 Gy (P<0.001). Again, higher irradiation doses (4–8 Gy) did not enhance radiosensitivity of these cells (Fig. 1A, right panel). 808 cells (Fig. 1A, lower panel), CaSki cells and 879 cells (Fig.1B) were almost completely resistant to irradiation in our experiments. Thus, the cervical carcinoma cells used in this study showed a heterogeneous responsiveness to irradiation.
Figure 1.
Cervical cancer cells demonstrate different radiosensitivity. (A) HeLa, SW756, 808 and (B) CaSki and 879 cells were treated with increasing doses of irradiation (0–8 Gy). After 48 h the cell viability was analyzed. The mean values from n=3 experiments performed in sextuplicates are indicated. **P<0.01 and ***P<0.001 vs. 0 Gy.
OSM signaling sensitizes cervical carcinoma cells for CCRT-induced cell death
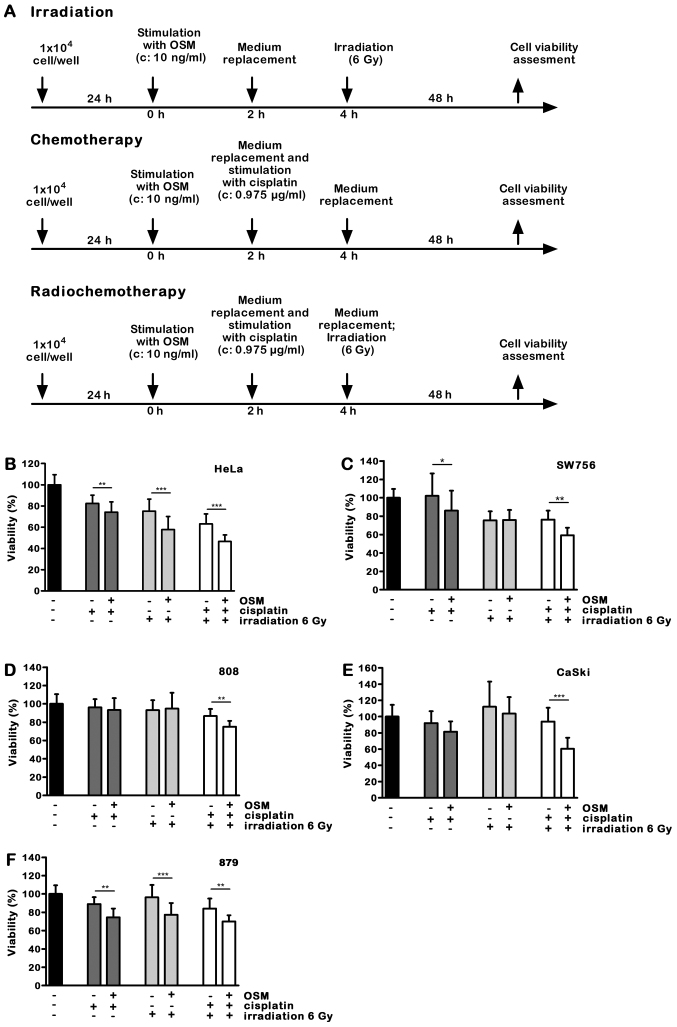
We previously described that OSM signaling sensitized cervical carcinoma cells to chemotherapeutic drug-induced cell death (10). Here we investigated the impact of OSM pre-treatment in cervical carcinoma cells on radio- or concurrent chemoradiotherapy. Cervical carcinoma cells were pre-treated with 10 ng/ml OSM or medium for 2 h. To mimic radio-, chemo- or chemoradiotherapy in vitro, cells were challenged with medium or 0.975 µg/ml cisplatin for 2 h and irradiated with a dose of 6 Gy or left untreated. The low cisplatin concentration of 0.975 µg/ml was selected to minimize its own effects on cancer cell viability but preserving its role as a radio sensitizer in chemoradiotherapy experiments. Cell viability was assessed after 48 h. A time schedule of the experimental procedure is shown in Fig. 2A.
Figure 2.
OSM sensitizes cervical cancer cells for chemoradiotherapy. (A) Time schedules of the experiments. (B) HeLa, (C) SW756, (D) 808, (E) CaSki and (F) 879 cells were treated with medium or OSM. Cells were incubated with medium or cisplatin, irradiated with 6 Gy or left untreated and the cell viability was assessed. n=3 experiments were performed in sextuplicates. *P<0.05, **P<0.01 and ***P<0.001. OSM, Oncostatin M.
While the low cisplatin dose of 0.975 µg/ml alone had only a minor effect on cell viability, OSM pretreatment for 2 h further increased cell death induction in 4 out of 5 cell lines. In 808 cells the selected combination of OSM and cisplatin had no effect on cell viability (Fig. 2B-F; dark grey bars).
We then studied the impact of OSM stimulation on irradiation induced cell death. Cell viability of HeLa cells irradiated with a dose of 6 Gy was 75.6%. OSM sensitized the HPV18 positive HeLa cells for irradiation-induced cell death (Fig. 2B, 18%, light grey bars; P<0.001)), whereas in SW756 and 808 cells OSM pretreatment did not affect cell viability in combination with irradiation (Fig. 2C and D). OSM pretreatment sensitized the HPV16 positive cell line CaSki only slightly (7%; Fig. 2E), whereas it significantly sensitized the 879 cells for irradiation-induced cell death (Fig. 2F; 19.1%, light grey bars; P<0.001). Thus, pre-treatment with OSM significantly sensitized two of five tested cervical cancer cells for irradiation-induced cell death. Furthermore, in the completely radioresistant 879 cells OSM pretreatment was sufficient to sensitize for irradiation.
Concurrent chemoradiotherapy is the standard treatment for advanced cervical cancers with FIGO>IIB. For this reason we analyzed the impact of OSM pretreatment on chemoradiotherapy-induced cell death. The HPV18 positive cell lines HeLa and SW756 as well as the more recently generated cells 808 were killed significantly more by OSM stimulation (Fig. 2B-D; 12–17%, white bars; P<0.01). OSM significantly sensitized the HPV16-positive CaSki cells for chemoradiotherapy-induced cell death up to 33% (Fig. 2E; white bars; P<0.001) and the more recently generated 879 cells, that were radio-resistant in our experiments, for enhanced cell death after combined chemoradiotherapy treatment (Fig. 2F; white bars; P<0.01).
Taken together, OSM stimulation of all five tested cervical carcinoma cells sensitized these cells for chemoradiotherapy-induced cell death. Notably, OSM treatment induced responsiveness to chemoradiotherapy-induced cell death in the irradiation-resistant cells 808, CaSki and 879. Table I summarizes our findings.
Table I.
OSM-induced cell death sensitization in different HPV-18- and HPV16-positive cervical cancer cells toward irradiation, chemo- and chemoradiotherapy.
| Response toward combined OSM pretreatment and | ||||
|---|---|---|---|---|
| HPV status | Cellsa | Irradiation | Chemotherapy | Chemoradiotherapy |
| HPV18-positive | HeLa | ++ | + | ++ |
| SW756 | − | + | ++ | |
| 808 | − | − | ++ | |
| HPV16-positive | CaSki | + | + | +++ |
| 879 | ++ | ++ | ++ | |
Cervical cancer cells were stimulated with OSM (10 ng/ml) or medium for 2 h and irradiated with 6 Gy (irradiation), treated with 0.975 µg/ml cisplatin (chemotherapy) or a combination of both (chemoradiotherapy). OSM-induced cell death sensitization in comparison with the medium treated cells was evaluated and indicated as follows: -, no OSM-mediated cell death sensitization; +, OSM-mediated cell death sensitization up to 10%; ++, OSM-mediated cell death sensitization from 10–20%; +++, OSM-mediated cell death sensitization >30%. HPV, human papilloma virus; OSM, Oncostatin M.
STAT3 mediates sensitization for CCRT-induced cell death by OSM signaling in cervical cancer cells
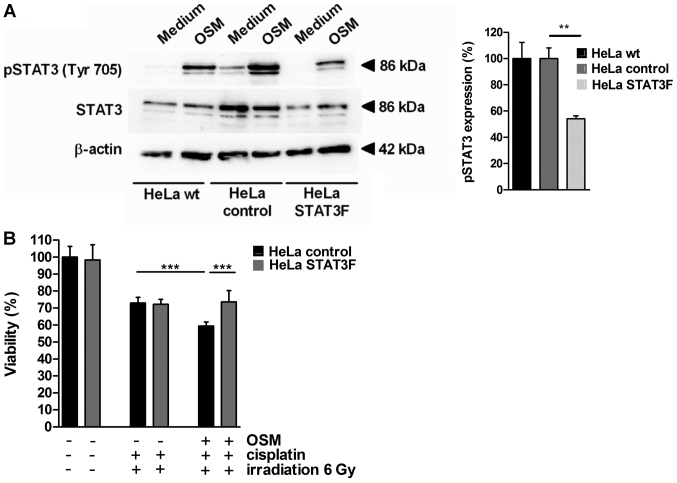
To investigate the molecular mechanism for CCRT-induced cell death sensitization by OSM in cervical cancer cells, HeLa cells were stably transfected with a dominant-negative version of STAT3 interfering with phosphorylation at Tyr705 (dnSTAT3-Y705F, HeLa STAT3F) or the empty vector as a control (HeLa control). OSM stimulation led to STAT3 phosphorylation at Tyrosin705 in HeLa wt and HeLa control cells (Fig. 3A) while in HeLa cells stably expressing STAT3F the pSTAT3 (Tyr705) phosphorylation was significantly decreased (45% reduction). In cell viability assays OSM pretreatment sensitized HeLa control cells for CCRT-induced cell death (18.7%, black bars, Fig. 3B). STAT3F overexpression completely abolished OSM-mediated sensitization (grey bars; P<0.001). Thus, our results provide evidence that chemoradiosensitisation by OSM depends on the pSTAT3 (Tyr705) signaling pathway in cervical cancer cells.
Figure 3.
STAT3 mediates OSM-induced cell death sensitization for chemoradio-therapy. (A) HeLa wild type cells or HeLa cells stably expressing pCAGGS-STAT3F or pCAGGS vector only were stimulated with medium or OSM for 15 min. Whole cell extracts were prepared and analyzed by western blot analysis using pTyr705-specific anti-STAT3 or non-phosphorylation-specific antibodies. Equal loading was controlled using β-actin specific monoclonal antibodies. The graph indicates the quantification of three independent western blots. (B) HeLa cells stably expressing pCAGGS-STAT3F or pCAGGS vector were stimulated with medium or OSM. Cells were incubated with medium or cisplatin, irradiated with 6 Gy or left untreated and cell viability was assessed. A total of n=2 experiments performed in sextuplicates were performed. **P<0.01 and ***P<0.001. OSM, Oncostatin M; STAT3, signal transducer and activator of transcription 3.
Discussion
Resistance of cervical cancer patients toward platinum-based radio/chemotherapy is a major clinical problem (9). For patients who do not respond to standard therapies new therapeutic strategies are needed. In our study we analyzed the impact of OSM pretreatment on the response of cervical cancer cells to radio/chemotherapy. Cervical cancer cells responded heterogeneously toward irradiation alone, three of the tested cells were resistant in our experiments. However, OSM pretreatment improved chemosensitivity for irradiation in all cervical cancer cells and even rendered two cell lines susceptible for irradiation that were otherwise completely resistant.
Over the past years, improved understanding in cancer pathogenesis gave rise to new treatment options to support standard cancer therapies based on surgery or radio/chemotherapy. This includes therapies that target tumor angiogenesis and cancer growth, as well as cancer immunotherapies that activate the patient immune system to support antitumor immunity (20,21). Targeted therapy strategies include several antibodies or inhibitors to block essential biochemical pathways required for tumor growth and survival, like EGFR, VEGF, BRAF or HER2 (21). Blockage of the inhibitory proteins CTLA-4, PD-1 or the ligand PD1-L with specific antibodies, checkpoint inhibitors (22), resulted in clinical benefit in several tumor types (23–25).
In cervical cancer the only approved targeted therapy so far is bevacizumab, an anti-VEGF antibody to inhibit angiogenesis, in combination with a cisplatin-based chemotherapy in patients with advanced, metastatic or recurrent cervical cancer (26,27). There is a strong need for new therapeutic strategies in cervical cancer patients because HPV interferes with local immunity suppressing the expression of inflammatory cytokines and chemokines in infected cells (28). Immunostimulatory cytokines like CCL2 and CCL20 are induced in the stromal compartment of invasive cervical carcinoma but they are involved in the generation of a pro-tumorigenic microenvironment (29–31). In contrast, the regulator of the adaptive immunity interleukin-12 is down-regulated in the cervical cancer microenvironment [own unpublished data and (32,33)]. One immunotherapeutic strategy might be usage of the synthetic viral dsRNA homolog polyinosinic:polycytidylic acid (PolyIC) that can stimulate necroptosis in cervical cancer cells expressing the kinase RIPK3 (34,35). Notably, this leads to enhanced interleukin-12 production of dendritic cells (34).
Another strategy might be the application of cell death sensitizers that employ the STAT3/IRF1 signaling pathway. We have recently shown that stimulation of cervical cancer cells with IL-6 in combination with the soluble IL-6R or OSM can potently activate STAT3 which leads to IRF1 up-regulation (10). Patients with high expression of the STAT3-regulated pro-apoptotic IRF1 in pretreatment cervical cancer biopsy cells showed in fact significantly higher responses to neoadjuvant chemo- and chemoradiotherapy (10). In line with this, the in vitro results from this study confirmed that cell death sensitization toward irradiation or chemoradiotherapy is induced by OSM pre-treatment of cervical carcinoma cells. As the underlying mechanism we identified the pSTAT3 (Tyr705) signaling pathway that sensitized cervical cancer cells for CCRT-induced cell death as shown via stable transfection of dominant-negative STAT3F (12). In this study we showed that OSM pre-treatment improved chemosensitivity for irradiation in all cervical cancer cells particularly in the initially radio-resistant cells 808, CaSki and 879 with up to 33% cell death enhancement. Sensitization by OSM stimulation for CCRT-induced cell death occurred in all tested cervical cancer cells irrespectively whether they were positive for HPV16 or HPV18. However, it appeared that HPV16 positive cervical cancer cells showed a slightly higher responsiveness towards OSM-mediated sensitization. This was particularly the case for CaSki cells. It can be speculated that their stronger response to OSM might be due to differences in the interaction between HPV16 and the OSM/STAT3 signaling pathway. Alternatively, the genetic or epigenetic alterations in these cells might affect their OSM-responsiveness. This will be subject of future studies.
OSM binds to the OSM receptor-β (OSM-R) which then associates with the receptor chain gp130 to activate the STAT3 signaling pathway (12,36). Recent studies indicate that OSM-R is overexpressed in advanced cervical squamous cell carcinomas making the cells susceptible for OSM signals. However, high expression of OSM-R in cervical cancers is associated with worse clinical outcome and OSM signals were described to initiate several pro-tumorigenic effects (37,38). For this reason, OSM-R is recommended as a candidate for antibody-mediated inhibition to block pro-malignant effects (38,39).
However, based on our findings [(10) and this study] blockage of OSM-R should be employed with caution. Indeed, inhibition of the OSM-R might block OSM-initiated pro-malignant effects but it would concurrently prevent sensitization of cervical cancer cells to chemo- or chemoradiotherapy. Thus OSM-R might have a dual role in cervical cancers and this may have major implications for personalization of cervical cancer therapy. In conclusion, based on our findings OSM pre-treatment might be an interesting option to improve the responsiveness of cervical cancer cells toward irradiation or chemoradiotherapy particularly in radioresistant cells. OSM-R blockage should therefore not be applied prior to irradiation or chemoradiotherapy.
Acknowledgements
The authors would like to thank Mr. Georg Blaß (Department of Radiotherapy and Radiation Oncology, Saarland University, Homburg, Germany) for their assistance in the irradiation experiments and Dr G.S. Stein (University of Massachusetts Medical School, Worcester, MA, USA), Dr T. Hirano, Dr M. Hibi (both Center for Developmental Biology, Kobe, Japan) and Dr K. Nakajima (Osaka City University, Osaka, Japan) for providing the cDNA constructs.
Funding
This work was supported by a grant from the Deutsche Krebshilfe (grant no. 109752) and the Saarland Staatskanzlei to SS (grant no. WT/2-LFFP 14/15).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
Conception and design: BWR, SS; Development of methodology: RS, BWR, JF, CR; Acquisition of data: RS, BWR; IJB, EFS; Analysis and interpretation of data: RS, BWR, SS; Writing, review, and/or revision of the manuscript: RS, BWR, CR, IJB, EFS, SS; Final approval of the version to be published: RS, BWR, JF, IJB, CR, EFS, SS; Administrative, technical, or material support: IJB, EFS, CR, SS; Study supervision: SS.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hausen Zur H. Papillomaviruses in the causation of human cancers-a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Barillot I, Horiot JC, Pigneux J, Schraub S, Pourquier H, Daly N, Bolla M, Rozan R. Carcinoma of the intact uterine cervix treated with radiotherapy alone: A French cooperative study: Update and multivariate analysis of prognostics factors. Int J Radiat Oncol Biol Phys. 1997;38:969–978. doi: 10.1016/S0360-3016(97)00145-4. [DOI] [PubMed] [Google Scholar]
- 3.Logsdon MD, Eifel PJ. Figo IIIB squamous cell carcinoma of the cervix: An analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys. 1999;43:763–775. doi: 10.1016/S0360-3016(98)00482-9. [DOI] [PubMed] [Google Scholar]
- 4.Perez CA, Camel HM, Kuske RR, Kao MS, Galakatos A, Hederman MA, Powers WE. Radiation therapy alone in the treatment of carcinoma of the uterine cervix: A 20-year experience. Gynecol Oncol. 1986;23:127–140. doi: 10.1016/0090-8258(86)90216-7. [DOI] [PubMed] [Google Scholar]
- 5.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J Clin Oncol. 2007;25:2952–2965. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 6.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 7.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, III, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 8.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 9.Peltenburg LT. Radiosensitivity of tumor cells. Oncogenes and apoptosis. Q J Nucl Med. 2000;44:355–364. [PubMed] [Google Scholar]
- 10.Walch-Ruckheim B, Pahne-Zeppenfeld J, Fischbach J, Wickenhauser C, Horn LC, Tharun L, Büttner R, Mallmann P, Stern P, Kim YJ, et al. STAT3/IRF1 Pathway activation sensitizes cervical cancer cells to chemotherapeutic drugs. Cancer Res. 2016;76:3872–3883. doi: 10.1158/0008-5472.CAN-14-1306. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. Embo J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 13.Wei LH, Kuo ML, Chen CA, Chou CH, Cheng WF, Chang MC, Su JL, Hsieh CY. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001;20:5799–5809. doi: 10.1038/sj.onc.1204733. [DOI] [PubMed] [Google Scholar]
- 14.Jee SH, Chiu HC, Tsai TF, Tsai WL, Liao YH, Chu CY, Kuo ML. The phosphotidyl inositol 3-kinase/Akt signal pathway is involved in interleukin-6-mediated Mcl-1 upregulation and anti-apoptosis activity in basal cell carcinoma cells. J Invest Dermatol. 2002;119:1121–1127. doi: 10.1046/j.1523-1747.2002.19503.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA, Cheng G, Hall BM, Lin J. Stat3 activation in human endometrial and cervical cancers. Br J Cancer. 2007;96:591–599. doi: 10.1038/sj.bjc.6603597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattillo RA, Hussa RO, Story MT, Ruckert AC, Shalaby MR, Mattingly RF. Tumor antigen and human chorionic gonadotropin in CaSki cells: A new epidermoid cervical cancer cell line. Science. 1977;196:1456–1458. doi: 10.1126/science.867042. [DOI] [PubMed] [Google Scholar]
- 17.Freedman RS, Bowen JM, Leibovitz A, Pathak S, Siciliano MJ, Gallager HS, Giovanella BC. Characterization of a cell line (SW756) derived from a human squamous carcinoma of the uterine cervix. In vitro. 1982;18:719–726. doi: 10.1007/BF02796428. [DOI] [PubMed] [Google Scholar]
- 18.Jones HW, Jr, McKusick VA, Harper PS, Wuu KD. George Otto Gey. (1899–1970). The HeLa cell and a reappraisal of its origin. Obstet Gynecol. 1971;38:945–949. [PubMed] [Google Scholar]
- 19.Brady CS, Bartholomew JS, Burt DJ, Duggan-Keen MF, Glenville S, Telford N, Little AM, Davidson JA, Jimenez P, Ruiz-Cabello F, et al. Multiple mechanisms underlie HLA dysregulation in cervical cancer. Tissue Antigens. 2000;55:401–411. doi: 10.1034/j.1399-0039.2000.550502.x. [DOI] [PubMed] [Google Scholar]
- 20.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 2016;37:462–476. doi: 10.1016/j.it.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Melero I, Berman DM, Aznar MA, Korman AJ, Gracia Pérez JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tewari KS, Sill MW, Long HJ, III, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuda N, Watari H, Ushijima K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin J Cancer Res. 2016;28:241–253. doi: 10.21147/j.issn.1000-9604.2016.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karim R, Meyers C, Backendorf C, Ludigs K, Offringa R, van Ommen GJ, Melief CJ, van der Burg SH, Boer JM. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6:e17848. doi: 10.1371/journal.pone.0017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroer N, Pahne J, Walch B, Wickenhauser C, Smola S. Molecular pathobiology of human cervical high-grade lesions: Paracrine STAT3 activation in tumor-instructed myeloid cells drives local MMP-9 expression. Cancer Res. 2011;71:87–97. doi: 10.1158/0008-5472.CAN-10-2193. [DOI] [PubMed] [Google Scholar]
- 30.Pahne-Zeppenfeld J, Schröer N, Walch-Rückheim B, Oldak M, Gorter A, Hegde S, Smola S. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9 expressing dendritic cells. Int J Cancer. 2014;134:2061–2073. doi: 10.1002/ijc.28549. [DOI] [PubMed] [Google Scholar]
- 31.Walch-Rückheim B, Mavrova R, Henning M, Vicinus B, Kim YJ, Bohle RM, Juhasz-Böss I, Solomayer EF, Smola S. Stromal fibroblasts induce CCL20 through IL6/C/EBPbeta to support the recruitment of Th17 cells during cervical cancer progression. Cancer Res. 2015;75:5248–5259. doi: 10.1158/0008-5472.CAN-15-0732. [DOI] [PubMed] [Google Scholar]
- 32.Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011;187:1157–1165. doi: 10.4049/jimmunol.1100889. [DOI] [PubMed] [Google Scholar]
- 33.Zijlmans HJ, Punt S, Fleuren GJ, Trimbos JB, Kenter GG, Gorter A. Role of IL-12p40 in cervical carcinoma. Br J Cancer. 2012;107:1956–1962. doi: 10.1038/bjc.2012.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt SV, Seibert S, Walch-Rückheim B, Vicinus B, Kamionka EM, Pahne-Zeppenfeld J, Solomayer EF, Kim YJ, Bohle RM, Smola S. RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1α release and efficient paracrine dendritic cell activation. Oncotarget. 2015;6:8635–8647. doi: 10.18632/oncotarget.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smola S. RIPK3-a predictive marker for personalized immunotherapy? Oncoimmunology. 2015;5:e1075695. doi: 10.1080/2162402X.2015.1075695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol. 2003;149:39–52. doi: 10.1007/s10254-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 37.Winder DM, Chattopadhyay A, Muralidhar B, Bauer J, English WR, Zhang X, Karagavriilidou K, Roberts I, Pett MR, Murphy G, Coleman N. Overexpression of the oncostatin M receptor in cervical squamous cell carcinoma cells is associated with a pro-angiogenic phenotype and increased cell motility and invasiveness. J Pathol. 2011;225:448–462. doi: 10.1002/path.2968. [DOI] [PubMed] [Google Scholar]
- 38.Caffarel MM, Coleman N. Oncostatin M receptor is a novel therapeutic target in cervical squamous cell carcinoma. J Pathol. 2014;232:386–390. doi: 10.1002/path.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucia-Tran JA, Tulkki V, Smith S, Scarpini CG, Hughes K, Araujo AM, Yan KY, Botthof J, Pérez-Gómez E, Quintanilla M, et al. Overexpression of the oncostatin-M receptor in cervical squamous cell carcinoma is associated with epithelial-mesenchymal transition and poor overall survival. Br J Cancer. 2016;115:212–222. doi: 10.1038/bjc.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.