Abstract
Circulating tumor cells (CTCs) dissemination is involved in tumor metastasis and is an independent prognostic factor in patients with primary and metastatic breast cancer. Regulatory T cells (Tregs) and cluster of differentiation (CD)8+ T lymphocytes are the main types of immune cells in the tumor microenvironment and exert opposite roles on the progression and outcome of breast cancer. The aim of the present study was to evaluate the associations between CTCs and intratumoral/peritumoral Tregs and CD8+ T lymphocytes in breast cancer. Peripheral CTCs were detected by a multi-marker quantitative polymerase chain reaction platform in 167 patients with invasive breast cancer. Intratumoral/peritumoral Tregs and CD8+ T lymphocytes were assessed by immunohistochemistry in 167 patients with invasive breast cancer to establish an association between these cell types and detection of peripheral CTCs. CTCs were detected in 55% of the patients with breast cancer. The prevalence of CTCs was positively associated with the number of intratumoral (P=0.002) and peritumoral Tregs (P=0.045), and the primary tumor size (P=0.012). This result was verified by analyzing intratumoral Tregs (P=0.044) and primary tumor size (P=0.044) with multivariate analysis, which indicated that the CTC-positive rate increased with an increasing number of intratumoral Tregs and a larger tumor size In the multivariate analysis, other variables including menopause, tumor-node-metastasis stage and peritumoral Tregs were not associated with the prevalence of CTCs. The prevalence of CTCs was inversely and weakly associated with the number of peritumoral CD8+ T lymphocytes using the univariate analysis, however this result was not statistically significant (P=0.470). In conclusion, regulatory T cells and CD8+ T lymphocytes may be involved, at least in part, in the CTCs dissemination of breast cancer, whereby Tregs appear to exert the dominant effect. Furthermore, the role of Tregs in the progression of breast cancer may be mediated by suppressing the dissemination of CTCs, which is primarily determined by intratumoral Tregs.
Keywords: breast cancer, circulating tumor cells, regulatory T cells, cluster of differentiation 8+ T lymphocytes
Introduction
Metastasis is a major cause of mortality in breast cancer. The metastatic cascade is composed of a series of biological steps that enables tumor cells to detach from the primary tumor, invade into the bloodstream and lymphatic system, extravasate into the parenchyma and establish a new metastatic lesion (1). This process may occur at the early stages of breast cancer. Circulating tumor cells (CTCs) may have a crucial role in the metastatic cascade and are a prognostic factor in patients with primary and metastatic breast cancer (2). CTC dissemination, as a part of metastasis, is a highly inefficient process, and only a minority of tumor cells that escape from local and systemic immune surveillance are capable of forming metastases (3–5). Therefore, immune changes in the primary tumor microenvironment may be involved in the dissemination of CTCs in breast cancer. Current evidence concerning the association between CTCs and the immune system in breast cancer is limited (6,7).
Tumor-infiltrating lymphocytes (TILs) are heterogeneous immune cells infiltrating in the tumor microenvironment and are considered to be the main components of the body's immune surveillance mechanism against cancer. In breast cancer, TILs are a heterogeneous population that may promote humoral and cellular antitumor responses (3,8). For example, as the predominant T lymphocytes infiltrate the tumor microenvironment, cluster of differentiation CD8+ T lymphocytes may exert cytotoxic activity towards tumor cells by triggering apoptosis (9). Therefore, CD8+ T lymphocytes are hypothesized to be the frontline defense against cancer. Several studies have observed an association between a high number of CD8+ TILs and favorable clinical outcomes (10,11). When CD8+ T lymphocytes fail to eliminate tumor cells, these tumors cells, which have reduced immunogenicity, may break through the primary tumor microenvironment (12). Accordingly, poor infiltration of CD8+ T lymphocytes may have a role in the initial dissemination of CTCs. Apart from a decrease in immune surveillance, several subsets of TILs are known to suppress anti-tumor immunity (3), which may participate in the dissemination of CTCs.
Considerable attention has been paid to regulatory T cells (Tregs), which have been identified as forkhead box (Foxp)3+ T cells (12,13). Tregs have an important role in immune evasion in cancer cells (14,15). Studies have confirmed that an increased number of intratumoral or peritumoral Tregs has a negative impact on the prognosis of breast cancer depending on the localization in the tumor microenvironment (16–18). Due to their opposing actions in tumor immunity and established use for breast cancer prognosis, a combined assessment of CD8+ T lymphocytes and infiltration of Tregs may be used to assess the impact of the immune microenvironment on the dissemination of CTCs in breast cancer (19–21).
The present study was conducted to assess the association between the number of tumor infiltrating CD8+ T lymphocytes/Tregs and the presence of CTCs in the peripheral blood. The TILs analyzed in the present study include the intratumoral and peritumoral cell types. A multi-marker quantitative polymerase chain reaction (qPCR) platform was established by using a combination of three widely used mRNA markers: Cytokeratin 19 (CK19), human mammaglobin (hMAM) and small breast epithelial mucin (SBEM) (21,22). In addition, the infiltrating CD8+ lymphocytes and Tregs were detected using antibodies against CD8 and Foxp3 by immunohistochemistry (IHC). Then, the association between the prevalence of CTCs and intratumoral/peritumoral Tregs or CD8+ T lymphocytes was analyzed.
Materials and methods
Patients
Between May 2015 and January 2017, 167 patients with invasive breast cancer, who were treated at the Department of Thyroid and Breast Surgery of The Affiliated Hospital of Nantong University (Nantong, China), were enrolled in the present study. The median age of the patients was 50 years (range, 26–84 years). The clinicopathological parameters evaluated for each patient included age, menstrual status, tumor size, tumor-node-metastasis (TNM) stage, histological grade, estrogen receptor (ER) and progesterone receptor (PR) status and human epidermal growth factor receptor 2 (HER2) status (Table I). For each patient, a detailed past medical history was recorded to exclude liver and kidney dysfunction, autoimmune disease, bone marrow disease and chronic infection. All tissue and blood samples were collected prior to chemotherapy, radiotherapy, endocrine therapy or any other treatment that could affect the immune state. A complete diagnostic evaluation, including history, physical examination, ultrasound, chest computed tomography (CT), abdominal CT, bone scan and tumor marker tests, was performed to exclude distant metastasis.
Table I.
Association between the CTC-positive rate in the peripheral blood of patients with breast cancer and clinicopathological parameters.
| Clinicopathological parameters | Total, n=167 | CTC-positive rate (%), n=92 | CTC-negative rate (%), n=75 | P-value |
|---|---|---|---|---|
| Age, years | 0.530 | |||
| ≤45 | 45 | 23 (51) | 22 (49) | |
| >45 | 122 | 69 (57) | 53 (43) | |
| Menopausal status | 0.084 | |||
| Premenopausal | 68 | 32 (47) | 36 (53) | |
| Postmenopausal | 99 | 60 (61) | 39 (39) | |
| Tumor sizea | 0.012 | |||
| T1 | 65 | 27 (42) | 38 (58) | |
| T2 | 98 | 62 (63) | 36 (37) | |
| T3 | 4 | 3 (75) | 1 (25) | |
| Lymph node stagea | 0.120 | |||
| pN0 | 102 | 52 (51) | 50 (49) | |
| pN1 | 34 | 20 (59) | 14 (41) | |
| pN2 | 15 | 7 (47) | 8 (53) | |
| pN3 | 16 | 13 (81) | 3 (19) | |
| Disease stagea | 0.095 | |||
| I | 43 | 18 (42) | 25 (58) | |
| II | 92 | 53 (58) | 39 (42) | |
| III | 32 | 21 (66) | 11 (34) | |
| Histological grade | 0.494 | |||
| 1 | 12 | 6 (50) | 6 (50) | |
| 2 | 98 | 51 (52) | 47 (48) | |
| 3 | 57 | 35 (61) | 22 (39) | |
| ER/PR statusb | 0.600 | |||
| Negative | 50 | 26 (52) | 24 (48) | |
| Positive | 117 | 66 (56) | 51 (44) | |
| HER2 statusc | 0.143 | |||
| Negative | 127 | 65 (51) | 62 (49) | |
| Positive | 40 | 27 (67) | 13 (33) |
Classified according to the Union for International Cancer Control.
ER/PR status was determined to be positive when the expression of one or both of the receptors are positive.
HER2 status was determined to be positive when a score of 3+ was attained in immunohistochemistry, or positive fluorescence was detected by in situ hybridization. CTC, circulating tumor cell; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
To define the baseline expression and positive threshold of the three markers (CK19, hMAM and SBEM), peripheral blood samples were also collected from control subjects. A total of 30 healthy female volunteers (aged 23–85 years; median, 44 years) and 60 patients with pathologically diagnosed benign breast disease (aged 23–72 years; median, 38 years) were enrolled between May 2015 and January 2017. The present study was approved by the Ethics Committee of Nantong University (Nantong, China) and The Affiliated Hospital of Nantong University (Nantong, China). All donors and patients provided written informed consent.
Blood processing
Following shedding of the first 2 ml of peripheral blood to reduce contamination by epithelial cells from the skin, 10 ml peripheral blood was collected in an EDTA-treated vacuum tube from each patient or control subject. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood by Ficoll density gradient centrifugation (Ficoll-Paque Plus; TBDscience, Tianjin, China) using standard procedures and frozen at −80°C until RNA extraction.
Reverse transcription-qPCR
Total RNA was isolated from PBMCs of patients with invasive breast cancer control subjects and 4 breast cancer tissue specimens. These were were selected as breast cancer tissues have been proven to express the genes CK19, hMAM and SBEM, it was chosen to verify the stability of the multi-maker RT-PCR system, and served as a positive control. Total RNA was isolated using TRIzol (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. The purity and quantification of RNA were assessed by measuring the optical density at 260 and 280 nm. The RNA quality was also verified by electrophoresis in a 1.5% non-denaturing agarose gel. The RNA integrity was assessed by qPCR amplification of the housekeeping gene, β-actin. Reverse transcription of the RNA was performed using the Prime-Script RT Master Mix system (Takara Biotechnology Co., Ltd.). cDNA was synthesized from 1.5 mg total RNA that was isolated from PBMCs in a total volume of 30 µl according to the manufacturer's protocol.
The primers for CK19, SBEM, hMAM and β-actin were designed using the software Primer version 5.0 (PREMIER, Palo Alto, CA, USA). All primers were synthesized by Invitrogen (Table II) (Invitrogen; Thermo Fisher Scientific, Inc.). All PCR reactions were performed using the ABI StepOnePlus real-time PCR system (Applied Biosystems, Thermo Fisher Scientific, Inc.) and fluorescent SYBR-Green I. qPCR was performed using the SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) according to the protocol in a final volume of 20 µl containing 10 µl 2× SYBR Premix Ex Taq, 0.4 µl PCR forward primer (10 µM), 0.4 µl PCR reverse primer (10 µM), 0.4 µl 50× ROX reference dye, 2 µl cDNA template and 6.8 µl dH2O. The thermocycling conditions consisted of 30 sec of predenaturation at 95°C and 40 cycles of PCR (denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec and extension at 72°C for 30 sec).
Table II.
Prime sequences.
| Gene | Sequence (5′-3′) |
|---|---|
| CK19 | |
| Sense | TCCGAACCAAGTTTGAGACG |
| Antisense | CCCTCAGCGTACTGATTTCCT |
| hMAM | |
| Sense | AGAACTGCAGGGTATGGTGAGAA |
| Antisense | ACATGTATAGCAGGTTTCAACAATTGT |
| SBEM | |
| Sense | GATCTTCAGGTCACCACCATG |
| Antisense | GGGACACACTCTACCATTCG |
| β-actin | |
| Sense | GCTGTGCTATCCCTGTACGC |
| Antisense | TGCCTCAGGGCAGCGGAACC |
CK19, cytokeratin 19; hMAM, human mammaglobin; SBEM, small breast epithelial mucin.
The relative expression of the marker genes was normalized to β-actin expression and calculated using the formula: 2−ΔΔCq (23). The relative gene expression value for the marker genes (CK19, hMAM and SBEM), in the peripheral blood of a healthy volunteer was set to 1.
qPCR positivity was defined as gene expression beyond the cut-off threshold, which was set for each gene marker at three standard deviations from the mean expression in the control subjects (24). Positivity of the multi-marker method was defined as positivity for at least one of the markers.
IHC staining and quantification of CD8 and Foxp3
Formalin-fixed (concentration 10%, room temperature, fixed for 24 h) paraffin sections (4 µm) of the tumor tissues were subjected to IHC staining. Briefly, the sections were deparaffinized and rehydrated in graded alcohol. Antigen retrieval was performed at 121°C for 2 min in citrate buffer (pH 6.0). After serial blocking with hydrogen peroxide and normal goat serum (Luoshen Biotechnologies, Inc., Shanghai, China, http://eastdiagno.bioon.com.cn/index_93476.html) at room temperature for 10 min, the sections were incubated with a primary monoclonal antibody against CD8 (catalog no, MA5-13473; clone C8/144B; dilution, 1:40; Thermo Fisher Scientific, Inc.) or Foxp3 (catalog no, ab20034; clone 236A/E7; dilution, 1:100; Abcam, Cambridge, UK) overnight at 4°C. Labeling was detected using the PV-6000 Polymer Detection system (OriGene Technologies, Inc., Beijing, China). Sections were counterstained with hematoxylin at room temperature for 3 min. Positive staining controls were performed in parallel with tonsil sections provided by patients in otolaryngology between October 2016 and January 2017, including 2 males and 3 females. A negative staining control was performed by omitting the use of the primary antibody.
The CD8+ T lymphocytes and Foxp3+ Tregs were quantified independently by two trained investigators, who were blinded to the clinical data and positivity of peripheral CTCs. A total of 5 intratumoral and 5 peritumoral areas were selected and imaged using an ×10 objective lens of a light microscope (Nikon 50i and Nikon DS-U3; Nikon Corporation, Tokyo, Japan). The images were obtained using an ×40 objective lens for cell counting. Due to the extensive heterogeneity in peritumoral infiltrating CD8+ T lymphocytes and Foxp3+ Tregs, a modified grading system for semi-quantitative scoring was used. In this system, G0 corresponded to the absence of lymphocytes. G1 corresponded to the mean number of CD8+ T lymphocytes ≤50 and Foxp3+ Tregs ≤25. G2 corresponded to the mean number of CD8+ T lymphocytes >50 and ≤100 and Foxp3+ Tregs >25 and ≤50. G3 corresponded to the mean number of CD8+ T lymphocytes >100 and Foxp3+ Tregs >50.
Statistical analysis
The associations between variables were evaluated by Pearson's χ2 test or Fisher's exact test if required. A univariate analysis was used to test if there are associations between CTC-positive rate and the number of Tregs, CD8+ T lymphocytes and clinicopathological parameters. A multivariate logistic regression model was also performed to analyze the independent factors associated with the CTC-positive rate. Statistical analysis was performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). In all analyses, a two-sided P<0.05 was considered statistically significant.
Results
Multi-marker qPCR analysis of CTCs in patients with breast cancer
The marker gene expression baseline was precisely quantified in 90 control subjects by qPCR. The median relative gene expression values for CK19, hMAM and SBEM in the control subjects were 1.18, 1.92 and 1.29, respectively. The cut-off threshold for marker gene positivity, that is, abnormal expression, was set at three standard deviations from the mean 2−ΔΔCq value for each gene. The cut-off thresholds for CK19, hMAM and SBEM were 4.92, 7.71 and 6.38, respectively. Of 167 patients, 62 (37%) were positive for CK19, 37 (22%) were positive for hMAM, and 40 (24%) were positive for SBEM (Table III). In total, 92 (55%) patients were positive for at least one marker. The sensitivities for the individual markers CK19, hMAM, and SBEM were 37, 22 and 24%, respectively, and the specificities were 100, 99 and 99%, respectively. The multi-marker qPCR platform yielded a sensitivity of 55% and a specificity of 98% (Table III).
Table III.
CTC-positive rate in controls and patients with breast cancer.
| CTC-positive rate of individual marker genes (%) | ||||
|---|---|---|---|---|
| Groups | CTC-positive rate of three marker genes (%) | CK19 | hMAM | SBEM |
| Control (n=90) | 2/90 (2.2) | 0/90 (0.0) | 1/90 (1.1) | 1/90 (1.1) |
| Breast cancer (n=167) | 92/167 (55) | 62/167 (37) | 37/167 (22) | 40/167 (24) |
CK19, cytokeratin 19; hMAM, human mammaglobin; SBEM, small breast epithelial mucin.
Detection of CD8+ T lymphocytes and Foxp3+ Tregs in the tumor microenvironment of breast cancer
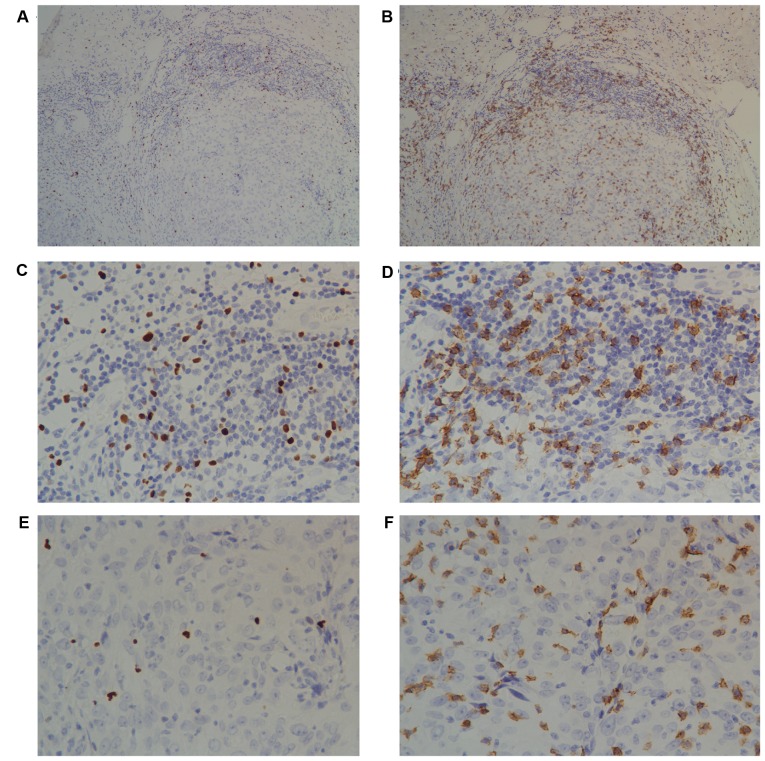
Infiltrating intratumoral CD8+ T lymphocytes and Foxp3+ Tregs presented with a diffuse pattern, and the peritumoral infiltration was more abundant and tended to form lymphoid aggregates (Fig. 1). There were significantly more CD8+ T lymphocytes (median, 79.03 vs. 25.00; range, 0–254.11 vs. 0–138.67) and Foxp3+ Tregs (median, 34.12 vs. 10.33; range 0–188.03 vs. 0–85.67) in the peritumoral than the intratumoral areas (data not shown).
Figure 1.
Immunohistochemical analyses of intratumoral and peritumoral lymphocytes. Immunohistochemical staining of tumor-infiltrating (A) Foxp3+ T cells and (B) CD8+ T lymphocytes in invasive breast cancer. A diffuse pattern of intratumoral lymphocytes was observed. By contrast, peritumoral lymphocytes formed a lymphoid aggregate (×10 objective lens). There were markedly more Foxp3+ Tregs in the (C) peritumoral area than the (E) intratumoral areas. There was an increased number of CD8+ lymphocytes in the (D) peritumoral area than the (F) intratumoral areas. A and B, ×10 objective lens; B-E, ×40 objective lens. CD, cluster of differentiation; Foxp3, forkhead box 3.
Association between CTC-positive rate and the clinicopathological parameters of patients with breast cancer
The peripheral CTC-positive rate was statistically significant between the different tumor sizes (P<0.05) and was associated with a larger tumor size (T1, 42%; T2, 63%; T3, 75%) (Table I). The peripheral CTC-positive rate was also weakly associated with a higher disease stage and histological grade, although this association did not reach significance (P>0.05). Associations between CTC-positive rate and the remaining clinicopathological parameters were not observed (P>0.05; Table I).
Association of CTC-positive rates with Foxp3 T cells but not with CD8+T cells in the tumor microenvironment
It was determined that increased numbers of intratumoral Tregs are associated with the CTC-positive rate in patients with breast cancer (Table IV). Patients with an increased number of intratumoral Tregs exhibited higher CTC-positive rates compared with those with a decreased number of Tregs (67 vs. 43%, P=0.002; Fig. 2). The same trend was observed for peritumoral infiltrating Tregs (G0, 48%; G1, 42%; G2, 57%; G3, 73%, P=0.045) (Table IV). Detection of peripheral CTCs was weakly and inversely associated with the number of infiltrating peritumoral CD8+ lymphocytes, although this association did not reach statistical significance (P=0.47; Table IV).
Table IV.
Association between the CTC-positive rate in the peripheral blood of patients with breast cancer and tumor-infiltrating CD8+ T lymphocytes and Foxp3+ Tregs in the tumor microenvironment.
| Infiltration of lymphocytes | CTC-positive rate (%) | CTC-negative rate (%) | P-value |
|---|---|---|---|
| Intratumoral Foxp3+ Tregsa | 0.002 | ||
| Low | 36 (43) | 47 (57) | |
| High | 56 (67) | 28 (33) | |
| Intratumoral CD8+ T lymphocytesa | 0.846 | ||
| Low | 44 (54) | 37 (46) | |
| High | 48 (56) | 38 (44) | |
| Peripheral Foxp3+ Tregsb | 0.045 | ||
| G0 | 24 (48) | 26 (52) | |
| G1 | 14 (42) | 19 (58) | |
| G2 | 27 (57) | 20 (43) | |
| G3 | 27 (73) | 10 (27) | |
| Peripheral CD8+ T lymphocytesb | 0.470 | ||
| G0 | 17 (65) | 9 (35) | |
| G1 | 22 (59) | 15 (41) | |
| G2 | 23 (55) | 19 (45) | |
| G3 | 30 (48) | 32 (52) |
Medium level was used as a cut-off (Foxp3+ Tregs, 10.33; and CD8+ T lymphocytes, 25.00).
G0 corresponded to the absence of lymphocytes. G1 corresponded to the mean number of CD8+ T lymphocytes ≤50 and Foxp3+ Tregs ≤25. G2 corresponded to CD8+ T lymphocytes >50 and ≤100 and Foxp3+ Tregs >25 and ≤50. G3 corresponded to CD8+ T lymphocytes >100 and Foxp3+ Tregs >50. CD, cluster of differentiation; Foxp3, forkhead box 3; Tregs, T regulatory cells.
Figure 2.
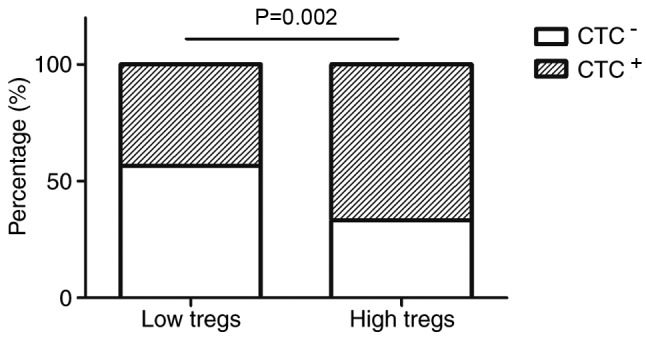
Association between CTCs in the peripheral blood of patients with breast cancer and infiltrating intratumoral Foxp3+ Tregs. The patients with breast cancer were divided into two groups according to the median (10.33). Patients with an increased number of intratumoral Foxp3+ Tregs exhibited a higher CTC-positive rate compared with those with a lower number of Foxp3+ Tregs (67 vs. 43%, P=0.002). CTC, circulating tumor cells; Tregs, regulatory T cells; Foxp3, forkhead box 3.
Multivariate analysis indicated that the prevalence of CTCs was independently associated with tumor size (OR=2.18, 95% CI, 1.02–4.64; P=0.044) and infiltrating intratumoral Tregs (OR=2.23, 95% CI, 1.02–4.87; P=0.044) (Table V).
Table V.
Multivariate analysis of the association between circulating tumor cells in the peripheral blood of breast cancer patients and clinicopathological parameters as well as Foxp3+ Tregsa.
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Menopausal status | 0.49 | 0.25–0.98 | 0.052 |
| Tumor size | 2.18 | 1.02–4.64 | 0.044 |
| TNM stage | 1.17 | 0.64–2.14 | 0.608 |
| Intratumoral Tregs | 2.23 | 1.02–4.87 | 0.044 |
| Peritumoral Tregs | 1.16 | 0.83–1.60 | 0.369 |
Parameters with P-value ≤0.1 in univariate analysis were selected for multivariate analysis. CI, confidence interval; Foxp3, forkhead box 3; OR, odds ratio; TNM, tumor node metastasis; Tregs, T regulatory cells.
Discussion
The association between CTCs and the immune system in breast cancer was reported (25,26). The effect of TILs on the prevalence of CTCs in patients with breast cancer is poorly understood. In the present study, it was demonstrated that the number and distribution of tumor-infiltrating Tregs were significantly associated with the presence of peripheral CTCs in patients with breast cancer.
CTCs can be identified and characterized in the peripheral blood of patients with breast cancer (27) and may represent the emergence of early hematological metastasis in breast cancer. CTCs have the ability to form clusters of microscopic tumors, and their detection, persistence or elevation is associated with poor prognosis (2). Therefore, it is essential to elucidate the factors that affect the dissemination of CTCs, which would provide a theoretical basis for improving breast cancer treatment. Immune cells, cytokines and other factors in the microenvironment are present throughout tumor development and have an impact on tumor progression (28); therefore, the effects of immune factors on disease progression may be partially mediated by the suppression of hematological micrometastasis in breast cancer.
Due to the heterogeneity of tumor cells and the instability of gene expression, there is no identified and specific standard for CTCs detection to date (29). Our center has established a multi-marker (CK19, hMAM, SBEM) qPCR platform for detection of CTCs in the peripheral blood of patients with breast cancer. The sensitivity and specificity of this method have been confirmed in several laboratories (30,31). CK19, hMAM and SBEM mRNA were detected in 37, 22 and 24% of the patients with breast cancer, respectively. When using the multi-marker platform (for which positivity was defined as positivity for at least one of the markers), the sensitivity was improved to 55%, which was greater compared with the values reported in similar studies (31,32). In addition, the specificity of the multi-marker platform was 98% due to contamination of only minor samples. Therefore, the multi-marker qPCR platform provides high sensitivity and specificity for the detection of CTCs.
TILs exert an important role in tumor immunity. Tregs were initially characterized as CD4+ CD25+ cells and are thought to be the main obstacles that hinder the efficacy of antitumor immunity and immunotherapy (30,33). Tregs effectively suppress the proliferation and activation of CD8+ T lymphocytes in a contact-dependent manner or via the release of cytokines (33). The impact of Tregs on the prognosis of breast cancer depends on their distribution and the expression of hormone receptors (34). The majority of studies have reported that an elevated number of intratumoral/peritumoral Tregs confers a poor clinical outcome (16–19) and is associated with disease progression in breast cancer (34). However, West et al (35) concluded that Tregs exhibit an antitumor activity as it may favor the survival time of patients with ER-negative breast cancer. Therefore, the present study investigated the association between the number of intratumoral/peritumoral Tregs and the presence of peripheral CTCs. Univariate analysis demonstrated that patients with an increased number of intratumoral/peritumoral Tregs exhibited a higher CTC-positive rate compared with those with a lower number of Tregs. Therefore, increasing the number of intratumoral/peritumoral Tregs may increase local immunosuppressive capabilities and reduced anti-tumor immunity, consequently forming an immunosuppressive microenvironment conducive to tumor growth, invasion and metastasis.
A local antitumor immunity deficiency may indicate a depressed systemic immune system (36,37). A depressed immunity in the tumor microenvironment may inefficiently stimulate tumor-antigen presentation or the immune response, which would enable breast cancer cells to escape from local or systemic immune surveillance and potentially form distant metastases (34). Previous studies regarding the prognostic value of Tregs in breast cancer were consistent with the results of the present study (16–19). In addition, the multivariate analysis in the present study demonstrated that an increasing number of intratumoral Tregs but not peritumoral Tregs was an independent risk factor for detection of CTCs. The prognosis data from Liu et al (19) supported the present findings that an increased number of intratumoral Tregs but not peritumoral Tregs was associated with a poorer clinical outcome (16,19). However, the present results were different from those reported by Mahmoud et al (17). One explanation for these contradictory results may be the tissue volume used (19). The tissue array used by Gobert et al (18) contained only a small volume of tumor tissue. The use of such small tissue fragments may not be optimal to obtain a sufficient number of cells for a reliable count of Tregs in different areas. In the present study full block tissue sections were used to enable the selection of areas with abundant intratumoral/peritumoral Tregs.
Rich infiltration by CD8+ T lymphocytes, which are a representative subset of lymphocytes that are associated with immune surveillance in the tumor microenvironment, may indicate strong anti-tumor immunity (37). The results of the present study revealed that an elevated grade for peritumoral CD8+ T lymphocytes was inversely associated with the detection of peripheral CTCs, although this association did not reach statistical significance. The hypothesis that CD8+ T lymphocytes suppress the dissemination of CTCs was not confirmed in the present study. This may be due to the following factors. First, either by a hematological or lymphangial route, breast cancer cells may invade the circulatory system and cause distant metastases. In the present study, the function of local immune suppression was examined in hematological micrometastasis rather than via the lymphangial route. In a study on human colorectal carcinoma, Chiba et al (38) observed that the effects of CD8+ T lymphocytes may be mediated by the suppression of lymphangial micrometastasis rather than growth suppression in the primary tumor. Secondly, the effect of CD8+ T lymphocytes on tumor progression depends not only on their number and distribution but also on their activity (3,39). Cytotoxic CD8+ T cells, which are merely active CD8+ T lymphocytes, may exert anti-tumor immunity. Although a significant association between tumor-infiltrating CD8+ T lymphocytes and the detection of peripheral CTCs was not observed, the possibility of this association cannot be ignored. Accumulating studies indicate that CD8+ T lymphocytes have antitumor activity, as they may favor the survival time of patients with breast cancer (9) Therefore, it is possible that CD8+ T lymphocytes may contribute to patient survival time via other mechanisms, and thus further research is required.
In conclusion, the results of the present study indicated that the role of Tregs in the progression of breast cancer may be mediated by the suppression of dissemination of CTCs, and the effect is mainly determined by intratumoral Tregs. Nevertheless, the impact of the phenotypic and functional qualities of Tregs or CD8+ T lymphocytes on hematological micrometastasis in breast cancer was not assessed comprehensively. Prospective studies are required to understand better the molecular mechanisms by which TILs affect the prevalence of CTCs and their potential role in breast cancer immunotherapy.
Acknowledgements
Not applicable.
Funding
The research was supported by The Nantong demonstration project for the people's livelihood (grant no. MS32015029).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributions
DX analyzed the data and was a major contributor in writing the manuscript. TX made substantial contribution in analysis and interpretation of the data. JW performed immunohistochemistry and cell counting. MC collected the information regarding the subjects enrolled in the present study and detected CTCs. SW had major role in designing the study. CZ designed the study and gave the final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Nantong University (Nantong, China) and The Affiliated Hospital of Nantong University (Nantong, China). All donors and patients gave informed consent.
Patient consent for publication
Informed consent was obtained from all patients included in this study for publication of the associated data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J, Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18:5701–5710. doi: 10.1158/1078-0432.CCR-12-1587. [DOI] [PubMed] [Google Scholar]
- 3.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med. 2013;91:411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, Al-Mehdi AB, Bernhard EJ, Muschel RJ. Apoptosis: An early event in metastatic inefficiency. Cancer Res. 2001;61:333–338. [PubMed] [Google Scholar]
- 5.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T, Wallwiener M, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 6.Santos MF, Mannam VK, Craft BS, Puneky LV, Sheehan NT, Lewis RE, Cruse JM. Comparative analysis of innate immune system function in metastatic breast, colorectal, and prostate cancer patients with circulating tumor cells. Exp Mol Pathol. 2014;96:367–374. doi: 10.1016/j.yexmp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Noman MZ, Messai Y, Muret J, Hasmim M, Chouaib S. Crosstalk between CTC, immune system and hypoxic tumor microenvironment. Cancer Microenviron. 2014;7:153–160. doi: 10.1007/s12307-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, Shin SW, Kim YH, Kim JS, Park KH. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol. 2013;36:224–231. doi: 10.1097/COC.0b013e3182467d90. [DOI] [PubMed] [Google Scholar]
- 9.Al-Saleh K, Abd El-Aziz N, Ali A, Abozeed W, Abd El-Warith A, Ibraheem A, Ansari J, Al-Rikabi A, Husain S, Nabholtz JM. Predictive and prognostic significance of CD8+ tumor-infiltrating lymphocytes in patients with luminal B/HER 2 negative breast cancer treated with neoadjuvant chemotherapy. Oncol Lett. 2017;14:337–344. doi: 10.3892/ol.2017.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 11.Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 13.Tang J, Yang Z, Wang Z, Li Z, Li H, Yin J, Deng M, Zhu W, Zeng C. Foxp3 is correlated with VEGF-C expression and lymphangiogenesis in cervical cancer. World J Surg Oncol. 2017;15:173. doi: 10.1186/s12957-017-1221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linehan DC, Goedegebuure PS. CD25+CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 15.Ménétrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 16.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;127:99–108. doi: 10.1007/s10549-010-0987-8. [DOI] [PubMed] [Google Scholar]
- 18.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130:645–655. doi: 10.1007/s10549-011-1647-3. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZK, Yang B, Liu H, Hu Y, Yang JL, Wu LL, Zhou ZH, Jiao SC. Regulatory T cells increase in breast cancer and in stage IV breast cancer. Cancer Immunol Immunother. 2012;61:911–916. doi: 10.1007/s00262-011-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andergassen U, Kölbl AC, Mahner S, Jeschke U. Real-time RT-PCR systems for CTC detection from blood samples of breast cancer and gynaecological tumour patients (Review) Oncol Rep. 2016;35:1905–1915. doi: 10.3892/or.2016.4608. [DOI] [PubMed] [Google Scholar]
- 22.Chong MH, Zhao Y, Wang J, Zha XM, Liu XA, Ling LJ, Du Q, Wang S. The dynamic change of circulating tumour cells in patients with operable breast cancer before and after chemotherapy based on a multimarker QPCR platform. Br J Cancer. 2012;106:1605–1610. doi: 10.1038/bjc.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Mikhitarian K, Martin RH, Ruppel MB, Gillanders WE, Hoda R, del Schutte H, Callahan K, Mitas M, Cole DJ. Detection of mammaglobin mRNA in peripheral blood is associated with high grade breast cancer: Interim results of a prospective cohort study. BMC Cancer. 2008;8:55. doi: 10.1186/1471-2407-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mego M, Gao H, Cohen EN, Anfossi S, Giordano A, Tin S, Fouad TM, De Giorgi U, Giuliano M, Woodward WA, et al. Circulating tumor cells (CTCs) are associated with abnormalities in peripheral blood dendritic cells in patients with inflammatory breast cancer. Oncotarget. 2017;8:35656–35668. doi: 10.18632/oncotarget.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mego M, Gao H, Cohen EN, Anfossi S, Giordano A, Sanda T, Fouad TM, De Giorgi U, Giuliano M, Woodward WA, et al. Circulating tumor cells (CTC) are associated with defects in adaptive immunity in patients with inflammatory breast cancer. J Cancer. 2016;7:1095–1104. doi: 10.7150/jca.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpé S, Van Marck E, Vermeulen PB, Dirix LY. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006;94:672–680. doi: 10.1038/sj.bjc.6602985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallergi G, Politaki E, Alkahtani S, Stournaras C, Georgoulias V. Evaluation of isolation methods for circulating tumor cells (CTCs) Cell Physiol Biochem. 2016;40:411–419. doi: 10.1159/000452556. [DOI] [PubMed] [Google Scholar]
- 30.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnamurthy S, Cristofanilli M, Singh B, Reuben J, Gao H, Cohen EN, Andreopoulou E, Hall CS, Lodhi A, Jackson S, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116:3330–3337. doi: 10.1002/cncr.25145. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Zou TN, Wu ZP, Zhou YC, Gu YL, Liu X, Jin CG, Wang XC. Detection of cytokeratin 19, human mammaglobin, and carcinoembryonic antigen-positive circulating tumor cells by three-marker reverse transcription-PCR assay and its relation to clinical outcome in early breast cancer. Int J Biol Markers. 2010;25:59–68. doi: 10.1177/172460081002500201. [DOI] [PubMed] [Google Scholar]
- 33.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014;16:432. doi: 10.1186/s13058-014-0432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, Watson PH. Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108:155–162. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 37.Li CH, Kuo WH, Chang WC, Huang SC, Chang KJ, Sheu BC. Activation of regulatory T cells instigates functional down-regulation of cytotoxic T lymphocytes in human breast cancer. Immunol Res. 2011;51:71–79. doi: 10.1007/s12026-011-8242-x. [DOI] [PubMed] [Google Scholar]
- 38.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: Possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91:163–171. doi: 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.