Abstract
The aim of the present study was to investigate the expression of long non-coding(lnc) RNA-extracellular matrix (ECM) in esophageal squamous cell carcinoma (ESCC) and its effect on ESCC metastasis. Using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), the expression of lncRNA-ECM in ESCC tissues was investigated and compared with that in corresponding adjacent tissues. In addition, the expression of lncRNA-ECM in the human ESCC cell lines TE-1, EC9706, KYSE150, Eca109 and KYSE30 was also detected and compared with that in the normal esophageal mucosal epithelial cell line HET-1A. The clinicopathological association between lncRNA-ECM and ESCC was assessed. Silencing and overexpression of lncRNA-ECM in ESCC TE-1 and Eca109 cells determined the correlation between lncRNA-ECM expression and ESCC invasion and metastasis. The possible target genes of lncRNA-ECM were predicted and verified by bioinformatics analysis and experimental results. The expression level of intercellular adhesion molecule 1 (ICAM1) was detected in ESCC tissues by RT-qPCR and the correlation between the expression of ICAM1 and lncRNA-ECM was analyzed. Changes in the expression of ICAM1 in ESCC TE-1 and Eca109 cell lines were evaluated after knocking down lncRNA-ECM and transfection of lncRNA-ECM overexpression plasmids. The expression level of lncRNA-ECM in the tissues of ESCC with lymph node metastasis were significantly increased compared with ESCC with no lymph metastasis (P<0.05). LncRNA-ECM silencing notably reduced the invasion and metastasis of TE-1 and Eca109 cells, while lncRNA-ECM overexpression promoted the invasion and metastasis of the two cell lines. The expression level of ICAMI was directly correlated with the expression of lncRNA-ECM, suggesting that ICAM1 may be the downstream target gene of lncRNA-ECM. LncRNA-ECM was revealed as being overexpressed in ESCC. LncRNA-ECM expression was positively correlated with metastasis and may affect the metastasis of ESCC through ICAMI regulation. These findings indicate that lncRNA-ECM may be promising as a novel biomarker for the diagnosis and prediction of prognosis for ESCC, and it may also serve as a novel therapeutic target for ESCC.
Keywords: long non-coding RNA ENST00000589379, esophageal squamous cell carcinoma, metastasis
Introduction
Esophageal cancer is one of the most common malignancies, with an incidence rate that ranks sixth in China and fourth among the causes of cancer-related mortality (1). Esophageal cancer is also one of the most common lethal tumors worldwide with the sixth highest mortality rate (2). Esophageal squamous cell carcinoma (ESCC) accounts for >90% of the cases of esophageal cancer, and has a high degree of aggressiveness and complexity of biological behavior, making its clinical course variable and heterogeneous. A considerable number of ESCC patients have already metastasized at diagnosis. The metastasis of ESCC is the main cause of poor prognosis and mainly involves lymph node and distant metastasis (3). Previous studies demonstrated that the 5-year survival rate of ESCC patients without metastasis was 70–92%, whereas it was only 18–47% in those with metastasis (3,4). Therefore, an in-depth understanding of the mechanism underlying ESCC metastasis is of high scientific and clinical value for improving the survival rate of ESCC.
Long non-coding (lnc)RNAs are a class of mature RNAs with a transcription length of >200 bases, which is significantly longer compared with that of mRNA. Despite having no coding ability, lncRNAs are involved in several processes, including gene imprinting, chromatin remodeling, cell cycle regulation, splicing regulation, mRNA degradation and translation regulation (5). They can regulate gene expression at the genetic, transcriptional and post-transcriptional level, and are widely implicated in most physiological as well as pathological processes (6). An increasing number of studies have demonstrated that differentially expressed lncRNAs are closely associated with tumor proliferation, invasion, metastasis and prognosis, and have great potential of becoming new targets for tumor therapy (7,8). It was demonstrated that lncRNAs are closely related to ESCC (9–11). However, little is known on their effect on ESCC metastasis. Therefore, it is crucial to identify differentially expressed lncRNAs in ESCC and investigate their function and underlying mechanism in ESCC metastasis.
LncRNA-ENST00000589379 has been found to be highly expressed in ESCC tissues with undefined function by gene chips (12). In the present study, this novel lncRNA is referred to as lncRNA-ECM. To further investigate the role of lncRNA-ECM in ESCC metastasis, we investigated the correlation between its expression and the clinical pathology of ESCC using real-time fluorescence quantitative PCR. Changes in cell invasion and migration capacity were detected following lncRNA-ECM knockdown/overexpression in the ESCC TE-1 and Eca109 cell lines by classical Transwell assay. Bioinformatics analysis and rescue experiments were used to examine the expression changes in the downstream geneintercellular adhesion molecule 1 (ICAM1) in order to elucidate the mechanism of action of lncRNA-ECM.
Materials and methods
Cases and specimens
Tissue samples from 62 cases with ESCC and matched paracancerous tissues were collected in Taizhou People's Hospital affiliated to Nantong University (Nantong, China) between September 2014 and December 2016. All the cases were confirmed by pathological examination. The patients included 43 men and 19 women, with a mean age at surgery of 65.75±6.39 years. None of the patients had received any radiotherapy or chemotherapy prior to surgery. The fresh specimens were immediately placed in liquid nitrogen; 8–10 min later, they were packed into RNAfixer (Takara, Dalian, China) and preserved at −80°C. The tumors were classified according to the 7th edition of the American Joint Committee on Cancer TNM staging guidelines for esophageal cancer. The study was approved by the Ethics Committee of Taizhou People's Hospital affiliated to Nantong University and the informed consent of the patients was obtained.
RNA extraction and RT-qPCR
Total RNA was extracted from ESCC samples and ESCC cell lines using the TRIzol reagent kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in accordance with the manufacturer's protocol; The total RNA quality and concentration was determined by UV spectrophotometry. Double-stranded complementary DNA was synthesized by reverse transcription in accordance with the cDNA synthesis kit; qPCR was performed using the Script SYBR Green PCR kit (both Toyobo, Osaka, Japan). The primer sequences of lncRNA-ECM were as follows: Forward, 5′-CAATATGTCCGTGGGACCCT-3′ and reverse, 5′-GGAGGCCAAACAACTGTGGA-3′. The primer sequences for ICAM1 were as follows: Forward, 5′-TTGAGGGCACCTACCTCTGT-3′ and reverse, 5′-GATAGGTTCAGGGAGGCGTG-3′. β-actin was used as reference (forward, 5′-CTGGGACGACATGGAGAAAA-3′ and reverse, 5′-AAGGAAGGCTGGAAGAGTGC-3′). Each experiment was duplicated three times. Relative expression levels were calculated using the 2−∆∆Ct method.
Microarray analysis (12)
LncRNA gene chip service was provided by Outdo Biotech [Agilent Human lncRNA Microarray V2.0 (4×180K; design ID 062918; containing 46,506 lncRNAs); Agilent Technologies, Inc., Santa Clara, CA, USA]. The operating process of the gene chip was as follows: First, RNA was prepared and double-stranded cDNA was synthesized by reverse transcription; next, a fluorescently labeled cRNA was synthesized with cyanine-3-CTP (Cy3). Then, chip hybridization between the cRNA and the chip was conducted after measuring their concentration and purity. After elution, the original image was obtained by scanning through the Agilent Scanner G2505C (Agilent Technologies, Inc.). The Feature Extraction Software (version 10.7.1.1; Agilent Technologies, Inc.) was used to process the original image and extract the data. The GeneSpring software (version 12.5; Agilent Technologies, Inc.) was used to analyze normalization and variance. Finally, all the differentially expressed lncRNAs were screened out with the selection criteria of fold change (FC)>2 and P<0.05.
Cell culture and transfection
The human ESCC cell lines TE-1, KYSE150, Eca109, KYSE30, EC9706 and the normal human esophageal mucosal epithelial HET-1A cell line were purchased from Shanghai Meixuan Biotechnology (Shanghai, China) and cultured in RPMI-1640 (Corning Inc., Corning, NY, USA) medium containing 10% fetal bovine serum (GE Healthcare Life Sciences, Logan, UT, USA) in 5% CO2 at 37°C. In the logarithmic growth phase, TE-1 and Eca109 cells were inoculated into 6-well plates (4×105 cells/ml). When the cell healing degree reached 90–95%, the plasmids of lncRNA-ECM silencing and overexpression were transfected into the cells by Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The lncRNA-ECM overexpression plasmids were synthesized according to the NCBI reference sequence. PcDNA3.1 vectors were used as null controls. After transfection for 48 h, the cells were used for the experiments. The sequences of si-lncRNA-ECM were as follows: si-lncRNA-ECM 1#, sense, 5′-CAGGAAAGCCAUACCAUGATT-3′ and antisense, 5′-UCAUGGUAUGGCUUUCCUGTT-3′; si-lncRNA-ECM 2# sense, 5′-CCUAUAGAGCGACUGUCAATT-3′ and antisense, 5′-UUGACAGUCGCUCUAUAGGTT-3′; si-lncRNA-ECM 3# sense, 5′-CAGCAAACUCGUAGGUCAATT-3′ and antisense, 5′-UUGACCUACGAGUUUGCUGTT-3′ (Sangon Biotech, Shanghai, China).
Cell invasion and migration assays
Transwell plates (Costar, New York, NY, USA) were used for the Transwell assays. After 12 h of transfection, the cells were inoculated into the 24-well Transwell plate (Corning Inc.) at an inoculation volume of 4×103/well. The membrane at the bottom of the upper chamber of the Transwell plate was coated with 100 µl (~25 µg) diluted Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and dried at 4°C; 50 µl serum-free culture medium was added to each well and cultured at 37°C for 30 min. To digest and collect transfected cells, the cells were suspended in serum-free medium at a density of 105/ml; 100 µl of the cell suspension was added to the upper chamber of the 24-well Transwell plate. Complete medium with 500 µl 20% FBS was added to the lower chamber. The plates were incubated in an incubator with 5% CO2 at 37°C for 24 h, washed with PBS twice, the upper layer of the cells was wiped with a cotton swab, fixed for 20 min in methanol, dyed for 10 min with 0.1% crystal violet solution, and rinsed with distilled water until there was no stain under the optical microscope. Finally, five random ×20 visual fields were observed, and the number of tumor cells was counted. The migration assay was performed in a similar manner, without the addition of Matrigel. All the experiments were performed in triplicate.
Statistical analysis
Student's t-test was used to evaluate the significance of the difference between the two groups of lncRNA-ECM expression in ESCC at the tissue level and the cellular level. One-way ANOVA was used to compare the means of the multiple groups data. When the result is significant, the LSD post hoc test was then used to compare the means between two pairs in this group. The clinicopathological characteristics were analyzed by the Chi-squared test or Wilcoxon's rank-sum test. The correlations were assessed by Pearson's correlation analysis. Data are expressed as mean ± standard deviation of three independent experiments. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed by SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
Microarray analysis
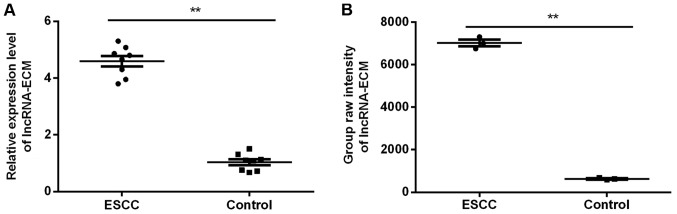
Among the 39 lncRNAs with significant differences (FC>10) reported previously (12), due to the significant between-group difference, non-significant in-group difference (Fig. 1A) and higher abundance compared with its origin (Fig. 1B) in the chip, lncRNA-ECM was selected.
Figure 1.
LncRNA-ECM analysis. (A) LncRNA-ECM were validated by reverse transcription-quantitative polymerase chain reaction in 8 patients with ESCC. (B) The chip original signal of lncRNA-ECM in 3 patients with ESCC. **P<0.01. ESCC, esophageal squamous cell carcinoma; Lnc, long-non coding; ECM, extracellular matrix.
Potential functional analysis of lncRNA-ECM
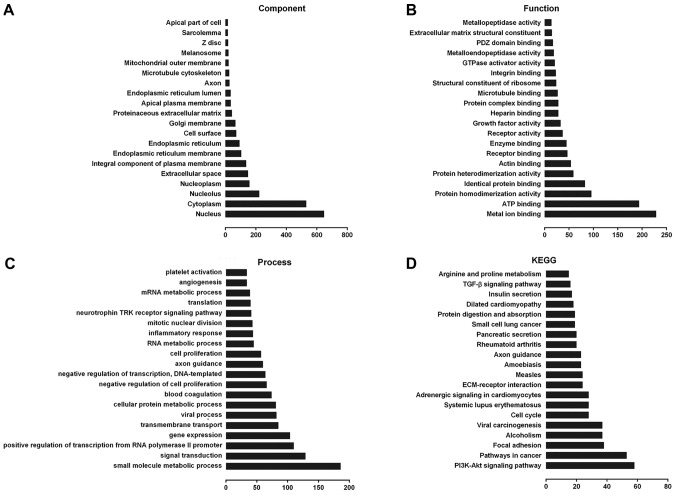
Using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, we found that lncRNA-ECM and its potential target genes are closely associated with cell cycle regulation, cell differentiation and other processes (Fig. 2A-C), including extracellular matrix receptor interaction, cell cycle regulation, adhesive junctions, and other multiple signaling pathways associated with cancer (Fig. 2D), and it may also be involved in the pathogenesis of ESCC.
Figure 2.
LncRNA-ECM GO and pathway analysis. (A) Cellular component of lncRNA-ECM GO terms. (B) Molecular function of lncRNA-ECM GO terms. (C) Biological process of lncRNA-ECM GO terms. (D) LncRNA-ECM pathway analysis. GO, Gene Ontology; Lnc, long-non coding; ECM, extracellular matrix.
Expression of lncRNA-ECM in ESCC tissues and association with clinicopathological parameters
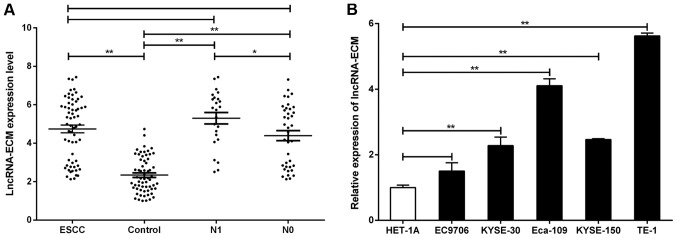
To further investigate the biological behavior of lncRNA-ECM in ESCC, we examined the expression of lncRNA-ECM in cancer tissues from 62 ESCC patients and corresponding paracancerous tissues by RT-PCR. The results demonstrated that the expression of lncRNA-ECM in ESCC tissues was significantly higher compared with that in adjacent tissues (Fig. 3A), which was in agreement with the microarray results. The experiments on cultured ESCC cell lines also yielded similar results: Compared with the normal human esophageal mucosal epithelial HET-1A cell line, the expression of lncRNA-ECM was significantly higher in all human ESCC cell lines (TE-1, KYSE150, Eca109 and KYSE30; Fig. 3B). The clinicopathological characteristics of the 62 ESCC cases are listed in Table I. LncRNA-ECM was significantly associated with lymph node metastasis (P=0.029; Table I and Fig. 3A) and TNM stage (P=0.036, Table I), but not with age, sex, tumor size, pathological differentiation or T stage (all P>0.05; Table I). These findings indicate that overexpression of lncRNA-ECM may be associated with ESCC metastasis.
Figure 3.
Expression of lncRNA-ECM in ESCC. (A) LncRNA-ECM was upregulated in ESCC tissues and patients with lymphatic metastasis (N1) had increased lncRNA-ECM expression. (B) Relative expression of lncRNA-ECM in a panel of ESCC cell lines. *P<0.05 and **P<0.01. Lnc, long-non coding; ECM, extracellular matrix; ESCC, esophageal squamous cell carcinoma.
Table I.
Association between long non-coding RNA-ECM expression and the clinicopathological features of patients with ESCC.
| Variable | Patients | Mean ± SD | P-value |
|---|---|---|---|
| Age (years) | 0.528 | ||
| ≥60 | 52 | 4.64±1.58 | |
| <60 | 10 | 5.26±1.68 | |
| Sex | 0.744 | ||
| Male | 43 | 4.91±1.51 | |
| Female | 19 | 4.35±1.76 | |
| Tumor size (cm) | 0.867 | ||
| ≤3 | 25 | 4.51±1.78 | |
| >3 | 37 | 4.89±1.47 | |
| Pathological differentiation grade | 0.609 | ||
| Well | 6 | 4.75±1.53 | |
| Moderately | 35 | 4.74±1.55 | |
| Poorly | 21 | 4.72±1.77 | |
| T stage | 0.471 | ||
| T1-2 | 30 | 4.37±1.61 | |
| T3-4 | 32 | 5.08±1.54 | |
| N stage | 0.029 | ||
| N0 | 38 | 4.20±1.56 | |
| N1 | 24 | 5.59±1.27 | |
| TNM stage | 0.036 | ||
| I–II | 43 | 4.23±1.54 | |
| III–IV | 19 | 5.87±1.07 |
SD, standard deviation; ECM, extracellular matrix; TNM, tumor, node, metastasis; ESCC, esophageal squamous cell carcinoma.
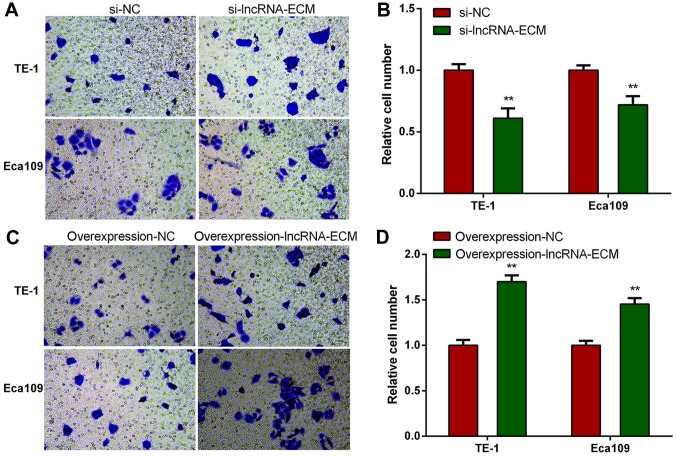
Knockdown of lncRNA-ECM decreases invasion and migration of ESCC cells
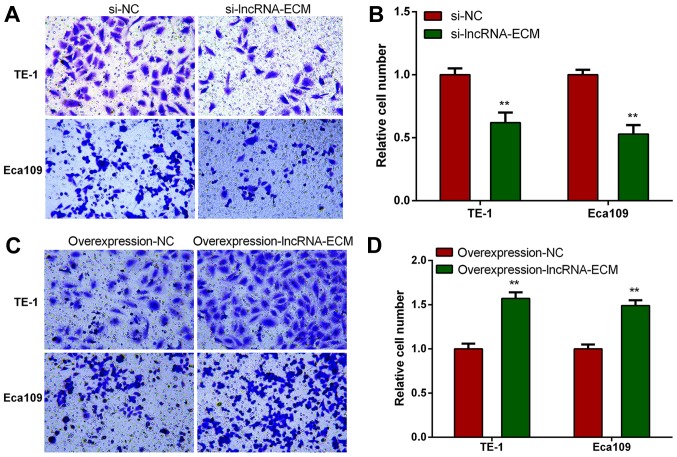
To explore the function of lncRNA-ECM in ESCC invasion and migration, we selected two highest cell lines of TE-1 and Eca109 to perform loss or gain of function assays. We transfected the specific siRNA of lncRNA-ECM into TE-1 and Eca109 cell lines and utilized a classical Transwell assay to assess the effect of lncRNA-ECM on the invasion and migration. The results revealed that the invasion and migration of ESCC cell lines decreased significantly after lncRNA-ECM silencing (Fig. 4). The result indicated that lncRNA-ECM is involved in the regulation of ESCC cell invasion and migration.
Figure 4.
Knockdown of lncRNA-ECM reduces the invasion and migration of ESCC cells. (A and B) The cell invasion ability decreased following knock-down of lncRNA-ECM. (C and D) The cell migration ability decreased following knock-down lncRNA-ECM. **P<0.01. Magnification, ×200. Lnc, long-non coding; ECM, extracellular matrix; NC, negative control; ESCC, esophageal squamous cell carcinoma.
Overexpression of lncRNA-ECM increases invasion and migration of ESCC cells
To elucidate whether lncRNA-ECM promotes ESCC cell invasion and migration, lncRNA-ECM overexpression plasmids were transfected into ESCC TE-1 and Eca109 cell lines, and the invasion and migration ability of the cell lines was evaluated after transfection. It was observed that the invasion and migration ability of ESCC cells was markedly increased in cells overexpressing lncRNA-ECM (Fig. 5). This result confirmed that lncRNA-ECM plays an important role in promoting ESCC cell migration.
Figure 5.
Overexpression of lncRNA-ECM increases the invasion and migration of ESCC cells. (A and B) The cell invasion ability increased following overexpression of lncRNA-ECM. (C and D) The cell migration ability increased following overexpression of lncRNA-ECM. **P<0.01. Magnification, ×200. Lnc, long-non coding; ECM, extracellular matrix; NC, negative control; ESCC, esophageal squamous cell carcinoma.
LncRNA-ECM may regulate ICAM1 in ESCC cells
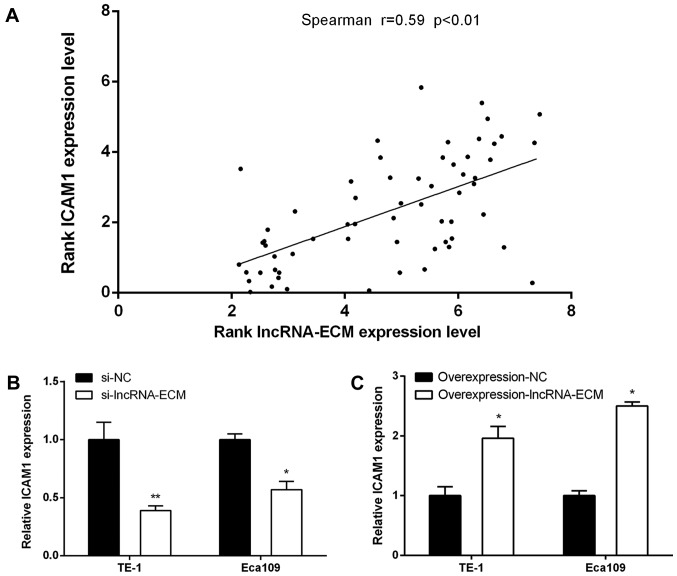
As genes with similar spatial location are often the targets of lncRNA regulation (6), the location of lncRNA-ECM and nucleic acid sequences was analyzed by UCSC and BLAST, and we found that ICAM1 (intercellular cell adhesion molecule-1) is adjacent to lncRNA-ECM. We detected the expression level of ICAM1 in the 62 cases of ESCC using RT-qPCR in the present study, and the results demonstrated that the expression level of ICAM1 was positively correlated with lncRNA-ECM expression (Fig. 6A; Pearson's correlation, R=0.59, P<0.01). We further investigated whether the expression of ICAM1 changed according to the level of lncRNA-ECM in ESCC cells. It was observed that, after knocking down lncRNA-ECM, the expression of ICAM1 also decreased, whereas the level of ICAM1 increased following transfection of lncRNA-ECM overexpression plasmids (Fig. 6B and C), suggesting that ICAM1 may be a downstream target of lncRNA-ECM.
Figure 6.
Correlation analysis between lncRNA-ECM and ICAM1. (A) Positive correlation between lncRNA-ECM and ICAM1 in ESCCs. (B) Following knock-down of lncRNA-ECM the level of ICAM1 decreases. (C) Following overexpression of lncRNA-ECM the level of ICAM1 increased. *P<0.05 and **P<0.01 vs. the NC group. Lnc, long-non coding; ECM, extracellular matrix; ICAM1, intercellular adhesion molecule 1; NC, negative control.
Discussion
Despite a slight decrease in its incidence in recent years, ESCC remains one of the most common malignant tumors in China. Due to its high invasiveness and propensity for metastasis, the prognosis of ESCC is not satisfactory. Over the last decades, the association between biomolecules and cancer has attracted extensive attention, and a number of studies on ESCC have been performed (13,14). However, only few sensitive and specific biomarkers for ESCC have been identified. Therefore, active research on the development of effective molecular markers will be beneficial in improving the diagnosis and prognosis of esophageal cancer.
With the rapid development of genome microarray and genome sequencing technology, a type of transcription without protein encoding, originally considered to be non-RNA genome encoding transcription ‘noise’, has become a research focus in recent years (15). Considering the abundance, variability, function and mechanism of lncRNAs, they may be the regulatory core of RNA, and an increasing number of studies have demonstrated that the differential expression of lncRNAs is closely associated with tumorigenesis and tumor development (7,8,16), which provides a new basis for understanding the mechanism underlying these processes. Since lncRNA AFAP1-AS1 was found to be highly expressed in esophageal adenocarcinoma and silencing its expression significantly inhibited the proliferation, invasion and metastasis of esophageal adenocarcinoma cell lines (17), the research of lncRNAs and ESCC has attracted the attention of many scholars, and a few types of lncRNAs have been identified in ESCC. For example, lncRNA AFAP1-AS1 (9), HOTAIR (10) and POU3F3 (18) are highly expressed in ESCC, and their high expression can promote the development of ESCC and adversely affect the prognosis. Following knockdown of these lncRNAs, the proliferation, invasion and migration ability of ESCC cells was significantly inhibited. In addition, the expression of lncRNA-LET (19), UCA1 (20) and LOC285194 (21) is low in ESCC tissues, and their expression level is inversely associated with tumor size, TNM stage, lymph node metastasis, distant metastasis, survival rate and prognosis, whereas their overexpression can inhibit the migration of ESCC cells.
LncRNA-ECM (lncRNA ENST00000589379) is a newly discovered lncRNA, which exhibited high expression in the ESCC gene chip (12). Sequence analysis demonstrated that lncRNA-ECM was composed of 3,218 bp, and there were a number of cis-regulatory elements, binding sites and hypersensitive sites of DNase I in the region of the chromosome, indicating that the transcription status of this region was relatively active. There are abundant DNA hypermethylation regions and epigenetic regulatory loci in this region, such as histone H3K4me1, H3K4me3 and H3K36me3, among others. To some extent, histone modification and DNA methylation play important roles in the development and progression of ESCC (22,23), suggesting the functional significance of lncRNA-ECM. In the present study, we investigated the expression of lncRNA-ECM in ESCC at the cellular and tissue level. Combining the lncRNA microarray data and RT-PCR results, the expression of lncRNA-ECM in ESCC tissues was found to be significantly higher compared with that in the corresponding paracancerous tissues and nomal control cells, indicating that lncRNA-ECM may be a biomarker for ESCC detection. In addition, we found that the expression level of lncRNA-ECM in cancers with lymph node metastasis was significantly higher. The results demonstrated that the expression of lncRNA-ECM was correlated with lymph node metastasis. We also investigated the association between the expression of lncRNA-ECM and TNM stage, and observed that a higher expression of lncRNA-ECM in patients with ESCC was associated with a more advanced TNM stage. Therefore, these findings suggest that lncRNA-ECM may be a potential marker for ESCC prognosis. Subsequently, we transfected siRNA plamids with lncRNA-ECM knockdown into ESCC TE-1 and Eca109 cells and found that the invasion and migration ability of the two cell lines was diminished; by contrast, after transfecting lncRNA-ECM overexpression plasmids, the invasion and migration ability of ESCC cells was enhanced, suggesting lncRNA-ECM plays an important role in ESCC metastasis and may be a characteristic molecule for diagnosing ESCC and predicting its prognosis.
LncRNA signaling is associated with integrin pathways, extracellular pathways and local adhesion pathways. ICAM1, a member of the immunoglobulin superfamily (IGSF), is an important adhesion molecule (24) involved in cell metastasis, differentiation and proliferation. Recently, ICAM1 was reported to serve as a liver cancer and ESCC stem cell marker (25,26); it can also promote ESCC epithelial-to-mesenchymal transition (EMT) (27) and cause metastasis of ESCC cells by regulating the expression of tumor metastasis-related genes, such as P53 (26). We predicted the possible role of ICAM1 as a target gene for lncRNA-ECM according to bioinformatics analysis. The expression of ICAM1 in ESCC tissues was detected by RT-PCR, and its expression level was found to be positively correlated with the expression of lncRNA-ECM. Furthermore, after knocking down lncRNA-ECM in ESCC cells, the level of ICAM1 decreased, while the expression level of ICAM1 increased following lncRNA-ECM overexpression. These data indicate that lncRNA-ECM plays a regulatory role in promoting lymph node metastasis in ESCC, and this regulation may be mediated by ICAM1.
In conclusion, we investigated the biological behavior of lncRNA-ECM in ESCC and found it to be overexpressed in ESCC tissues. It may be deduced that lncRNA-ECM plays an important role in oncogenesis and progression of ESCC by regulating ICAM1, and it may promote ESCC metastasis. Therefore, lncRNA-ECM may be a new biomarker for diagnosing ESCC and predicting patient prognosis, and it may also represent a novel molecular target for the treatment of ESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu Provincial Medical Innovation Team (grant no. CXTDA2017042) of Jiangsu Province, and the 333 Plan Foundation (grant no. BRA2017173) of Jiangsu Province, China.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JY was responsible for writing the manuscript and performing the lncRNA-ECM cell experiments. XS was responsible for collecting the ESCC and matched para-cancerous tissues and performing the RT-qPCR experiments. HL, JX and SS were responsible for collecting the ESCC and matched para-cancerous tissues. JXH wrote the paper and performed the bioinformatics analysis. ML assisted with the experiments and revised the paper.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Taizhou People's Hospital affiliated to Nantong University and written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
References
- 1.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.1186/s40880-015-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633–641. doi: 10.1097/SLA.0b013e3181656d07. [DOI] [PubMed] [Google Scholar]
- 4.Kayani B, Zacharakis E, Ahmed K, Hanna GB. Lymph node metastases and prognosis in esophageal carcinoma-a systematic review. Eur J Surg Oncol. 2011;37:747–753. doi: 10.1016/j.ejso.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 6.Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D, et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Xing Z, Lin A, Li C, Liang K, Wang S, Liu Y, Park PK, Qin L, Wei Y, Hawke DH, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell. 2014;159:1110–1125. doi: 10.1016/j.cell.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou XL, Wang WW, Zhu WG, Yu CH, Tao GZ, Wu QQ, Song YQ, Pan P, Tong YS. High expression of long non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog. 2016;55:2095–2105. doi: 10.1002/mc.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu ZL, Zhou GZ, Cao G, Jin L, Xie HW, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52:908–915. doi: 10.1002/mc.21944. [DOI] [PubMed] [Google Scholar]
- 11.Song W, Zou SB. Prognostic role of lncRNA HOTAIR in esophageal squamous cell carcinoma. Clin Chim Acta. 2016;463:169–173. doi: 10.1016/j.cca.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Yao J, Huang JX, Lin M, Wu ZD, Yu H, Wang PC, Ye J, Chen P, Wu J, Zhao GJ. Microarray expression profile analysis of aberrant long noncoding RNAs in esophageal squamous cell carcinoma. Int J Oncol. 2016;48:2543–2557. doi: 10.3892/ijo.2016.3457. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Hu Y, Zhang MF, Luo KJ, Xie XY, Wen J, Fu JH, Yang H. MMP1 promotes tumor growth and metastasis in esophageal squamous cell carcinoma. Cancer Lett. 2016;377:97–104. doi: 10.1016/j.canlet.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Lin C, Liang W, Wu S, Liu A, Wu J, Zhang X, Ren P, Li M, Song L. TBL1×R1 promotes lymphangiogenesis and lymphatic metastasis in esophageal squamous cell carcinoma. Gut. 2015;64:26–36. doi: 10.1136/gutjnl-2013-306388. [DOI] [PubMed] [Google Scholar]
- 15.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG, et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z, Protivankova M, et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Bhagat TD, Yang X, Song JH, Cheng Y, Agarwal R, Abraham JM, Ibrahim S, Bartenstein M, Hussain Z, et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology. 2013;144(956–966):e4. doi: 10.1053/j.gastro.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Zheng J, Deng J, You Y, Wu H, Li N, Lu J, Zhou Y. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology. 2014;146(1714–1726):e5. doi: 10.1053/j.gastro.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Wang PL, Liu B, Xia Y, Pan CF, Ma T, Chen YJ. Long non-coding RNA-Low Expression in Tumor inhibits the invasion and metastasis of esophageal squamous cell carcinoma by regulating p53 expression. Mol Med Rep. 2016;13:3074–3082. doi: 10.3892/mmr.2016.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Gao Z, Liao J, Shang M, Li X, Yin L, Pu Y, Liu R. lncRNA UCA1 inhibits esophageal squamous-cell carcinoma growth by regulating the Wnt signaling pathway. J Toxicol Environ Health A. 2016;79:407–418. doi: 10.1080/15287394.2016.1176617. [DOI] [PubMed] [Google Scholar]
- 21.Tong YS, Zhou XL, Wang XW, Wu QQ, Yang TX, Lv J, Yang JS, Zhu B, Cao XF. Association of decreased expression of long non-coding RNA LOC285194 with chemoradiotherapy resistance and poor prognosis in esophageal squamous cell carcinoma. J Transl Med. 2014;12:233. doi: 10.1186/s12967-014-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu C, Liu M, Zhang W, Xu Q, Ma K, Chen L, Wang Z, He S, Zhu H, Xu N. Upregulation of KLF4 by methylseleninic acid in human esophageal squamous cell carcinoma cells: Modification of histone H3 acetylation through HAT/HDAC interplay. Mol Carcinog. 2015;54:1051–1059. doi: 10.1002/mc.22174. [DOI] [PubMed] [Google Scholar]
- 23.Kishino T, Niwa T, Yamashita S, Takahashi T, Nakazato H, Nakajima T, Igaki H, Tachimori Y, Suzuki Y, Ushijima T. Integrated analysis of DNA methylation and mutations in esophageal squamous cell carcinoma. Mol Carcinog. 2016;55:2077–2088. doi: 10.1002/mc.22452. [DOI] [PubMed] [Google Scholar]
- 24.Kluger HM, Hoyt K, Bacchiocchi A, Mayer T, Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A, Halaban R. Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res. 2011;17:2417–2425. doi: 10.1158/1078-0432.CCR-10-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J, Wang J, Zhang D, Cheng S, Liu S. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology. 2013;144(1031–1041):e10. doi: 10.1016/S0016-5085(13)60032-3. [DOI] [PubMed] [Google Scholar]
- 26.Tsai ST, Wang PJ, Liou NJ, Lin PS, Chen CH, Chang WC. ICAM1 is a potential cancer stem cell marker of esophageal squamous cell carcinoma. PLoS One. 2015;10:e0142834. doi: 10.1371/journal.pone.0142834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.