Abstract
Central neurocytoma (CN) is a rare periventricular tumor of the central nervous system in young adults. Typically, patients with CN exhibit a favorable prognosis, but in certain cases the clinical course is more aggressive. Therefore, investigating effective therapeutic approaches is important. Oridonin has attracted attention due to its antitumor activities. However, the role of oridonin in tumorigenesis and progression remains unknown. The present study examined the antitumor function of oridonin in CN cells, and investigated the underlying molecular mechanism. An MTT assay suggested that treatment with oridonin was able to significantly inhibit the proliferation of CN cells. The annexin V-fluorescein isothiocyanate/propidium iodide assay and western blot analysis demonstrated that oridonin was able to induce apoptosis and alter the expression of apoptosis-associated proteins by downregulating anti-apoptotic protein, B-cell lymphoma-2 (Bcl-2), and upregulating pro-apoptosis proteins, Bcl-2-like protein 4, cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase 1. Subsequently, the Wnt/β-catenin signaling pathway was examined. Western blot analysis indicated that oridonin markedly decreased the expression of β-catenin, cyclin D1 and v-myc avian myelocytomatosis viral oncogene homolog. Furthermore, β-catenin was silenced by small interference RNA or overexpressed in CN cells, and the effect on cell proliferation was examined. The results indicated that silencing of β-catenin enhanced the inhibitory effect of oridonin on cell growth, whereas the overexpression of β-catenin attenuated this effect. These data indicated that oridonin inhibited proliferation and induced apoptosis to exert its antitumor activity in CN cells by repressing Wnt/β-catenin signaling. Therefore, the present study suggested that oridonin might be an effective adjuvant agent, and that the Wnt/β-catenin signaling pathway may be a potent target for the therapy in CN.
Keywords: oridonin, proliferation, apoptosis, central neurocytoma, Wnt/β-catenin signaling
Introduction
Central neurocytoma (CN) is a rare but usually benign neuronal tumor composed of uniform round cells with neuronal differentiation and typically located in the supratentorial ventricles in young adults (1,2). Surgery and radiotherapy are widely considered as the most important therapeutic approaches for CN. Patients with CN commonly exhibit a good prognosis, but in certain cases the clinical course is more aggressive or is followed by recurrence (3). Chemotherapy may be useful for recurrent CN that cannot be resected and has been radiated. Therefore, it is necessary to develop effective antitumor drugs for adjuvant therapy of CN. In particular, the combination of antitumor drugs with molecular target therapy may greatly improve the prognosis of CN.
Oridonin, a diterpenoid isolated from the Chinese medicinal herb Rabdosia rubescens, exhibits potential anti-inflammatory, anti-tumor, pro-apoptotic and neurological effects (4,5). Oridonin has been demonstrated to inhibit proliferation and induce apoptosis in a variety of human cancer cells, including colon, pancreatic, breast, lung and liver cancer (6–10). However, the underlying mechanisms remain poorly understood. Previously, oridonin has been demonstrated to exert antitumor activities through several signaling pathways that are associated with cell proliferation and apoptosis, including c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) and protein kinase B (Akt) signaling pathways (11,12). Although oridonin exhibits a significant antitumor function in multiple types of cancer, its exact effect on CN and the underlying mechanism remain unclear.
The Wnt/β-catenin signaling pathway is a canonical Wnt pathway, which serves a critical role in regulating cell growth, apoptosis, motility, polarity and differentiation (13). Dysregulation of the pathway is identified in several types of cancer, including liver, colon and several types of brain tumors (14). The underlying molecular mechanisms of CN have been largely examined, but it has been suggested that the receptors and effectors of the Wnt pathway are differentially overexpressed in CN cells (15). Additionally, it has also been demonstrated that oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt signaling (16). Therefore, the present study hypothesized whether oridonin may inhibit the growth of CN cells by affecting Wnt/β-catenin signaling transduction.
In the present study, the roles of oridonin in the proliferation and apoptosis of CN cells as well as the potential molecular mechanisms were investigated. It was indicated that oridonin was able to inhibit proliferation and induce apoptosis, which might be mediated by repressing the Wnt/β-catenin signaling pathway in CN cells.
Materials and methods
Cell culture and treatment
Central neurocytoma tissue was obtained from 1 patient diagnosed with CN for cell culture following resection at the First Affiliated Hospital of Jiamusi University (Jiamusi, China). Resected tissues were stored in Mg2+/Ca2+-free Hank's Balanced Salt Solution (HBSS), and then cut into small pieces in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) solution (20 mM PIPES, 25 mM glucose, 5 mM KCl, 120 mM NaCl) and treated as previously described (17–19). The tissue samples were resuspended in Dulbecco's modified Eagle's medium/F-12/N2 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 5% fetal bovine serum (FBS; Lonza Group, Ltd., Basel, Switzerland), 100 U/ml penicillin, 100 µg/ml streptomycin, 10 ng/ml epidermal growth factor (EGF) and 20 ng/ml basic fibroblast growth factor (bFGF). A total of 1×106 dissociated cells/well were plated into collagen IV (BD Biosciences, Franklin Lakes, NJ, USA) or fibronectin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) precoated 6-well culture plate. The cells were cultured in a 37°C incubator with 5% CO2. The present study design was approved by the Ethics Committee of First Affiliated Hospital of Jiamusi University, and all patients provided written informed consent.
RNA interference and overexpression of β-catenin
Small interfering RNA (siRNA) targeting β-catenin (siβ-catenin) and recombinant adenoviruses expressing β-catenin (Ad-β-ctn) were obtained as previously described using the AdEasy technology (20–22). The sequence of β-catenin siRNA was 5′-CAGGGGGUUGUGGUUAAGCUCUU-3′. A scramble siRNA sequence (5′-TTCTCCGAACGTGTCACGT-3′) was used as a control (Gima Biol Engineering Inc., Shanghai, China). A total of 2×104 cultured central neurocytoma cells were seeded into each well of a 12-well plate and were cultured to 80% confluence. Cell transfections were performed using 100 nmol siRNA and 5 µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cells were further cultured for 48 h following transfection, and cells were subsequently lysed and analyzed for the protein expression of β-catenin by western blotting. In addition, cells were treated with oridonin, and then cell proliferation was detected.
Proliferation assay
Cell proliferation was assessed by MTT assay. Briefly, following transfection, 104 cells were seeded in a 96-well flat bottom plate. The cells were cultured in a 5% CO2 incubator at 37°C. Once cell confluence reached 80%, the supernatant was replaced with fresh medium and the cells were treated with 0, 5, 10, 15, 20 or 25 µM oridonin (Xi'an Hao-Xuan Bio-tech Co., Ltd., Xi'an, China) dissolved in dimethyl sulfoxide (DMSO) for 24, 48 or 72 h followed by an additional 4 h subsequent to the addition of 20 µl MTT (5 mg/ml) into each well. A total of 200 µl DMSO was added to the wells for cell lysis. Absorbance was detected using an ELISA spectrophotometer at 490 nm.
Apoptosis assay
A total of 1×106 central neurocytoma cells were seeded on 60 mm dishes and cultured in Dulbecco's modified Eagle's/Nutrient Mixture F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 5% fetal bovine serum (Lonza Group, Ltd., Basel, Switzerland), 100 U/ml penicillin and 100 µg/ml streptomycin. When cells reached 80% confluence, they were treated with 0, 10, 15 or 20 µM oridonin for the indicated time. Apoptosis was quantified by Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) assay (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer's protocol. Briefly, cells were collected by trypsinization and pooled with cell supernatants from corresponding culture dishes; 500 µl of this cell suspension was incubated with 2 µl of Annexin-V-FITC stock solution for 15 min in the dark. After a short centrifugation (300 × g, 5 min), cells were washed in PBS before being resuspended in 0.5 ml of 1× binding buffer supplemented with 1 µl PI (1 µg/ml final concentration). The Annexin V-FITC/PI assay detects the amount of phosphatidylserine on the outer surface of the plasma membrane (a biochemical alteration unique to membranes of apoptotic cells) and the amount of PI, a dye that easily enters dead cells or cells in the late stages of apoptosis and binds DNA but does not bind with the plasma membrane of viable cells. Fluorescence was detected using a FACSCalibur flow cytometer by fluorescence activated cell sorter (FACS) analysis, and data were analyzed using CellQuestPro version 5.2 software (BD Biosciences, San Jose, CA, USA). The cells with phosphatidylserine on their surface were considered to be apoptotic.
Western blot analysis
The cells were washed twice with PBS and were lysed with lysis buffer (50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1% NP40, 150 mM NaCl, 10 mM NaF and 1 mM Na3VO4) containing a protease inhibitor cocktail (Roche Molecular Diagnostics, Branchburg, NJ, USA). Following centrifugation at 12,000 × g for 10 min at 4°C, the supernatant was collected and quantified using a bicinchoninic acid quantification kit (Beyotime Institute of Biotechnology, Haimen, China). 50 µg proteins were loaded per lane onto 10% SDS-PAGE gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and transferred to Immobilon-P membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk in Tris-buffered saline containing 0.05% Tween 20 for 1 h at room temperature, and incubated with the following specific primary antibodies overnight at 4°C. The antibodies against B-cell lymphoma-2 (Bcl-2; 1:500; catalogue no. sc7382; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Bcl-2-like protein 4 (Bax; 1:500; cat no. sc493; Santa Cruz Biotechnology, Inc.), cleaved caspase-3 (1:1,000; cat no. 9661S; Cell Signaling Technology, Inc., Danvers, MA, USA) and cleaved poly (ADP-ribose) polymerase 1 (cleaved PARP; 1:1,000; catalogue no. 9541; Cell Signaling Technology, Inc.), β-catenin (1:500; catalogue no. sc1496; Santa Cruz Biotechnology, Inc.), cyclin D1 (1:1,000; catalogue no. sc20044; Santa Cruz Biotechnology, Inc.), v-myc avian myelocytomatosis viral oncogene homolog (c-Myc; 1:1,000; catalogue no. sc788; Santa Cruz Biotechnology, Inc.) and β-actin (1:1,000; catalogue no. sc47778; Santa Cruz Biotechnology, Inc.) were used for detection. This was followed by incubation with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:2,000; catalogue no. sc-2005; Santa Cruz Biotechnology, Inc.) and anti-rabbit immunoglobulin G antibody (1:2,000; catalogue no. sc-2004; Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. The blots were visualized using enhanced chemiluminescence detection reagent (GE Healthcare Life Sciences, Little Chalfont, UK). The gray value of the targeted bands was quantified with QuantityOne software version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) following incubation, with β-actin used as the internal reference.
Quantitative reverse-transcriptase polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent according to the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was generated with reverse transcription using the RevertAid™ First Strand cDNA synthesis kit (Fermentas, Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) and was amplified using a TaqMan® Gene Expression Assay (Applied biosystems; Thermo Fisher Scientific, Inc.) with fluorogenic carboxyfluorescein-labeled probes using specific primers for target proteins. The specific primers for PCR were forward, 5′-ACCAGTGGATTCTGTGTTGTT-3′ and reverse, 5′-ATTTGAAGGCAGTCTGTCGTA-3′ for β-catenin and forward, 5′-GATCCCTCCAAAATCAAGTG-3′ and reverse, 5′-GAGTCCTTCCACGATACCAA-3′ for GAPDH. Real-time fluorescence detection was performed with the ABI PRISM 7700 Sequence Detector (Applied Biosystems; Thermo Fisher Scientific, Inc.). PCR involved 40 amplification cycles of 94°C for 10 sec, 53°C for 30 sec and 72°C for 40 sec, followed by final extension at 72°C for 10 min. β-catenin expression was normalized to GAPDH expression and calculated using the 2−ΔΔCt formula (23). The relative level of β-catenin mRNA was presented as a percentage of the control.
Statistical analysis
All of the experiments were repeated at least 3 times. SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA) was used to analyze the experimental data. One-way analysis of variance was used to assess the differences between the groups. Duncan's multiple range test was employed for pairwise comparison and followed by Bonferroni correction. The data are presented as the mean ± standard error of the mean. P<0.05 (two-tailed) was considered statistically significant difference.
Results
Oridonin suppresses the proliferation of CN cells
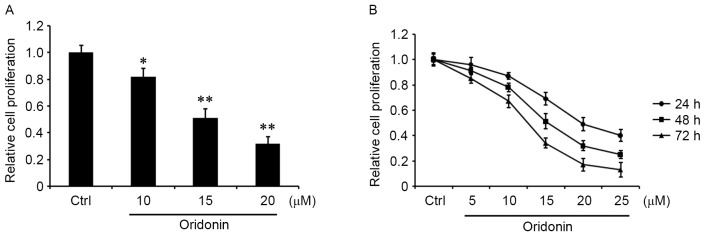
To determine the role of oridonin in CN cells, CN tissue was obtained from one patient diagnosed with CN following resection, and a CN cell line was generated by primary culture (17–19). The cells were grown in the medium for 24 h, and then treated with 10, 15 and 20 µM oridonin for 48 h or were treated with 5, 10, 15, 20 or 25 µM oridonin for 24, 48 or 72 h. An MTT assay was performed to evaluate cell proliferation. The results demonstrated that oridonin was able to markedly decrease the proliferation of CN cells in a concentration- and time-dependent manner relative to the DMSO-treated control (Fig. 1A and B).
Figure 1.
Effect of oridonin on the proliferation of central neurocytoma cells. The cells were seeded in 96-well plates. Following 24 h incubation, the cells (A) were treated with 10, 15 and 20 µM oridonin or DMSO for 48 h or (B) 5, 10, 15, 20 and 25 µM oridonin or DMSO for 24, 48 and 72 h. Cell proliferation was evaluated by MTT assay and compared with DMSO-treated control cells. The assays were performed in triplicate. *P<0.05 and **P<0.01 vs. control. Data are presented as the mean ± standard error of the mean. DMSO, dimethyl sulfoxide; Ctrl, control cells.
Oridonin induces the apoptosis of CN cells
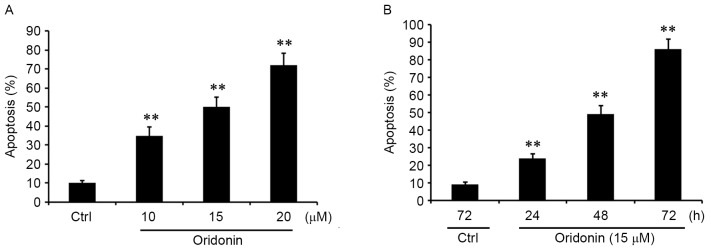
To confirm whether the inhibition of proliferation that is mediated by oridonin is associated with cell apoptosis, the cells were treated with 10, 15 and 20 µM oridonin for 48 h. Apoptosis was quantified by FACS analyses. The results demonstrated that oridonin treatment was able to significantly promote the apoptosis of CN cells in a concentration-dependent manner (Fig. 2A). Additionally, CN cells were treated with 15 µM oridonin for 24, 48 and 72 h. It was identified that apoptosis was induced by oridonin in a time-dependent manner (Fig. 2B). These results were consistent with the pattern observed with the inhibition of proliferation, suggesting that oridonin acted as a potent apoptotic inducer in CN cells.
Figure 2.
Oridonin induces the apoptosis of central neurocytoma cells in a concentration- and time-dependent manner. (A) CN cells were treated with 10, 15 and 20 µM oridonin or DMSO for 48 h. The effect of oridonin on apoptosis was determined by FACS assay. (B) The cells were treated with 15 µM oridonin for 24, 48 and 72 h, and DMSO-treated control cells were cultured for 72 h. The proapoptotic property of oridonin was assessed by FACS assay. The assays were performed in triplicate. **P<0.01 vs. control, mean ± standard error of the mean. Ctrl, DMSO-treated control cells; FACS, fluorescence activated cell sorter.
Oridonin affects the expression of apoptosis-associated proteins in CN cells
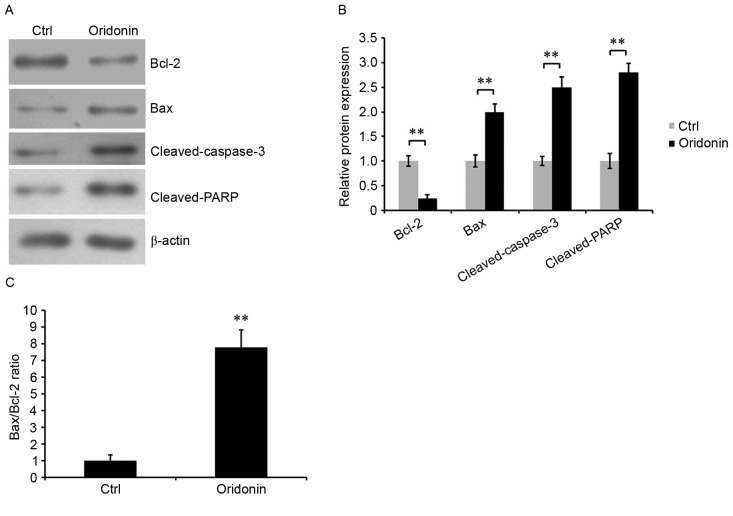
To additionally determine the role of oridonin in the apoptosis of CN cells, western blotting was performed to assess the expression levels of apoptosis-associated proteins: Bcl-2, Bax, cleaved caspase-3 and cleaved PARP. The results demonstrated that the expression of Bcl-2, an anti-apoptotic protein, was significantly reduced in oridonin-treated CN cells compared with control cells. By contrast, the expression levels of pro-apoptosis protein Bax, cleaved caspase-3 and cleaved PARP were increased (Fig. 3A and B), and the Bax/Bcl-2 ratio was increased (Fig. 3C). The Bax/Bcl-2 ratio is a key factor in determining the occurrence and level of apoptosis (24). These results indicated that treatment with oridonin was able to alter cell apoptosis, potentially by modulating Bcl-2, Bax, and cleaved caspase-3 and cleaved PARP.
Figure 3.
Oridonin alters the expression of apoptosis-associated proteins in central neurocytoma cells. (A) The levels of Bcl-2, Bax, cleaved caspase-3 and cleaved PARP proteins in oridonin-treated cells and DMSO-treated control cells were detected by western blotting using respective specific antibodies. β-actin was used as the loading control. (B) Protein levels were quantified. **P<0.01. (C) The Bax/Bcl-2 ratio was calculated by dividing the value for Bax expression by the value for Bcl-2 expression. The assay was performed in triplicate. **P<0.01 vs. ctrl. Mean ± standard error of the mean. DMSO, dimethyl sulfoxide; Bcl-2, B-cell lymphoma 2; Bax, BCL-2-like protein 4; PARP, poly(ADP-ribose) polymerase 1; Ctrl, DMSO-treated control cells.
Oridonin downregulates the Wnt/β-catenin signaling pathway
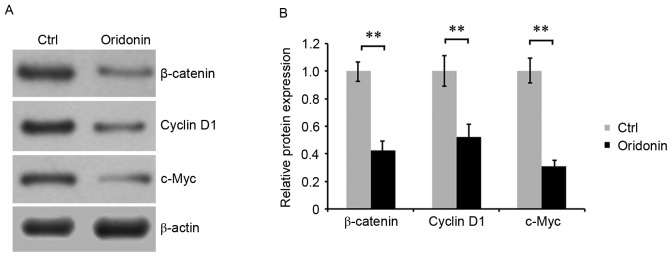
To investigate the molecular mechanism underlying the effect of oridonin on the growth and apoptosis of CN cells, the Wnt/β-catenin signaling pathway was evaluated by detecting the expression of key proteins of this pathway. CN cells were treated with 15 µM oridonin for 48 h. Western blot analysis was performed, and the results indicated that treatment with oridonin was able to downregulate the accumulation of β-catenin, cyclin D1 and c-Myc in CN cells. Densitometric analysis of the western blot bands confirmed these results (Fig. 4A and B), suggesting that oridonin regulated the growth and apoptosis of CN cells, which is likely to be mediated by Wnt signaling.
Figure 4.
Oridonin downregulates the Wnt/β-catenin signaling pathway. (A) The levels of β-catenin, cyclin D1 and c-Myc proteins in central neurocytoma cells that were treated with or without oridonin as evaluated by western blotting using respective specific antibodies. β-actin was detected as the internal reference. (B) Target protein expression normalized to β-actin expression is indicated by the bar graph. The assay was performed in triplicate. **P<0.01. Data are presented as the mean ± standard error of the mean. c-Myc, v-myc avian myelocytomatosis viral oncogene homolog; Ctrl, dimethyl sulfoxide-treated control cells.
Silencing and overexpressing β-catenin in CN cells
To verify the involvement of Wnt/β-catenin signaling pathway in oridonin-mediated inhibition on proliferation of CN cells, β-catenin was knocked down using siRNA or was overexpressed by infecting cells with recombinant adenoviruses that expressed β-catenin in CN cells. The RNA expression level of β-catenin was examined by RT-qPCR, and protein expression was detected by western blot analysis. The results indicated that the relative β-catenin mRNA expression was markedly decreased in cells that were treated with β-catenin siRNA and the residual protein expression of β-catenin in the cells was markedly reduced compared with the control siRNA-treated cells (Fig. 5A and B). Additionally, western blot analysis indicated that β-catenin was significantly elevated in cells that overexpressed β-catenin (Fig. 5C).
Figure 5.
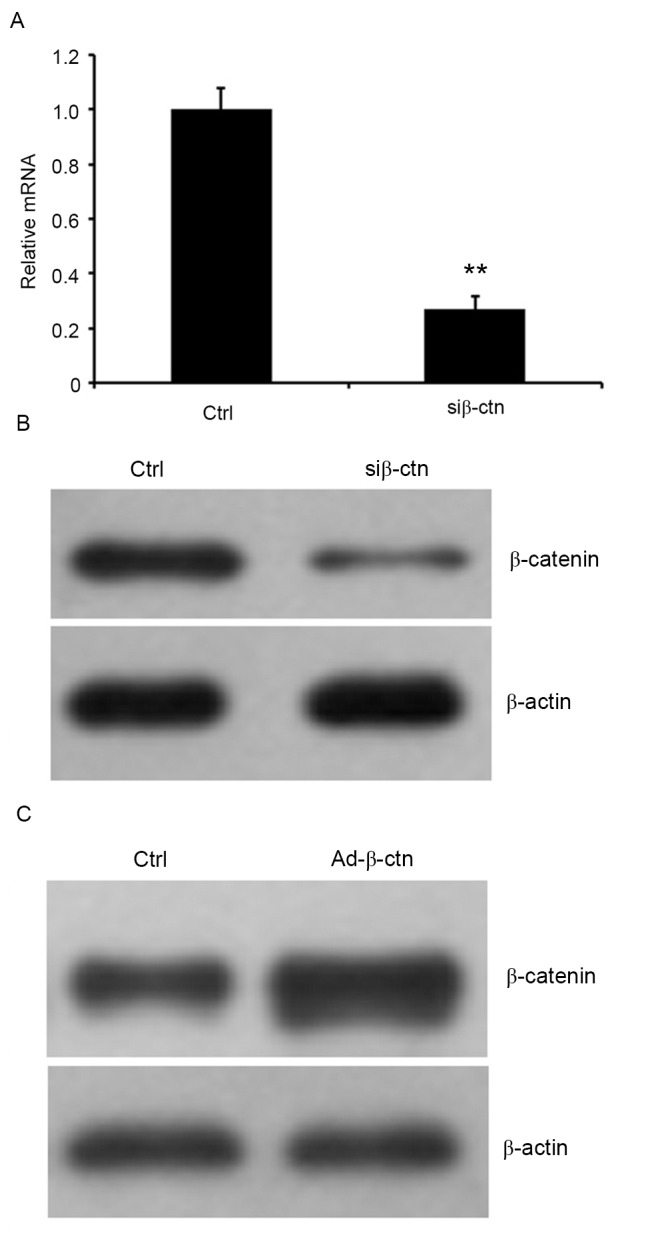
Silencing and overexpressing β-catenin in CN cells. β-catenin expression was downregulated by si-β-catenin-infected cells (si-β-ctn), and was upregulated in Ad-β-catenin-infected CN (central neurocytoma) cells (Ad-β-ctn). (A) Reverse transcription-quantitative polymerase chain reaction and (B) western blotting were performed to detect mRNA and protein expression of β-catenin in β-catenin-silenced cells (si-β-ctn) and control cells (ctrl). (C) The expression of β-catenin protein in β-catenin-overexpressed cells and ctrl cells were assessed by western blot analysis. **P<0.01 vs. control. The data are presented as the mean ± standard error of the mean.
Role of β-catenin in oridonin-mediated inhibition of cell proliferation
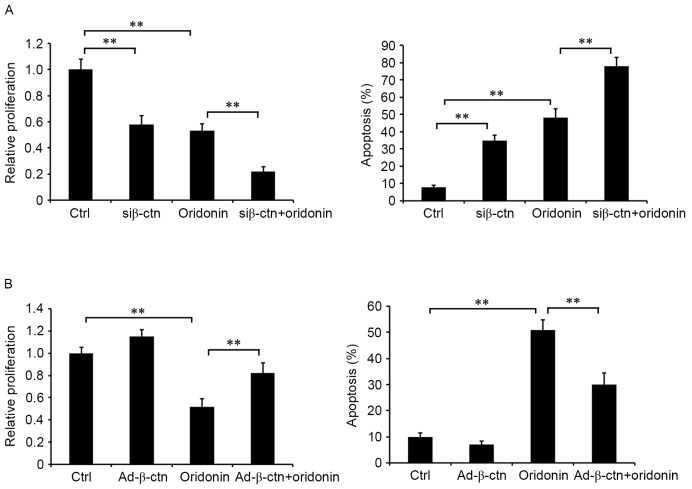
The effect of β-catenin, a key member of the Wnt signaling pathway, on oridonin-mediated inhibition of cell proliferation in CN cells was examined in the aforementioned cell lines. β-catenin-silenced and non-silenced CN cells were treated with or without 15 µM oridonin for 48 h. Detection of cell proliferation indicated that treatment with oridonin and silencing of β-catenin inhibited cell proliferation and induced apoptosis (Fig. 6A). The combination of oridonin treatment and silencing of β-catenin markedly augmented the effects on cell proliferation and apoptosis compared with oridonin single treatment (Fig. 6A). Additionally, the overexpression of β-catenin attenuated the effects of oridonin on proliferation and apoptosis (Fig. 6B). These data suggested that the Wnt/β-catenin signaling pathway served an important role in the antitumor activities of oridonin in CN cells, and that oridonin regulated the growth of CN cells, which might be via the Wnt/β-catenin signaling pathway.
Figure 6.
Role of β-catenin in oridonin-mediated inhibition on the proliferation of CN cells. The cells that were infected with (A) siβ-catenin adenovirus or (B) Ad-β-catenin were seeded in 96-well plates and were incubated with or without 15 µM oridonin. At 48 h following treatment, cell proliferation and apoptosis were detected and quantified. The assays were performed in triplicate. **P<0.01. The data are presented as the mean ± standard error of the mean. CN, central neurocytoma; siβ-ctn, recombinant adenovirus expressing β-catenin-siRNA; Ad-β-ctn, β-catenin-expressing adenovirus; Ctrl, ctrl, control cells.
Discussion
Oridonin is a diterpenoid compound isolated from the Chinese traditional medicine herb Rabdosia rubescens (25), which has attracted attraction due to its antitumor activities (26). Oridonin has been demonstrated to exert antitumor effects by inhibiting cell growth, proliferation and inducing apoptosis in multiple types of human cancer (27). However, the role of oridonin in the regulation of biological function of CN cells and the underlying molecular mechanisms remain unclear. The molecular regulatory mechanisms in CN cells are predominantly unexplored. In the present study, the data suggested that oridonin may suppress cell proliferation and induce apoptosis in CN cells, and that the function may be mediated by altering the Wnt/β-catenin signaling pathway. To investigate the molecular mechanisms underlying the inhibition of cell proliferation and induction of apoptosis in CN cells, the expression levels of apoptosis-associated proteins, Bcl-2, Bax, cleaved caspase-3 and cleaved PARP, were detected by western blotting in the present study.
Anti-apoptotic Bcl-2 and pro-apoptotic Bax as well as Bcl-2 family proteins regulate mitochondrial permeability to alter apoptosis via an intrinsic pathway (28). In the present study, treatment with oridonin was able to decrease Bcl-2 expression and increase Bax expression in CN cells. Furthermore, the Bax/Bcl-2 ratio (an important parameter to measure the occurrence and levels of apoptosis) was significantly elevated.
Cleaved caspase-3, also known as mature or activated caspase-3, is a critical mediator of cell apoptosis (29). Pro-caspases require cleavage after aspartic acid residues, which result in one large and one small subunit. These subunits associate into an a2b2 tetramer to form the active enzyme (30,31). Caspase-3 is able to cleave PARP to a specific 85-kDa form, which is observed during apoptosis (30,31). In the present study, treatment with oridonin was able to increase the levels of cleaved caspase-3 and cleaved PARP, which was consistent with the promotion of apoptosis. These data indicated that oridonin-induced apoptosis in CN cells is associated with a decrease in Bcl-2 expression and an increase in Bax expression and activation of caspase-3.
The canonical Wnt signaling pathway serves an important role in the regulation of cell proliferation and apoptosis. It has been indicated that downstream target genes of the Wnt/β-catenin pathway, including c-Myc and cyclin D1, are associated with apoptosis (32,33). The Wnt signaling pathway is activated by interactions between the Wnt ligand and the Frizzled family receptor and the low-density lipoprotein receptor-related protein 5/6. The interaction leads to an accumulation of β-catenin in the cytoplasm and translocation into the nucleus due to the inactivation of the β-catenin destruction complex, Axin/adenomatous polyposis coli/glycogen synthase kinase-3β (34–37). Subsequently, β-catenin interacts with TCF4/LEF to activate the transcription of the target genes Bcl-2, cyclin D1 and c-Myc, which control the transition from G1 to S, resulting in abnormal cellular proliferation and apoptosis (38–40). The deregulation of Wnt signaling has been identified to be involved in tumorigenesis and the development of various types of cancer (41). It has been demonstrated that the Wnt pathway receptor, Frizzled-1, and the effector, T cell transcription factor 4 (TCF4), are highly expressed in CN cells and involved in the origin and expansion of neurocytoma from native subependymal progenitor cells (15), suggesting that Wnt/β-catenin signaling is activated in CN cells. Combined with the reported finding that oridonin inhibits Wnt signaling in osteosarcoma cells (16), it is possible that oridonin may affect Wnt signaling in CN cells.
The stability of β-catenin is commonly used to evaluate the activity of the Wnt/β-catenin signaling pathway. In the present study, it was revealed that oridonin was able to downregulate the level of β-catenin protein in CN cells in a concentration- and time-dependent manner. It has been previously reported that β-catenin is able to bind TCFs to stimulate cellular growth and proliferation in tumorigenesis by triggering the cell cycle regulator cyclin D1 (42). c-Myc, as the target of β-catenin protein, also serves a critical role in tumor prognosis (43). In the present study, it was demonstrated that cyclin D1 and c-Myc, the downstream targets of β-catenin, were reduced in CN cells, indicating that the Wnt/β-catenin pathway was inhibited. Additionally, silencing β-catenin was able to augment oridonin-mediated inhibition of proliferation, whereas the overexpression of β-catenin was able to attenuate these effects in CN cells. These findings indicated that the antitumor activity of oridonin is mediated via the Wnt/β-catenin signaling pathway in CN cells.
The potential molecular mechanism underlying the antitumor activities of oridonin has been investigated. Several studies indicated that certain signaling pathways and key genes associated with cell apoptosis and cells cycle were regulated by oridonin (44). For instance, oridonin is able to induce autophagy and apoptosis by upregulating p21 in prostate cancer cells (43) or can downregulate the phosphoinositide 3-kinase/Akt signaling pathway to suppress proliferation and induce caspase-dependent apoptosis in cervical cancer HeLa cells (45). In addition, oridonin may downregulate the activities of ERK and Akt and stimulate c-Jun and MAPK pathways to suppress proliferation and induce apoptosis in osteosarcoma cells (46). Oridonin has also been suggested to suppress the protein tyrosine kinase-Ras-Raf-JNK survival pathway and activate the ERK-p53 apoptotic pathway, resulting in cell cycle arrest and apoptosis in murine fibrosarcoma cells (47). Therefore, whether other signaling pathways or proteins take part in the regulation of antitumor activities of oridonin in CN cells remains to be elucidated.
In conclusion, the results of the present study indicated that oridonin exerts its antitumor function in CN cells by downregulating the activity of the Wnt/β-catenin signaling pathway, suggesting that oridonin and other compounds from Chinese herbal medicines targeting the Wnt/β-catenin signaling may be alternative drugs for CN therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key Research and Development Program of China (grant no. 2016YFE0126000), China National Natural Science Foundation (grant no.81570392), China Postdoctoral Science Foundation funded project (grant no.2016M591937), Natural Science Fund for Colleges and Universities in Jiangsu Province (grant no.16KJB320017), and High Level Talent Support Program of Yangzhou University (grant no. 137080077).
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL and YW were major contributors in the conception and design of the research and revision of the manuscript. Acquisition of data was performed by WW, SG and LW were the major contributors in the analysis and interpretation of data and statistical analysis. Drafting the manuscript was performed by JL.
Ethics approval and consent to participate
Ethics Committee of Jiamusi University approved this study.
Patient consent for publication
Written informed consent was obtained from patient for publication of this research article and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kim DG, Paek SH, Kim IH, Chi JG, Jung HW, Han DH, Choi KS, Cho BK. Central neurocytoma: The role of radiation therapy and long term outcome. Cancer. 1997;79:1995–2002. doi: 10.1002/(SICI)1097-0142(19970515)79:10<1995::AID-CNCR22>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Schild SE, Scheithauer BW, Haddock MG, Schiff D, Burger PC, Wong WW, Lyons MK. Central neurocytomas. Cancer. 1997;79:790–795. doi: 10.1002/(SICI)1097-0142(19970215)79:4<790::AID-CNCR16>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt MH, Gottfried ON, von Koch CS, Chang SM, McDermott MW. Central neurocytoma: A review. J Neurooncol. 2004;66:377–384. doi: 10.1023/B:NEON.0000014541.87329.3b. [DOI] [PubMed] [Google Scholar]
- 4.Kuo LM, Kuo CY, Lin CY, Hung MF, Shen JJ, Hwang TL. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules. 2014;19:3327–3344. doi: 10.3390/molecules19033327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadota S, Basnet P, Ishii E, Tamura T, Namba T. Antibacterial activity of trichorabdal A from Rabdosia trichocarpa against Helicobacter pylori. Zentralblatt fur Bakteriologie: Int J Med Microbiol. 1997;286:63–67. doi: 10.1016/S0934-8840(97)80076-X. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Jiang H, Wang C, Yang B, Zhao L, Hu D, Qiu G, Dong X, Xiao B. Oridonin triggers apoptosis in colorectal carcinoma cells and suppression of microRNA-32 expression augments oridonin-mediated apoptotic effects. Biomed Pharmacother. 2015;72:125–134. doi: 10.1016/j.biopha.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Wang YF, Liu TG, Xiang XL, Huang SL. Experimental study on anti-pancreatic cancer effect of oridonin. Zhong Yao Cai. 2014;37:1230–1233. (In Chinese) [PubMed] [Google Scholar]
- 8.Li Y, Wang Y, Wang S, Gao Y, Zhang X, Lu C. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells. Med Oncol. 2015;32:365. doi: 10.1007/s12032-014-0365-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang YY, Lv YF, Lu L, Cai L. Oridonin inhibits mTOR signaling and the growth of lung cancer tumors. Anticancer Drugs. 2014;25:1192–1200. doi: 10.1097/CAD.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 10.Bohanon FJ, Wang X, Ding C, Ding Y, Radhakrishnan GL, Rastellini C, Zhou J, Radhakrishnan RS. Oridonin inhibits hepatic stellate cell proliferation and fibrogenesis. J Surg Res. 2014;190:55–63. doi: 10.1016/j.jss.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li Y, Cui JH. Oridonin induces apoptosis in SW1990 pancreatic cancer cells via p53- and caspase-dependent induction of p38 MAPK. Oncol Rep. 2014;31:975–982. doi: 10.3892/or.2013.2888. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T. Oridonin induces a caspase-independent but mitochondria- and MAPKdependent cell death in the murine fibrosarcoma cell line L929. Biol Pharm Bull. 2004;27:1527–1531. doi: 10.1248/bpb.27.1527. [DOI] [PubMed] [Google Scholar]
- 13.Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477–487. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 14.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim FJ, Keyoung HM, Goldman JE, Kim DK, Jung HW, Roy NS, Goldman SA. Neurocytoma is a tumor of adult neuronal progenitor cells. J Neurosci. 2006;26:12544–12555. doi: 10.1523/JNEUROSCI.0829-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ, Huang J, Wu K, Luo JY, Zuo GW, Chen L, et al. Oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt/β-catenin signaling. Int J Oncol. 2014;45:795–803. doi: 10.3892/ijo.2014.2456. [DOI] [PubMed] [Google Scholar]
- 17.Roy NS, Benraiss A, Wang S, Fraser RA, Goodman R, Couldwell WT, Nedergaard M, Kawaguchi A, Okano H, Goldman SA. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000;59:321–331. doi: 10.1002/(SICI)1097-4547(20000201)59:3<321::AID-JNR5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Kim JE, Paek SH, Keyoung HM, Kim DG, Jung HW. Primary cell culture of central neurocytomas. J Korean Neurosurg Soc. 2003;34:238–244. [Google Scholar]
- 20.Wu K, Yang Q, Mu Y, Zhou L, Liu Y, Zhou Q, He B. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/beta-catenin signaling. Int J Oncol. 2012;41:292–298. doi: 10.3892/ijo.2012.1423. [DOI] [PubMed] [Google Scholar]
- 21.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13:2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Feng M, Li J, Wang J, Ma C, Jiao Y, Wang Y, Zhang J, Sun Q, Ju Y, Gao L1, Zhao Y. High glucose increases LPS-induced DC apoptosis through modulation of ERK1/2, AKT and Bax/Bcl-2. BMC Gastroenterol. 2014;14:98. doi: 10.1186/1471-230X-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abelson PH. Medicine from plants. Science. 1990;247:513. doi: 10.1126/science.2300807. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Qian C, Shen Z. Anti-tumor activity of oridonin on SNU-5 subcutaneous xenograft model via regulation of c-Met pathway. Tumour Biol. 2014;35:9139–9146. doi: 10.1007/s13277-014-2178-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S, Ikejima T. Fas/FasL signaling allows extracelluar-signal regulated kinase to regulate cytochrome c release in oridonin-induced apoptotic U937 cells. Biol Pharm Bull. 2006;29:1873–1879. doi: 10.1248/bpb.29.1873. [DOI] [PubMed] [Google Scholar]
- 28.Jin S, Shen JN, Wang J, Huang G, Zhou JG. Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther. 2007;6:261–268. doi: 10.4161/cbt.6.2.3621. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490:70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Stennicke HR, Salvesen GS. Caspases-controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477:299–306. doi: 10.1016/S0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- 31.Gray DC, Mahrus S, Wells JA. Activation of specific apoptotic caspases with an engineered small molecule-activated protease. Cell. 2010;142:637–646. doi: 10.1016/j.cell.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Wharton KA., Jr Runnin' with the Dvl: Proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 34.Rao TP, Kühl M. An updated overview on Wnt signaling pathways: A prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 35.Minde DP, Anvarian Z, Rüdiger SG, Maurice MM. Messing up disorder: How do missense mutations in the tumor suppressor protein APC lead to cancer? Mol Cancer. 2011;10:101. doi: 10.1186/1476-4598-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minde DP, Radli M, Forneris F, Maurice MM, Stefan Rüdiger GD. large extent of disorder in adenomatous polyposis coli offers a strategy to guard Wnt signalling against point mutations. PLoS One. 2013;8:e77257. doi: 10.1371/journal.pone.0077257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez J, Tait SW. Mitochondrial apoptosis: Killing cancer using the enemy within. Br J Cancer. 2015;112:957–962. doi: 10.1038/bjc.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen GM. Caspases: The executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 41.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 42.Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal JF, Zhurinsky J, Geiger B, Oren M. Excess beta-catenin promotes accumulation of transcriptionally active p53. EMBO J. 1999;18:3054–3063. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 44.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 45.Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: An active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–704. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Li X, Wang J, Ye Z, Li JC. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int J Biol Sci. 2012;8:901–912. doi: 10.7150/ijbs.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu HZ, Yang YB, Xu XD. Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol Sin. 2007;28:1819–1826. doi: 10.1111/j.1745-7254.2007.00667.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.