Abstract
Circulating follicular helper T (cTfh) cells are a novel subset of cluster of differentiation (CD)4+ helper T cells. Interleukin (IL)-21 and C-X-C motif chemokine ligand (CXCL)13 are the principal effectors and chemotactic regulatory factors of Tfh. However, the roles of IL-21 and CXCL13 in gastric cancer have not yet been completely elucidated. The aim of the present study was to investigate the distribution of cTfh cells, and the expression of IL-21 and CXCL13 in patients with gastric cancer was evaluated in order to ascertain the significance and potential mechanisms of these effectors in gastric cancer. A total of 50 patients with gastric cancer were enrolled as the study subjects, with 30 healthy individuals selected as controls. The percentage of cTfh cells (cTfh%) in the peripheral blood was calculated using flow cytometry. They are identified in the present study as CD4+ chemokine C-X-C receptor (CXCR)5+ inducible T cell co-stimulator (ICOS)+ cells. The serum levels of IL-21 and CXCL13 were determined by ELISA. The cTfh% in the peripheral blood and the concentration of IL-21 and CXCL13 in the serum were significantly higher in patients with gastric cancer compared with the control group. cTfh% was significantly higher in patients with lymph node metastasis, Tumor-Node-Metastasis (TNM) stage III–IV and low differentiation. The concentrations of IL-21 and CXCL13 in patients with lymph node metastasis and/or TNM III–IV were significantly higher than in those without lymph node metastasis or with TNM I–II. There was a positive correlation between cTfh%/CXCL13 and IL-21/CXCL13, while there was no correlation between cTfh%/IL-21. cTfh cells and associated factors (IL-21/CXCL13) may be involved in the development and progression of gastric cancer. There may be mutual regulation among cTfh cells, IL-21 and CXCL13.
Keywords: follicular helper T cells, gastric cancer, interleukin-21, chemokine C-X-C ligand 13, circulating
Introduction
Gastric cancer is a common malignant tumor of the gastrointestinal tract (1). Recent statistics from China reported the incidence of gastric cancer to be the second highest of all malignancies in 2015, while the mortality rate of gastric cancer increased from the third to the second highest in China (2). Chronic inflammation contributes to the pathogenesis of gastric cancer. A number of immune cells and cytokines causing chronic inflammation, such as Th1 cells, Th2 cells, Th17 cells, regulatory T cells and interleukin (IL)-17, have been reported to be involved in the pathogenesis of gastric cancer (3–5).
Follicular helper T (Tfh) cells, were identified in human tonsils by Schaerli et al (6) and belong to a class of T cells with B cell helper functions. The characteristic cell phenotype of Tfh cells is cluster of differentiation (CD)4+ chemokine C-X-C receptor (CXCR)5+ inducible T cell co-stimulator (ICOS)+ (7). Tfh cells interact with B cells through the ICOS and CD40 ligand on the surface of the cell, which not only results in Tfh cell differentiation into effector Tfh cells, but also promotes the proliferation and differentiation of B cells into plasma cells (8). Circulating Tfh (cTfh) cells exist in human peripheral blood and have the same approximate cellular phenotype as Tfh cells (9). Unlike Tfh cells in follicles, CD69, ICOS and programmed cell death 1 (PD-1) exhibit a low expression on the surfaces of cTfh cells, suggesting that cTfh cells may function as resting or memory Tfh cells (10). cTfh cells also share functional properties with Tfh cells in secondary lymphoid organs (11). The percentage of cTfh cells is increased in infectious diseases, including chronic hepatitis C infection, autoimmune diseases, such as Sjögren's syndrome and systemic lupus erythematosus, and certain malignancies, including non-small cell lung cancer, and was associated with the severity of disease, indicating that cTfh cells may serve a role in the progression of these diseases (12–14). However, the role of cTfh cells in gastric cancer has not, to the best of our knowledge, been reported.
IL-21 is the major effector of cTfh cells that is critical for plasma cell generation and normal immunoglobulin production (15,16). C-X-C motif chemokine ligand (CXCL) 13 may be one of the most important factors in the homing, migration and accumulation of B lymphocytes by specifically binding to CXCR5 (17). The CXCL13/CXCR5 interaction also promotes the homing of Tfh cells to lymphoid follicles, facilitating contact between Tfh cells and B cells (18). IL-21 and/or CXCL13 also serve a role as biomarker of progression and prognosis in various types of cancer, including breast cancer, cervical cancer and colon cancer (19–21); however, few studies have been performed examining their effect on gastric cancer.
The aim of the present study was to investigate the distribution of cTfh cells and serum concentrations of associated cytokines (IL-21 and CXCL13) in patients with gastric cancer. Correlations between the percentage of cTfh cells, IL-21 and CXCL13 were also investigated. The results of the present study attempted to elucidate the effects of these cells and molecules in gastric cancer and their probable mechanisms of action.
Materials and methods
Patients
The study group included 50 patients with gastric cancer recruited from Qingdao Municipal Hospital (Qingdao, China) from January 2013 to December 2013. The patients, including 32 males and 18 females, were aged between 40 to 78 years with a median age of 62 years. Diagnosis was confirmed by two independent pathologists blinded to the data from Qingdao Municipal Hospital based on histological criteria by samples from endoscopic biopsy and/or surgery resection. None of the patients had undergone surgery, chemotherapy or radiotherapy prior to enrollment in the present study. A total of 30 healthy controls were enrolled during the aforementioned period. The controls were aged between 45 to 74 years with a median age of 60 years. No obvious inflammatory or ulcerative lesions were identified by gastroscopy or biopsy histology. All controls were matched with patients in terms of age and sex (Table I). Tumor-Node-Metastasis (TNM) staging system by American Joint Committee on Cancer was used to evaluate the tumor stage (22). Informed consent was obtained from all study participants according to the Declaration of Helsinki. The present study was approved by the ethics committee of Qingdao Municipal Hospital (Qingdao, China).
Table I.
Patient characteristics.
| Characteristics | Gastric cancer (n=50) | Controls (n=30) | P-value |
|---|---|---|---|
| Age, years | 62.8±1.3 | 59.8±1.5 | 0.137 |
| Sex | 0.721 | ||
| Male | 32 | 18 | |
| Female | 18 | 12 | |
| TNM stage | |||
| I | 11 | ||
| II | 9 | ||
| III | 21 | ||
| IV | 9 |
TNM, tumor-node-metastasis.
Isolation of peripheral blood mononuclear cells (PBMCs)
PBMCs were obtained by Ficoll-Hypaque (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the manufacturer's protocol following centrifugation at 800 × g at 25°C for 10 min of heparinized blood. PBMCs were then resuspended in RPMI-1640 tissue culture medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Flowcytometry
Human PBMCs were incubated with Fc receptor blocking reagent (1:5; cat no. 14-9161-73; eBioscience; Thermo Fisher Scientific, Inc.) for 20 min at 25°C and then stained with antibody of phycoerythrin (PE)-conjugated anti-human CXCR5 (1:40; cat no. 12-9185-42; eBioscience; Thermo Fisher Scientific, Inc.), fluorescein isothiocyanate-conjugated anti-human ICOS (1:5; cat no. 11-9948-42; eBioscience; Thermo Fisher Scientific, Inc.), PE-Texas Red (ECD)-conjugated anti- human CD4 (1:100; cat no. 6604727; Beckman Coulter, Inc., Brea, CA, USA) or isotype-matched IgG1, including PE-conjugated IgG1 (cat no. 12-4714-82; Invitrogen; Thermo Fisher Scientific, Inc.), FITC-conjugated IgG1 (cat no. 11-4714-42; Invitrogen; Thermo Fisher Scientific, Inc.) and ECD-conjugated IgG1 (cat no. A07797; Beckman Coulter, Inc.) as the control at 25°C away from light for 15 min. These antibodies were used for both groups. Following washing with 1× PBS twice, the cells were then subjected to analysis using a CytoFLEX cytometer and CytExpert software version 2.0 (Beckman Coulter, Inc.). The cells were gated on the forward scatter of living cells and then centered on CD4+CXCR5+ICOS+ T cells.
ELISA
Following fasting for 8 h, 3 ml blood was taken from the subjects and then centrifuged at 1,600 × g at 25°C for 10 min. Serum was collected and stored at −20°C for further examination. ELISA kits were used for detecting IL-21 (cat no. CHE0056; 4A Biotec Co., Ltd, Beijing, China) and CXCL13 (cat no. A0336; Westang Bio-TECH Co., Ltd, Shanghai, China). The procedures were performed in strict accordance with the manufacturer's protocol.
Statistical analysis
SPSS17.0 software (SPSS, Inc., Chicago, IL, USA) was used for data processing. The measurement data are expressed as the mean ± standard error. Comparisons between 2 groups were made by unpaired Student's t-test and between ≥3 groups by one-way analysis of variance followed by a Least-Significance-Difference post-hoc test. Linear correlation analysis was performed to calculate the Pearson's correlation coefficient. Categorical data were compared by χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Distribution of cTfh cells in patients with gastric cancer and its association with the examined clinicopathological features
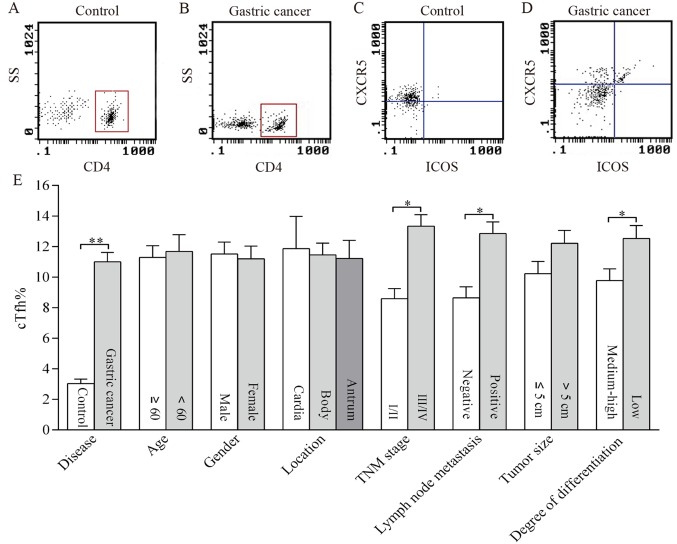
cTfh cells were identified as CXCR5+ ICOS+ T cells within the CD4+ T cell population by flow cytometry (Fig. 1A and B). The percentage of cTfh cells (cTfh%) was quantified by calculating the proportion of cells in the right upper quadrant as depicted in Fig. 1C and D. The cTfh% represented by density of the point in the right upper quadrant was higher in the gastric cancer group (Fig. 1D) than in the control group (Fig. 1C). The cTfh% in patients with gastric cancer and in the control group were 11.00±0.62 and 3.03±0.29, respectively. The comparison of the mean cTfh% between different groups is depicted in Fig. 1E. The cTfh% in patients with lymph node metastasis, TNM III–IV and low differentiation were significantly higher than in those without lymph node metastasis, TNM I–II and well differentiated tumors (P<0.01). There were no significant differences in cTfh% between individuals of different age, sex, tumor location or tumor size (Fig. 1E).
Figure 1.
Distribution of cTfh in the peripheral blood of patients with gastric cancer and its association with clinicopathological features. cTfh cells were identified as CXCR5+ ICOS+ T cells within the CD4+ T cell population by flow cytometry. As presented in representative dotplots, CD4+ cells were distinguished by setting the gate in (A) controls and in (B) the patients. Then cTfh cells were recognized as CXCR5+ ICOS+ cells shown in the right upper quadrant in (C) the controls and in (D) the patients. The percentage of cTfh cells was compared in patients with gastric cancer and controls as well as in patients with different clinicopathological characteristics including age, sex, location, TNM stage, lymph node metastasis, tumor size, and degree of differentiation (E). *P<0.01; **P<0.001. cTfh, circulating follicular helper T cells; TNM, Tumor-Node-Metastasis; CXCR5, Chemokine C-X-C receptor 5; ICOS, inducible T cell co-stimulator; SS, side scatter.
Association between the concentration of IL-21 and clinicopathological features in patients with gastric cancer
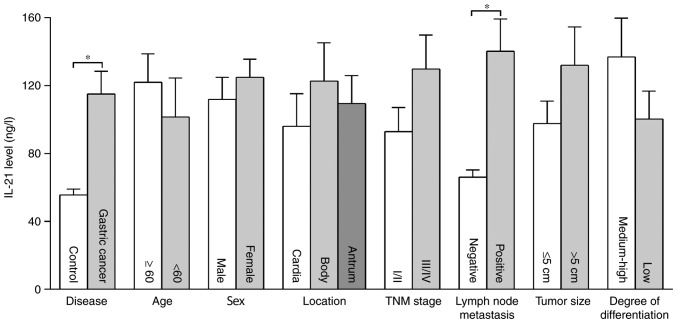
The serum levels of IL-21 in patients with gastric cancer were significantly higher than those in the control group, and the serum concentrations of IL-21 in the two groups were 114.95±13.51 and 55.66±3.39 ng/l, respectively (P<0.01). The concentration of IL-21 in patients with lymph node metastasis was higher than in those without lymph node metastasis (P<0.01). There were no significant differences in serum IL-21 levels among individuals of different age, sex, tumor location, TNM stage, histological differentiation and tumor size (Fig. 2).
Figure 2.
Association between the concentration of IL-21 and clinicopathological features in patients with gastric cancer. *P<0.01. IL-21, interleukin 21; TNM, Tumor-Node-Metastasis.
Association between the concentration of CXCL13 and clinicopathological features in patients with gastric cancer
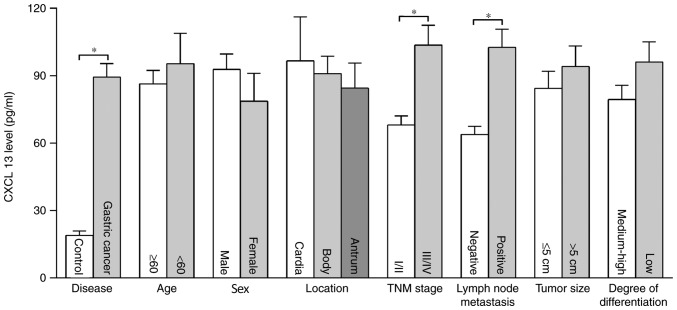
The concentration of serum CXCL13 in patients with gastric cancer was significantly higher than in the control group (P<0.01), and the concentrations of CXCL13 in the two groups were 89.41±6.00 and 18.91±1.99 pg/ml, respectively. The concentration of CXCL13 in patients with lymph node metastasis and TNM III–IV was significantly higher than those without lymph node metastasis and TNM I–II (P<0.01). There were no significant differences in concentration of CXCL13 among patients of different age, sex, tumor location, histological differentiation and tumor size (Fig. 3).
Figure 3.
Association between the concentration of CXCL13 and clinicopathological features in patients with gastric cancer. *P<0.01. CXCL13, C-X-C motif chemokine ligand 13; TNM, Tumor-Node-Metastasis.
Correlation between cTfh%, IL-21 and CXCL13 in patients with gastric cancer
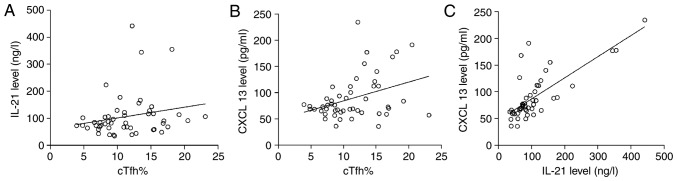
The results of linear correlation analysis demonstrated that cTfh% had no correlation with the concentration of IL-21 in patients with gastric cancer (r=0.217, P=0.130), while a significant correlation was observed between cTfh% and CXCL13 (r=0.368, P=0.008), as well as between IL-21 and CXCL13 (r=0.748, P=0.000; Fig. 4).
Figure 4.
Correlation between cTfh%, IL-21 and CXCL13 in patients with gastric cancer. The association between (A) cTfh% and IL-21, (B) cTfh% and CXCL13, and (C) IL-21 and CXCL13 are shown. cTfh, circulating follicular helper T cells; IL-21, interleukin 21; CXCL13, C-X-C motif chemokine ligand 13.
Discussion
In the present study, the distribution of cTfh cells and the concentrations of IL-21 and CXCL13 in the peripheral blood of patients with gastric cancer were investigated. Compared with the control group, the cTfh% in the peripheral blood and the concentration of IL-21 and CXCL13 in the serum were significantly higher in patients with gastric cancer. A higher cTfh% and a higher concentration of IL-21 and CXCL13 were also associated with the characteristics of tumor progression, including the late tumor stage, lymph node metastasis and poor differentiation.
cTfh may serve a role in the pathogenesis and progression of various types of malignancies. A study undertaken by Cha et al (16), reported that in primary diffuse large B cell lymphoma (DLBCL), CD4+ CXCR5+ cells in the peripheral blood promoted the proliferation and inhibited the apoptosis of DLBCL cells. Additionally, a study undertaken by Shi et al (14) demonstrated the percentage of cTfh to be significantly higher in non-small cell lung cancer (NSCLC) and in advanced-stage cases. An additional study reported that when Crohn's disease was accompanied by colorectal cancer, the number of cTfh cells was increased 1.59-fold (23). To the best of our knowledge, at present, there are no reports on the role of cTfh in gastric cancer. The results of the present study demonstrated that the percentage of cTfh in patients with gastric cancer was significantly higher than in the control group, and was associated with lymph node metastasis, advanced stages of cancer and a low degree of differentiation. Therefore, cTfh may be considered as an indicator of diagnosis/prognosis in patients with gastric cancer.
The leading studies of IL-21 in tumors have focused on its inhibitory effect on tumor growth, and consequently IL-21 has become a target for immunotherapy (24–26). However, in breast cancer, the IL-21 gene polymorphism may be an important index for assessing the prognosis of disease (27). While the role of IL-21 in gastric cancer has rarely been reported, a previous study reported that the concentration of IL-21 in gastric cancer tissues has been increased (28). The serum concentration of IL-21 in patients with gastric cancer was demonstrated to be significantly higher when compared with the control group. By comparing the levels of IL-21 expression between different clinicopathological features, it was identified that the serum concentration of IL-21 in patients with lymph node metastasis was significantly higher than in those without lymph node metastasis. This suggested that IL-21 may serve a role in the development and progression of gastric cancer.
The impact of CXCL13/CXCR5 on various types of cancer, including breast cancer, colorectal cancer and prostate cancer has been the focus of a number of studies (29–31). CXCL13 and CXCR5 may be associated with unfavorable clinical characteristics in tumors, including invasive pathological type, positive lymph node, metastasis and the relapse of cancer at an advanced stage of disease. The results of the present study demonstrated that the concentration of CXCL13 in the peripheral blood of patients with gastric cancer was significantly higher, when compared with the control group, and the concentration of CXCL13 in patients with lymph node metastasis and TNM III–IV was higher than in those without lymph node metastasis and TNM I–II. These results suggested that the increased concentration of CXCL13 may be associated with the progression of gastric cancer.
IL-21 is a well-established effector of Tfh cells, and can also bind with IL-21R on the surface of Tfh cells to promote the expression of CXCR5 and ICOS through the autocrine signaling pathway, thereby inducing further differentiation in Tfh cells (32). As the principal ligand of CXCR5, CXCL13 can induce the homing and migration of Tfh cells by combining with CXCR5 (33). In this way, there may be a correlation between Tfh, IL-21 and CXCL13. In the present study, the correlations between these three parameters were examined. A study undertaken by An et al (34), reported that the proportion of Tfh cells in the peripheral blood was positively associated with the concentration of IL-21 in patients with unexplained infertility. In the present study, no correlation was determined between the percentage of cTfh and the concentration of IL-21 in serum of patients with gastric cancer. The result of no correlation between cTfh and IL-21 in peripheral blood may be associated with the multiple sources of IL-21 in the serum. In addition to Tfh cells, Th17 cells and natural killer T cells are capable of secreting a small amount of IL-21 (35,36). Th17 cells in the peripheral blood appeared to be notably amplified in gastric cancer (29). Whether this affects the synthesis and secretion of IL-21 remains to be established. The association between Tfh cells and CXCL13, as well as CXCL13 and IL-21, has not, to the best of our knowledge, been reported previously. The results of the present study demonstrated a positive association between cTfh% and CXCL13, and a positive correlation between CXCL13 and IL-21. CXCL13 and IL-21 are the key factors in the survival of the Tfh/B cell axis. CXCL13 can direct Tfh cell migration into B cell follicles through binding to CXCR5 on the surface of Tfh (37). Tfh cells located in lymphoid follicles express ICOS, PD-1 and IL-21, which not only provide markers for identification of Tfh cells but also serve important functions in their interactions with B cells (18,38,39). The association between cTfh, CXCL13 and IL-21 was determined in peripheral blood. Whether this association can indirectly reflect the interaction between these three parameters in lymphoid follicles remains to be studied.
To conclude, the findings of the present study demonstrated that an increased percentage of cTfh cells, IL-21 and CXCL13 were observed in patients with gastric cancer, and that all three factors may be involved in the development and progression of cancer. Furthermore, there may be mutual regulation among cTfh cells, IL-21 and CXCL13; however, the direct association between cTfh cells and associated factors was not reflected in the present study. In the future, the direct associations between them may be analyzed through intervention experiments in vitro in order to fully elucidate their effects on gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
XM and CZ produced the concept of the present study. XM and QD designed the present study. CZ and QD supervised the present study. XM, XY and JM provided the materials. XM, QX, XY, XX and JL conducted data collection and/or processing.XM, XY and JM conducted analysis and/or interpretation. XM, JM and XY conducted the literature review. XM and XY wrote the manuscript. QD and CZ critically reviewed the manuscript.
Ethics approval and consent to participate
Informed consent was obtained from all study participants according to the Declaration of Helsinki. The present study was approved by the ethics committee of Qingdao Municipal Hospital (Qingdao, China).
Patient consent for publication
The patients provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B, Ai J, Zhang Z, Song J, Shan B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep. 2013;30:1215–1222. doi: 10.3892/or.2013.2570. [DOI] [PubMed] [Google Scholar]
- 4.Meng XY, Zhou CH, Ma J, Jiang C, Ji P. Expression of interleukin-17 and its clinical significance in gastric cancer patients. Med Oncol. 2012;29:3024–3028. doi: 10.1007/s12032-012-0273-1. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Gao J, Su Z, Dai X, Li Y, Liu Y, Chen J, Tong J, Zhang Y, Wu C, et al. Downregulation of Hlx closely related to the decreased expressions of T-bet and Runx3 in patients with gastric cancer may be associated with a pathological event leading to the imbalance of Th1/Th2. Clin Dev Immunol. 2012;2012:949821. doi: 10.1155/2012/949821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Zhou Y, Yu Q, Zhao Z, Wang H, Luo X, Chen Y, Zhu Z, Chen G, Wu M, Qiu L. Higher frequency of CD4+CXCR5+ICOS+PD1+ T follicular helper cells in patients with infectious mononucleosis. Medicine (Baltimore) 2015;94:e2061. doi: 10.1097/MD.0000000000002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2014;92:64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 11.Forcade E, Kim HT, Cutler C, Wang K, Alho AC, Nikiforow S, Ho VT, Koreth J, Armand P, Alyea EP, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127:2489–2497. doi: 10.1182/blood-2015-12-688895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Zhang L, Li H, Chen Z, Luo A, Liu B, Chen M, Peng M, Ren H, Hu P. Circulating T follicular helper cells are associated with rapid virological response in chronic hepatitis C patients undergoing peginterferon therapy. Int Immunopharmacol. 2016;34:235–243. doi: 10.1016/j.intimp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Szabo K, Papp G, Szanto A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjogren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2016;183:76–89. doi: 10.1111/cei.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi W, Li X, Cha Z, Sun S, Wang L, Jiao S, Yang B, Shi Y, Wang Z, Wu Z, Dai G. Dysregulation of circulating follicular helper T cells in nonsmall cell lung cancer. DNA Cell Biol. 2014;33:355–360. doi: 10.1089/dna.2013.2332. [DOI] [PubMed] [Google Scholar]
- 15.Davis MR, Zhu Z, Hansen DM, Bai Q, Fang Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015;358:107–114. doi: 10.1016/j.canlet.2014.12.047. [DOI] [PubMed] [Google Scholar]
- 16.Cha Z, Qian G, Zang Y, Gu H, Huang Y, Zhu L, Li J, Liu Y, Tu X, Song H, Qian B. Circulating CXCR5+CD4+ T cells assist in the survival and growth of primary diffuse large B cell lymphoma cells through interleukin 10 pathway. Exp Cell Res. 2017;350:154–160. doi: 10.1016/j.yexcr.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Dupuis J, Boye K, Martin N, Copie-Bergman C, Plonquet A, Fabiani B, Baglin AC, Haioun C, Delfau-Larue MH, Gaulard P. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30:490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, Yu D, Li X, Yu N, Li X, Wang Y, Wang Y. CD4+CXCR5+ follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjogren's syndrome. Int J Clin Exp Pathol. 2014;7:1988–1996. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu S, Lin J, Qiao G, Wang X, Xu Y. Tim-3 identifies exhausted follicular helper T cells in breast cancer patients. Immunobiology. 2016;221:986–993. doi: 10.1016/j.imbio.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Tian Y, Yuan C, Ma D, Zhang Y, Liu Y, Zhang W, Hou F, Cui B. IL-21 and IL-12 inhibit differentiation of Treg and TH17 cells and enhance cytotoxicity of peripheral blood mononuclear cells in patients with cervical cancer. Int J Gynecol Cancer. 2011;21:1672–1678. doi: 10.1097/IGC.0b013e3182358955. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 2015;400:287–295. doi: 10.1007/s11010-014-2285-y. [DOI] [PubMed] [Google Scholar]
- 22.Deng J, Zhang R, Zhang L, Liu Y, Hao X, Liang H. Negative node count improvement prognostic prediction of the seventh edition of the TNM classification for gastric cancer. PLoS One. 2013;8:e80082. doi: 10.1371/journal.pone.0080082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Wang Z, Diao Y, Qian X, Zhu N, Dong W. Circulating follicular helper T cells in Crohn's disease (CD) and CD-associated colorectal cancer. Tumour Biol. 2014;35:9355–9359. doi: 10.1007/s13277-014-2208-2. [DOI] [PubMed] [Google Scholar]
- 24.Sondergaard H, Skak K. IL-21: Roles in immunopathology and cancer therapy. Tissue Antigens. 2009;74:467–479. doi: 10.1111/j.1399-0039.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 25.Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21 combination strategies for cancer therapy. Nat Rev Drug Discov. 2008;7:231–240. doi: 10.1038/nrd2482. [DOI] [PubMed] [Google Scholar]
- 26.Kannappan V, Butcher K, Trela M, Nicholl I, Wang W, Attridge K. Interleukin 21 inhibits cancer-mediated FOXP3 induction in naïve human CD4 T cells. Cancer Immunol Immunother. 2017;6:637–645. doi: 10.1007/s00262-017-1970-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You Y, Deng J, Zheng J, Hu M, Li N, Wu H, Li W, Lu J, Zhou Y. IL-21 gene polymorphism is associated with the prognosis of breast cancer in Chinese populations. Breast Cancer Res Treat. 2013;137:893–901. doi: 10.1007/s10549-012-2401-1. [DOI] [PubMed] [Google Scholar]
- 28.Su Z, Sun Y, Zhu H, Liu Y, Lin X, Shen H, Chen J, Xu W, Xu H. Th17 cell expansion in gastric cancer may contribute to cancer development and metastasis. Immunol Res. 2014;58:118–124. doi: 10.1007/s12026-013-8483-y. [DOI] [PubMed] [Google Scholar]
- 29.Biswas S, Sengupta S, Chowdhury Roy S, Jana S, Mandal G, Mandal PK, Saha N, Malhotra V, Gupta A, Kuprash DV, Bhattacharyya A. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Res Treat. 2014;143:265–276. doi: 10.1007/s10549-013-2811-8. [DOI] [PubMed] [Google Scholar]
- 30.Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, Wu HR. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014;18:1916–1924. [PubMed] [Google Scholar]
- 31.El-Haibi CP, Singh R, Gupta P, Sharma PK, Greenleaf KN, Singh S, Lillard JW., Jr Antibody microarray analysis of sSignaling networks regulated by cxcl13 and cxcr5 in prostate cancer. J Proteomics Bioinform. 2012;5:177–184. doi: 10.4172/jpb.1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spolski R, Leonard WJ. IL-21 and T follicular helper cells. Int Immunol. 2010;22:7–12. doi: 10.1093/intimm/dxp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Bélanger S, Kasturi SP, Landais E, Akondy RS, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci USA. 2016;113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An LF, Zhang XH, Sun XT, Zhao LH, Li S, Wang WH. Unexplained infertility patients have increased serum IL-2, IL-4, IL-6, IL-8, IL-21, TNFα, IFNγ and increased Tfh/CD4 T cell ratio: Increased Tfh and IL-21 strongly correlate with presence of autoantibodies. Immunol Invest. 2015;44:164–173. doi: 10.3109/08820139.2014.932377. [DOI] [PubMed] [Google Scholar]
- 35.Duan MC, Huang Y, Zhong XN, Tang HJ. Th17 cell enhances CD8 T-cell cytotoxicity via IL-21 production in emphysema mice. Mediators Inflamm. 2012;2012:898053. doi: 10.1155/2012/898053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 37.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 38.Hardtke S, Ohl L, Förster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 39.Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol. 2010;32:183–196. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.