Abstract
SPARC-related modular calcium binding 1 (SMOC1) represents a vital member of the SPARC matricellular protein family that regulates cell matrix interaction through binding to cell-surface receptors. The present study aimed to investigate the roles and molecular mechanisms of SMOC1 silencing on the fibrosis of myocardial fibroblasts (MFBs). Cell Counting kit-8 and flow cytometry assays were performed to determine cell viability and reactive oxygen species (ROS) content, respectively. ELISA was performed to detect the expression of associated cytokines and matrix proteins. Western blot analysis and reverse transcription-quantitative polymerase chain reaction assays were used to evaluate the expression of associated proteins and mRNAs, respectively. The results revealed that SMOC1 silencing suppressed the cell viability of angiotensin II (Ang II)-treated MFBs. SMOC1 silencing reduced the ROS content and oxidative stress in MFBs in response to Ang II. Furthermore, SMOC1 silencing downregulated the expression levels of fibrosis-associated proteins in Ang II-treated MFBs. SMOC1 silencing affected the bone morphogenetic protein 2 (BMP2)/Smad signaling pathway in Ang II-treated MFBs. In conclusion, the results of the present study suggested that SMOC1 silencing suppressed the Ang II-induced myocardial fibrosis of mouse MFBs through affecting the BMP2/Smad signaling pathway.
Keywords: SPARC-related modular calcium binding 1, myocardial fibrosis, bone morphogenetic protein 2, Smad, oxidative stress, transforming growth factor β1, collage-I, collagen-III
Introduction
Heart failure affected 40 million people worldwide in 2015 (1). In developing countries, the morbidity and mortality of cardiovascular disease continue to increase annually, which threatens the lives and quality of life of patients (2). Until now, ventricular remodeling has been considered as an important pathological process in the occurrence and development of heart failure (3). The main pathological manifestations of ventricular remodeling are myocardial microcirculation, apoptosis and necrosis of myocardial cells, progressive myocardial collagen network remodeling and myocardial interstitial fibrosis (4). Myocardial fibrosis represents excessive deposition of extracellular matrix in normal myocardial tissue caused by various pathological factors. The characteristics of myocardial fibrosis are numerous, including enhanced collagen concentration and volume fraction, the imbalance in the proportion of various types of collagen, and disordered arrangement of collagen (5). The pathological basis of myocardial fibrosis is that the percentage of myocardial interstitium in myocardial tissue is increased. Cardiac muscle fiber connective tissue is mainly comprised of fibroblasts, myocardial fibroblasts, valvular mesenchymal cells and extracellular matrix. Additionally, extracellular matrix of the myocardium, such as collagen, is mainly synthesized by fibroblasts (6,7). However, in the past, research has focused on the study of myocardial cells, ignoring the importance of myocardial fibroblasts (MFBs). MFBs have a strong ability to split and to secrete matrix proteins, collagen-I (COL-I) and COL-III under various pathological conditions, including hypoxic-ischemic injury, local inflammatory cytokine stimulation and neuroendocrine factor secretion (8,9). Therefore, MFBs serve an important role in the myocardial fibrosis of MFBs.
Bone morphogenetic proteins (BMPs) serve as a multi-potent family of proteins regulating the growth and differentiation of cells. Studies have demonstrated that BMPs may reverse fibrosis progression (10,11). BMP2 overexpression significantly suppressed the fibrosis induced by transforming growth factor β1 (TGF-β1) in renal interstitial fibroblasts (12). Furthermore, it has been proven that upregulation of BMP2 may enhance myocardial fibrotic signaling through activation of the Smurf1/Smad6 complex (13). Additionally, a large volume of evidence has suggested that the Smad family is associated with the pathological mechanisms underlying fibrosis (14–16). Therefore, it was hypothesized that BMP2 and Smad may participate in the development and progression of myocardial fibrosis.
SPARC-related modular calcium binding 1 (SMOC1) represents a vital member of the SPARC matricellular protein family that regulates cell-matrix interactions through binding to cell-surface receptors, including growth factors and the components of extracellular matrix (17,18). SMOC1 is widely expressed in numerous tissue types, and it has been revealed that SMOC1 is mainly located at the basement membrane (18,19). A previous study has demonstrated that Xenopus SMOC protein, also known as the ortholog of human SMOC1, acted as a BMP antagonist (20). These results suggested that human SMOC1 may also regulate BMP signaling. However, knowledge is insufficient regarding the biological function of SMOC1 in BMP pathway regulation in myocardial fibrosis.
The present study analyzed the association between SMOC1 silencing and the fibrosis of mouse MFBs. Furthermore, the exact roles and molecular mechanisms of SMOC1 silencing and the BMP2/Smad pathway in the fibrosis of angiotensin II (Ang II)-treated MFBs were also investigated.
Materials and methods
Cell culture, genes and plasmids
Mouse MFBs (MIC-iCell-c002, iCell Bioscience Inc., Shanghai) were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 atmosphere at 37°C. The cells were observed with an inverted microscope (×40 magnification). si-SMOC1 (GGGAAGTCAAGGTCAGTACG) or si-negative control (NC) (GGGAAGTCAAGGTCAGTACG), was cloned into the psiRNA-h7SK vector (Biovector Science Lab, Inc.). The plasmid (1 µg) was transfected into the cells with Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). The cells were incubated at 37°C for 6 h; and then the cells were transferred into fresh DMEM and maintained for 18 h at 37°C. Then the cells were used to perform the following experiments.
Grouping
Six treatment groups were created in the present research, including a control group (MFBs without treatment), a negative control (NC) group (MFBs transfected with an empty vector), an si-SMOC1 group (MFBs transfected with si-SMOC1), an Ang II group [MFBs treated with 1 µM Ang II (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 18 h at 37°C], an Ang II+NC group (MFBs transfected with an empty vector and then treated with 1 µM Ang II) and an Ang II+si-SMOC1 group (MFBs transfected with si-SMOC1 and then treated with 1 µM Ang II).
Cell viability analysis
A Cell Counting kit-8 assay (CCK-8; Beyotime Institute of Biotechnology, Haimen, China) was performed to measure cell viability. Approximately 6×104 MFBs/ml in the logarithmic phase were seeded into 96-well plates and maintained in an incubator at 37°C in 5% CO2 for 12 h. Subsequently, cells were divided into the aforementioned treatment groups. Cells were maintained for 12, 24 and 48 h, respectively. Subsequently, 10 µl CCK-8 reagent was added to each well and cells were maintained for 3 h at 37°C. A microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to measure the absorbance at 450 nm. Cell viability was evaluated by the percentage of surviving cells compared with the control.
Flow cytometry
MFBs were trypsinized by 0.25% trypsin (Beyotime Institute of Biotechnology) and collected in Eppendorf tubes. Cells were then washed with PBS. Subsequently, cells were re-suspended with serum-free Dulbecco's modified Eagle medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2′-7′-dichlorofluorescin diacetate (D6883, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a density of 1×106 cells/ml and incubated for 0.5 h at 37°C. Following centrifugation at 224 × g for 1 min at room temperature, the supernatant was discarded and cells were collected. Cells were re-suspended with PBS and a FACSCalibur flow cytometer with CellQuest software version 3.3 (BD Biosciences, Franklin Lakes, NJ, USA) was used to assess the reactive oxygen species (ROS) content.
Detection of malondialdehyde (MDA), lactate dehydrogenase (LDH) and superoxide dismutase (SOD)
The following kits were used: LDH assay kit (Abcam, Cambridge, UK), MDA assay kit (Beyotime Institute of Biotechnology, Haimen, China) and SOD assay kit (Sigma-Aldrich; Merck KGaA). All assays were performed according to the manufacturer's protocol.
ELISA
The expression of TGF-β1, COL-I and COL-III were determined by ELISA kits: Mouse TGF-β1 ELISA Kit (E-EL-M0051c; Elabscience, Wuhan, Hubei, China), COL1 ELISA kit (E-EL-M0325c; Elabscience) and COL-III ELISA kit (E-EL-M0316; Elabscience). According to the manufacturer's protocol, MFBs (6×103/well) were added into 96-well plate. The plates were sealed with adhesive tape and maintained at 37°C for 90 min. Subsequently, 100 µl biotinylated antibody fluids were added. The plates were sealed with adhesive tape and maintained at 37°C for 60 min. Next, 100 µl HRP conjugate enzyme binding solutions were added. The plates were sealed with adhesive tape and maintained at 37°C for 30 min. Substrate regent (100 µl) was added and plates were maintained for 10–15 min in the dark at 37°C. Subsequently, stop solution was added and mixed in for 10 min immediately. The OD450 value was detected using a microplate reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
The proteins were isolated with NP40 lysis buffer (Beyotime Institute of Biotechnology). The protein concentration was measured by bicinchoninic assay protein assay kit (Pierce; Thermo Fisher Scientific Inc.). 20 µg protein was separated by 12% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk at room temperature for 2 h. The primary antibodies were incubated with the membranes at 4°C overnight. Western blotting was performed using the following specific antibodies: Rabbit anti-mouse anti-SMOC1 (dilution, 1:500; catalog no., ab200219; Abcam, Cambridge, MA, USA), rabbit anti-mouse anti-fibronectin (FN) (dilution, 1:1,000; catalog no., ab131390; Abcam), rabbit anti-mouse anti-TGF-β1 (dilution, 1:1,000; catalog no., ab92486; Abcam), rabbit anti-mouse anti-COL-I (dilution, 1:500; catalog no., ab64883; Abcam), rabbit anti-mouse anti-COL-III (dilution, 1:5,000; catalog no., ab7778; Abcam), rabbit anti-mouse anti-BMP2 (dilution, 1:500; catalog no., ab14933; Abcam), rabbit anti-mouse anti-Smad2 (dilution, 1:1,000; catalog no., ab33875; Abcam), rabbit anti-mouse anti-p-Smad2 (dilution, 1:1,000; catalog no., ab53100; Abcam), and rabbit anti-mouse anti-actin (dilution, 1:5,000; catalog no., ab179467; Abcam). The membranes were subsequently incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (dilution, 1:2,000; catalog no., ab205718; Abcam) at room temperature for 1 h. Enhanced chemiluminescent (ECL) reagents (EMD Millipore) and an ECL system (GE Healthcare, Chicago, IL, USA) were used to assess the results. The density of the blots was analyzed by Quantity One software version 4.6.9 (Bio-Rad Laboratories).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured MFBs using TRIzol reagent (Thermo Fisher Scientific, Inc.). RNA was reverse transcribed to cDNA using a Reverse Transcription kit (Sigma-Aldrich; Merck KGaA), according to the manufacturer's protocols. RT-qPCR was performed using SYBR-Green PCR master mix (Vazyme, Piscataway, NJ, USA) on ABI 7500 Thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: 5 min pretreatment at 95°C; 94°C for 15 sec, 60°C for 45 sec (35 cycles); final extension at 76°C for 10 min and maintenance at 4°C. Actin was used as the control of the input RNA level. The method of quantification was according to 2−∆∆Cq method (21). The primers used, designed by Invitrogen; Thermo Fisher Scientific, Inc., were as follows: SMOC1 forward, 5′-CCAAGCCCAAGAAATGTGCC-3′ and reverse, 5′-AGTCCTGTCTCCTCGGAGTT-3′ (227 bp); FN forward, 5′-TGACAACTGCCGTAGACCTG-3′ and reverse, 5′-CACTGGGGTGTGGATTGACC-3′ (232 bp); TGF-β1 forward, 5′-GTCCAAACTAAGGCTCGCCA-3′ and reverse, 5′-ATAGATGGCGTTGTTGCGGT-3′ (202 bp); COL-I forward, 5′-ATTTGTGCGTCGGTTGGGTA-3′ and reverse, 5′-GTTGTGTTCTGAAGCCACGG-3′ (298 bp); COL-III, forward, 5′-GCTACAGGCCTTTTGTTGGC-3′ and reverse, 5′-CCACAGAATGGGTGGGAGAC-3′ (222 bp); and actin, forward, 5′-TGCCCGGTGCTTTAGACTAC-3′ and reverse, 5′-AAATAATGAACCCAGCCAGCC-3′ (171 bp).
Statistical analysis
The results of the present study are presented as the mean ± standard error of the mean of at least three independent experiments. All of the experimental data were analyzed by one-way analysis of variance following Tukey's test. P<0.05 was considered to indicate a statistically significant difference. GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) was used for data analysis.
Results
Identification of mouse MFBs
Over the course of the present study, mouse MFBs were cultured with DMEM containing 10% FBS. As demonstrated in Fig. 1, the morphology of the MFBs was spindle-shaped and polygonal. Furthermore, large nuclei and clear cytoplasms were observed in the MFBs. Therefore, MFBs were harvested and used for the subsequent experiments.
Figure 1.
Identification of mouse MFBs. The MFBs were observed by inverted fluorescence microscopy at magnifications of (A) ×100 and (B) ×200. MFBs, myocardial fibroblasts.
SMOC1 silencing suppresses the cell viability of Ang II-treated MFBs
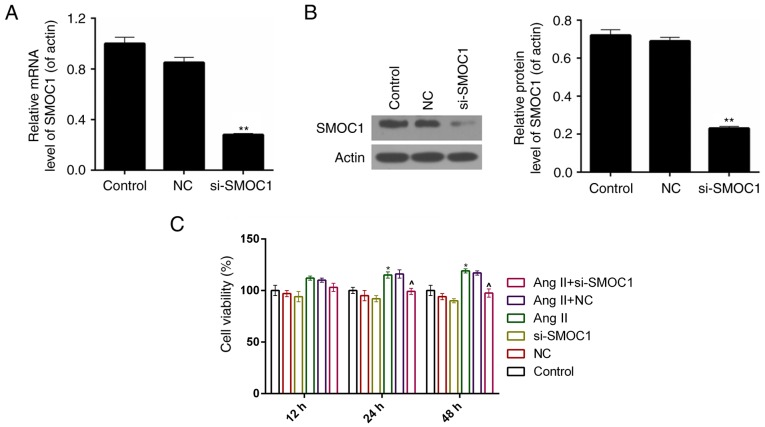
A siRNA vector targeting SMOC1, si-SMOC1, was constructed in the present study. The knockdown efficiency was ~75% in MFBs following stable transfection with si-SMOC1 (P<0.01; Fig. 2A). According to the results of western blot analysis, it was revealed that, following transfection with si-SMOC1, the protein expression level of SMOC1 was significantly reduced (P<0.01; Fig. 2B). Therefore, a CCK-8 assay was performed to measure the cell viability of MFBs separated into the six treatment groups described earlier. The results demonstrated that, the cell viability was inhibited in si-SMOC1 group compared to Ang II group (Fig. 2C).
Figure 2.
SMOC1 silencing suppresses the cell viability of Ang II-induced MFBs. (A) Reverse transcription-quantitative polymerase chain reaction and (B) western blot analysis were performed on the expression level of SMOC1 in MFBs transfected with empty vector or si-SMOC1. **P<0.01 vs. NC. (C) A Cell Count kit-8 assay was performed to measure the cell viability of MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1. *P<0.05 vs. control. ^P<0.05 vs. Ang II. SMOC1, SPARC-related modular calcium binding 1; MFBs, myocardial fibroblasts; si, small interfering RNA; Ang II, angiotensin II; NC, negative control.
SMOC1 silencing reduces the ROS content in Ang II-treated MFBs
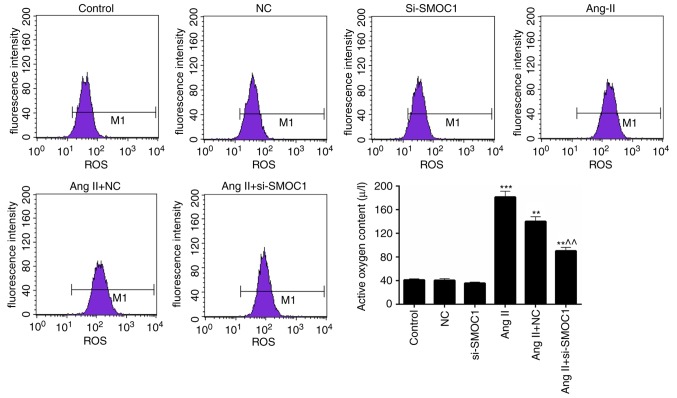
Additionally, the ROS content in MFBs of the previously described treatment groups was also assessed. A significant increase in ROS content was observed in the Ang II group compared with the NC group (P<0.01; Fig. 3). However, it was also revealed that the ROS content in Ang II-induced MFBs was significantly reduced by transfection with si-SMOC1 (P<0.01; Fig. 3). Therefore, it has been determined that SMOC1 silencing is able to reduce the production of ROS in MFBs treated with Ang II.
Figure 3.
SMOC1 silencing reduces the ROS content in Ang II-induced MFBs. Flow cytometry was performed on the ROS content in MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1. **P<0.01 and ***P<0.001 vs. NC; ^^P<0.01 vs. Ang II+NC. SMOC1, SPARC-related modular calcium binding 1; ROS, reactive oxygen species; MFBs, myocardial fibroblasts; si, small interfering RNA; Ang II, angiotensin II; NC, negative control.
SMOC1 silencing mitigates oxidative stress in MFBs treated with Ang II
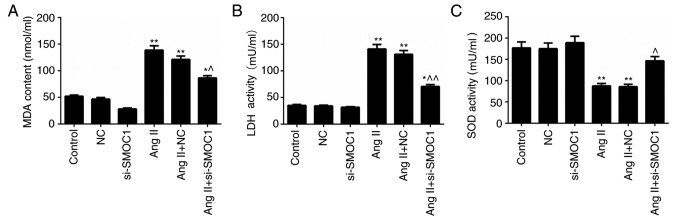
The MDA and LDH content in MFBs treated with Ang II were significantly higher than in the NC group (P<0.01; Fig. 4A-B). However, significant decrease in MDA and LDH content in Ang II-treated MFBs transfected with si-SMOC1 were observed (P<0.05; Fig. 4A-B). Nevertheless, Ang II was revealed to inhibit the activity of SOD in MFBs. In the SMOC1-silenced group, the SOD activity in Ang II-treated MFBs was significantly enhanced (P<0.05; Fig. 4C). In summary, it was confirmed that SMOC1 silencing reduced the MDA and LDH content, whereas enhancing SOD activity in Ang II-induced MFBs. Therefore, SMOC1 silencing mitigated oxidative stress in MFBs induced by Ang II.
Figure 4.
SMOC1 silencing mitigates the oxidative stress in MFBs induced by Ang II. ELISA was performed to evaluate the (A) MDA content, (B) LDH content and (C) SOD activity in MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1. *P<0.05 and **P<0.01 vs. NC; ^P<0.05 and ^^P<0.01 vs. Ang II+NC. SMOC1, SPARC-related modular calcium binding 1; MFBs, myocardial fibroblasts; Ang II, angiotensin II; MDA, malondialdehyde; LDH, lactate dehydrogenase; SOD, superoxide dismutase; si, small interfering RNA; NC, negative control.
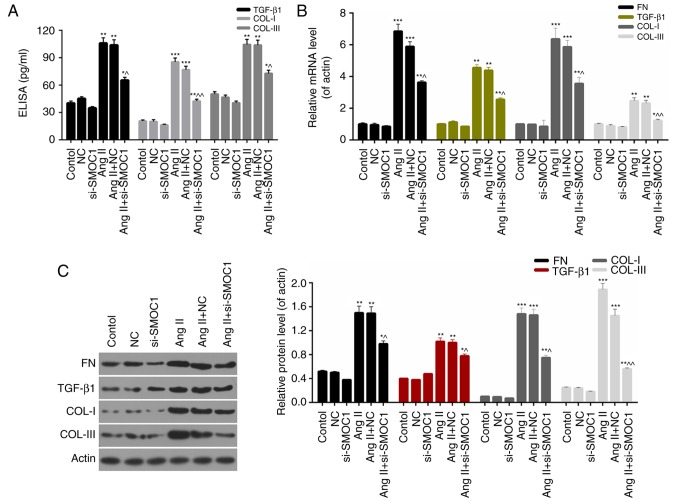
SMOC1 silencing downregulates the expression levels of fibrosis-associated proteins
Furthermore, the present study investigated the molecular mechanisms underlying fibrosis in MFBs and the expression levels of fibrosis-associated proteins, including FN, TGF-β1, COL-I and COL-III in MFBs. On the basis of ELISA data, it was revealed that SMOC1 silencing significantly decreased the expression of TGF-β1, COL-I and COL-III in Ang II-treated MFBs (P<0.05; Fig. 5A). Furthermore, the RT-qPCR results indicated that the expression levels of FN, TGF-β1, COL-I and COL-III in Ang II-treated MFBs were significantly downregulated in response to si-SMOC1 (P<0.05; Fig. 5B). Western blot analysis results also revealed similar trends in the expression of fibrosis-associated proteins in MFBs from each group (Fig. 5C). Based on these results, it was confirmed that SMOC1 silencing suppressed fibrosis of Ang II-treated MFBs through downregulating the expression levels of FN, TGF-β1, COL-I and COL-III.
Figure 5.
SMOC1 silencing downregulates the expression levels of fibrosis-associated proteins. (A) ELISA was performed to measure the TGF-β1, COL-I and COL-III expression in MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1. (B) Reverse transcription-quantitative polymerase chain reaction and (C) western blot analysis were performed on the expression levels of FN, TGF-β1, COL-I and COL-III in MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1. *P<0.05, **P<0.01, and ***P<0.001 vs. NC; ^P<0.05 and ^^P<0.01 vs. Ang II+NC. SMOC1, SPARC-related modular calcium binding 1; FN, fibronectin; TGF-β1, transforming growth factor β1; COL, collagen; Ang II, angiotensin II; MFBs, myocardial fibroblasts; si, small interfering RNA; NC, negative control.
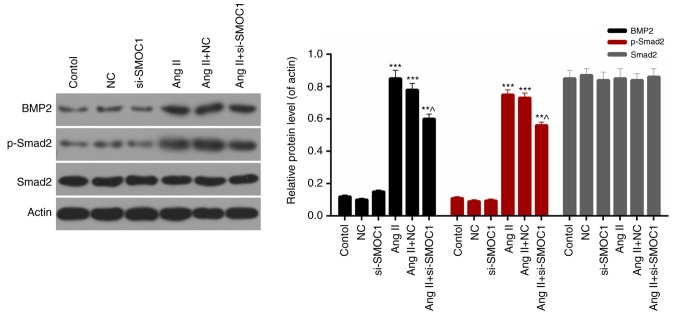
SMOC1 silencing affects the BMP2/Smad signaling pathway
Finally, the expression levels of BMP2, phosphorylated Smad2 and Smad2 in MFBs from each group were evaluated. Western blot analysis results revealed that the expression levels of BMP2 and phosphorylated Smad2 in Ang II-treated MFBs were significantly downregulated in response to si-SMOC1 (P<0.05; Fig. 6). Therefore, it was determined that SMOC1 silencing was able to suppress the phosphorylation of Smad2 in Ang II-treated MFBs. Additionally, there was no significant difference in the Smad2 expression in MFBs from each group (Fig. 6). Therefore, it was concluded that SMOC1 silencing affected the BMP2/Smad pathway in Ang II-treated MFBs.
Figure 6.
SMOC1 silencing affects the BMP2/Smad pathway. Western blot analysis was performed to measure the expression levels of BMP2, Smad2 and p-Smad2 in MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1. **P<0.01 and ***P<0.001 vs. NC; ^P<0.05 vs. Ang II+NC. SMOC1, SPARC-related modular calcium binding 1; BMP2, bone morphogenetic protein 2; p-, phosphorylated; MFBs, myocardial fibroblasts; si, small interfering RNA; Ang II, angiotensin II; NC, negative control.
Discussion
Ang II serves as the most crucial component of the renin-angiotensin system, which is usually associated with hypertension and renal failure (22). It has been demonstrated that Ang II is able to increase the expression level of TGF-β1, promote the DNA synthesis of fibroblasts and facilitate the proliferation of fibroblasts (23,24). Numerous studies have demonstrated that Ang II not only induced atherosclerosis by promoting the migration of human umbilical vein endothelial cells to the intima (25), but also led to hepatic fibrosis via promoting hepatic stellate cell migration and myocardial fibrosis via accelerating the migration of MFBs (26,27). Therefore, Ang II was selected as the inducer to establish the model of myocardial fibrosis on mouse MFBs.
SMOC1, a member of the matricellular protein family, is mainly expressed in the basement membrane of different tissues (18,19,28,29). Knowledge is limited regarding the roles of SMOC1 in physiology or pathophysiology, while studies have proven that the expression of SMOC1 was enhanced in several types of cancer (30,31). To the best of our knowledge, the function of SMOC1 in the prevention and therapy of myocardial fibrosis has not yet been studied. In the present study, the plasmid cloned with SMOC1 siRNA was prepared and named si-SMOC1. The knockdown efficiency of SMOC1 was ~75% in MFBs following stable transfection with si-SMOC1, according to the RT-qPCR and western blot analyses. Initially, the cell viability of untreated MFBs, MFBs transfected with an empty vector, and Ang II-induced MFBs transfected with an empty vector or si-SMOC1 was assessed. The results revealed that Ang II markedly enhanced the cell viability of MFBs, while SMOC1 silencing suppressed the cell viability of Ang II-induced MFBs.
Oxidative stress refers to the overproduction of highly reactive molecules, including ROS, when the organism is subjected to various harmful stimuli, which exceeds the scavenging activity of the organism and further results in tissue damage (32). As a second messenger of intracellular signal transduction, ROS is often involved in cell proliferation, apoptosis and accumulation. In vivo, physiological quantities of ROS can destroy pathogenic microorganisms and possess defensive physiological functions. However, sustained high concentrations of ROS may cause oxidation reactions with the surrounding macromolecular substances, and further impair the structure and function of cells (33,34). In the cardiovascular system, Ang II has been identified to cause the activation of NADPH oxidase to produce ROS, and to be involved in the migration of vascular smooth muscle cells (35). Therefore, the content of ROS and the levels of oxidative stress markers in the MFBs from all the treatment groups were evaluated. Based on the results, it was confirmed that SMOC1 silencing significantly reduced the ROS content in Ang II-induced MFBs. In addition, it was revealed that SMOC1 silencing also lessened the content of MDA and LDH, and strengthened the activity of SOD in Ang II-induced MFBs. According to these results, it was concluded that SMOC1 silencing reduced oxidative stress in Ang II-induced MFBs.
In order to investigate the accurate roles and mechanisms of SMOC1 in myocardial fibrosis, several fibrosis-associated proteins were further selected as the objects of the present study. Based on previous studies, the expression levels of FN, TGF-β1, COL-I and COL-III in MFBs treated with Ang II and transfected with si-SMOC1 were assessed (34–37). In accordance with the ELISA data, it was revealed that SMOC1 silencing markedly decreased the TGF-β1, COL-I and COL-III expression levels in Ang II-induced MFBs. Additionally, RT-qPCR and western blot analysis results also suggested that SMOC1 silencing markedly downregulated the expression levels of FN, TGF-β1, COL-I and COL-III in Ang II-induced MFBs. Therefore, it was confirmed that SMOC1 silencing was able to downregulate the expression levels of fibrosis-associated proteins in Ang II-induced MFBs. Therefore, it was determined that SMOC1 silencing suppressed the fibrosis of MFBs induced by Ang II. Studies have reported that BMPs and Smad family proteins were associated with the development and progression of myocardial fibrosis (13,36–38). However, the regulatory mechanism of the BMP2/Smad pathway in MFBs remains unclear. Therefore, the present study measured the expression levels of BMP2, phosphorylated Smad2 and Smad2 in MFBs treated with Ang II and si-SMOC1. The western blot results indicated that Ang II increased the expression level of BMP2 and the phosphorylation of Smad2 in MFBs. Additionally, it was revealed that SMOC1 silencing decreased the expression of BMP2 and the phosphorylation of Smad2 in MFBs in Ang II-induced MFBs. However, no significant difference in Smad2 expression was observed. Taken together, these results indicated that SMOC1 silencing affected the BMP2/Smad pathway in Ang II-induced MFBs. Based on the aforementioned results, it was hypothesized that SMOC1 silencing suppressed the fibrosis of MFBs induced by Ang II by affecting the BMP2/Smad pathway. Nevertheless, the present study only investigated the effects of SMOC1 silencing on Ang II-induced myocardial fibrosis. At present, knowledge regarding the roles and mechanisms of SMOC1 overexpression on Ang II-induced myocardial fibrosis remains insufficient. Future studies should consider the effects of overexpressing SMOC1.
Taken together, the results of the present study demonstrated that SMOC1 silencing suppressed the Ang II-induced myocardial fibrosis of MFBs through affecting the BMP2/Smad pathway. Additionally, the observations of the present study provided novel insight for comprehending the pathogenesis of myocardial fibrosis and enabling an alternative approach for the therapy of myocardial fibrosis.
In summary, the present study indicated that SMOC1 silencing suppressed the Ang II-induced myocardial fibrosis of MFBs through affecting the BMP2/Smad pathway. The results of the present study have crucial influence on the mechanisms of SMOC1 and Ang II-induced MFBs. The potential effects of SMOC1 on the myocardial fibrosis of Ang II-induced MFBs suggested that SMOC1 may be an effective target for myocardial fibrosis therapies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study are included in this published article
Authors' contributions
YW wrote the main manuscript and analyzed the data. XW performed the experiments. YW designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL. Understanding the epidemic of heart failure: Past, present, and future. Curr Heart Fail Rep. 2014;11:404–415. doi: 10.1007/s11897-014-0220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. Embo J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 2014;35:5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol. 2009;195:321–338. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 6.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev. 2014;19:173–185. doi: 10.1007/s10741-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 8.Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, Wang X, Oudit GY, Kassiri Z. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res. 2014;103:268–280. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Halade GV, Lindsey ML. Extracellular matrix and fibroblast communication following myocardial infarction. J Cardiovasc Transl Res. 2012;5:848–857. doi: 10.1007/s12265-012-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YL, Liu YS, Chuang LY, Guh JY, Lee TC, Liao TN, Hung MY, Chiang TA. Bone morphogenetic protein-2 antagonizes renal interstitial fibrosis by promoting catabolism of type I transforming growth factor-beta receptors. Endocrinology. 2009;150:727–740. doi: 10.1210/en.2008-0090. [DOI] [PubMed] [Google Scholar]
- 11.Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Muller GA, Kalluri R. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003;285:F1060–F1067. doi: 10.1152/ajprenal.00191.2002. [DOI] [PubMed] [Google Scholar]
- 12.Yang YL, Ju HZ, Liu SF, Lee TC, Shih YW, Chuang LY, Guh JY, Yang YY, Liao TN, Hung TJ, Hung MY. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial-mesenchymal transition. J Cell Biochem. 2011;112:2558–2565. doi: 10.1002/jcb.23180. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Sun A, Li L, Zhao G, Jia J, Wang K, Ge J, Zou Y. Up-regulation of BMP-2 antagonizes TGF-β1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/Smad6 complex. J Cell Mol Med. 2012;16:2301–2310. doi: 10.1111/j.1582-4934.2012.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bornstein P, Sage EH. Matricellular proteins: Extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 18.Vannahme C, Smyth N, Miosge N, Gosling S, Frie C, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem. 2002;277:37977–37986. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- 19.Gersdorff N, Müller M, Schall A, Miosge N. Secreted modular calcium-binding protein-1 localization during mouse embryogenesis. Histochem Cell Biol. 2006;126:705–712. doi: 10.1007/s00418-006-0200-7. [DOI] [PubMed] [Google Scholar]
- 20.Thomas JT, Canelos P, Luyten FP, Moos M., Jr Xenopus SMOC-1 Inhibits bone morphogenetic protein signaling downstream of receptor binding and is essential for postgastrulation development in Xenopus. J Biol Chem. 2009;284:18994–19005. doi: 10.1074/jbc.M807759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Curr Opin Investig Drugs. 2002;3:569–577. [PubMed] [Google Scholar]
- 23.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161:1999–2004. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- 24.Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: Autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des. 2007;13:1247–1256. doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- 25.Zhu M, Chen D, Li D, Ding H, Zhang T, Xu T, Zhang Y. Luteolin inhibits angiotensin II-induced human umbilical vein endothelial cell proliferation and migration through downregulation of Src and Akt phosphorylation. Circ J. 2013;77:772–779. doi: 10.1253/circj.CJ-12-0310. [DOI] [PubMed] [Google Scholar]
- 26.Rosin NL, Sopel M, Falkenham A, Myers TL, Legare JF. Myocardial migration by fibroblast progenitor cells is blood pressure dependent in a model of angII myocardial fibrosis. Hypertens Res. 2012;35:449–456. doi: 10.1038/hr.2011.217. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Zhu QJ, Zhou W, Ye J, Qian W, Zhu R, Hu TH, Hou XH. Effect of beta-elemene on the proliferation, migration and RhoA expression of hepatic stellate cells induced by angiotensin II. Zhonghua Gan Zang Bing Za Zhi. 2008;16:748–751. (In Chinese) [PubMed] [Google Scholar]
- 28.Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–488. doi: 10.1016/j.biocel.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YA, Lim J, Kim KM, Acharya B, Cho JY, Bae YC, Shin HI, Kim SY, Park EK. Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J Proteome Res. 2010;9:2946–2956. doi: 10.1021/pr901110q. [DOI] [PubMed] [Google Scholar]
- 30.Boon K, Edwards JB, Eberhart CG, Riggins GJ. Identification of astrocytoma associated genes including cell surface markers. BMC Cancer. 2004;4:39. doi: 10.1186/1471-2407-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brellier F, Ruggiero S, Zwolanek D, Martina E, Hess D, Brown-Luedi M, Hartmann U, Koch M, Merlo A, Lino M, Chiquet-Ehrismann R. SMOC1 is a tenascin-C interacting protein over-expressed in brain tumors. Matrix Biol. 2011;30:225–233. doi: 10.1016/j.matbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17:48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montezano AC, Callera GE, Yogi A, He Y, Tostes RC, He G, Schiffrin EL, Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 36.Sun B, Huo R, Sheng Y, Li Y, Xie X, Chen C, Liu HB, Li N, Li CB, Guo WT, et al. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension. 2013;61:352–360. doi: 10.1161/HYPERTENSIONAHA.111.00562. [DOI] [PubMed] [Google Scholar]
- 37.Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD. Induction of cardiac fibroblast lysyl oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 2011;55:90–97. doi: 10.1016/j.cyto.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Hao J, Jones SC, Yee MS, Roth JC, Dixon IM. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H1685–H1696. doi: 10.1152/ajpheart.00266.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article