Abstract
Ailanthone, which is extracted from the traditional Chinese medicinal plant Ailanthus altissima, has been thoroughly demonstrated to have anti-tumor, anti-HIV, anti-inflammatory, anti-malarial, anti-allergic and anti-microbial activities. However, the anti-proliferative effects of ailanthone on HL-60 cells and potential mechanisms underlying those effects have not been reported. In the present study, we demonstrated the potent cytotoxicity of ailanthone against HL-60 cells. Annexin V-APC/7-ADD staining assay indicated that ailanthone increased the number of apoptotic cells in a dose-dependent manner. PI staining showed that ailanthone increased the percentage of G0/G1-phase cells in a dose-dependent manner. Acridine orange staining suggested that ailanthone induced the formation of acidic vesicular organelles in HL-60 cells and pretreatment with BaF-A1 could attenuate this process. Western blotting showed that ailanthone up-regulated the protein expression levels of beclin-1 and LC3-II and down-regulated those of LC3-I and p62 in a dose-dependent manner. Use of BaF-A1 showed that the anti-proliferative effects of ailanthone on HL-60 cells may be partly attributable to the induction of autophagy-mediated apoptosis by MTT assay and annexin V-APC/7-ADD staining assay.
Keywords: ailanthone, apoptosis, autophagy, HL-60 cells, beclin-1, LC3I/II, p62
Introduction
Leukemia makes up about 1/3 of the case of cancer in people younger than 15 years old and is not uncommon in adults (1). Despite significant advances in the chemotherapeutic management of leukemia, post-remission relapse occur frequently in most patients (2). In this way, it is imperative to find more efficient low-toxicity agents to use in chemo-preventive therapies for leukemia.
Ailanthone, which is extracted from traditional Chinese medicinal plant Ailanthus altissima (3), has been well-demonstrated to have anti-tumor, anti-HIV, anti-inflammatory, anti-malarial, anti-allergic, and anti-microbial activities (4,5). The growth-inhibitory effect of ailanthone on varieties of tumor cells (He La, Jurkat, Hep G2, Hep3B, R-Hep G2, MCF-7, MDA-MB-231, Huh7 and A549) in vitro has been reported (6–9). However, the cytotoxicity of ailanthone on human HL-60 leukemia cells and its underlying molecular mechanism are poorly understood.
Apoptosis, type I programmed cell death, is a series of physiological changes that are mediated by genes and proteins, and the cells depend on this mechanism to activate their own destruction. If pathological interference take place during the process of apoptosis, malignant tumors may form (10–12). Autophagy, type II programmed cell death, is a conserved decomposition process that allows the degradation and recycling of cytoplasm, aggregated proteins, and excess or defective organelles (13). Autophagy is mainly a response to the stress of irradiation (14), chemotherapeutic drugs (15), or starvation (16). Despite its contribution to cell survival, previous studies have demonstrated that several anti-tumor agents induce cell death with autophagic features in various cancer cells (17–19).
In the present study, we sought to investigate the cytotoxicity of ailanthone in human HL-60 leukemia cells in vitro and to elucidate the mechanisms that may underlie its actions. We are searching for a new natural anti-tumor drug that is efficient and has minimal toxicity.
Materials and methods
Materials
The pure ailanthone used in this study was extracted from Ailanthus altissima. The ailanthone sample (purity >98%) was provided by the Institute of Traditional Chinese Medicine and Natural Products, Jinan University (Guangzhou, China). Dimethyl sulfoxide (DMSO) was purchased from Shanghai Joey Chemical Reagent Co., Ltd., (Shanghai, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide (MTT) was purchased from Amresco, LLC, (Solon, OH, USA). Propidium iodide (PI) and acridine orange (AO) was purchased from Nanjing Key Gen Biotech Co., Ltd., (Nanjing, China) RPMI-1640 medium was purchased from Gibco; Thermo Fisher Scientific, Inc., (Waltham, MA, USA). Fetal bovine serum (FBS), penicillin/streptomycin solution (PS), and antibodies for LC3I/II, beclin-1, p62, GAPDH were obtained from Nanjing KeyGen Biotech Co., Ltd. Autophagy inhibitor Bafilomycin-A1 (BaF-A1) was purchased from Boster Biological Technology Co., Ltd., (Wuhan, China).
Cell line and culture
Human promyelocytic leukemia HL-60 cells were obtained from Nanjing Key Gen Biotech Co., Ltd. HL-60 cells were cultured in RPMI-1640 medium with 10% FBS and 1% PS solution in a humidifying thermostat with 5% CO2 at 37°C. Stock solutions of ailanthone were prepared in DMSO and stored at −20°C and diluted to the required concentration with RPMI-1640 complete medium.
MTT assay of cell viability
The cytotoxicity of ailanthone in human HL-60 cells was assessed by using the MTT assays. Exponentially growing HL-60 cells (5×104 cells/well) were seeded and cultured in 96-well plates for 24 h and then treated with various concentrations of ailanthone (1.25, 2.5, 5, 10, and 20 µM) or 0.1% DMSO (control group) for 24, 48 and 72 h at 37°C. 20 µl MTT (5 mg/ml) was added to each well of the plate and then cultured for 4 h at 37°C. Subsequently, cells were washed in PBS followed by the addition of 150 µl DMSO. Micro-plate spectrophotometer (RT-6000; Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, China) was used to measure the optical densities at 490 nm spectral wavelength.
Flow cytometric analysis of cell apoptosis
HL-60 cells (5×105 cells/well) were seeded into 6-well plates and cultured for 24 h at 37°C, and were then treated with various concentrations of ailanthone (5, 10, and 20 µM) or 0.1% DMSO (control group) for 48 h at 37°C, respectively. Cells were washed twice in cold PBS, and then the cell pellets were mixed with annexin V APC/7-ADD (Nanjing Key Gen Biotech Co., Ltd.) for 15 min at 37°C in the dark. Cell apoptosis was were evaluated using a FACSCalibur Flow Cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and the rate of cell death was analyzed using a FACSCalibur internal software system (BD Biosciences).
Flow cytometric analysis of cell cycle
HL-60 cells (5×105 cells/well) were seeded into 6-well plates and cultured for 24 h at 37°C, and then treated with various concentrations of ailanthone (5, 10, and 20 µM) or 0.1% DMSO (control group) for 48 h at 37°C, respectively. Cells were fixed and rendered permeable with 70% cold ethanol at 4°C. After this interval, cells were treated with 1% RNase and stained with PI solution for 30 min at 4°C. Cell cycle phase distribution of cells was established by FACSCalibur flow cytometer (BD Biosciences) using the cell cycle analysis software (FlowJo LLC, Ashland, OR, USA).
Detection of autophagy by AO staining
AO staining was used to detect the presence of acidic vesicular organelles (AVOs) after ailanthone treatment. HL-60 cells (5×105 cells/well) were treated with ailanthone at concentrations of 10 µM or 0.1% DMSO (control group) for 48 h at 37°C, and then cells were washed twice in cold PBS and fixed with 4% formaldehyde for 10 min. After the treatment, cells were stained with 5 µg/ml AO solution for 15 min at 37°C in the dark and observed using an inverted fluorescence microscope (OlympusIX5; Olympus Corporation, Tokyo, Japan).
Western blotting
HL-60 cells (5×106 cells/well) were washed twice in cold PBS and suspended in radio immune-precipitation assay lysis buffer (Nanjing Key Gen Biotech Co., Ltd.) on ice for 30 min, then treated with various concentrations of ailanthone (5, 10, and 20 µM) or 0.1% DMSO (control group) for 48 h at 37°C, respectively. The lysates were then cleared by centrifugation at 14,000 × g for 15 min at 4°C. Subsequently, a bicinchoninic acid protein assay kit (Nanjing Key Gen Biotech Co., Ltd.) was used to measure the total protein concentration of each sample. The Protein samples (30 µg) were separated by 15% SDS-PAGE in each group and then transferred onto polyvinylidene difluoride membranes (Pall Life Sciences, Port Washington, NY, USA). Membranes were stuck with 5% (w/v) non-fat dry milk dissolved in TBS containing 0.05% Tween-20 (TBST) at room temperature for 1 h, and then washed three times with TBST. Subsequently, membranes were incubated with primary antibodies against beclin-1 (1:200), p62 (1:200), LC3I/II (1:200), and GAPDH (1:400) for 12 h at 4°C. The members were washed three times with TBST, and then incubated with HRP-conjugated goat anti-rabbit (1:2,000) IgG (Nanjing Key Gen Biotech Co., Ltd.) secondary antibodies at room temperature for 1 h. The immune-reactive bands were visualized with enhanced chemiluminescent substrates (Thermo Fisher Scientific, Inc.) using an X-ray film processor (Kodak, Rochester, NY, USA). GAPDH served as a loading control. The experiment was independently repeated three times, and Quantity One software (v.4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to analyze the densitometry of each band.
Statistical analysis
Data are here expressed as means ± SD. With SPSS software for windows v.17.0 (SPSS, Inc., Chicago, IL, USA), one way ANOVA followed by Newman-Keuls multiple comparison test was used for analysis of variance and the student's t-test was used for pair-wise comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Ailanthone inhibits cell proliferation in HL-60 cells
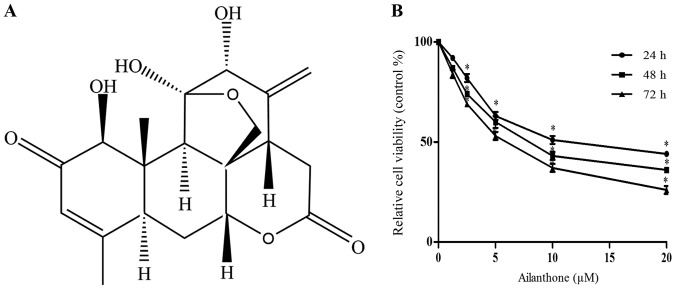
Ailanthone share its chemical structure with the triterpenoids. Triterpenoids have many physiological activities, including anti-tumor activities (Fig. 1A). The MTT assay was used to assess the cytotoxicity of ailanthone in HL-60 cells. Treatment with ailanthone reduced the viability of HL-60 cells in a dose- and time-dependent manner (Fig. 1B), with half maximal inhibitory concentration (IC50) values of 12.18, 8.497, and 5.986 µM after 24, 48, and 72 h of treatment, respectively.
Figure 1.
(A) Structure of ailanthone. (B) Ailanthone inhibited growth of HL-60 cells in a time- and dose-dependent manner. *P<0.05 vs. 1.25 µΜ groups.
Ailanthone induces apoptosis and cell cycle arrest in HL-60 cells
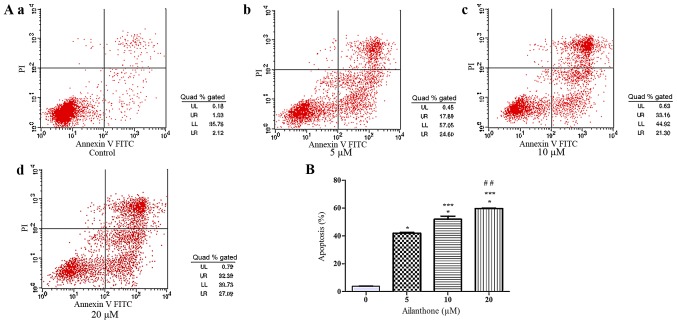
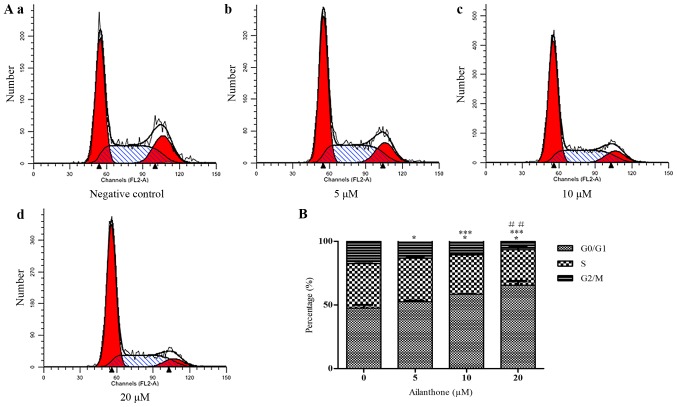
An annexin V-APC/7-ADD staining assay was used to confirm that ailanthone induced apoptosis in HL-60 cells. In the right quadrants of flow cytometry graphs, ailanthone significantly increased the number of apoptotic cells (Fig. 2A). After treatment with 5, 10, and 20 µM ailanthone for 48 h at 37°C, the rate of apoptosis among the cells was increased significantly from 42.02 to 59.68% and the percentage of apoptotic cells was 42.02±0.54, 52.05±2.27 and 59.69±0.25%, which was significantly higher than in the control group (3.92±0.14%; P<0.05; Fig. 2B). In order to determine whether the anti-proliferative effect of ailanthone on HL-60 cells was attributable to cell cycle arrest, we measured DNA content as an indicator of the cell cycle phase ratio by flow cytometry with PI staining (Fig. 3A). The percentage of G0/G1 cells increased significantly in a dose-dependent manner and cells in G0/G1 phase was 53.54±0.88, 58.42±0.31, and 65.57±3.16% in the 5, 10, and 20 µM groups, respectively. Ailanthone treatment induced significant G0/G1-phase accumulation not observed in the control group (47.5±2.54%; P<0.05; Fig. 3B).
Figure 2.
Ailanthone induced apoptosis in HL-60 cells. (A) Ailanthone induced apoptosis in HL-60 cells, which were stained by annexin V-APC/7-AAD fluid and assessed using flow cytometry analysis. (a) Control cells; Cells treated with (b) 5, (c) 10, and (d) 20 µM ailanthone for 48 h at 37°C, respectively. Lower left (LL) quadrants: Viable cells. Lower right (LR) quadrants: Early apoptotic cells. Upper right (UR) quadrants: Late apoptotic cells. Upper left (UL) quadrants: Nonviable cells. (B) The data showed that ailanthone increased the percentage of apoptotic cells in a dose-dependent manner, ANOVA using Newman-Keuls multiple comparison test. *P<0.05 vs. control, ***P<0.05 vs. 5 µM groups, ##P<0.05 vs. 10 µM groups.
Figure 3.
Ailanthone induced G0/G1 arrest in HL-60 cells. (A) Ailanthone induced G0/G1 arrest of HL-60 cells. Ailanthone induced cycle arrest in HL-60 cells, which were stained by PI fluid, and cell cycle distribution was analyzed by flow cytometry. (a) Control cells; Cells treated with (b) 5, (c) 10, and (d) 20 µM ailanthone for 48 h at 37°C, respectively. (B) The data showed that ailanthone increased the percentage of G0/G1-phase cells in a dose-dependent manner, ANOVA using Newman-Keuls multiple comparison test. *P<0.05 vs. control, ***P<0.05 vs. 5 µM groups. ##P<0.05 vs. the 10 µM groups. PI, propidium iodide.
Ailanthone induces autophagy in HL-60 cells
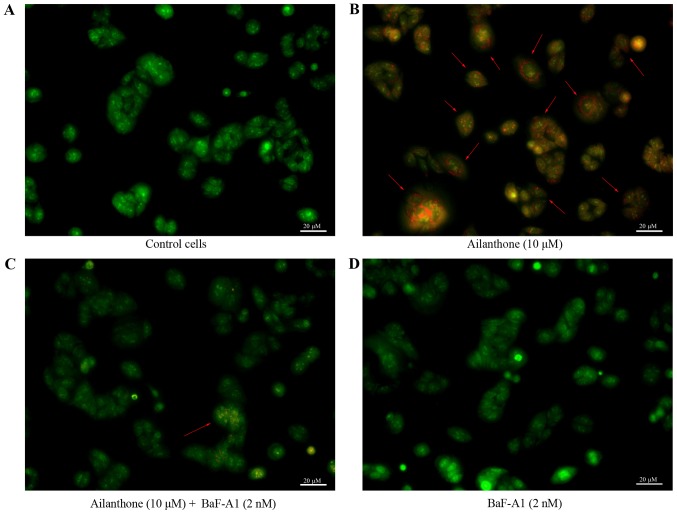
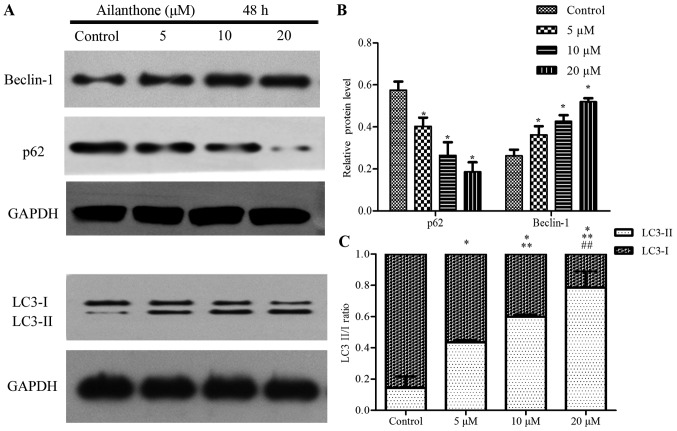
AO staining was used to observe the formation of acidic vesicular organelles, the main characteristics of autophagy (Fig. 4). Ailanthone induced noticeable formation of AVOs, which displayed red fluorescence in the lysosomal compartments of HL-60 cells (Fig. 4B). Control cells treated with 1% DMSO displayed green fluorescence in the cytosolic and nuclear compartments, indicating the lack of AVOs (Fig. 4A). Those results showed that AVOs to be present in ailanthone-treated HL-60 cells, which suggest that ailanthone may induce autophagy in HL-60 cells. In addition, pretreatment with BaF-A1 displayed sporadic red fluorescence in the cells suggests that ailanthone-induced formation of AVOs in HL-60 cells were attenuated (Fig. 4C). Pretreatment with BaF-A1 without ailanthone also did not indicate the formation of AVOs (Fig. 4D). To investigate the effect of ailanthone on the protein expression levels in HL-60 cells that were associated with autophagy, we performed Western blot analysis to measure the levels of beclin-1, p62, and LC3-I/II (Fig. 5A). Treatment with ailanthone markedly increased beclin-1 levels but decreased and p62 levels (Fig. 5B). The ratio of LC3-I/II showed that treatment with ailanthone markedly increased LC3-II levels but decreased and LC3-I levels (Fig. 5C).
Figure 4.
Inverted fluorescence microscope detection of AVOs in HL-60 cells. (A) Control cells. (B) Cells treated with ailanthone (10 µM) for 48 h at 37°C. Red arrows indicate characteristic autophagy. (C) Cells were pretreated with BaF-A1 (2 nM) for 30 min, followed by treatment with ailanthone (10 µM) for an additional 48 h at 37°C. The red arrow indicates that autophagy is suppressed. (D) Cells were treated with BaF-A1 (2 nM) for 48 h at 37°C without ailanthone. These images suggest that ailanthone may induce HL-60 cell autophagy and pretreatment with BaF-A1 could attenuate this process. Scale bar, 20 µm. AVOs, acidic vesicular organelles.
Figure 5.
Protein expression levels in HL-60 cells. (A) Effects of ailanthone on the protein expression levels of beclin-1, p62, and LC3I/II in HL-60 cells were determined by western blotting after treatment with 5, 10, and 20 µM ailanthone for 48 h at 37°C. (B) The data showed the protein expression levels of beclin-1 to be up-regulated and down-regulated of p62 in a dose-dependent manner. (C) The results showed the protein expression levels of LC3-I to be down-regulated and LC3-II up-regulated in a dose-dependent manner, ANOVA using Newman-Keuls multiple comparison test. *P<0.05 vs. control. **P<0.05 vs. 5 µM groups. ##P<0.05 vs. 10 µM groups.
Autophagy is associated with ailanthone-mediated apoptosis of HL-60 cells
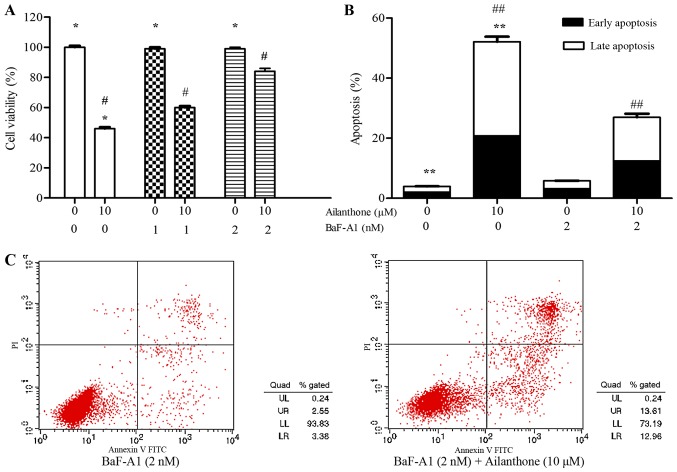
To investigate the role of autophagy in ailanthone-induced apoptosis, autophagy inhibitor BaF-A1 (1 or 2 nM) was used. Treatment with ailanthone significantly suppressed the viability of HL-60 cells, and the suppression was significantly attenuated by BaF-A1 (Fig. 6A). The rate of apoptotic in HL-60 cells was conspicuously reduced by BaF-A1 pretreatment (Fig. 6B and C). After treatment with 0 and 10 µM ailanthone for 48 h at 37°C, the rate of apoptosis among the cells pretreated with 2 nM BaF-A1 was increased from 5.77±0.25 to 26.93±1.74%, which were significantly lower than in the group without pretreated with BaF-A1 (from 3.92±0.14 to 52.05±2.27%; P<0.05′). Those results suggested that the anti-proliferative effects of ailanthone on HL-60 cells may be partially due to the induction of autophagy-mediate apoptosis.
Figure 6.
Autophagy is associated with ailanthone-mediated apoptosis of HL-60 cells. (A) Cells were pretreated with and without BaF-A1 (1 or 2 nM) for 30 min, following treatment with and without ailanthone (10 µM) for 48 h at 37°C. MTT assay was used to determine the cell viability. (B) Cells were pretreated with and without BaF-A1 (2 nM) for 30 min, following by treatment with and without ailanthone (10 µM) for 48 h at 37°C, (C) annexin V-FITC staining assay was used to analyze the cell apoptosis, ANOVA using Newman-Keuls multiple comparison test. *P<0.05 vs. control, #P<0.05 vs. (0, 10 µM) groups, **P<0.05 vs. control, ##P<0.05 vs. (0, 10 µM) groups. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide.
Discussion
Ailanthone, which is extracted from the traditional Chinese medicinal plant Ailanthus altissima (3), has been thoroughly demonstrated to have anticancer activity in previous studies (4–9). However, the anti-proliferative effects of ailanthone on HL-60 cells and mechanisms that may underlie these effects have been poorly understood. The present study is the first to demonstrate the potent-cytotoxicity of ailanthone against HL-60 cells. The mechanisms that underlie these effects may involve induction of autophagic cell death in HL-60 cells.
In the present study, we found that the rate of apoptosis in ailanthone-treated HL-60 cells to increase in a dose-dependent manner and this effect may be associated with an increase in the number of cells arrested at the G0/G1 phase. The cell cycle is the overall process of the cell from the beginning of a division to the end of the next division that allows the cell to proliferate, and is divided into four consecutive phases, known as G0/G1, S, and G2/M phases (20). Although G0 is often referred to as the quiescent phase, G0-phase cells are still quite with respect to cellular growth and are strictly regulated to determine when the cell will enter other stages of the cell cycle (21). The mitogenic signaling mediated by the RAS/RAF/MAPK pathway promotes this shift, whose endpoint is the stimulation of D-type cyclin production (22). Our study suggested that ailanthone induces cell cycle arrest of HL-60 cells at the G0/G1 phase. In addition, a previous study found that ailanthone significantly induced cell cycle arrest at the G1/S phase in Huh-7 hepatocellular carcinoma cells (9). In eukaryotic cells, the process of G2/M cell cycle is regulated by protein B (cyclinB)-p34cdc2, G2/M phase arrest takes place mainly in a p53-dependent manner. Watson et al (23), investigated a p53 wild-type MCF-7 cell line and p53 mutated MDA-MB-231 cell line. They found that the cell cycle of p53 wild-type MCF-7 cell line was arrested at the G1 and G2 phases under ionizing radiation, while p53 mutated MDA-MB-231 cells were arrested at the G2/M phase. Drug-induced cell cycle modulation not only varies between the same cell line treated with different drugs, but also in different cells after treatment with the same drug. Lavhale et al (24), found ailanthus excelsa chloroform extract-1 extracted from Ailanthus excels could induce S/G2-M cell cycle arrest in MDA-MB-231, MCF-7, and PC3 cells and a G1 arrest in B16F10 cells. Treatment with AECHL-1 results in a significant decrease in the levels of c-Myc, CDK-4, and cyclin D1 in B16F10 cells, while the expression level of p21 was increased. p21 forms a complex with CDK2/CDK4/CDK6 and inhibits the CDK-cyclin kinase activity phase and arrests cells in the G1 phase (24). Therefore, we believe that the induction of cell cycle arrest at different phase by compounds extracted from ailanthus may be associated with a variety of factors, such as mutation of p53 gene and expression level of p21 in tumor cells and so on. In conclusion, these results demonstrated that the anti-proliferative effects of ailanthone on HL-60 cells were partially due to the induction of apoptosis and G0/G1 phase cell cycle arrest.
A previous study showed that some anti-cancer chemotherapy drugs can induce autophagic apoptosis in malignant tumor cells, thus inhibiting the proliferation of tumor cells (25,26). In autophagy, targeted cytoplasm constituents are isolated from other parts of the cell, which forms a double membrane called autophagosome. Then, the autophagosome enters the lysosome through the cytoplasm, and the two organelles fuse. In the lysosome, the contents of the autophagosome are degraded by acidic lysosome hydrolase (27,28). Our experiment indicated the presence of acidic vesicular organelles in HL-60 cells after treatment with ailanthone by AO staining, which suggested that ailanthone may induce HL-60 cells autophagy. Bafilomycin-A1 is a V-type ATPase inhibitor, which can prevent acidification and alters the membrane potential of some specific layers. Treatment with Baf-A1 will eventually lead to block in fusion of autophagosomes with lysosomes, thus preventing the maturity of autophagy (29,30). There are several autophagy inhibitory reagents different from Baf-A1, such as E64d/pepstatin-A or chloroquine. E-64d is a membrane-permeable cysteine protease inhibitor, which can block the activity of a subset of lysosomal hydrolases. Pepstatin-A is an aspartyl protease inhibitor to inhibit lysosomal protein degradation. E-64d should be used in combination with pepstatin-A to inhibit lysosomal protein degradation (31). Chloroquine is lysosomal compounds, and elevate/neutralize the lysosomal/vacuolar pH. Chloroquine improve the lysosomal pH value and the ultimately inhibition of autophagosomes and lysosome fusion, thereby preventing autophagosome maturation of autophagosomes into autoly-sosomes (32). Using E64d/pepstatin-A or chloroquine may better interpret the autophagy flux. However, our experiment refers whether or not autophagy is activated. Our currently study using Baf-A1 could clear to see the formations of autophagic vacuoles were suppressed under microscope.
We then investigated ailanthone-induced autophagy in HL-60 cells. The autophagy levels of the cells were found to be very low in a physiological environment; the major function of autophagy is degradation and recycling of senescent organelles and longevity proteins, which allow the cells to live longer (14–16). The role of autophagy in cells is paradoxical and when cells are faced with physiological or pathological stresses, the autophagy levels can increase significantly, which can lead to cell death (33,34). Autophagy is associated with molecular regulation of autophagy associated protein, such as LC3, beclin-1, and p62. LC3 is an important protein in the autophagy process, during which cytoplasmic pattern LC3 (LC3-I) is converted to autophagosomal membrane LC3 (LC3-II), resulting in elevated LC3-II levels (35). The beclin-1 gene is located on chromosome 17q21, which is homologous with the yeast autophagy gene Apg6/Vps30 and involves in the composition of the class III PI3K complex (36). Beclin-1 is a critical protein in the formation of autophagosome encoded by beclin-1 gene. Beclin-1 may mediate cell apoptosis by binding to the apoptosis-associated protein bcl-2 (37). p62 is a multifunctional ubiquitin protein, participating in the ubiquitin-proteasome system and the autophage system. With the occurrence of autophagy, p62 protein is incorporated into autophagosomes and then degraded (38). We found that ailanthone increased beclin-1 and LC3-II levels but decreased and p62 levels in a dose-dependent manner. Those results indicated that beclin-1, p62, and LC3 may participate in ailanthone inducted-autophagy in HL-60 cells. A recent paper found that ailanthone inhibits the proliferation activity of vestibular schwannoma cells by blocking the Ras/Ras/Raf/MEK/ERK and mTOR pathways through down-regulating the expression of mir-21. They observed that ailanthone reduced CyclinD1 protein levels, indicating that ailanthone may block vestibular schwannom cells in the G1 phase of cell cycle. Furthermore, their experiment also found that ailanthone enhanced the protein expression of LC3-II and Beclin-1, reduced the expression of p62, indicating that ailanthone may induce vestibular schwannom cells autophagy (39). Unlike this, our study is the first to demonstrate the potent-cytotoxicity of ailanthone against HL-60 cells. We observed the formation of acidic vesicular organelles by AO staining, visually see the characteristic performance of autophagy. In addition, we investigated whether ailanthone-induced autophagy is associated with apoptotic cell death in HL-60 cells. The MTT assay and annexin V-FITC staining assay suggested that the anti-proliferative and pro-apoptotic effects of ailanthone on HL-60 cells were significantly reversed following pretreatment of autophagy inhibitor BaF-A1. These results suggested that ailanthone-induced autophagy may play a pivotal role in apoptotic cell death in human promyelocytic leukemia cells.
In summary, the present study is the first to demonstrate that ailanthone extracted from Ailanthus altissima has anti-proliferative effects on HL-60 cells in vitro. We further found that these effects were partially due to the induction of apoptosis and G0/G1 phase cell cycle arrest. We found that ailanthone induces HL-60 cell autophagy possibly through the modulation of beclin-1, p62, and LC3 proteins expression. In addition, we found that the anti-proliferative and pro-apoptotic effects of ailanthone on HL-60 cells were significantly reversed by inhibiting autophagy. These results suggest that ailanthone-induced autophagy in the HL-60 cells likely involves autophagic cell death. However, considering the potential fluctuation of p62 and LC3-II levels during the process, the absence of data at other time points besides 48 h would be a limitation of the present study. Further studies are need to determine the details of the mechanism underlying autophagic and apoptotic cell death induced by ailanthone in leukemia cells, and to evaluate the anti-proliferative effects of ailanthone on leukemia cells in vivo. Our results revealed the pharmacological activity of ailanthone on HL-60 cells and suggest that ailanthone could be a suitable therapeutic agent for the treatment of leukemia.
Acknowledgements
The authors would like to thank the Institute of Traditional Chinese Medicine and Natural Products, Jinan University for providing the pure sample of ailanthone.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81241102).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZJ and CW designed the study. CW was a major contributor in writing the manuscript. CC and YC performed cell culture and MTT assay experiments. LZ and YW performed the experiments and analyzed the data regarding ailanthone-induced apoptosis and cell cycle arrest in HL-60 cells. CL performed AO staining. YH and ZY performed western blotting. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Routledge DJ, Bloor AJ. Recent advances in therapy of chronic lymphocytic leukaemia. Br J Haematol. 2016;174:351–367. doi: 10.1111/bjh.14184. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wang WJ, Su C, Zhang DM, Xu LP, He RR, Wang L, Zhang J, Zhang XQ, Ye WC. Cytotoxic quassinoids from Ailanthus altissima. Bioorg Med Chem Lett. 2013;23:654–657. doi: 10.1016/j.bmcl.2012.11.116. [DOI] [PubMed] [Google Scholar]
- 4.kundu P, Laskar S. A brief resume on the genus Ailanthus: Chemical and pharmacological aspects. Phytochem Rev. 2010;9:pp379–412. doi: 10.1007/s11101-009-9157-1. [DOI] [Google Scholar]
- 5.Okunade AL, Bikoff RE, Casper SJ, Oksman A, Goldberg DE, Lewis WH. Antiplasmodial activity of extracts and quassinoids isolated from seedlings of Ailanthus altissima (Simaroubaceae) Phytother Res. 2003;17:675–677. doi: 10.1002/ptr.1336. [DOI] [PubMed] [Google Scholar]
- 6.Fukamiya N, Lee KH, Muhammad I, Murakami C, Okano M, Harvey I, Pelletier J. Structure-activity relationships of quassinoids for eukaryotic protein synthesis. Cancer Lett. 2005;220:37–48. doi: 10.1016/j.canlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Rosati A, Quaranta E, Ammirante M, Turco MC, Leone A, De Feo V. Quassinoids can induce mitochondrial membrane depolarisation and caspase 3 activation in human cells. Cell Death Differ. 2004;11(Suppl 2):S216–S218. doi: 10.1038/sj.cdd.4401534. [DOI] [PubMed] [Google Scholar]
- 8.Yang XL, Yuan YL, Zhang DM, Li F, Ye WC. Shinjulactone O, a new quassinoid from the root bark of Ailanthus altissima. Nat Prod Res. 2014;28:1432–1437. doi: 10.1080/14786419.2014.909418. [DOI] [PubMed] [Google Scholar]
- 9.Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng L, Yao N, Peng Q, Chen Z, Ye W, Zhang D. Ailanthone inhibits Huh7 cancer cell growth via cell cycle arrest and apoptosis in vitro and in vivo. Sci Rep. 2015;5:16185. doi: 10.1038/srep16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 11.Kondo S, Tanaka Y, Kondo Y, Hitomi M, Barnett GH, Ishizaka Y, Liu J, Haqqi T, Nishiyama A, Villeponteau B, et al. Antisense telomerase treatment: Induction of two distinct pathways, apoptosis and differentiation. FASEB J. 1998;12:801–811. doi: 10.1096/fasebj.12.10.801. [DOI] [PubMed] [Google Scholar]
- 12.Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: A review. Biochim Biophys Acta. 2011;1813:238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Arozena Acevedo A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 15.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 16.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 18.Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Xia Z, Zhang H, Xu D, Lao Y, Fu W, Tan H, Cao P, Yang L, Xu H. Xanthones from the leaves of garcinia cowa induce cell cycle arrest, apoptosis, and autophagy in cancer cells. Molecules. 2015;20:11387–11399. doi: 10.3390/molecules200611387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GK, Shah MA. Targeting the cell cycle: A new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 21.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherr CJ. The Pezcoller lecture: Cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 23.Watson NC, Di YM, Orr MS, Fornari FA, Jr, Randolph JK, Magnet KJ, Jain PT, Gewirtz DA. Influence of ionizing radiation on proliferation, c-myc expression and the induction of apoptotic cell death in two breast tumour cell lines differing in p53 status. Int J Radiat Biol. 1997;72:547–559. doi: 10.1080/095530097143059. [DOI] [PubMed] [Google Scholar]
- 24.Lavhale MS, Kumar S, Mishra SH, Sitasawad SL. A novel triterpenoid isolated from the root bark of Ailanthus excelsa Roxb (Tree of Heaven), AECHL-1 as a potential anti-cancer agent. PLoS One. 2009;4:e5365. doi: 10.1371/journal.pone.0005365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wang ZH, Peng ZL, Duan ZL, Liu H. Expression and clinical significance of autophagy gene Beclin 1 in cervical squamous cell carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:860–863. (In Chinese) [PubMed] [Google Scholar]
- 26.Xu MY, Lee DH, Joo EJ, Son KH, Kim YS. Akebia saponin PA induces autophagic and apoptotic cell death in AGS human gastric cancer cells. Food Chem Toxicol. 2013;59:703–708. doi: 10.1016/j.fct.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 28.Česen MH, Pegan K, Spes A, Turk B. Lysosomal pathways to cell death and their therapeutic applications. Exp Cell Res. 2012;318:1245–1251. doi: 10.1016/j.yexcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 30.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–850. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- 31.Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem Sci. 1991;16:150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 32.Seglen PO, Grinde B, Solheim AE. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979;95:215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 33.Phadwal K, Watson AS, Simon AK. Tightrope act: Autophagy in stem cell renewal, differentiation, proliferation, and aging. Cell Mol Life Sci. 2013;70:89–103. doi: 10.1007/s00018-012-1032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 35.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Yang P, Sun D, Jiang F. Ailanthone promotes human vestibular schwannoma cells apoptosis and autophagy by down-regulation of miR-21. Oncol Res. 2018 Jan 3; doi: 10.3727/096504018X15149775533331. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.