Abstract
Triple-negative breast cancer (TNBC) is a subtype of breast cancer with a poor prognosis and limited effective treatment. The rise in immunotherapeutic strategies prompted the establishment of a genetic vaccine against TNBC in vitro using a possible biological marker of TNBC. In the present study, different detection methods were used to evaluate the distribution and expression of runt-associated transcription factor 2 (Runx2) in various breast cancer cell lines. Following the development of the Runx2-dendritic cell (DC) vaccine using a lentivirus, the transfection efficacy was recorded. The T lymphocytes co-cultured with the vaccine were collected to assess the antitumor potency. Increased levels of Runx2 were expressed in breast cancer cells; however, different breast cancer cell lines expressed various levels of Runx2. Runx2 demonstrated particularly high expression in TNBC cells, compared with non-TNBC cells. A Runx2 lentivirus transfection system was successfully engineered, and Runx2 was transduced into dendritic cells whilst maintaining stable expression. The sustained and stable cytotoxic T cells induced in the transfected group had higher and more specific antitumor efficacy against TNBC, compared with the other cell lines. Runx2 may be a novel target for TNBC treatment. The Runx2-DC vaccine may induce specific and efficient antitumor effects in TNBC in vitro.
Keywords: dendritic cell vaccine, runt-associated transcription factor 2, immunotherapy, triple-negative breast cancer
Introduction
Breast cancer, a heterogeneous tumor, may be divided into different subtypes with various manifestations. Among them, triple-negative breast cancer (TNBC) involves tumors negative for estrogen receptor (ER), progesterone receptor and human epidermal growth factor receptor 2. The primary characteristics of TNBC include: High incidence in young females; poor histological differentiation; early visceral metastasis; reduced disease-free survival; and high mortality rates (1,2). Despite the aggressive clinical behavior and poor overall prognosis of TNBC, the treatment of patients with TNBC remains inferior due to a lack of known target genes and hormone receptors.
Immunotherapy, which aims to strengthen the capacity of the immune system rather than methods that attack cancer cells directly, such as chemotherapy and radiotherapy, is expected to prolong patient survival. It is considered that immunotherapy may revolutionize the treatment of cancer.
The provision of antigen-presenting cells (APCs) and co-stimulation signals are essential in the activation of T lymphocytes. Dendritic cells (DCs) are one of the most powerful APCs and serve an important role in activating resting T lymphocytes (3). The major histocompatibility complex (MHC) combines with the surface proteins of tumor antigens to form peptide-MHC, and DCs assist T cells in recognizing the antigen, thus stimulating a cluster of differentiation (CD)8+ cytotoxic T cell (CTL) response and a CD4+ helper T cell response (4). DCs have the ability to activate T cells by highly expressing MHC I/II molecules and by expressing a variety of co-stimulating molecules; however, in patients with cancer, the tumor microenvironment competes with their immune system to maintain the growth and reproduction of cancer cells (5). The normal immune function becomes damaged and exhibits the following features: Absence or dysfunction of the MHC molecule; decline in the number of DCs and their presentation capacity; and inhibition of T lymphocytes (5). All of these features contribute to an imbalance of the immune system, and DC-based immunotherapy assists in the restoration of this balance, providing a novel cancer treatment option (6,7).
Different surface antigens were determined between tumor cells and healthy cells in the 1960s, and these tumor-associated antigens became the basis of cancer treatment (8). Currently, DC vaccines for breast cancer primary include a DC-fusion vaccine and a specific antigen-loaded DC vaccine (9–12). Fusion vaccines present all of the antigens on the surface of breast cancer cells to T cells, in order to induce an immune response, whilst antigen-loaded DC vaccines stably present antigens and continuously stimulate DCs to provide a stronger antitumor effect (13,14). Overall, identifying a specific antigen marker may result in a novel breakthrough in TNBC treatment.
Runt-associated transcription factor 2 (Runx2) is a type of oncogene that is unusually increased in prostate cancer and breast cancer, and is associated with cancer progression (15–17). Runx2 is expressed in various mammary epithelial cell lines indicating a potential role in the development of mammary glands; however, downregulation is required in pregnancy for full differentiation (18). Abnormal Runx2 expression drives epithelial to mesenchymal transition in normal mammary epithelial cells (19), disturbing normal mammary gland structures, such that the normal breast tissue exhibits malignant features and promoting the development of breast cancer by enhancing tumor cell proliferation (20,21). Runx2 has the ability to also directly influence downstream effectors, including matrix metalloproteinase (MMP) 9, MMP13 (22–24), vascular endothelial growth factor (VEGF), parathyroid hormone-associated protein levels (25), bone sialoprotein and osteopontin (22), which results in a more aggressive breast cancer (26,27). In addition, the pro-angiogenic effect of Runx2 serves an important role in promoting migration and invasion in tumors (28,29). Runx2 is also associated with hormones in breast cancer (26,30). The loss of ERα may lead to enhanced invasiveness in breast cancer cells (31); therefore, ERα is considered to be a key protective factor for breast cancer cells, whilst Runx2 and ERα antagonize each other through the ERαDNA binding domain and partially through SNAI2 (26,27,30). In a study on 123 patients with breast cancer, Runx2 was more active in patients with c-erbB2 (HER2/c-neu)-negative breast cancer than the positive ones (39 vs. 17%) (32). These data may indicate that Runx2 may be a novel biomarker for patients with TNBC.
In the present study, Runx2 served as a target to induce immunotherapy against TNBC by preparing a Runx2-DC vaccine. The aims were to evaluate the efficacy of the vaccine in activating autologous lymphocytes in vitro and to observe the specific anti-TNBC effects of the vaccine with the goal of providing a novel therapeutic strategy for patients with TNBC.
Materials and methods
The use of human subjects was specifically approved by the Clinical Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Guangzhou Blood Center (Guangzhou, China) supplied the blood and recorded the informed consent. Prior to donating blood, the volunteers were informed and provided written informed consent for the scientific research use of blood samples.
Cell cultures
MDA-MB-231 cells exhibited greater Runx2 expression than non-TNBC cell lines in previous studies (32–34), thus MDA-MB-231 was selected as the focus of the present study. Human breast epithelial cell line MCF10A was purchased from the National Infrastructure of Cell Line Resource (China; http://www.cellresource.cn/index.aspx). The cell line was cultured in D/F 12 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 5% horse serum (Gibco; Thermo Fisher Scientific, Inc.), insulin (10 µg/ml), hydrocortisone (0.5 µg/ml) and epidermal growth factor (20 ng/ml) (PeproTech China, Suzhou, China). The TNBC cell line MDA-MB-231 and the MCF7 cell line were purchased from the American Type Culture Collection (ATCC) and cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.). 293FT cells (purchased from the ATCC) were also cultured in DMEM containing 10% FBS. All the cell lines were negative for mycoplasma and were maintained in a humidified environment at 37°C with 5% CO2.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from all three cell lines (MDA-MB-231, MCF7 and MCF10A) were extracted by trizol (Invitrogen; Thermo Fisher Scientific, Inc.) and transcribed into cDNA according to the reverse transcription kit (PrimeScript RT Master Mix Perfect Real Time; Takara Biotechnology Co., Ltd., Dalian, China) protocols. The resultant cDNA mixed with the SYBR® Green PCR mix (Takara Biotechnology Co., Ltd.) and primers of the target genes were amplified and analyzed with the Applied Biosystems 7500 FAST system (Thermo Fisher Scientific, Inc., Waltham MA, USA) according to the manufacturer's protocol. The reaction conditions were as follows: 95°C for 30 sec; followed by 95°C for 3 sec and 60°C for 30 sec for 40 cycles. GAPDH was used as an internal control. The relative quantification 2−∆∆Cq method was used to analyze the PCR data (35). The primers were the following: Runx2 forward, 5′-CGGCCCTCCCTGAACTCT-3′ and reverse, 5′-TGCCTGCCTGGGGTCTGTA-3′; GAPDH forward, 5′-ATGTTCGTCATGGGTGTGAA-3′ and reverse, 5′-TGTGGTCATGAGTCCTTCCA-3′. All experiments were repeated in triplicate.
Western blot analysis
Runx2 protein expression was evaluated in all three cell lines. Cells were lysed in lysis buffer (Sigma-Aldrich; Merck KGaA, Damstadt, Germany) and quantified via a bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). A total of 20 µg of extract was loaded and resolved on a 10% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane using the Bio-Rad protein transferring apparatus (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PVDF membrane was removed and blocked with 5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for 1–2 h at room temperature (RT). The membrane was incubated with a monoclonal antibody against Runx2 (1:1,000; cat. no. ab769560; Abcam, Cambridge, UK) overnight at 4°C, followed by secondary antibody incubation (Rabbit Anti-mouse horseradish peroxidase conjugated; 1:2,000; cat. no. bs-0296R-HRP; Beijing Boaosen Biotechnology Co., Ltd., Beijing, China) at RT for 1 h and SuperSignal West Pico Trial Kit for subsequent detection (Thermo Fisher Scientific, Inc.). The internal reference was GAPDH. The protein expression were detected by ChemiDoc MP V3 Western Workflow for Midi Gels (Bio-Rad Laboratories, Inc.) and analyzed by GraphPad Prism v.5.0 (GraphPad Software, USA).
Immunocytochemistry
A total of 2×104 cells/ml in each cell line group were seeded on disinfected coverslips in 6-well plates, followed by fixation with 4% poly-stained formaldehyde for 15 min at RT until the cells were grown to 60–70% confluence. Following this, the catalase was eliminated from the cells by incubating with 0.5% Trion X-100 at RT for 20 min, followed by 3% H2O2 at 37°C fo between 10 and 15 min. The cells were blocked with 5% goat serum (Beijing Boaosen Biotechnology Co., Ltd.) for 5–10 min at RT. Anti-Runx2 mouse mAb (cat. no. ab76956; Abcam, Cambridge, UK) at a 1:1,000 dilution was used as the primary antibody for the treatment group while antibody diluent (Beijing Boaosen Biotechnology Co., Ltd.) was added in negative group, and incubated at 37°C for 1 h. Next, the sections were incubated with a secondary antibody (ChemMate EnVision Detection kit, horseradish peroxidase/DAB conjugated, rabbit/mouse, cat. no. GK500705/10; Dako; Agilent Technologies, Inc., Santa Clara, Clara, CA, USA) for 30 min at 37°C, processed with the 3,3′-diaminobenzidine provided in the manufacturer's kit and counterstained using hematoxylin for 1–2 mins at RT. Electron microscopy (Olympus Corporation, Tokyo, Japan) was used to observe the cells at a magnification of ×100.
Generation and culture of DCs
Human peripheral venous blood was obtained from the Guangzhou Blood Center and heparinized under aseptic conditions. Ficoll-Hypaque gradient centrifugation at 450 × g, 22°C for 25 min was used to isolate peripheral blood mononuclear cells (PBMCs) from peripheral blood. The isolated cells were replenished in sterile 6-well plates of 1×105 cells/well with serum-free RPMI-1640 medium (Gibico, USA) at 37°C in a 5% CO2 incubator for 2 h. Non-adherent cells were collected for culture reserves. Adherent cells were cultured in RPMI-1640 medium containing recombinant human granulocyte macrophage-colony stimulating factor (100 ng/ml) (Peprotech China) and recombinant human interleukin-4 (rhIL-4; 50 ng/ml) (Peprotech China). The medium was changed every other day. On day five, the pro-inflammatory mediator tumor necrosis factor (TNF-α; 20 ng/ml) (Peprotech China) was added, and the cells were incubated for another two days. Finally, the suspension of cells were collected as mature DCs.
Flow cytometry analysis
All of the day-7 DCs were collected and washed with PBS, followed by blocking with 5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for 20 min on ice, and then incubating with 1:100 dilutions of antibodies against CD11c-phycoerythrin (PE; cat. no. 555392; BD Biosciences, Franklin Lakes, NJ, USA), CD83-fluorescein isothiocyanate (FITC; cat. no. 556910; BD Biosciences), CD86-FITC (cat. no. 557343; BD Biosciences) and human leukocyte antigen-antigen D related (HLA-DR)-PE (cat. no. 555561; BD Biosciences) and their isotype controls IgG-1k-PE (cat. no. 562538; BD Biosciences) and IgG-1k-FITC (cat. no. 555786; BD Biosciences) for 30 min at 4°C. Cells were analyzed by a FACScan flow cytometer with CellQuest Pro software (version 5.1; BD Biosciences) following a wash with PBS.
DC transfection and detection
Runx2 lentivirus vector was purchased from Novo Biological Company (Shanghai, China), and amplified according to the plasmid extraction kit (Endo-free Plasmid Mini Kit II; Omega Bio-Tek, Guangzhou, China). Plenti6 plasmids (150 ng/ul; Invitrogen; Thermo Fisher Scientific, Inc.) were used to pack Runx2 lentivirus according to the manufacturer's specification. After 48–72 h, the supernatant was collected following centrifuging at 500 × g for 15 min at 4°C and passing through a 0.45 um filter. The 293FT cells were cultured in sterile 96-well plates of 1×105/ml in a humidified environment at 37°C with 5% CO2. According to the expected titer of the virus, 8 sterile EP tubes were prepared and 90 µl serum-free DMEM was added to each tube. In the first tube, 10 µl virus was added. A total of 10 µl was taken from the first tune and mixed with the second tube, and this was repeated in the same manner until the last tube. Next, 90 µl diluted virus from each tube was joined with the 293FT cells at day 2. Subsequent to 4 days, the results were observed under a fluorescence microscope, then the last two wells in which fluorescence could be observed were selected and the number of fluorescent cells were counted. With Y being the number of fluorescent cells counted in the last well as Y and X being the number of fluorescent cells in the second-to-last well, the titer (TU/ml)=(X+Y*10)*1,000/2/the virus fluid content (µl) of X. The prepared day-2 DCs were divided into three groups as follows: Runx2 lentivirus was added to one group (transfected group); 293 cell lysate (Abgent, USA) was added to another group (control group); and no treatment was added to the last group (blank control group). The cells were placed in RPMI-1640 medium 24 h following infection, and DCs were cultured as aforementioned. After 48–72 h, green fluorescent protein was observed by a fluorescence microscope at ×40 magnification. Transfection efficiency was assessed by RT-qPCR and western blot analysis using the methods aforementioned.
Autologous T-lymphocyte culture
Nylon wool was used to purify the non-adherent PBMCs as previously described, and B and NK cells were then removed, leaving autologous T lymphocytes. T cells were cultured in RPMI-1640 medium containing 10% FBS and rhIL-2 (20 ng/ml) at 37°C in a 5% CO2 atmosphere.
Cytokine detection by ELISA
The cells were divided into TNBC groups (DC-transfection group+MDA-MB-231; control group+MDA-MB-231; and blank control group+MDA-MB-231), luminal breast cancer groups (DC-transfection group+MCF7; control group+MCF7; and blank control group+MCF7), normal breast cell groups (DC-transfection group+MCF10A; control group+MCF10A; and blank control group+MCF10A), effector cell groups (DC-transfection group; control group; and blank control group) and target cell groups (MDA-MB-231; MCF7; and MCF10A). The cells in each group were co-cultured with T lymphocytes extracted previously at a ratio of 1:20 for 72 h in 24-well plates. The supernatant was collected to detect the secretion of IL-12 and interferon (IFN)-γ using an ELISA kit (cat. no. SEA111Hu for IL-12, SEA049Hu for IFN-γ; Wuhan USCN Business Co., Ltd., Wuhan, China) in triplicate.
Cytotoxicity assay
The tumor-specific CTLs were stimulated following the co-culture of each group of cells with T lymphocytes, and nylon wool was used to harvest the CTLs again. MDA-MB-231 was selected as the research focus, and the CTLs of the TNBC groups were incubated with MDA-MB-231 at a ratio of 20:1 in a 96-well plate for 72 h as the reactive cells. In order to understand what proportion of the effective target ratio is more appropriate, the ratio was also set to 5:1 and 10:1. At the time, the sole target cell group, sole effector cell group were being cultured. PBS was used to dissolve the purple formazan 2–3 times. Following the addition of MTT in each group for 4 h at 37°C, the supernatant was removed. Absorbance optical density (OD) values were detected using an enzyme-labeled instrument at 490 nm. Detection in the MCF7 and MCF10A groups was performed in a similar manner. The cytotoxicity rate was calculated by the following formula: [1-(OD value of reactive cell well-OD value of effector cell well)/(OD value of target cell well)] ×100%.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) software was used to analyze all the measured data. The data of western blot were analyzed by GraphPad Prism v.5.0 (GraphPad Software, USA). The other statistical differences were calculated by a two-way analysis of variance and LSD post hoc test between groups. The data is presented as the mean ± standard error of the mean, and three repeats were conducted. P<0.05 was considered to indicate a statistically significant difference.
Results
Runx2 expression in breast cells
Runx2 is considered to be associated with a particular subtype of breast cancer cells (36); however, whether it can be regarded as a biomarker of TNBC has not yet been concluded. Therefore, RT-qPCR was used to detect the expression of Runx2 in various breast cancer cell lines. Fig. 1A demonstrates the results of Runx2 mRNA expression. Runx2 expression in the TNBC cell line MDA-MB-231 was significantly higher compared with the other cell lines, followed by the luminal breast cell line MCF7 and then the normal breast cell line MCF10A (P<0.05). Following this, western blot analysis was performed to examine whether Runx2 protein expression followed the same patterns. Runx2 protein levels remained significantly higher in the TNBC cells, compared with the other two cell lines (P<0.05; Fig. 1B and C); Runx2 expression was also greater in MCF7 cells, compared with MCF10A cells. Next, immunocytochemistry was used to elucidate the distribution of the Runx2 protein (Fig. 1D). All of the cell lines demonstrated positive staining for Runx2; however, the levels and distribution were different. Normal breast cells indicated reduced Runx2 expression, compared with the other breast cancer cell lines, and Runx2 was situated in the cell membrane, similar to MCF7. Runx2 in the MDA-MB-231 cell line was almost completely distributed throughout the nucleus with a high positive rate.
Figure 1.
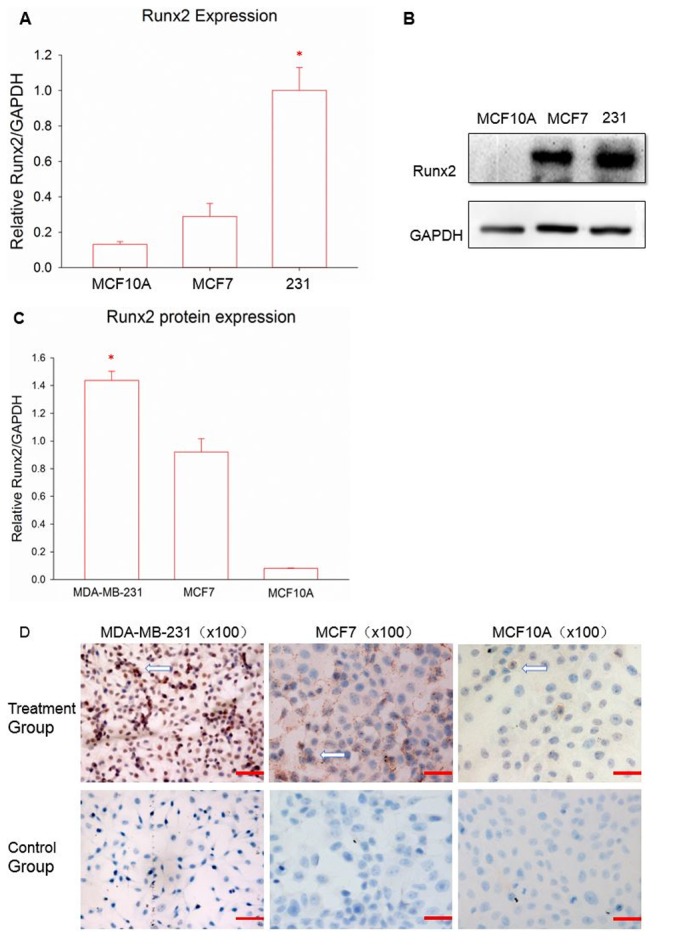
(A) Expression of Runx2 in different breast cells. Data are reported as the mean ± standard error of the mean of the three groups. (B) Western blot analysis was used to measure the distribution of the Runx2 protein. (C) The quantitative results for the western blot analysis. (D) Red staining represents Runx2 protein expression, marked with arrows. Magnification, ×100; scale bar, 100 µm. *P<0.05 vs. MCF7 and MCF10A according to analysis of variance and an LSD test. Runx2, runt-associated transcription factor 2.
Morphology and phenotype of DCs
The DCs were all extracted from the PBMCs of healthy volunteers, and were supplied and isolated at the Guangzhou Blood Center. In the culture process, pro-inflammatory cytokines stimulated DC maturation. When observed under a microscope, immature DCs exhibited adherent growth, and clusters consisted of small, round cells with relatively little cytoplasm (Fig. 2A). DCs gradually transformed from an adherent to a semi-suspended state when cultured for 3 days, and they began to have a small amount of fine outgrowths on their surface (Fig. 2B). The typical morphology of mature DCs acquired following 7 days of incubation revealed a dispersed suspension state with irregular cell morphology and a coarse dendritic surface, with a significantly larger volume and more abundant cytoplasm, compared with immature DCs. Flow cytometry was used to analyze the phenotype of the DCs. The monoclonal antibodies CD83, CD86, HLA-DR and CD11c were selected to stain the DCs, and all of them were present at high levels (Fig. 2C); thus indicating that mature DCs were successfully obtained.
Figure 2.
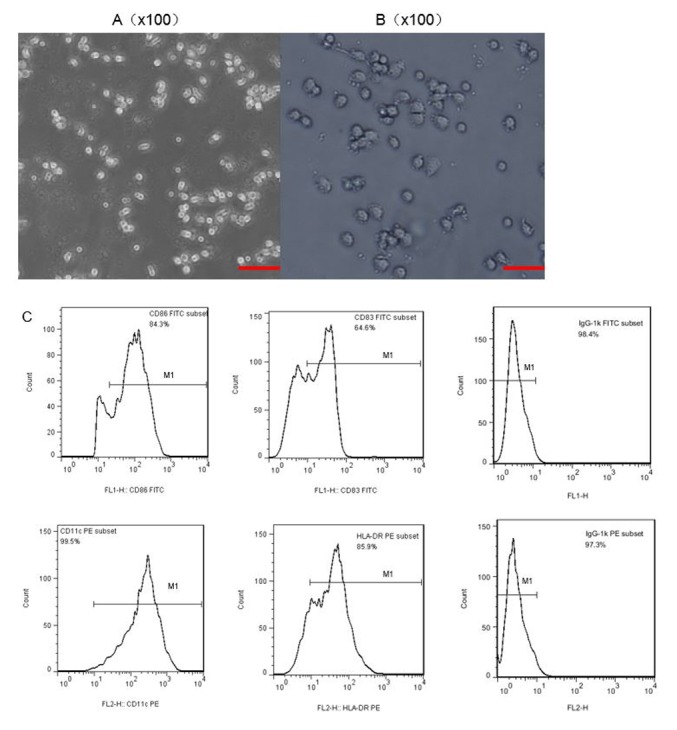
Morphology and characteristics of DCs in different phases of culture. Microscopy was used to observe (A) immature DCs cultured for 3 days. (B) Subsequent to adding tumor necrosis factor (TNF-α; 20 ng/ml) on day 5 and culturing for a further two days, mature DCs could be observed on day 7. Magnification, ×100; scale bar, 100 µm. (C) Characteristic phenotypes of mature DCs were observed by flow cytometry. DCs, dendritic cells.
DC transfection and identification of transfection efficiency
To prepare the Runx2-DC vaccine, a Runx2 plasmid was used to package the lentivirus and infect the DCs. Eventually, a lentivirus was added to day-2 immature DCs, due to there being no influence on the state of maturity, the ability to stimulate an immune response and also due to the highest transfection efficiency achieved in day-2 DCs (37). Following transfection for 48 h, significant expression of green fluorescent protein was observed using a fluorescence microscope (Fig. 3A and B). The expression was more notable following 72 h, indicating successful transduction. Following 7 days of incubation, the transfected DCs demonstrated typical mature cell morphology with suspended growth and clear dendritic protrusions on the cell surface (Fig. 3C). Whether Runx2 was stably expressed in DCs has not been previously confirmed; therefore, RT-qPCR and western blot (Fig. 3D and E) analysis were used to assess the Runx2 expression of transfected groups at the mRNA and protein levels. It was concluded that Runx2 was successfully integrated into the DCs at the nucleic acid level due to the transfected group expressing a significant amount of Runx2 and due to the control groups expressing almost no Runx2 (P<0.05). In addition, the Runx2 protein was notably expressed in the transfected group, but almost no significant expression was observed in the other two control groups. The Runx2-DC vaccine was then established.
Figure 3.
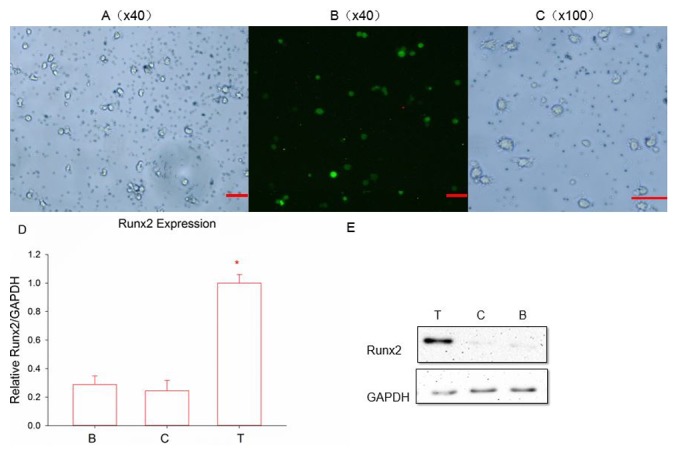
(A) Fluorescence signals and Runx2 emitted by infected cells. Magnification, ×40; scale bar, 100 µm. (B) Following 48 h of transfection. Magnification, ×40; scale bar, 100 µm. (C) Following 7 days of culture, transfected DCs became mature. Magnification, ×100; scale bar, 100 µm. Reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively, were used to detect the (D) mRNA and (E) protein expression of Runx2. *P<0.05 vs. the two control groups according to analysis of variance and an LSD-post hoc test. T, transfected group; C, negative control group; B, blank control group; Runx2, runt-associated transcription factor 2.
Secretion of IL-12 and IFN-γ in T cells stimulated with the Runx2-DC vaccine
To detect T cell activation, the secretion of IL-12 and IFN-γ was measured in each group with an ELISA kit (Fig. 4). In the previous experiments, it was determined that cytokine secretions reach a peak when co-cultured with T lymphocytes and the vaccine or other control groups for 5 days. The supernatants were collected for detection using an ELISA kit following 5 days of co-culture. The expression of IL-12 (pg/ml) was as follows in each group: 170.33±5.03 (DC-Runx2); 42.43±2.48 (DC-control); and 75.00±3.00 (231-DC) (P<0.05). The secretion of IFN-γ in each of the three groups was as follows: 517.15±5.88 (DC-Runx2); 195.63±5.51 (DC-control); and 232.57±4.73 (231-DC) (P<0.05). Thus, the Runx2-DC vaccine may stimulate T lymphocytes to secrete more cytokines (P<0.05).
Figure 4.
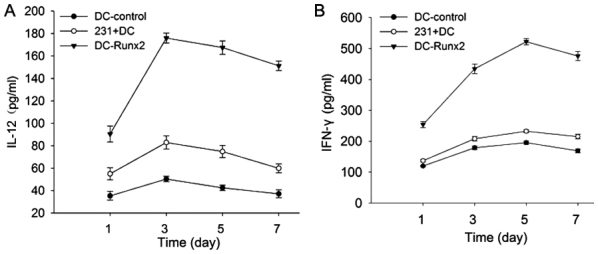
Secretion of (A) IL-12 and (B) IFN-γ in T cells stimulated by different groups. Following co-culture with T cells and each group for 7 days at a ratio of 20:1, the supernatant at different times in each group was collected. *P<0.05 vs. control group and mixed cell group according to analysis of variance and an LSD test. IL-12, interleukin-12; DC, dendritic cells; Runx2, runt-associated transcription factor 2; IFN-γ, interferon-γ; 231, MDA-MB-231.
Cytotoxicity against TNBC provided by the Runx2-DC vaccine
An objective of the present study was to determine whether the Runx2-DC vaccine would be efficacious for cytotoxicity against TNBC; therefore, the cytotoxicity rates for different cell lines were analyzed. For MDA-MB-231, the cytotoxicity rates were positively associated with the effective target ratio, and at the ratio of 20:1 the cytotoxicity rates of the transfected, control and blank group were 60.02±3.51, 25.57±3.64 and 26.45±1.30%, respectively (Fig. 5A). The transfected group indicated a significant increase in cytotoxicity efficiency in MDA-MB-231 cells, compared with the non-transfected groups (P<0.01); thus, the Runx2-DC vaccine may induce more powerful cytotoxicity of CTLs.
Figure 5.
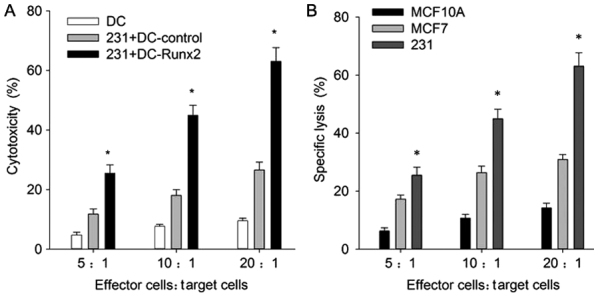
(A) Cytotoxicity of CTLs against tumor cells. The CTLs induced by the Runx2-DC vaccine, mixed cells and DCs alone were incubated with target cells (MDA-MB-231) at a ratio of 5:1, 10:1 and 20:1. *P<0.05 vs. control group and mixed cell group according to two-way ANOVA and an LSD test. (B) Similar methods were used for MDA-MB-231, MCF7 and MCF10A. *P<0.05 vs. MCF7 and MCF10A according to two-way ANOVA. CTL, cytotoxic T cell; Runx2, runt-associated transcription factor 2; DC, dendritic cells; ANOVA, analysis of variance; 231, MDA-MB-231.
To ensure that the anti-TNBC cytotoxicity was specific, the transfected CTLs were co-cultured with MCF7 and MCF10A, and the following cytotoxicity rates at the effective target ratio of 20:1 were calculated: 30.91±1.70 and 14.27±1.60, respectively (Fig. 5B). The cytotoxicity rates showed the same correlation with effective target ratio. These results revealed that the vaccine had significantly stronger specific cytotoxicity effects against TNBC, compared with the non-TNBC and normal breast cell groups (P<0.05). It is notable that the vaccine has strong cytotoxicity against breast cell lines, but also affects normal breast cells.
Discussion
Based on microarray and proteomic studies, Runx2 was determined to be one of the most upregulated genes in invasive breast cancers (38,39). Runx2 has the ability to promote cell proliferation and inhibit apoptosis caused by P53 in tumor cells, thus advancing the progression of breast cancer (21). Runx2 has also been reported to mediate the invasion of the human breast cancer cell lines MDA-MB-231 and MCF7 (36). Following Runx2 inhibition or knockout in breast cancer, the tumor phenotypes move gradually toward normal phenotypes, with suppression of tumor cell proliferation and growth (20,21). It was previously determined that the expression of Runx2 was lower in the normal breast cells and significantly increased in breast cancer cells (20,21); therefore, abnormal expression of Runx2 in tumors may be associated with tumor progression. McDonald et al (36) described abundant Runx2-specific expression in TNBC using microarray analysis and determined that it was associated with a lower survival rate; however, other research has indicated that Runx2 expression is more notable in ER-positive cells (32). Based on these characteristics, Pratap et al (20) demonstrated that Runx2 had a higher expression in breast cancer cells MDA-MB-231 compared with that in MCF10A cells (20). Comparing between MDA-MB-231 and MCF7 cells, higher expression of Runx2 was present in the highly invasive MDA-MB-231 cells (32,33). With a higher Runx2 expression level in MDA-MB-231 cells, compared with non-TNBC cell lines demonstrated in previous studies (32–34), MDA-MB-231 was selected as the research focus. Similar to these previous data, higher Runx2 expressions was demonstrated in the breast cancer cell lines compared with the normal breast cancer cell line, and a stronger expression of Runx2 in TNBC was determined, compared with the MCF7 and MCF10A cell lines, indicating that Runx2 may be used as a novel target for TNBC immunotherapy.
In the present study, Runx2 indicated a wide variety of distribution in different breast cancer cells. Positive expression of Runx2 in the cytoplasm and nucleus was observed in breast cancer cells. Runx2 primarily existed in the nucleus in TNBC and in the cytoplasm in luminal breast cancer cells, whilst Runx2 was typically present in the cytoplasm in normal breast cell lines. Different distributions may be associated with the function of Runx2. As a transcription factor, Runx2 was enriched in terminal end buds, which were essential for the branching of the developing gland in mammary epithelial and breast cancer cell lines (40). It was considered that Runx2 expressed in the cytoplasm of normal breast cells promoted differentiation; therefore, Runx2 may exert more functions, including tumor cell proliferation, migration and invasion, in the nucleus.
As they are concentrated in the periphery of carcinomas, DCs may come into contact and identify tumor cell-specific antigens with dendrites on their surface (41). Their major functions include: Intake, processing and presentation of antigens; participation in T-lymphocyte differentiation; promotion of B-lymphocyte proliferation directly or indirectly; and regulation of innate immunity (42). However, patients with cancer lack DC and co-stimulatory signals, genetic instability and heterogeneity of tumor cell antigens, which all contribute to a valid T cell activation and an effective immune response (42). Transfusion or vaccination of patients with cancer with antigen-sensitized DCs in vitro may assist in breaking down these barriers.
The method of transducing Runx2 into DCs is challenging. As non-dividing cells, DCs are difficult to transduce with viral vectors, and their phenotype and function may change following transfection (43–45). A previous study indicated that lentivirus may lead to efficient transfection and preservation of cell maturation and function with day-2 immature DCs (46). Unlike the adenoviral vector, lentiviruses may also avoid vector-mediated immune stimulation (47). The present data confirmed that Runx2 may be loaded into DCs without affecting cell morphology and maturity, and that lentivirus vector transfection provided stable expression of Runx2.
A valid CD8+ T cell response is associated with positive prognosis for tumor control and clearance (48). CD8+ T cells do not have the ability to produce the cytokines IFN-γ, IL-2 and TNF, which means that they gradually deplete in the tumor microenvironment, thus losing the ability to eliminate tumor cells (49). Following effective identification of tumor antigen presented by DCs, CD4+ T cells have the ability to activate the CTL response (50). CD4+ T cells differentiate into Th1 and Th2 cells, Th1 cells effectively stimulate and mediate cellular immunity, which is primarily induced by IL-12. Under normal circumstances, Th1 and Th2 cells are in equilibrium. Changes to the equilibrium help the tumor cells to escape the immune response, thus leading to tumor growth (51,52). DCs drive naive T cells to differentiate into various phenotypes, including Th1, Th2, Th17, Treg or CTLs (53). The unique ability to regulate specific types of cellular immune responses gives priority to DCs in cancer immunotherapy, where Th1-induced tumor-specific CTLs are particularly desirable (54,55).
Integrated DCs and T cells secrete IL-12 and IL-18 to promote T cell proliferation and aggregation, and release anti-angiogenic factors, including IL-12 and IFN-γ, to inhibit tumor angiogenesis (56). The cytokine IFN-γ strengthens cellular immunity by inducing increased MHC expression and promoting T-lymphocyte differentiation (57). For example, CD8+ T cell differentiation is partially dependent on IFN-γ (48,58). It is also important for innate immunity due to its actions in enhancing monocyte-macrophage activation, the efficiency of natural killer (NK) cells and stimulating VEGF secretion. IL-12 serves an essential role in promoting cell division of T lymphocytes, inducing Th1 cell differentiation, and stimulating NK cell and B cell activity (57,59). This may reflect a change in the quantity and function of T cells, reaching a peak value following 24–72 h in vitro according to a previous study (60).
In the present study, the transfection group secreted more IL-12, activating and polarizing Th1 cells and inducing NK cells, compared with the non-transfection group. Subsequently, Th1 cells have the ability to effectively activate CTLs, providing a large amount of IFN-γ, perforin and granzyme, enhancing the cytotoxic effect on tumor cells. This result was confirmed in subsequent experiments, with the sustained expression of the Runx2 antigen significantly enhancing the antitumor effect on TNBC by inducing the CTL and Th1 response. Different immune responses in anti-MDA-MB-231 and anti-MCF7 cells were induced by the Runx2-DC vaccine, and anti-MDA-MB-231 cells demonstrated the strongest effect among the different cell lines. The vaccine may induce specific T cell-mediated immune responses against TNBC following targeting and recognizing TNBC. To further confirm the data, additional cell lines should be used. A more troublesome problem is the damage to normal breast tissue caused by the vaccine in vitro. The cytotoxicity against normal tissue requires further study in vivo, and the use of regulatory T cells and suppressor cells may potentially prevent this phenomenon.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TNBC
triple-negative breast cancer
- ER
estrogen receptor
- DCs
dendritic cells
- APC
antigen-presenting cells
- MHC
major histocompatibility complex
- CTL
cytotoxic T cell
- MMP
matrix metalloproteinase
- VEGF
vascular endothelial growth factor
- FBS
fetal bovine serum
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- RT
room temperature
Funding
The present study was supported by the Guangdong Natural Science Foundation (grant no. 2014A030313193).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MT, YL and YH designed the study; QCZ, PZ and JKW analyzed the data; and JNW and YR drafted the manuscript and helped to analyzed the data.
Ethics approval and consent to participate
The use of human subjects was specifically approved by the Clinical Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). Guangzhou Blood Center (Guangzhou, China) supplied the blood and recorded the informed consent. Prior to donating blood, the volunteers were informed and provided written informed consent for the scientific research use of blood samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 2.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 3.Bonasio R, von Andrian UH. Generation, migration and function of circulating dendritic cells. Curr Opin Immunol. 2006;18:503–511. doi: 10.1016/j.coi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kichler-Lakomy C, Budinsky AC, Wolfram R, Hellan M, Wiltschke C, Brodowicz T, Viernstein H, Zielinski CC. Deficiences in phenotype expression and function of dendritic cells from patients with early breast cancer. Eur J Med Res. 2006;11:7–12. [PubMed] [Google Scholar]
- 6.Bennaceur K, Chapman J, Brikci-Nigassa L, Sanhadji K, Touraine JL, Portoukalian J. Dendritic cells dysfunction in tumour environment. Cancer Lett. 2008;272:186–196. doi: 10.1016/j.canlet.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Bosco MC, Puppo M, Blengio F, Fraone T, Cappello P, Giovarelli M, Varesio L. Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Rifkind RA, Hsu KC, Morgan C, Seegal Bc, Knox Aw, Rose Hm. Use of ferritin-conjugated antibody to localize antigen by electron microscopy. Nature. 1960;187:1094–1095. doi: 10.1038/1871094a0. [DOI] [PubMed] [Google Scholar]
- 9.Koido S, Nikrui N, Ohana M, Xia J, Tanaka Y, Liu C, Durfee JK, Lerner A, Gong J. Assessment of fusion cells from patient-derived ovarian carcinoma cells and dendritic cells as a vaccine for clinical use. Gynecol Oncol. 2005;99:462–471. doi: 10.1016/j.ygyno.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Homma S, Kikuchi T, Ishiji N, Ochiai K, Takeyama H, Saotome H, Sagawa Y, Hara E, Kufe D, Ryan JL, et al. Cancer immunotherapy by fusions of dendritic and tumour cells and rh-IL-12. Eur J Clin Invest. 2005;35:279–286. doi: 10.1111/j.1365-2362.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 11.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: Mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer J. 2003;104:92–97. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 14.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 15.Blyth K, Vaillant F, Jenkins A, McDonald L, Pringle MA, Huser C, Stein T, Neil J, Cameron ER. Runx2 in normal tissues and cancer cells: A developing story. Blood Cells Mol Dis. 2010;45:117–123. doi: 10.1016/j.bcmd.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Pratap J, Lian JB, Stein GS. Metastatic bone disease: Role of transcription factors and future targets. Bone. 2011;48:30–36. doi: 10.1016/j.bone.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shore P. A role for Runx2 in normal mammary gland and breast cancer bone metastasis. J Cell Biochem. 2010;96:484–489. doi: 10.1002/jcb.20557. [DOI] [PubMed] [Google Scholar]
- 18.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 19.Owens TW, Rogers RL, Best S, Ledger A, Mooney AM, Ferguson A, Shore P, Swarbrick A, Ormandy CJ, Simpson PT, et al. Runx2 is a novel regulator of mammary epithelial cell fate in development and breast cancer. Cancer Res. 2014;74:5277–5286. doi: 10.1158/0008-5472.CAN-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratap J, Imbalzano KM, Underwood JM, Cohet N, Gokul K, Akech J, van Wijnen AJ, Stein JL, Imbalzano AN, Nickerson JA, et al. Ectopic runx2 expression in mammary epithelial cells disrupts formation of normal acini structure: Implications for breast cancer progression. Cancer Res. 2009;69:6807–6814. doi: 10.1158/0008-5472.CAN-09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki T, Wu D, Sugimoto H, Nagase H, Nakagawara A. Runt-related transcription factor 2 (RUNX2) inhibits p53-dependent apoptosis through the collaboration with HDAC6 in response to DNA damage. Cell Death Disease. 2013;4:e610. doi: 10.1038/cddis.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza-Villanueva D, Deng W, Lopez-Camacho C, Shore P. The Runx transcriptional co-activator, CBFbeta, is essential for invasion of breast cancer cells. Mol Cancer. 2010;9:171. doi: 10.1186/1476-4598-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, Lian JB. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol. 2005;25:8581–8591. doi: 10.1128/MCB.25.19.8581-8591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvamurugan N, Kwok S, Partridge NC. Smad3 interacts with JunB and Cbfa1/Runx2 for transforming growth factor-beta1-stimulated collagenase-3 expression in human breast cancer cells. J Biol Chem. 2004;279:27764–27773. doi: 10.1074/jbc.M312870200. [DOI] [PubMed] [Google Scholar]
- 25.Pratap J, Wixted JJ, Gaur T, Zaidi SK, Dobson J, Gokul KD, Hussain S, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Runx2 transcriptional activation of Indian Hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 2008;68:7795–7802. doi: 10.1158/0008-5472.CAN-08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalid O, Baniwal SK, Purcell DJ, Leclerc N, Gabet Y, Stallcup MR, Coetzee GA, Frenkel B. Modulation of Runx2 activity by estrogen receptor-alpha: Implications for osteoporosis and breast cancer. Endocrinology. 2008;149:5984–5995. doi: 10.1210/en.2008-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chimge NO, Baniwal SK, Luo J, Coetzee S, Khalid O, Berman BP, Tripathy D, Ellis MJ, Frenkel B. Opposing effects of Runx2 and estradiol on breast cancer cell proliferation: In vitro identification of reciprocally regulated gene signature related to clinical letrozole responsiveness. Clin Cancer Res. 2012;18:901–911. doi: 10.1158/1078-0432.CCR-11-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Vitolo M, Passaniti A. Runt-related gene 2 in endothelial cells: Inducible expression and specific regulation of cell migration and invasion. Cancer Res. 2001;61:4994–5001. [PubMed] [Google Scholar]
- 29.Pierce AD, Anglin IE, Vitolo MI, Mochin MT, Underwood KF, Goldblum SE, Kommineni S, Passaniti A. Glucose-activated RUNX2 phosphorylation promotes endothelial cell proliferation and an angiogenic phenotype. J Cell Biochem. 2012;113:282–292. doi: 10.1002/jcb.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chimge NO, Baniwal SK, Little GH, Chen Y, Kahn M, Tripathy D, Borok Z, Frenkel B. Regulation of breast cancer metastasis by Runx2 and estrogen signaling: The role of SNAI2. Breast Cancer Res. 2011;13:R127. doi: 10.1186/bcr3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 32.Das K, Leong DT, Gupta A, Shen L, Putti T, Stein GS, van Wijnen AJ, Salto-Tellez M. Positive association between nuclear Runx2 and oestrogen-progesterone receptor gene expression characterises a biological subtype of breast cancer. Eur J Cancer. 2009;45:2239–2248. doi: 10.1016/j.ejca.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito K, Inoue M, Putti TC, Loh M, Ko TK, Huang C, et al. Runx3 is frequently inactivated by dual mechanisms of protein mislocalization and promoter hypermethylation in breast cancer. Cancer Res. 2006;66:6512–6520. doi: 10.1158/0008-5472.CAN-06-0369. [DOI] [PubMed] [Google Scholar]
- 34.Tandon M, Chen Z, Pratap J. Runx2 activates PI3K/Akt signaling via mTORC2 regulation in invasive breast cancer cells. Breast Cancer Res. 2014;16:R16. doi: 10.1186/bcr3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.McDonald L, Ferrari N, Terry A, Bell M, Mohammed ZM, Orange C, Jenkins A, Muller WJ, Gusterson BA, Neil JC, et al. RUNX2 correlates with subtype-specific breast cancer in a human tissue microarray, and ectopic expression of Runx2 perturbs differentiation in the mouse mammary gland. Dis Model Mech. 2014;7:525–534. doi: 10.1242/dmm.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraja GM, Othman M, Fox BP, Alsaber R, Pellegrino CM, Zeng Y, Khanna R, Tamburini P, Swaroop A, Kandpal RP. Gene expression signatures and biomarkers of noninvasive and invasive breast cancer cells: Comprehensive profiles by representational difference analysis, microarrays and proteomics. Oncogene. 2006;25:2328–2338. doi: 10.1038/sj.onc.1209265. [DOI] [PubMed] [Google Scholar]
- 39.Barnes GL, Javed A, Waller SM, Kamal MH, Hebert KE, Hassan MQ, Bellahcene A, Van Wijnen AJ, Young MF, Lian JB, et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63:2631–2637. [PubMed] [Google Scholar]
- 40.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillenbrand EE, Neville AM, Coventry BJ. Immunohistochemical localization of CD1a-positive putative dendritic cells in human breast tumours. Br J Cancer. 1999;79:940–944. doi: 10.1038/sj.bjc.6690150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 43.Hegde NR, Chevalier MS, Johnson DC. Viral inhibition of MHC class II antigen presentation. Trends Immunol. 2003;24:278–285. doi: 10.1016/S1471-4906(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 44.Alcami A, Ghazal P, Yewdell JW. Viruses in control of the immune system. Workshop on molecular mechanisms of immune modulation: Lessons from viruses. EMBO Rep. 2002;3:927–932. doi: 10.1093/embo-reports/kvf200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yewdell JW, Hill AB. Viral interference with antigen presentation. Nature Immunol. 2002;3:1019–1025. doi: 10.1038/ni1102-1019. [DOI] [PubMed] [Google Scholar]
- 46.Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/S0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- 47.Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, Logar AJ, Robbins PD, Falo LD, Thomson AW. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/JVI.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang ML, Lukens JR, Bullock TN. Cognate memory CD4+ T cells generated with dendritic cell priming influence the expansion, trafficking, and differentiation of secondary CD8+ T cells and enhance tumor control. J Immunol. 2007;179:5829–5838. doi: 10.4049/jimmunol.179.9.5829. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romagnani S. Human TH1 and TH2 subsets: Doubt no more. Immunol Today. 1991;12:256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 52.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 Polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 53.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang H, Hao S, Li F, Ye Z, Yang J, Xiang J. CD4+ Th1 cells promote CD8+ Tc1 cell survival, memory response, tumor localizationand therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007;120:148–159. doi: 10.1111/j.1365-2567.2006.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savai R, Schermuly RT, Pullamsetti SS, Schneider M, Greschus S, Ghofrani HA, Traupe H, Grimminger F, Banat GA. A combination hybrid-based vaccination/adoptive cellular therapy to prevent tumor growth by involvement of T cells. Cancer Res. 2007;67:5443–5453. doi: 10.1158/0008-5472.CAN-06-3677. [DOI] [PubMed] [Google Scholar]
- 57.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 58.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 59.Zobywalski A, Javorovic M, Frankenberger B, Pohla H, Kremmer E, Bigalke I, Schendel DJ. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med. 2007;5:18. doi: 10.1186/1479-5876-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang P, Yi S, Li X, Liu R, Jiang H, Huang Z, Liu Y, Wu J, Huang Y. Preparation of triple-negative breast cancer vaccine through electrofusion with day-3 dendritic cells. PLoS One. 2014;9:e102197. doi: 10.1371/journal.pone.0102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


