Abstract
Gastric cancer can be a fatal tumor and therefore represents one of the primary challenges in modern oncology. Survivin and X-linked inhibitor of apoptosis protein (XIAP) are members of the IAP family, which exerts a strong inhibitory effect on cellular apoptosis. In previous studies, the expression levels of survivin and XIAP have been demonstrated to influence the prognosis of patients with gastric cancer; therefore, the present study investigated the effect of silencing survivin and XIAP on the biological activity of the gastric cancer HGC-27 cell line. It was demonstrated that the expression levels of survivin and XIAP were significantly increased in gastric cancer tissues, compared with the adjacent non-tumor tissues. Furthermore, it was observed that the expression levels of survivin and XIAP were similarly elevated in gastric cancer HGC-27 cells, compared with normal gastric epithelial GES-1cells. Furthermore, small interfering RNA-mediated surviving- or XIAP-knockdown, in addition to the dual knockdown of survivin and XIAP, inhibited the proliferation and promoted the apoptosis of HGC-27 cells. Simultaneous inhibition of XIAP and survivin expression was more effective, compared with inhibition of XIAP or survivin alone. These results indicated that the dual knockdown of survivin and XIAP may be an effective strategy for treating gastric cancer in the future.
Keywords: gastric cancer, X-linked inhibitor of apoptosis protein, survivin, HGC-27, proliferation, apoptosis
Introduction
Gastric cancer is the second leading cause of cancer-associated mortality globally (1). Although improvements have been made in the diagnosis and treatment of patients with early stage gastric cancer, the prognosis of patients with advanced stages of disease remains poor (2). Surgery is the primary curative treatment for gastric cancer (3). Therapeutic endoscopy, surgery, radiotherapy, chemotherapy, interventional therapy, biotherapy and immunotherapy provide limited benefits to patients diagnosed with an advanced stage of gastric cancer (4); therefore, it is necessary to investigate novel avenues in the treatment of gastric cancer, including gene therapy, to determine a feasible strategy.
Gastric cancer is a complex disease with multiple genetic aberrations; therefore, a single gene approach to the development of novel therapies may not be as effective as a multiple gene approach. Resistance to apoptosis is a common phenomenon in malignant tumors (5,6). Previously, numerous signaling molecules involved in the regulation of apoptosis and the proliferation of tumor cells have been identified (7), and of these, the proteins of the inhibitor of apoptosis (IAP) family exert a strong inhibitory effect on cellular apoptosis. Thus far, 8 types of apoptosis inhibitory proteins have been identified in the IAP family, namely X-linked IAP (XIAP), survivin, IAP-like protein 2, cellular IAP (cIAP) 1, cIAP2, baculoviral IAP repeat-containing ubiquitin-conjugating enzyme, NLR family apoptosis inhibitory protein and melanoma-IAP (8). It has been demonstrated that a poor prognosis is associated with increased survivin and XIAP expression levels in patients with gastric cancer (9,10); therefore, survivin and XIAP are considered to be promising targets for anticancer therapy (11–13).
The survivin gene was isolated and identified in 1997 (14). Since then, studies (15,16) have determined that it has a role in the inhibition of apoptosis and the regulation of cell proliferation. Furthermore, its overexpression has been observed in numerous human malignant tumor types, including gastric cancer (17–21). XIAP was first identified in 1996 (22), and it has been identified as an IAP that may be induced by various triggers (23). Additionally, it may be the most potent member of the IAP gene family (24). Following its induction, XIAP binds to and inhibits the activity of caspases 3, 7 and 9, and ultimately suppresses caspase-mediated cellular apoptosis (25).
In addition, it has been demonstrated that survivin and XIAP form a heterocomplex when apoptosis is induced in vivo (26). This interaction promotes cell survival, due to it enhancing the stability of XIAP and its resistance to proteasomal degradation. This interaction is involved in the reduction of apoptosome-mediated cell death, an effect that is abolished in XIAP−/−cells; therefore, the dual knockdown of survivin and XIAP may result in enhanced apoptosis that is more prominent, compared with the knockdown of a single gene, in gastric cancer cells.
The present study investigated the effect of the knockdown of survivin and XIAP on the apoptosis and growth of the gastric cancer HGC-27 cell line. The results demonstrated that the simultaneous inhibition of survivin and XIAP expression may be a potential target for the development of novel gastric cancer treatments for clinical application.
Materials and methods
Tissue samples
A total of 144 patients underwent surgical resection for gastric cancer between May 2016 and March 2017 at the Third Affiliated Hospital of Harbin Medical University (Harbin, China). There were 87 male patients and 57 female patients, ranging in age from 23 to 75 years with a mean age of 52 years old. All these cases were collected for the detection of clinical characteristics, whilst, 26 gastric cancer and matched peritumoral tissue samples were randomly collected from the 144 for further mRNA and protein of XIAP and survivin analysis. All these cases were diagnosed by pathologists in the Department of Pathology of the Third Affiliated Hospital of Harbin Medical University (Harbin, China) who were blinded to the study and no patients had received radiotherapy, chemotherapy or adjuvant therapy prior to surgery. Data of the clinicopathological features of the patients were collected from surgical and pathological reports based on the Tumor-Node-Metastasis staging system (27). The present study was approved by the Ethical Committee of the Third Affiliated Hospital of Harbin Medical University and written informed consent was obtained from all patients.
Immunohistochemical staining and analysis
The tissues were fixed with 4% paraformaldehyde for 24 h at room temperature. Immunohistochemical staining of the 4-µm thick sections of paraffin-embedded human gastric cancer tissues and matched peritumoral tissue samples were performed by the Department of Pathology of the Third Affiliated Hospital of Harbin Medical University (Harbin, China). In brief, following deparaffinization in xylene at room temperature, and then rehydrated in graded concentrations of ethyl alcohol (100, 95 and 75%), and antigen retrieval at 95°C for 32 min, the sections were blocked with 10% bovine serum albumin (Beyotime Institute of Biotechnology, Shanghai, China) in PBS for 60 min at room temperature and incubated with anti-XIAP (1:500; cat no. ab2541) and anti-survivin (1:500; cat no. ab469; both Abcam, Cambridge, UK) overnight at 4°C. Subsequently, the sections were washed 3 times in PBS and incubated with a horseradish peroxidase-conjugated Mouse Anti-rabbit IgG secondary antibody (1:200; cat no. bs-0295M-HRP; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) for 30 min at 37°C. Immunocomplexes were detected with 3,3′-diaminobenzidine (Beyotime Institute of Biotechnology). Finally, the sections were counterstained with hematoxylin for 3 min at room temperature, rehydrated in a descending series of ethanol (85% for 2 min, 95% for 2 min and 100% for 5 min), finally mounted using xylene for 1 min examined under the light microscope (E100; magnification, ×400; Nikon Corporation, Tokyo, Japan).
Cell culture
The gastric cancer HGC-27 cell line was provided by Dr Qiang Yao from Harbin Medical University (Harbin, China), which was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The human gastric mucosal GES-1 and gastric cancer MGC-803 cell lines were obtained from the Genetics Laboratory of Harbin Medical University. All these cell lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin-G and 100 µg/ml streptomycin. The cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C. Cells were digested with 0.25% pancreatin for subculture 2–3 times/week.
Survivin and XIAP small interfering (si)RNA-mediated knockdown
Previous studies demonstrated that siRNA has the ability to mediate the intracellular degradation of targeted mRNA, thereby affecting the expression of targeted mRNA at the translational level (28,29). According to the gene sequences of survivin and XIAP, specific siRNA and non-sense siRNA were designed and synthesized in vitro (Invitrogen; Thermo Fisher Scientific, Inc.) as follows: Survivin siRNA (+), 5′-CACCGCAUCUCUACAUUCATTAdTdT-3′; XIAP siRNA (+), 5′-GCAGGUUGUAGAUAUAUCATTAdTdT-3′; and control non-sense siRNA (−), 5′-UUCUCCGAACGUGUCACGUTTAdTdT-3′. HGC-27 cells were harvested and counted one day prior to transfection and seeded overnight at 37°C at a concentration of 8×103 cells/well on a 96-well plate (used for the cell proliferation assay) and 2×105 cells/well on a 24-well plate (for all other assays). Transfection was performed using 100 nM siRNA (for survivin and/or XIAP, 100 nM siRNA of each was used when the two were combined) and X-tremeGENE siRNA Transfection Reagent (Roche Diagnostics, Basel, Switzerland). After 24 h, the cells (transfected with si-survivin, si-XIAP, and a combination of si-survivin and XIAP) were collected.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The mRNA expression of survivin and XIAP from gastric cancer and matched peritumoral tissues was analyzed by RT-qPCR. Total RNA from tissues or HGC-27 cells was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Reverse transcription (Transcriptor First Strand cDNA Synthesis kit; cat no. 04 379 012 001; Roche Diagnostics GmbH, Mannheim, Germany) and PCR detection (FastStart Universal SYBR-Green Master; cat no. 04 673 484 001; Roche Diagnostics GmbH) were performed according to the kit protocols. The primer sequences used were as follows: Survivin forward, 5′-AGGACCACCGCATCTCTACAT-3′ and reverse, 5′-AAGTCTGGCTCGTTCTCAGTG-3′; XIAP forward, 5′-ACCGTGCGGTGGTGCTTTAGTT-3′ and reverse, 5′-TGCGTGGCACTATTTTCAAGATA-3′; and GAPDH forward, 5′-CATCTTCCAGGAGCGAGA-3′ and reverse, 5′-TGTTGTCATACTTCTCA-3′ (Invitrogen; Thermo Fisher Scientific, Inc.). The ABI7500 fast real-time qPCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to perform RT-qPCR. A total of 1 µl first-strand cDNA was used per 10 µl reaction volume. A total of 0.4 µl 50× ROX Reference Dye II (FastStart Universal SYBR-Green Master; cat no. 04 673 484 001; Roche Diagnostics GmbH), 0.4 µl survivin or XIAP upstream primer, 0.4 µl survivin or XIAP downstream primer and 6.8 µl deionized water was used. The amplification conditions were in accordance with the manufacturer's protocol of PCR detection (FastStart Universal SYBR-Green Master): Denaturation at 95°C for 5 min, followed by 40 amplification cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec (30). The 2−ΔΔCq method was used to determine the relative mRNA expression level (31). GAPDH amplification was consistent with that of survivin and XIAP.
Western blot analysis
Total proteins of HGC-27 cells were extracted in cold lysis buffer (Beyotime Institute of Biotechnology) containing 1 µl proteinase inhibitor per 1 ml. Protein quantity was assessed using the bicinchoninic acid assay (Beyotime Institute of Biotechnology, Haimen, China). Subsequently, proteins (100 µg) were mixed with 4× SDS buffer (Beyotime Institute of Biotechnology) and resolved by 15% SDS-PAGE electrophoresis at 100 V for 1 h. Following this, proteins were transferred onto polyvinylidene membranes, which were washed in 100% methanol for 40 sec. Membranes were then blocked with Tris buffered saline (TBS) containing 5% skimmed milk for 1 h at room temperature, and incubated overnight at 4°C with anti-human survivin primary antibody (1:2,000; cat. no. ab76424) and anti-human XIAP primary antibody (1:500; cat. no. ab21278) or anti-human Actin primary antibody (1:2,000; cat. no. ab8227) or anti-human GAPDH primary antibody (1:2,000; cat. no. ab8245; all Abcam) in TBS with 0.5% Tween-20 containing 5% skimmed milk. Following washing 3 times with PBS with 0.5% Tween-20, membranes were incubated with Goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:1,000; cat. no. TA130003; OriGene Technologies, Inc., Beijing, China) for 2 h at room temperature. Finally, the membranes were visualized using enhanced chemiluminescence reagent purchased from Beyotime Institute of Biotechnology (cat. no. P0018) and scanned using AlphaView image analysis software version 2.0 (ProteinSimple, San Jose, CA, USA).
Cellular apoptosis detection
The Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (Beyotime Institute of Biotechnology) was used to detect apoptosis. HGC-27 cells were detached using trypsin and collected 48 h after transfection with survivin and XIAP siRNA. Subsequently, the cells were washed twice with PBS and treated with 10 µl propidium iodide, 5 µl Annexin V-FITC and 195 µl V-FITC binding buffer for 20 min at room temperature, and protected from the light. A flow cytometer (Cytomics FC500 flow cytometer, Beckman Coulter Inc., Brea, CA, USA) and CXP Analysis software (version CR; Beckman Coulter Inc.) were used to analyze and quantify cells within 30 min of the incubation. The apoptosis rate was calculated using the following formula: Apoptotic rate= (number of apoptotic cells/total number of cells) ×100.
Cell Counting kit (CCK)-8 assay
HGC-27 cells were trypsinized, washed twice in PBS, seeded into 96-well plates at a concentration of 2×105 cells/ml and incubated at 37°C in serum-free RPMI-1640 medium for 12 h. The cells were divided into six groups: Untransfected cells grown in RPMI-1640 medium, cells transfected with control siRNA, cells transfected with survivin siRNA, cells transfected with XIAP siRNA, and cells transfected with survivin and XIAP siRNAs. Subsequently, HGC-27 cells were transfected with 6 µg survivin or XIAP siRNA (sequences as previously mentioned; initial concentration, 10 µM) and 5 µl X-tremeGENE siRNA Transfection reagent (Roche Diagnostics), and incubated for 24 h. For the control siRNA group, HGC-27 cells were transfected with scrambled sequence siRNA. A total of 10 µl CCK-8 solution (Beyotime Institute of Biotechnology) was added to each well and the mixture was incubated at 37°C for 1 h. A microplate reader was used to measure the absorbance at a wavelength of 450 nm. The inhibition rate was calculated using the following formula: Inhibition rate (%)=(Acontrol group-Aexperimental group)/(Acontrol group-Ablank group) ×100, where A is the absorbance, the control group is the cells transfected with control siRNA, the blank group is the untransfected cells grown in RPMI-1640 medium and the experimental group is the group being tested.
Bromodeoxyuridine (BrdU) incorporation analysis
Cell proliferation was concurrently analyzed using a BrdU assay kit (Cell Signaling Technology, Inc., Danvers, MA, USA). The steps were performed according to the manufacturer's protocol. In brief, HGC-27 cells were incubated at 37°C with 20 µM BrdU for 40 min prior to being harvested. Subsequently, the cells were treated with 1× fixing solution (cat no. 6813; Cell Signaling Technology, Inc.) at room temperature for 30 min, followed by incubation with anti-BrdU antibody (cat no. 6813; 1:1,000; Cell Signaling Technology, Inc.) at 37°C for 1 h. Finally, the optical absorbance was measured with a multimode microplate reader at dual wavelengths of 450/550 nm.
Statistical analysis
Statistical analysis was performed using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard error of the mean. Data in different groups were analyzed using one-way analysis of variance followed by the Student-Newman-Keuls post hoc test. The χ2 or Fisher's exact tests were performed to identify statistical differences in the clinicopathological variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between the survivin or XIAP protein expression level and clinicopathological factors of gastric cancer
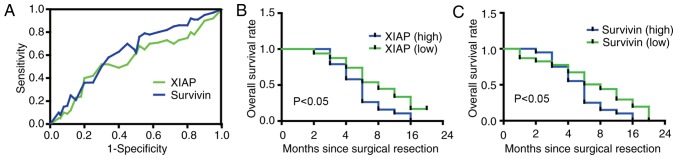
In the present study, the receiver operating characteristic (ROC) curves were produced to determine the cut-off values (negative or positive expression) of XIAP and surviving (32). Fig. 1A depicts the ROC curve of XIAP and survivin, which demonstrated that the cut-off for the XIAP expression levels was 0.6178 [95% confidence interval (CI), 0.4749–0.7607], whilst the cut-off for the survivin expression levels was 0.5433 (95% CI, 0.3948–0.6918). Fig. 1B depicts the survival curves used to assess the malignant potential of high and low XIAP expression; whilst Fig. 1C depicts the survival curves used to assess the malignant potential of high and low survivin expression. Clinical characteristics from 144 patients were all collected and recorded for subsequent analysis. The mean age of patients was 52 years (range, 23–75 years), 79.8% patients exhibited lymph node metastasis and the median tumor size was 5.8 cm. The results of the univariate analysis demonstrated that the XIAP expression level was significantly associated with tumor size, serosal invasion and lymph node metastasis (P<0.05; Table I), whilst the survivin expression level was significantly associated with tumor size and lymph node metastasis (P<0.05; Table II).
Figure 1.
Association between survivin/XIAP and clinicopathological factors of gastric cancer. (A) The receiver operating characteristics curves. The cutoffs for XIAP and survivin expression levels were 0.6178 and 0.5433, respectively. (B) The survival curve for patients with gastric cancer with different expression levels of XIAP (P<0.05). (C) The survival curve for patients with gastric cancer with different expression levels of survivin (P<0.05). XIAP, X-linked inhibitor of apoptosis protein.
Table I.
Association between XIAP expression levels and clinicopathological features of gastric cancer.
| XIAP expression | |||
|---|---|---|---|
| Variable | Low | High | P-value |
| Sex | 0.63a | ||
| Male | 20 | 56 | |
| Female | 16 | 52 | |
| Age, years | 0.70a | ||
| ≤50 | 19 | 30 | |
| >50 | 17 | 78 | |
| Location | 0.26b | ||
| Upper | 20 | 66 | |
| Middle | 11 | 22 | |
| Lower | 5 | 13 | |
| Whole | 0 | 7 | |
| Tumor size, cm | <0.01a | ||
| <5 | 13 | 18 | |
| ≥5 | 23 | 90 | |
| Histological type | 0.60b | ||
| WMD | 14 | 36 | |
| PD | 16 | 65 | |
| SRC | 4 | 2 | |
| MC | 2 | 5 | |
| Serosal invasion | <0.01a | ||
| Absent | 20 | 21 | |
| Present | 16 | 87 | |
| Lymph node metastasis | 0.02a | ||
| Negative | 15 | 14 | |
| Positive | 21 | 94 | |
| Total | 36 | 108 | |
χ2
Fisher's exact test was used for statistical analysis. XIAP, X-linked inhibitor of apoptosis protein; WMD, well or moderately differentiated adenocarcinoma; PD, poorly differentiated adenocarcinoma; SRC, signet ring cell carcinoma; MC, mucinous adenocarcinoma.
Table II.
Association between survivin expression levels and clinicopathological features of gastric cancer.
| Survivin expression | |||
|---|---|---|---|
| Variable | Low | High | P-value |
| Sex | 0.70a | ||
| Male | 17 | 50 | |
| Female | 19 | 58 | |
| Age, years | 0.86a | ||
| ≤50 | 12 | 35 | |
| >50 | 24 | 73 | |
| Location | 0.11b | ||
| Upper | 18 | 54 | |
| Middle | 17 | 32 | |
| Lower | 0 | 13 | |
| Whole | 0 | 9 | |
| Tumor size, cm | <0.05a | ||
| <5 | 19 | 45 | |
| ≥5 | 17 | 63 | |
| Histological type | 0.84b | ||
| WMD | 8 | 45 | |
| PD | 20 | 58 | |
| SRC | 6 | 0 | |
| MC | 2 | 5 | |
| Serosal invasion | 0.93a | ||
| Absent | 18 | 57 | |
| Present | 18 | 51 | |
| Lymph node metastasis | 0.04a | ||
| Negative | 16 | 18 | |
| Positive | 22 | 90 | |
| Total | 36 | 108 | |
χ2
Fisher's exact test was used for statistical analysis. WMD, well or moderately differentiated adenocarcinoma; PD, poorly differentiated adenocarcinoma; SRC, signet ring cell carcinoma; MC, mucinous adenocarcinoma.
Expression levels of surviving or XIAP in vivo and in vitro
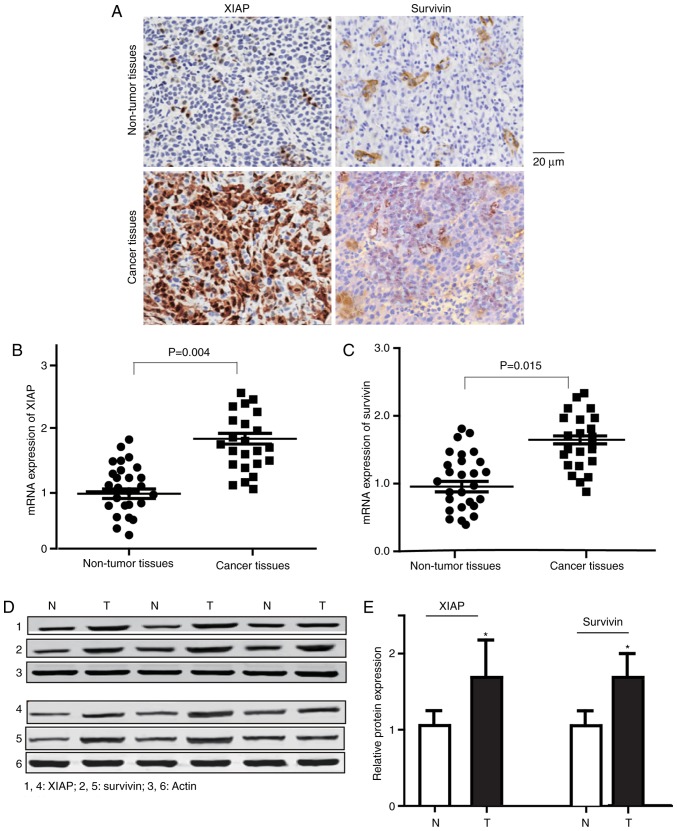
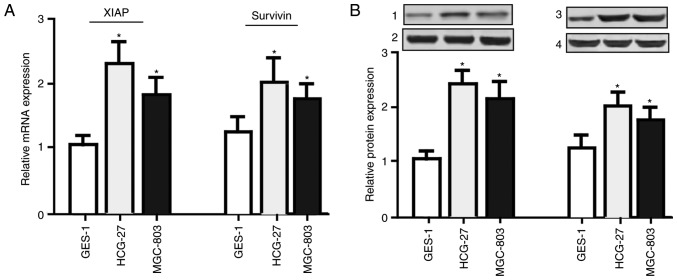
In order to detect the expression levels of survivin and XIAP, RT-qPCR and western blotting were performed. As presented in Fig. 2, increased expression levels of survivin and XIAP were observed in gastric cancer tissues, compared with the matched non-tumor tissues (mRNA, P<0.01; protein, P<0.05). Additionally, the expression levels of survivin and XIAP were significantly elevated (Fig. 3) in the HGC-27 and MGC803 gastric cancer cells, compared with the GES-1 normal gastric epithelial cells (P<0.05).
Figure 2.
Expression levels of survivin and XIAP in gastric cancer tissues, compared with matched non-tumor tissues. (A) Representative immunohistochemical stain results of XIAP and survivin expression in gastric carcinoma tissues and non-tumor tissues. (B) mRNA expression levels of XIAP in gastric cancer and non-tumor tissues. P<0.01. (C) mRNA expression levels of survivin in gastric cancer and non-tumor tissues. P<0.05. (D) Representative western blot analysis results of survivin and XIAP in gastric cancer and non-tumor tissues (n=26). (E) Bar graph of the relative survivin and XIAP protein expression levels. The protein expression levels of survivin and XIAP were significantly elevated in gastric cancer tissues compared with non-tumor tissues. Data are expressed as the mean ± standard error of the mean. *P<0.05 vs. non-tumor tissues (one-way analysis of variance with the Student-Newman-Keuls post hoc test). XIAP, X-linked inhibitor of apoptosis protein; N, non-tumor tissues; T, tumor tissues.
Figure 3.
Expression levels of survivin and XIAP in HGC-27 and MGC803 gastric cancer cells, and normal gastric epithelial GES-1 cells. (A) mRNA expression levels of survivin and XIAP were increased in the HGC-27 and MGC803 gastric cancer cell lines, compared with the normal gastric epithelial GES-1 cells, as determined by reverse transcription-quantitative polymerase chain reaction. (B) Protein expression levels of survivin and XIAP were increased in the HGC-27 and MGC803 gastric cancer cell lines, compared with the normal gastric epithelial GES-1 cells, as determined by western blotting. 1, XIAP; 2, Actin; 3, survivin; 4, Actin. Data are expressed as the mean ± standard error of the mean. n=26. *P<0.05 vs. GES-1 group (one-way analysis of variance with the Student-Newman-Keuls post hoc test). XIAP, X-linked inhibitor of apoptosis protein.
Knockdown of survivin and/or XIAP in HGC-27 cells
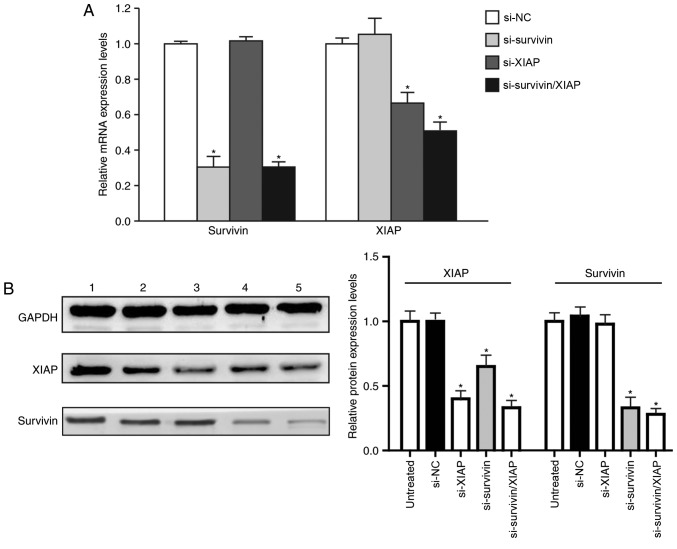
The siRNA-mediated surviving- and XIAP-knockdown was evaluated by RT-qPCR and western blotting. The mRNA expression of survivin was significantly reduced in the HGC-27 cells transfected with survivin siRNA, in addition to those transfected with survivin/XIAP siRNA (P<0.05). Compared with the negative control group, the survivin mRNA expression was reduced by 65.31 and 69.44% in these two groups, respectively (P<0.05). The expression level of XIAP was also significantly reduced in cells transfected with XIAP siRNA, in addition to those transfected with survivin/XIAP siRNA, by 33.38 and 49.01%, respectively, compared with the negative control group (P<0.05). These results indicated that survivin siRNA, XIAP siRNA and survivin/XIAP siRNA were effective in silencing survivin and/or XIAP expression in HGC-27 cells. Compared with the negative control group, the expression of survivin in the XIAP siRNA-transfected cells was not significantly altered. Furthermore, the expression of XIAP in cells transfected with survivin siRNA remained unaltered (Fig. 4A).
Figure 4.
Knockdown of survivin and/or XIAP in HGC-27 cells. (A) Reverse transcription-quantitative polymerase chain reaction analysis of survivin and XIAP mRNA expression in HGC-27 cells transfected with control non-sense, survivin, XIAP or survivin/XIAP siRNA. (B) Western blot analysis of XIAP, survivin and GAPDH protein expression levels. Lane 1, untreated HGC-27 cells; lane 2, control nonsense siRNA-treated HGC-27 cells; lane 3, HGC-27 cells transfected with XIAP siRNA; lane 4, HGC-27 cells transfected with survivin siRNA; and lane 5, HGC-27 cells transfected with survivin/XIAP siRNA (n=3). Data are expressed as the mean ± standard error of the mean. *P<0.05 vs. control group (one-way analysis of variance). XIAP, X-linked inhibitor of apoptosis protein; si, small interfering RNA; NC, negative control (transfected with control non-sense siRNA).
The protein expression levels of survivin and XIAP exhibited no significant difference between the blank control and negative control groups; however, in the survivin siRNA- and survivin/XIAP siRNA-transfected cells, the survivin expression was significantly reduced (P<0.05). In the XIAP siRNA- and survivin/XIAP siRNA-transfected cells, the XIAP expression was also significantly reduced (P<0.05). Additionally, the XIAP protein expression was also reduced in the survivin siRNA-transfected cells, indicating that survivin siRNA reduced XIAP expression at the protein level, which implied that survivin-mediated pathophysiological alterations in the HGC-27 cells were partially associated with XIAP (Fig. 4B).
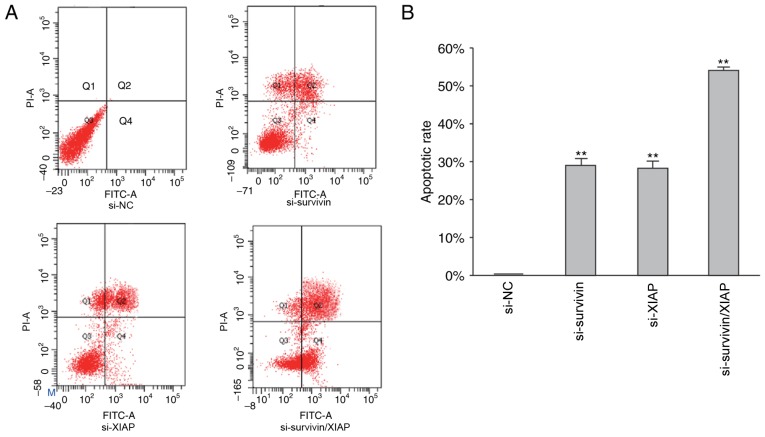
Dual knockdown of survivin and XIAP further promotes cellular apoptosis
Following siRNA-mediated survivin- and XIAP-knockdown, cellular apoptosis was examined using flow cytometry. Apoptosis was notably increased in survivin siRNA-, XIAP siRNA-and survivin/XIAP siRNA-transfected cells. As demonstrated in Fig. 5, in the cells transfected with survivin, XIAP and survivin/XIAP siRNA, the apoptotic rates were 28.29, 26.68 and 53.59%, respectively. These results indicated that dual knockdown of survivin and XIAP promoted cellular apoptosis to a greater extent than the knockdown of survivin or XIAP alone. As demonstrated in Fig. 4B, the knockdown of survivin simultaneously reduced XIAP protein expression, which indicated that unknown crosstalk between survivin and XIAP may be involved in the process of cellular apoptosis.
Figure 5.
Dual knockdown of survivin and XIAP further promotes cellular apoptosis. (A) Apoptosis of HGC-27 cells in different transfection groups and (B) the corresponding statistical results. Data are expressed as the mean ± standard error of the mean. **P<0.01 vs. NC group (one-way analysis of variance with the Student-Newman-Keuls post hoc test), n=3. XIAP, X-linked inhibitor of apoptosis protein; si, small interfering RNA; FITC, fluorescein isothiocyanate; NC, negative control (transfected with control non-sense siRNA); PI, propidium iodide.
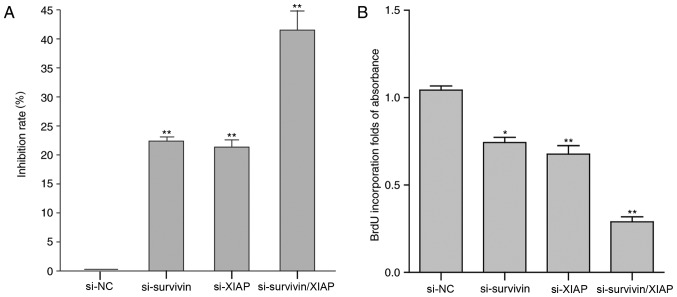
Dual knockdown of survivin and XIAP further suppresses cell proliferation
Following siRNA-mediated survivin-and XIAP-knockdown, cell viability and proliferation were examined using a CCK-8 kit and BrdU incorporation analysis. Cell proliferation was significantly inhibited following cell transfection with survivin siRNA, XIAP siRNA or survivin/XIAP siRNA (P<0.05). The CCK-8 assay results indicated that in the cells transfected with survivin, XIAP and survivin/XIAP siRNAs, the inhibition rates were 22.01, 21.01 and 40.39%, respectively (Fig. 6A). Furthermore, cell proliferation was determined by BrdU incorporation analysis. The results were in accordance with the results of the CCK-8 assay (Fig. 6B). Dual knockdown of survivin and XIAP suppressed cell proliferation to a greater extent (41.12%), compared with the knockdown of survivin (25.37%) or XIAP (27.11%) alone. These data indicated that dual knockdown of these two genes may be a potential target for the treatment of gastric cancer in the future.
Figure 6.
Dual knockdown of survivin and XIAP further suppresses cell proliferation. (A) Effect of siRNA-mediated knockdown of survivin and/or XIAP on HGC-27 cell viability. (B) Proliferation of cells transfected with survivin, XIAP or survivin/XIAP siRNAs. Data are expressed as the mean ± standard error of the mean. n=3. *P<0.05, **P<0.01 vs. NC group (one-way analysis of variance with the Student-Newman-Keuls post hoc test). XIAP, X-linked inhibitor of apoptosis protein; si, small interfering RNA; NC, negative control (transfected with control nonsense siRNA); BrdU, bromodeoxyuridine.
Discussion
Resistance to apoptotic signals in malignant cells is one of the most important factors in the failure of anticancer therapy in clinical settings (33); therefore, inhibition of cancer cell apoptosis has important biological and clinical significance (34,35). Therapeutic targeting of antagonists of the XIAP-survivin complex may be used as a potentially beneficial tool against apoptosis resistance in tumors, and to protect cancer cells from inactivation by ubiquitination or protease hydrolysis (36). Inhibition of survivin and/or XIAP expression may have potential therapeutic effects in gastric cancer. The results of the present study indicated that multi-target treatment options may be effective strategies for the treatment of gastric cancer in a clinical setting.
The results of the present study demonstrated that the expression levels of survivin and XIAP were elevated in vivo and in vitro, which is consistent with the results of a previous study (37). Furthermore, HGC-27 cells were transfected with siRNA targeting surviving and/or XIAP. Following transfection, expression levels were markedly reduced at the mRNA and protein levels. In XIAP-siRNA transfected cells, the expression of survivin mRNA did not change, compared with the negative control (non-sense siRNA-treated) cells. Similar results were observed for XIAP mRNA expression in survivin siRNA-transfected cells. These results indicated that silencing of survivin or XIAP had no influence on the mRNA expression of the other gene in HGC-27 cells; however, the expression of survivin mRNA in survivin siRNA-transfected cells was decreased, compared with survivin/XIAP siRNA-transfected cells. Additionally, XIAP mRNA expression was increased in XIAP siRNA-transfected cells, compared with survivin/XIAP siRNA-transfected cells. These results indicated that survivin and XIAP may be regulated through different mechanisms, and that a potential crosstalk may exist between these apoptotic regulatory signaling pathways.
The results of the western blot analysis revealed that the expression of XIAP was also reduced in survivin siRNA-transfected cells. This result indicated that survivin siRNA reduced XIAP expression at the protein level, via indirect mechanisms, which require further investigation. The results of flow cytometry indicated that the proportion of apoptotic cells was significantly increased in cells transfected with survivin, XIAP and survivin/XIAP siRNA, compared with the negative control cells. In addition, the apoptosis rate was increased in survivin/XIAP siRNA-transfected cells, compared with survivin siRNA- or XIAP siRNA-transfected cells. The results of the CCK-8 assay and BrdU incorporation analysis demonstrated that in survivin siRNA-, XIAP siRNA- and survivin/XIAP siRNA-transfected cells, cell growth was inhibited. In survivin/XIAP siRNA-transfected cells, the growth inhibition rate was significantly increased, compared with cells transfected with survivin or XIAP siRNAs. This maybe due to the reduced formation of the survivin/XIAP heterocomplex in response to cell death stimulation, and the potential crosstalk between these two IAPs (28). Furthermore, a survivin/XIAP complex reciprocally controls survivin stability and, as a XIAP-associated molecule, XIAP-associated factor-1 promotes proteasomal degradation of the protein (38); therefore, there may be an enhanced therapeutic benefit when the two factors are inhibited simultaneously (39). It was hypothesized that simultaneous inhibition of XIAP and survivin may strongly induce the apoptosis of HGC-27 cells and inhibit their growth by unknown mechanisms.
Previous studies (40–42) have demonstrated that targeting of XIAP and survivin inhibits the growth of various cancer cell types in vitro, and that the apoptotic fraction is enhanced by dual knockdown of survivin and XIAP. Preclinical studies in lung cancer have indicated that a high expression of XIAP and survivin is associated with an advanced clinical stage and a poor prognosis, whereas inhibition of XIAP and survivin expression increases the sensitivity of cancer cells to radiotherapy and chemotherapy (43–45). Further studies have indicated that survivin and XIAP regulate the response of colorectal cancer cells to radiation (39), and simultaneous inhibition of XIAP and survivin markedly decreases the proliferation of pancreatic cells (46).
There are a number of limitations to the present study; for example, there maybe other signaling pathways involved in the process of inhibiting survivin and XIAP expression levels that are unknown. Additionally, there are currently no inhibitors of XIAP and survivin available; therefore, the prognostic impact and treatment response from the clinic could not be measured. Thus, further in vitro and in vivo experiment should be performed in the future.
In conclusion, the results of the present study demonstrated that simultaneous inhibition of survivin and XIAP expression induces the apoptosis of HGC-27 cells, and inhibits cell growth to a greater extent, compared with the inhibition of XIAP or survivin alone. Therefore, simultaneous inhibition of survivin and XIAP expression may be a beneficial target for the development of novel gastric cancer treatments.
Acknowledgements
The authors would like to thank Professor Li Hui (Department of Genetics, Harbin Medical University, Harbin, China) for providing experimental guidance.
Funding
The present study was supported by Harbin Technological Innovation Special Fund Research Projects (grant no. 2014RFXGJ044). The funding source had no role in the study design, collection, analysis or interpretation of data, in writing the report or in the decision to submit the article for publication.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YL was a major contributor in designing the research and writing the manuscript. WG was a major contributor in conducting the western blotting and RT-qPCR. YM was a major contributor in conducting the immunohistochemistry. GZ was a major contributor in conducting the cell culture, transfection and flow cytometry. FC was a major contributor in designing the research and conducting the statistical analysis. HQ was a major contributor in designing the study and providing the experimental funding.
Ethics approval and consent to participate
All procedures involving human participants in the present study were performed in accordance with the ethical standards of the Ethical Committee of Harbin Medical University. The present study was approved by the Ethical Committee of the Third Affiliated Hospital of Harbin Medical University and written informed consent was obtained from all patients.
Patient consent for publication
All the patients recruited in the present study provided written informed consent for the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ueda SM, Mao TL, Kuhajda FP, Vasoontara C, Giuntoli RL, Bristow RE, Kurman RJ, Shih IeM. Trophoblastic neoplasms express fatty acid synthase, which may be a therapeutic target via its inhibitor C93. Am J Pathol. 2009;175:2618–2624. doi: 10.2353/ajpath.2009.081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian J, Qian Y, Wang J, Gu B, Pei D, He S, Zhu F, Røe OD, Xu J, Liu L, et al. A clinical prognostic scoring system for resectable gastric cancer to predict survival and benefit from paclitaxel-or oxaliplatin-based adjuvant chemotherapy. Drug Des Devel Ther. 2016;10:241–258. doi: 10.2147/DDDT.S88743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: The way forward. Cancer Treat Rev. 2014;40:692–700. doi: 10.1016/j.ctrv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Marrero Y, Spinner S, Kaufmann T, Jost PJ. Survival control of malignant lymphocytes by anti-apoptotic MCL-1. Leukemia. 2016;30:2152–2159. doi: 10.1038/leu.2016.213. [DOI] [PubMed] [Google Scholar]
- 6.Perini GF, Ribeiro GN, Neto Pinto JV, Campos LT, Hamerschlak N. BCL-2 as therapeutic target for hematological malignancies. J Hematol Oncol. 2018;11:65. doi: 10.1186/s13045-018-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang XL, Cui GH, Zhou KY. Correlation of PI3K-Akt signal pathway to apoptosis of tumor cells. Ai Zheng. 2008;27:331–336. (In Chinese) [PubMed] [Google Scholar]
- 8.Urrutia R. The IAP: More international than ever. Pancreatology. 2009;9:III–IV. doi: 10.1159/000293602. [DOI] [PubMed] [Google Scholar]
- 9.Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG. Prognostic value of survivin in patients with gastric cancer: A systematic review with meta-analysis. PLoS One. 2013;8:e71930. doi: 10.1371/journal.pone.0071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MA, Lee HE, Lee HS, Yang HK, Kim WH. Expression of apoptosis-related proteins and its clinical implication in surgically resected gastric carcinoma. Virchows Arch. 2011;459:503–510. doi: 10.1007/s00428-011-1150-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Beitler JJ, Huang W, Chen G, Qian G, Magliocca K, Patel MR, Chen AY, Zhang J, Nannapaneni S, et al. Honokiol radiosensitizes squamous cell carcinoma of the head and neck by downregulation of survivin. Clin Cancer Res. 2018;24:858–869. doi: 10.1158/1078-0432.CCR-17-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/S1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 13.Werner TA, Tamkan-Olcek Y, Dizdar L, Riemer JC, Wolf A, Cupisti K, Verde PE, Knoefel WT, Krieg A. Survivin and XIAP: Two valuable biomarkers in medullary thyroid carcinoma. Br J Cancer. 2016;114:427–434. doi: 10.1038/bjc.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Krishnakumar S, Kanwar RK, Cheung CH, Kanwar JR. Clinical aspects for survivin: A crucial molecule for targeting drug-resistant cancers. Drug Discov Today. 2015;20:578–587. doi: 10.1016/j.drudis.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Bi Y, Zhang Y, Cui C, Ren L, Jiang X. Gene-silencing effects of anti-survivin siRNA delivered by RGDV-functionalized nanodiamond carrier in the breast carcinoma cell line MCF-7. Int J Nanomedicine. 2016;11:5771–5787. doi: 10.2147/IJN.S117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia Y, Hu R, Li P, Zheng Y, Wang Y, Ma X. DEC1 is required for anti-apoptotic activity of gastric cancer cells under hypoxia by promoting Survivin expression. Gastric Cancer. 2017 doi: 10.1007/s10120-017-0780-z. (Epub Ahead of Print) [DOI] [PubMed] [Google Scholar]
- 18.Tsuburaya A, Noguchi Y, Yoshikawa T, Saito A, Doi C, Okamoto T, Fukuzawa K. An anti-apoptosis gene, survivin and telomerase expression in gastric cancer. Hepatogastroenterology. 2002;49:1150–1152. [PubMed] [Google Scholar]
- 19.Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004;10:1984–1988. doi: 10.3748/wjg.v10.i13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XY, Wang MW, Wang GS, You WD. Expression of human anti-apoptotic gene survivin and its splice in normal human gastric tissue and gastric cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20:75–76. (In Chinese) [PubMed] [Google Scholar]
- 21.Cao W, Yang W, Li H, Lou G, Jiang J, Geng M, Xi W, Ren R, Qu Q, Jin X, et al. Using detection of survivin-expressing circulating tumor cells in peripheral blood to predict tumor recurrence following curative resection of gastric cancer. J Surg Oncol. 2011;103:110–115. doi: 10.1002/jso.21777. [DOI] [PubMed] [Google Scholar]
- 22.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE, MacKenzie A, Korneluk RG. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 23.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang DG, Sun YB, Ye F, Li W, Kharbuja P, Gao L, Zhang DY, Suo J. Anti-tumor activity of the X-linked inhibitor of apoptosis (XIAP) inhibitor embelin in gastric cancer cells. Mol Cell Biochem. 2014;386:143–152. doi: 10.1007/s11010-013-1853-x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell. 2001;8:613–621. doi: 10.1016/S1097-2765(01)00341-0. [DOI] [PubMed] [Google Scholar]
- 26.Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, et al. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- 27.Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678–1685. doi: 10.1245/s10434-013-3466-8. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Narang AS, Mahato RI. Subcellular fate and off-target effects of siRNA, shRNA, and miRNA. Pharm Res. 2011;28:2996–3015. doi: 10.1007/s11095-011-0608-1. [DOI] [PubMed] [Google Scholar]
- 29.Kaur IP, Chopra K, Rishi P, Puri S, Sharma G. Small RNAs: The qualified candidates for gene manipulation in diverse clinical pathologies. Crit Rev Ther Drug Carrier Syst. 2014;31:305–329. doi: 10.1615/CritRevTherDrugCarrierSyst.2014007943. [DOI] [PubMed] [Google Scholar]
- 30.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Xuan Q, Ji H, Tao X, Xu Y, Zhang Q. Quantitative assessment of HER2 amplification in HER2-positive breast cancer: Its association with clinical outcomes. Breast Cancer Res Treat. 2015;150:581–588. doi: 10.1007/s10549-015-3334-2. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Zhang W, Liu K, Wang Y, Ji B, Liu Y. Synergistic effects of co-expression plasmidbased ADAM10-specific siRNA and GRIM-19 on hepatocellular carcinoma in vitro and in vivo. Oncol Rep. 2014;32:2501–2510. doi: 10.3892/or.2014.3503. [DOI] [PubMed] [Google Scholar]
- 34.Fulda S. Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: Molecular mechanisms and therapeutic opportunities. Leukemia. 2014;28:1414–1422. doi: 10.1038/leu.2014.56. [DOI] [PubMed] [Google Scholar]
- 35.Chene P. Inhibiting the p53-MDM2 interaction: An important target for cancer therapy. Nat Rev Cancer. 2003;3:102–109. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- 36.Rajalingam K, Sharma M, Paland N, Hurwitz R, Thieck O, Oswald M, Machuy N, Rudel T. IAP-IAP complexes required for apoptosis resistance of C. Trachomatis-infected cells. PLoS Pathog. 2006;2:e114. doi: 10.1371/journal.ppat.0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatakeyama K, Yamakawa Y, Fukuda Y, Ohshima K, Wakabayashi-Nakao K, Sakura N, Tanizawa Y, Kinugasa Y, Yamaguchi K, Terashima M, Mochizuki T. A novel splice variant of XIAP-associated factor 1 (XAF1) is expressed in peripheral blood containing gastric cancer-derived circulating tumor cells. Gastric Cancer. 2015;18:751–761. doi: 10.1007/s10120-014-0426-3. [DOI] [PubMed] [Google Scholar]
- 38.Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282:26202–26209. doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- 39.Hehlgans S, Petraki C, Reichert S, Cordes N, Rodel C, Rodel F. Double targeting of Survivin and XIAP radiosensitizes 3D grown human colorectal tumor cells and decreases migration. Radiother Oncol. 2013;108:32–39. doi: 10.1016/j.radonc.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Ouyang J, Ouyang L, Chen Y. Inhibition of cell proliferation and increase of chemosensitivity by simultaneous knockdown of XIAP and survivin in pancreatic carcinoma cells. Oncol Res. 2013;21:43–50. doi: 10.3727/096504013X13793555706722. [DOI] [PubMed] [Google Scholar]
- 41.Lee SH, Lee JY, Jung CL, Bae IH, Suh KH, Ahn YG, Jin DH, Kim TW, Suh YA, Jang SJ. A novel antagonist to the inhibitors of apoptosis (IAPs) potentiates cell death in EGFR-overexpressing non-small-cell lung cancer cells. Cell Death Dis. 2014;5:e1477. doi: 10.1038/cddis.2014.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Moraes Nestal G, Delbue D, Silva KL, Robaina MC, Khongkow P, Gomes AR, Zona S, Crocamo S, Mencalha AL, Magalhães LM, et al. FOXM1 targets XIAP and Survivin to modulate breast cancer survival and chemoresistance. Cell Signal. 2015;27:2496–2505. doi: 10.1016/j.cellsig.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 44.Krepela E, Dankova P, Moravcikova E, Krepelova A, Prochazka J, Cermak J, Schützner J, Zatloukal P, Benkova K. Increased expression of inhibitor of apoptosis proteins, survivin and XIAP, in non-small cell lung carcinoma. Int J Oncol. 2009;35:1449–1462. doi: 10.3892/ijo_00000464. [DOI] [PubMed] [Google Scholar]
- 45.Yang D, Song X, Zhang J, Ye L, Wang S, Che X, Wang J, Zhang Z, Wang L, Shi W. Therapeutic potential of siRNA-mediated combined knockdown of the IAP genes (Livin, XIAP, and Survivin) on human bladder cancer T24 cells. Acta Biochim Biophys Sin (Shanghai) 2010;42:137–144. doi: 10.1093/abbs/gmp118. [DOI] [PubMed] [Google Scholar]
- 46.Yi XP, Han T, Li YX, Long XY, Li WZ. Simultaneous silencing of XIAP and survivin causes partial mesenchymal-epithelial transition of human pancreatic cancer cells via the PTEN/PI3K/Akt pathway. Mol Med Rep. 2015;12:601–608. doi: 10.3892/mmr.2015.3380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.