Abstract
Circular RNAs (circRNAs) may serve as biomarkers for a potentially non-invasive diagnosis of cancer. To understand their diagnostic performance, a systematic meta-analysis of the published literature was conducted to review the diagnostic efficiency of circRNAs in patients with cancer. Eligible studies published up to November 30, 2017, on PubMed and EMBASE, were selected for the meta-analysis. All studies were carefully and independently reviewed by two researchers based on their titles and abstracts, following which full texts were perused for potential eligibility. All statistical analyses were performed by STATA 13.0 statistical software and Meta-DiSc 1.4. A total of 10 eligible studies were included. The pooled diagnostic odds ratio was 7.265. The pooled sensitivity was 0.708 and the pooled specificity was 0.722. The positive likelihood and negative likelihood ratios were 2.483 and 0.372, respectively. The area under the curve was 0.793. circRNA was determined to be a notably effective assistant diagnostic biomarker for cancer.
Keywords: circular RNA, cancer, diagnostic value, meta-analysis
Introduction
Cancer is a major public health problem globally. Cancer is a class of diseases that undergoes uncontrollable cell proliferation and differentiation. Based on the 2015 cancer statistics, it is currently the second leading cause of mortality in numerous countries (including China, Europe and the USA), and is expected to surpass heart diseases as the leading cause of mortality in the near future (1). Although the risk of succumbing to cancer has decreased by ~20% from its maximum in 1991–2011 (1), it must be diagnosed with high sensitivity and specificity in order to determine the appropriate therapy and prognosis. Recently, a number of biomarkers with diagnostic and prognostic potential value have been demonstrated in numerous cancer types, including the tumor markers human epididymis secretory protein 4 and cancer antigen 125 in endometrial (2) and ovarian cancer types (3). Additionally, mutant genes have been used in the selection of an appropriate therapy, including epidermal growth factor receptor mutation in non-small cell lung cancer (4), Kirsten rat sarcoma viral oncogene homolog in colorectal cancer (5) and v-raf murine sarcoma viral oncogene homolog B1 mutation in melanoma (6); however, reliable and convenient biomarkers are required to evaluate the diagnostic and prognostic significance of different cancer types.
Classic biomarkers present with potentially limiting factors, including cost, availability and reproducibility (7). Utility is compromised by different disease heterogeneities, specific genetics and proteomics, and the influence of lifestyle; therefore, a number of serum or tissue biomarkers, including non-coding RNAs (ncRNAs), have been developed for clinical experiments. ncRNAs have notable potential for future biomarker approaches. Numerous studies have reported the use of ncRNAs, including microRNAs and long ncRNAs (lncRNAs), in the early detection and prognosis of various cancer types (8,9). Previously, a number of studies focused on a novel class of ncRNAs that is endogenously expressed as single-stranded, covalently-closed circular molecules, also known as circular RNAs (circRNAs) (10–12). circRNAs were demonstrated to be antagonists of specific microRNAs by functioning as microRNA sponges (10,13), and they are also known as stable molecules, as demonstrated by their long half-lives in cells (14). These observations resulted in the consideration that circRNAs could serve as potential biomarkers for the non-invasive diagnosis of numerous diseases, including disorders of the central nervous system (15), cancer (16) and a number of forms of cardiovascular diseases (17).
To determine if circRNA could serve as a sensitive and specific biomarker for cancer, a systematic meta-analysis of the published literature was performed in the present study, in order to review the diagnostic efficiency of circRNA in patients with cancer from the available data and to identify a novel non-invasive biomarker for cancer diagnosis.
Materials and methods
Search strategy
This meta-analysis was conducted in accordance with the guidelines of diagnostic meta-analysis as follows: Eligible studies published up to November 30, 2017, on PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (https://www.elsevier.com/solutions/embase-biomedical-research), were selected for the meta-analysis. Non-English studies were excluded. No restriction was placed on the year of publication or publishing status. The key words employed for literature retrieval included the following: ‘circular RNA’ or ‘circRNA’, and ‘tumor’ or ‘neoplasm’, or ‘cancer’ or ‘carcinoma’. Additionally, the reference lists of eligible articles were manually searched to obtain additional sources.
Selection of publications
All studies were carefully and independently reviewed by two researchers based on their titles and abstracts, following which full texts were perused for potential eligibility. Any disagreement was resolved by a full discussion, until consensus was achieved. All publications included in the meta-analysis were required to meet the following criteria: i) Studies should analyze the association between circRNA and patients with any cancer type; ii) studies should contain sensitivity and specificity data (or the possibility of deriving such values from the data); and iii) studies should have enrolled ≥20 patients and matched controls. Studies were excluded if they involved any of the following parameters: i) Duplicate studies; ii) letters, editorials, meeting abstracts, case reports and reviews; iii) patients and control subjects that did not qualify, in which the patients sample size was low or the disease cannot be defined; iv) studies with missing data, and v) No-English studies. If the same author reported that their results were acquired from overlapping populations, only the first study published or the most complete study was included.
Data extraction and quality assessment
The following parameters were collected from each study: Author name, publication year, country and ethnicity, sample type, normalization control, sample size and data for two-by-two tables (sensitivity and specificity). The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist (http://www.bristol.ac.uk/population-health-sciences/projects/quadas/) was used to systematically assess the quality of the articles included in the diagnostic meta-analysis. Specifically, 14 items from the QUADAS checklist were applied to each article, and an answer of ‘Yes’, ‘No’ or ‘Unclear’ was determined. Only ‘Yes’ resulted in a score.
Statistical analysis
All statistical analyses were performed using the STATA 13.0 statistical software (StataCorp LLC, TX, USA) and Meta-DiSc 1.4 (Unit of Clinical Biostatistics, Ramón y Cajal Hospital, Madrid, Spain). Data from each study (true-positives, false-positives, true-negatives and false-negatives) were extracted to obtain the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and their 95% confidence interval (CI), summary receiver operator characteristic (SROC) curve and area under the curve (AUC), in order to determine the overall performance of the detection method. P<0.05 (two-sided) was considered to indicate a statistically significant difference. Additionally, heterogeneity across studies was assessed using Cochran's Q and I2 statistics, where I2>50% indicated the existence of significant heterogeneity. Finally, evaluation of the threshold effect (Spearman's rank correlation) and publication bias (funnel plots) were also undertaken.
Results
Literature search
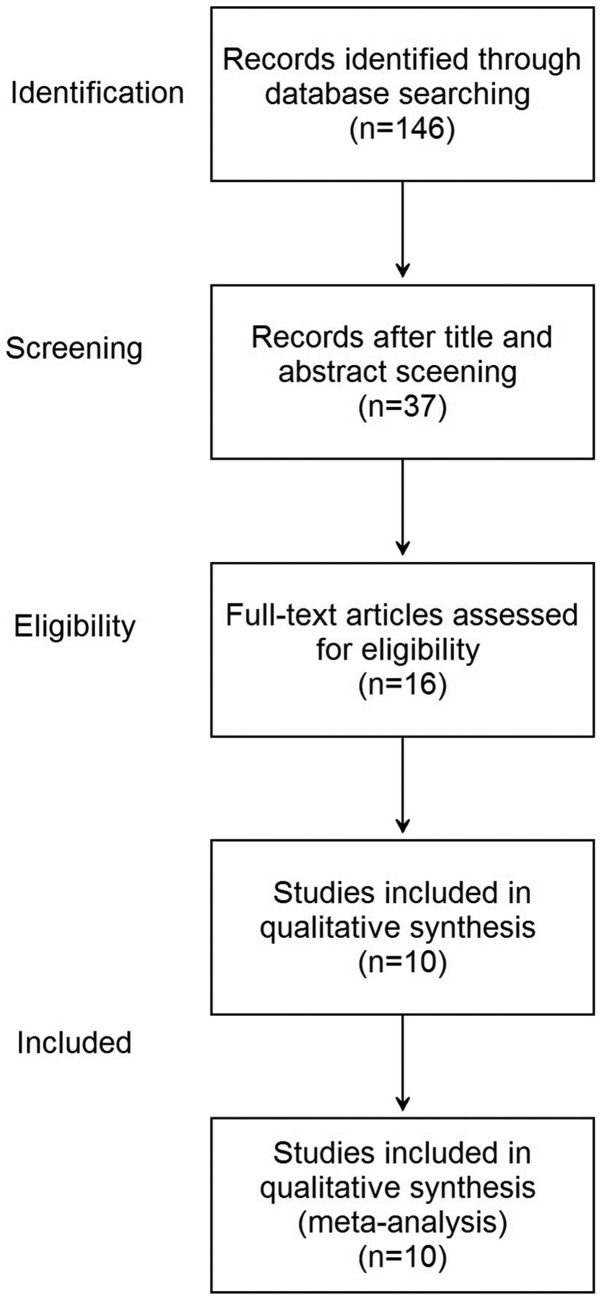
Electronic and manual searches yielded a total of 146 potentially eligible articles. The steps involved in screening the articles for the meta-analysis is depicted as a flow chart in Fig. 1. Screening titles and abstracts resulted in the exclusion of 109 articles. A further 21 articles were excluded following more detailed assessment of the full text. Finally, 10 eligible studies (12 tests) were included in the meta-analysis (18–27).
Figure 1.
Flow chart of studies included in the meta-analysis following implementation of exclusion criteria.
Study characteristics
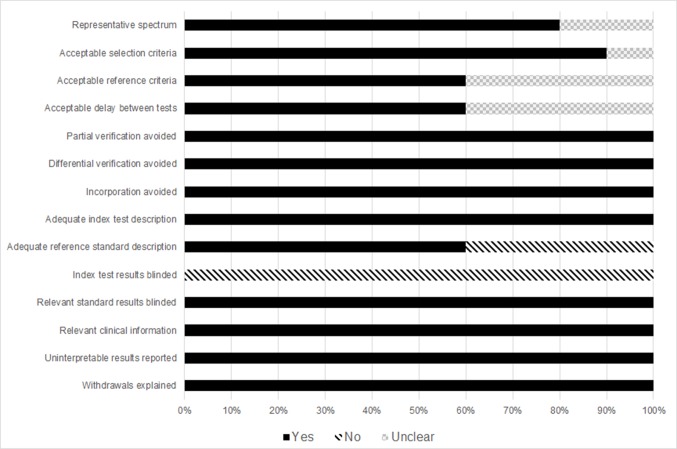
The characteristics of the 10 eligible studies are summarized in Table I (18–27). A total of 799 patients with different cancer types and adjacent controls were involved in these 12 tests. Assessment using QUADAS indicated that the studies were of high quality, with positive results in 13/14 items (Fig. 2). Additionally, the mean impact factor was calculated to be 2.59.
Table I.
Characteristics of 10 studies included in the meta-analysis.
| First author | Year | Disease | circRNA used for detection | Case no. | Control no. | Region | TP | FP | FN | TN | IF | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al | 2015 | Gastric cancer | hsa_circ_002059 | 101 | 101 | China | 82 | 38 | 19 | 63 | 2.799 | (18) |
| Qin et al | 2016 | Hepatocellular carcinoma | hsa_circ_0001649 | 89 | 89 | China | 72 | 28 | 17 | 61 | 1.736 | (19) |
| Wang et al | 2015 | Colorectal cancer | hsa_circ_001988 | 31 | 31 | China | 21 | 8 | 10 | 23 | 1.581 | (20) |
| Shang et al | 2016 | Hepatocellular carcinoma | hsa_circ_0005075 | 30 | 30 | China | 25 | 3 | 5 | 27 | 2.133 | (21) |
| Chen et al | 2017 | Gastric cancer | hsa_circ_0000190 | 104 | 104 | China | 75 | 30 | 29 | 74 | 2.799 | (22) |
| Huang et al | 2017 | Gastric cancer | hsa_circ_0000745 | 60 | 60 | China | 51 | 33 | 9 | 27 | 3.365 | (23) |
| Fu et al | 2017 | Hepatocellular carcinoma | hsa_circ_0003570 | 107 | 107 | China | 48 | 14 | 59 | 93 | 1.521 | (24) |
| Yin et al | 2017 | Breast cancer | hsa_circ_0001785 | 20 | 20 | China | 16 | 5 | 4 | 15 | 2.871 | (25) |
| Breast cancer | hsa_circ_0108942 | 20 | 20 | China | 16 | 10 | 4 | 10 | 2.871 | |||
| Breast cancer | hsa_circ_0068033 | 20 | 20 | China | 14 | 8 | 6 | 12 | 2.871 | |||
| Yao et al | 2017 | Hepatocellular carcinoma | cirZKSCAN1 | 102 | 102 | China | 84 | 28 | 18 | 74 | 5.314 | (26) |
| Zhao et al | 2017 | Gastric cancer | hsa_circ_0000181 | 115 | 115 | China | 62 | 17 | 53 | 98 | 1.521 | (27) |
TP, true-positive; FP, false-positive; FN, false-negative; TN, true-negative; IF, impact factor; circRNA, circular RNA.
Figure 2.
Quality assessment of included studies based on the Quality Assessment of Diagnostic Accuracy Studies tool.
Meta-analysis
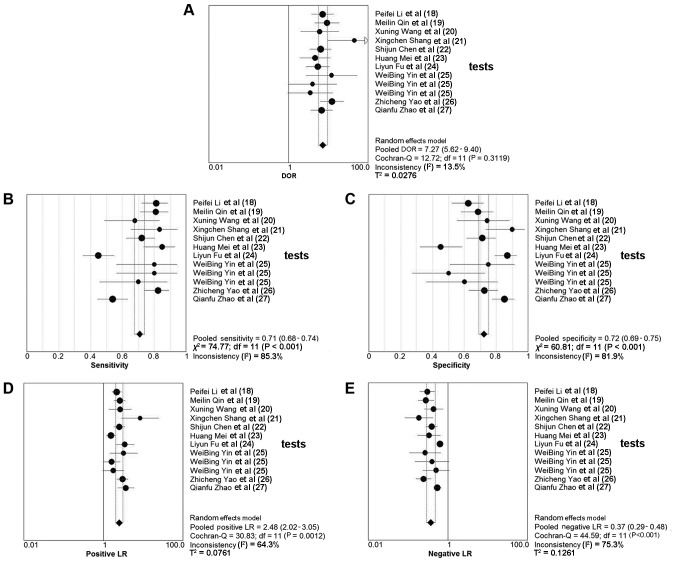
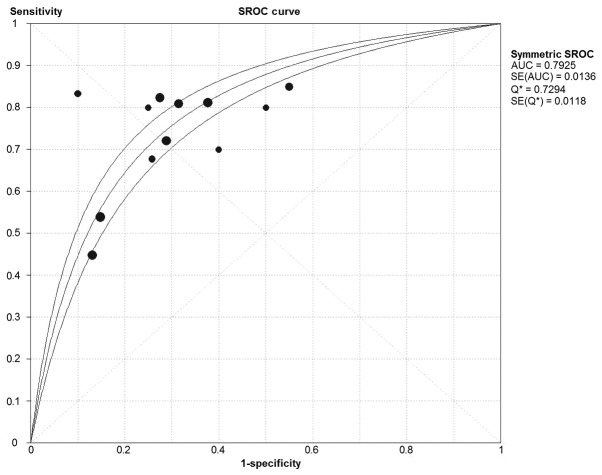
Overall, 10 studies involving 799 patients with various cancer types reported the detection performances of circRNA (Table II). The sensitivity of circRNA detection testing ranged from 0.449–0.855, and the reported specificity ranged from 0.450–0.900. The pooled DOR was 7.265 (95% CI, 5.616–9.398; Q=12.72; P=0.312; I2=13.5%). The pooled sensitivity was 0.708 (95% CI, 0.676–0.740; Q=74.77; P<0.001; I2=85.3%) and the pooled specificity was 0.722 (95% CI, 0.690–0.753; Q=60.81; P<0.001; I2=81.9%). The PLR and NLR were 2.483 (95% CI, 2.019–3.054; Q=30.83; P=0.001; I2=64.3%) and 0.372 (95% CI, 0.289–0.479; Q=44.59; P<0.001; I2=75.3%), respectively. The AUC was 0.793. The forest plots and SROC are depicted in Figs. 3 and 4, respectively.
Table II.
Detection performances of circular RNA reported by 10 studies.
| First author | Diagnostic OR (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive LR (95% CI) | Negative LR (95% CI) | (Refs.) |
|---|---|---|---|---|---|---|
| Li et al | 7.16 (3.77–13.59) | 0.81 (0.72–0.88) | 0.62 (0.52–0.72) | 2.16 (1.65–2.82) | 0.30 (0.20–0.46) | (18) |
| Qin et al | 9.23 (4.62–18.44) | 0.81 (0.71–0.88) | 0.69 (0.58–0.78) | 2.57 (1.86–3.55) | 0.28 (0.18–0.44) | (19) |
| Wang et al | 6.04 (2.01–18.17) | 0.68 (0.49–0.83) | 0.74 (0.55–0.88) | 2.63 (1.38–5.00) | 0.43 (0.25–0.75) | (20) |
| Shang et al | 45.00 (9.73–208.08) | 0.83 (0.65–0.94) | 0.90 (0.73–0.98) | 8.33 (2.81–24.67) | 0.19 (0.08–0.42) | (21) |
| Chen et al | 6.38 (3.49–11.66) | 0.72 (0.62–0.80) | 0.71 (0.61–0.80) | 2.50 (1.81–3.46) | 0.39 (0.28–0.55) | (22) |
| Huang et al | 4.64 (1.94–11.09) | 0.85 (0.73–0.93) | 0.45 (0.32–0.58) | 1.55 (1.20–1.99) | 0.33 (0.17–0.65) | (23) |
| Fu et al | 5.40 (2.70–53.33) | 0.45 (0.35–0.55) | 0.87 (0.79–0.93) | 3.43 (2.01–5.83) | 0.63 (0.53–0.76) | (24) |
| Yin et al | 12.00 (2.70–53.33) | 0.80 (0.56–0.94) | 0.75 (0.51–0.91) | 3.20 (1.45–7.05) | 0.27 (0.11–0.66) | (25) |
| 4.00 (0.98–16.27) | 0.80 (0.56–0.94) | 0.50 (0.27–0.73) | 1.60 (0.98–2.61) | 0.40 (0.15–1.07) | ||
| 3.50 (0.94–12.97) | 0.70 (0.46–0.88) | 0.60 (0.36–0.81) | 1.75 (0.95–3.22) | 0.50 (0.23–1.07) | ||
| Yao et al | 12.33 (6.31–24.09) | 0.82 (0.74–0.89) | 0.73 (0.63–0.81) | 3.00 (2.16–4.16) | 0.24 (0.16–0.38) | (26) |
| Zhao et al | 6.74 (3.58–12.69) | 0.54 (0.44–0.63) | 0.85 (0.77–0.91) | 3.65 (2.28–5.84) | 0.54 (0.44–0.67) | (27) |
OR, odds ratio; LR, likelihood ratio; CI, confidence interval.
Figure 3.
Forests plot of the accuracy of circRNAs for the diagnosis of cancer. (A) DOR forest plot of the circRNAs. (B) Sensitivity forest plot of the circRNAs. (C) Specificity forest plot of the circRNAs. (D) Positive LR forest plot of the circRNAs. (E) Negative LR forest plot of the circRNAs. DOR, diagnostic odds ratio; LR, likelihood ratio; circRNAs, circular RNAs; df, degrees of freedom.
Figure 4.
SROC of circular RNAs for cancer diagnosis. SROC, summary receiver-operating characteristic; AUC, area under curve; SE, standard error; Q*, Q statistic.
Investigation of the threshold effect
Spearman's rank correlation was also performed to confirm the threshold effect. No indication of a threshold effect was determined in the studies [Spearman's correlation coefficient (ρ), 0.340; P=0.280]. Additionally, the slope (b) of the regression equation did not differ from zero (P=0.852), implying no heterogeneity between the studies.
Publication bias
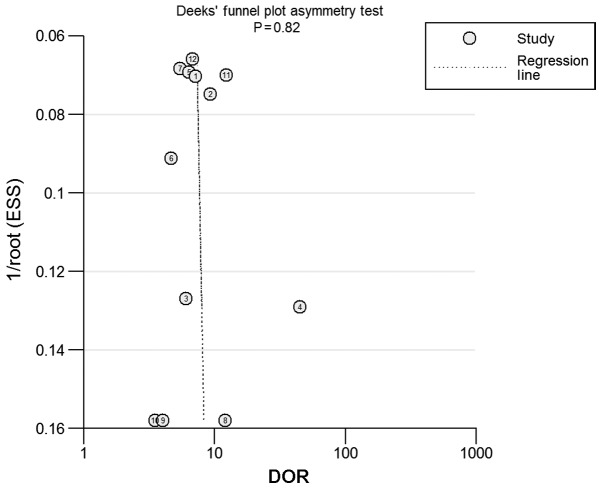
Finally, the presence of a statistically significant slope coefficient (P<0.05) was considered to indicate a possible publication bias. Funnel plots were produced (Fig. 5). No publication bias was observed in the included studies (P=0.82) and the regression line represented a symmetrical curve.
Figure 5.
Funnel plot of the 12 tests in the 10 included studies. DOR, diagnostic odds ratio; ESS, effective sample size.
Discussion
There are an increasing number of molecular biomarkers, including microRNAs and lncRNAs, being used in cancer diagnostics. circRNAs are widely expressed in human cells (28). Highly conserved sequences and a high degree of stability in mammalian cells are two of their most important properties (10,13); thus, circRNAs have the potential to be ideal biomarkers in the diagnosis of cancer. Numerous studies have evaluated the performance of circRNAs in cancer diagnosis (18–27); however, no systematic evaluation of circRNAs has been performed. The differences in the performances were too large and hence, to the best of our knowledge, the present study is the first meta-analysis to provide precise and controlled data on the diagnostic performance of circRNAs in cancer.
A total of 10 eligible, high-quality studies were included in the present meta-analysis. The present study demonstrated the varying sensitivities and specificities of circRNAs in the diagnosis of cancer; however, the range of their sensitivity and specificity was large and their diagnostic performance cannot be evaluated. The pooled sensitivity and specificity were observed to be slightly high (70.8 and 72.2%), which demonstrated that circRNAs could be used as assistant indicators in the diagnosis of cancer. The SROC curve and DOR indicated that circRNAs exhibited a moderate diagnostic performance. The pattern of the data points in the SROC curve did not indicate a ‘shoulder-arm’ shape, which indicates no threshold effect was determined in these studies, and the AUC of the SROC was 0.793. Cumulatively, these results indicated that circRNA had a moderate level of overall diagnostic accuracy for cancer diagnosis.
In the present study, heterogeneity was not determined in the pooled DOR of the circRNAs (P=0.852). Furthermore, publication bias and Spearman's rank correlation were also performed. No statistical difference was determined using Spearman's rank correlation, which meant that no threshold effect among these studies was observed. No publication bias was observed either in the included studies.
However, a number of limitations in this meta-analysis should be noted. Firstly, all included studies were reported by Chinese researchers. For this reason, the diagnostic performance of circRNAs may be not be all-sided, in spite of the absence of heterogeneity, threshold effect and publication bias; Therefore, further research regarding circRNAs, particularly in relation to the other countries' projects, as a biomarker in cancer diagnosis is required. Secondly, only the integral diagnostic performance of circRNAs on cancer was evaluated. The performance may be cursory on a specific type of circRNA for specific cancer types. Since the aim of the present study was to evaluate the likelihood of circRNAs performing for the diagnosis of cancer, the integral performance of circRNA in cancer was sufficient. Finally, the moderate levels of circRNA sensitivity and specificity could be attributed to technological, instrumental and staffing limitations; however, there is not sufficient data to evaluate these parameters. The cut-off value of circRNA efficiency in different cancer types remains controversial, and investigating its clinical significance may improve the diagnostic performance of circRNAs.
In conclusion, circRNA is a moderately effective assistant diagnostic biomarker for cancer; however, its diagnostic performance remains to be determined and further research of specific circRNA types for specific cancer types is required in order to determine this.
Acknowledgements
The authors thank Dr Si Chen (Department of Clinical Laboratory, Beijing Anzhen Hospital) and Dr Chuiwen Deng (Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital) who gave positive advice regarding this study.
Glossary
Abbreviations
- circRNAs
circular RNAs
- QUADAS
quality assessment of diagnostic accuracy studies
- PLR
positive likelihood ratio
- NLR
negative likelihood ratio
- DOR
diagnostic odds ratio
- CI
confidence interval
- SROC
summary receiver operator characteristic
- AUC
area under the curve
Funding
The present study was supported by the Talent Training Project of High Level Health Technology from Beijing Health System (grant no. 2015-3-052) and the National Natural Science Foundation of China (grant no. 81500332).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YL and XZ carried out the conception and design, acquisition of data, analysis of data, and drafting the manuscript. HY performed the acquisition of data, and the drafting and revising of the manuscript. JH and SZ aided with acquisition of data. HC aided with the statistical analysis. QS and NJ participated in the design and coordination of the study and helped to revise the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bian J, Sun X, Li B, Ming L. Clinical significance of serum HE4, CA125, CA724, and CA19-9 in patients with endometrial cancer. Technol Cancer Res Treat. 2017;16:435–439. doi: 10.1177/1533034616666644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karakaya BK, Başer E, Bildacı B, Cömert EÇ, Bayraktar N, Dursun P, Kuşçu E, Ayhan A. Alternative tumor markers in the diagnosis of ovarian cancer. Ginekol Pol. 2016;87:565–769. doi: 10.5603/GP.2016.0045. [DOI] [PubMed] [Google Scholar]
- 4.Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R, Ward C, Mayne K, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chien Chang CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 6.Gear H, Williams H, Kemp EG, Roberts F. BRAF mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci. 2004;45:2484–2488. doi: 10.1167/iovs.04-0093. [DOI] [PubMed] [Google Scholar]
- 7.Bodzin AS, Busuttil RW. Hepatocellular carcinoma: Advances in diagnosis, management, and long term outcome. World J Hepatol. 2015;7:1157–1167. doi: 10.4254/wjh.v7.i9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu AX, Huang ZY, Zhang L, Shen J. Potential prognostic long non-coding RNA identification and their validation in predicting survival of patients with multiple myeloma. Tumour Biol. 2017;39:1010428317694563. doi: 10.1177/1010428317694563. [DOI] [PubMed] [Google Scholar]
- 9.Song T, Liang Y, Cao Z, Du W, Li Y. Computational analysis of specific MicroRNA biomarkers for noninvasive early cancer detection. Biomed Res Int. 2017;2017:4680650. doi: 10.1155/2017/4680650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 11.Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW, Zeillinger R, Pils D. Correlation of circular RNA abundance with proliferation-exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5:8057. doi: 10.1038/srep08057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 13.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 15.Lukiw WJ. Circular RNA (circRNA) in Alzheimer's disease (AD) Front Genet. 2013;4:307. doi: 10.3389/fgene.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010;6:e1001233. doi: 10.1371/journal.pgen.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z, Yang J, Fan J, Liu L, Qin W. Hsa_circ_0001649: A circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark. 2016;16:161–169. doi: 10.3233/CBM-150552. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhang Y, Huang L, Zhang J, Pan F, Li B, Yan Y, Jia B, Liu H, Li S, Zheng W. Decreased expression of hsa_circ_001988 in colorectal cancer and its clinical significances. Int J Clin Exp Pathol. 2015;8:16020–16025. [PMC free article] [PubMed] [Google Scholar]
- 21.Shang X, Li G, Liu H, Li T, Liu J, Zhao Q, Wang C. Comprehensive circular RNA profiling reveals that hsa_circ_0005075, a new circular RNA biomarker, is involved in hepatocellular crcinoma development. Medicine (Baltimore) 2016;95:e3811. doi: 10.1097/MD.0000000000003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi: 10.1016/j.cca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Huang M, He YR, Liang LC, Huang Q, Zhu ZQ. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol. 2017;23:6330–6338. doi: 10.3748/wjg.v23.i34.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu L, Wu S, Yao T, Chen Q, Xie Y, Ying S, Chen Z, Xiao B, Hu Y. Decreased expression of hsa_circ_0003570 in hepatocellular carcinoma and its clinical significance. J Clin Lab Anal. 2018;32 doi: 10.1002/jcla.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin WB, Yan MG, Fang X, Guo JJ, Xiong W, Zhang RP. Circulating circular RNA hsa_circ_0001785 acts as a diagnostic biomarker for breast cancer detection. Clin Chim Acta: S0009-8981(17)30407-2. 2017 doi: 10.1016/j.cca.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, Zhang P, Xiong Z, He C, Huang Z, et al. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017;11:422–437. doi: 10.1002/1878-0261.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Q, Chen S, Li T, Xiao B, Zhang X. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal. 2018;32:e22333. doi: 10.1002/jcla.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.