Abstract
The objective of the present study was to investigate the tumor-associated vascular changes in hepatocellular carcinoma (HCC) following treatment with transarterial chemoembolization (TACE) combined with sorafenib. The data of 20 patients were retrospectively analyzed. Patients underwent treatment depending on their chosen regimens (orally administered sorafenib was recommended, however the cost prevented some study articipants from selecting this course). Based on this, the patients were divided into TACE combined with sorafenib (TS) (n=10) and TACE-only treatment groups (n=10). Digital subtraction angiography images of all patients were analyzed by 2 radiologists who were blind to the type of treatment administered. The diameters of the hepatic and proper hepatic arteries, and hepatic artery branches (tumor-associated arteries), the splenic, left gastric and gastroduodenal arteries or portal veins (non-tumor-associated arteries) and the number of microvascular vessels were compared prior to and following sorafenib treatment in the TS group, between the first and second sessions of TACE in the TACE-only group and between the TS and TACE-only groups. In the TS group, the diameters of the hepatic and proper hepatic arteries, their branches and the number of microvascular vessels were significantly decreased following sorafenib treatment (P<0.05), while the diameters of the splenic, gastroduodenal and left gastric arteries were not significantly altered (P>0.05). In the TACE-only group, the diameters of the hepatic, proper hepatic, splenic, left gastric and gastroduodenal arteries were not significantly different between the first and second TACE sessions (P>0.05), while the diameters of the hepatic artery branches and the number of microvascular vessels were significantly altered (P<0.05). TACE combined with sorafenib significantly decreased the diameters of the tumor-associated arteries and the number of tumor microvascular vessels when compared with TACE treatment alone (P<0.05). No significant difference in the diameters of the portal vein and its branches between the two groups was observed (P>0.05). Treatment with TACE combined with sorafenib may significantly affect the tumor-associated vasculature compared with treatment with TACE alone in HCC.
Keywords: tumor-associated vasculature, transarterial chemoembolization, sorafenib, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most commonly diagnosed types of cancer worldwide (1). While there are specific differences in its etiology associated with different regional effects for example, in western countries, alcoholic hepatitis is the main reason for HCC. However, in China, a large number of HCC cases arise as a consequence of Hepatitis B virus (HBV) infection (2). HCC is a complicated disease and represents a significant cause of morbidity and mortality in China (2,3).
Previous advances in treatment methods for HCC have improved the clinical outcomes of patients with HCC. Liver transplantation, curative surgical resection, percutaneous ethanol injection and radiofrequency ablation are considered curative treatments, while transarterial treatments, including transarterial chemoembolization (TACE) in particular, are currently recognized as treatments that may prolong survival time (3).
TACE has been suggested to treat Barcelona Clinic Liver Cancer (BCLC) B- or C-stage HCC through 2 potential methods (4). Firstly, arterial embolization may stop blood supply to the HCC tissue and inhibit tumor growth until neovascularization occurs (5). Secondly, the controlled release of chemotherapeutic agents allows the delivery of a stable concentration of drug to the lesions, and decreases the severity of systemic side effects (6). However, a previous study demonstrated that TACE may trigger cancer angiogenesis by upregulating the expression of certain cytokines and growth factors, including basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1α (7) Furthermore, additional studies have demonstrated that the expression levels of these cytokines and growth factors were markedly decreased in patients treated with surgery alone compared with those that underwent pre-surgical TACE (8).
Sorafenib is an orally-administered small molecule drug (9) that inhibits multiple protein kinases, including VEGF receptors 2 and 3 and Platelet-derived growth factor (PDGF) receptor B (9), which are involved in tumor angiogenesis, and the family of Raf kinases, which facilitates the proliferation of cancer cells (10). Sorafenib is globally accepted and widely used as the first-line treatment for advanced-stage HCC (11). However, the effects of treatment with sorafenib alone on overall survival (OS) have not been satisfactory in patients with advanced HCC (12).
Novel therapies are urgently required in order to improve the OS of patients with advanced HCC. At present, sorafenib is the only approved systemic drug for patients with advanced-stage disease (BCLC C-stage) (13). TACE is suggested as the primary treatment method for BCLC B-stage HCC in the BCLC guidelines (14). Physicians have demonstrated that TACE was also effective for patients with BCLC C-stage HCC with vascular invasion or metastases (14,15). Furthermore, previous data have indicated that combined therapy, including sorafenib combined with TACE, may achieve significant improvements in the treatment of advanced HCC compared with single TACE treatment (16).
At present, clinical studies generally use the modified Response Evaluation Criteria In Solid Tumors (mRECIST) to evaluate the effect of sorafenib and TACE treatment on HCC (17). However, hemodynamic digital subtraction angiography (DSA) images and the structure of the vascular network, which reflect the hemodynamic features of liver cancer, have been proposed as priority concerns by interventional radiology physicians for analyzing the effects of treatments including TACE prior and subsequent to initiation of treatment (18). To the best of our knowledge, few studies have been performed investigating the arterial characteristics of HCC following treatment with sorafenib combined with TACE. Therefore, the aim of the present study was to compare the changes in the tumor vasculature of HCC following the use of TACE alone compared with its use in combination with sorafenib. The results of the present study may provide insight into this combination treatment method as a therapy for advanced HCC.
Patients and methods
In the present study, 20 patients (5 females and 15 males; median age 51 years, age range, 37–74 years) with HBV-associated HCC admitted to Zhongshan Hospital Affiliated to Xiamen University (Xiamen, China) our hospital between January 2011 and August 2016 were reviewed. All patients exhibited advanced HCC according to the BCLC staging system (BCLC C-stage) (19), confirmed by abdominal enhanced computed tomography (CT) or magnetic resonance imaging (MRI). None of the patients had been previously treated for HCC. A total of 10 patients were included in the TACE combined with sorafenib treatment (TS) group. All of these patients were initially treated with TACE, followed by treatment with sorafenib (200 mg orally; Bayer AG, Leverkusen, Germany) at a dose of 400 mg twice daily, for 1 week. After 6 weeks, the patients in the TS group were treated with a second session of TACE. The first angiography was performed prior to each session of TACE and prior to sorafenib treatment; the angiography was then repeated during the second session of TACE and following sorafenib treatment. The remaining 10 patients were treated with TACE only, with at least two sessions of TACE. The data from the first TACE session were then compared with those from the second TACE session using the angiography data collected. The angiography was performed following each TACE session in the TACE-only group, then the first angiography was compared with the second angiography.
In the present study, grade ≤3 hepatic artery branches were defined as microvascular vessels. The patient inclusion criteria are summarized in Table I and exclusion criteria are described in Table II. The baseline information of patients is summarized in Table III. The present study was approved by the Ethics Committee of Zhongshan Hospital Affiliated to Xiamen University (Xiamen, China). Patients provided written informed consent to participate prior to treatment, and for publication. TACE was performed according to a standardized approach.
Table I.
Patient inclusion criteria.
| Criteria | Cut-off |
|---|---|
| BCLC grade | C |
| Liver function | Child-Pugh class A or B (39) |
| Total bilirubin, µmol/l | <34.2 (3.4–17.1) |
| Alanine transaminase level, U | <100 (7–40) |
| Aspartate aminotransferase level, U | <90 (13–35) |
| Prothrombin time activity, sec | <22 (9–13) |
| Serum creatinine level, µmol/l | 1.5-fold the normal value (53–97) |
| Neutrophil count, /l | 4-10×109 (4–10) |
| Hemoglobin level, g/l | ≥90 (115–150) |
| Cardiopulmonary | NA |
| Cerebral function | NA |
| Estimated life expectancy, weeks | >12 |
BCLC, Barcelona Clinic Liver Cancer.
Table II.
Patients exclusion criteria.
| Criteria | Cut-off |
|---|---|
| Tumor volume/total liver volume, % | >70 |
| Portal vein patency | Main portal vein/right branch of portal vein completely obstructed |
| Previous treatment (surgical resection, ablation or TAI) | Yes |
TAI, transhepatic artery-infusion chemotherapy.
Table III.
Comparison of the baseline information between the TS and TACE groups.
| Parameters | TS group | TACE group |
|---|---|---|
| Sex, male/female | 8/2 | 7/3 |
| Average age, years | 55 | 50 |
| BCLC grade | C | C |
| Prothrombin time activity, sec | 13.9 (12.7–16.2) | 14.9 (13.2–16.6) |
| Total bilirubin, µmmol/l | 16.3 (8.6–32.8) | 16.9 (7.9–29.0) |
| Hemoglobin, g/l | 127.0 (106.0–177.0) | 124.0 (104.0–140.0) |
| Alanine transaminase, U | 78.2 (38.0–95.5) | 72.4 (54.3–89.2) |
| Aspartate aminotransferase, U | 57.1 (25.0–84.0) | 60.1 (33.0–83.8) |
BCLC, Barcelona Clinic Liver Cancer grade; TACE, transarterial chemoembolization; TS group, TACE combined with sorafenib. Data are presented as mean (range).
The TACE procedure was performed. Firstly, a catheter was implanted into the celiac artery using the Seldinger technique, and then a hepatic angiography was performed via a common femoral approach using a 4 Fr angiographic catheter (Terumo Medical Co., Tokyo, Japan). Subsequent to locating the lesion by selective arteriography using a 2.0–2.7 Fr microcatheter (Terumo Medical Co.), an emulsion consisting of lipiodol (Guerbet, Roissy, France), epirubicin (Pfizer, Inc., New York, NY, USA) and oxaliplatin (Jiangsu Aosaikang Pharmaceutical Industry Ltd., Co., Nanjing, China) was infused via the feeding arteries.
The angiography was then performed to observe the tumor and to identify all the feeding arteries of the tumor. The nonionic contrast iopromide (Bayer AG) was injected into each hepatic artery at 5 ml/sec using an automated power injector. The duration of the arterial injection was 11 sec. Subsequently, the diameters of the branches of the celiac trunk, including the hepatic and proper hepatic arteries, hepatic arterial branches, splenic, gastroduodenal and left gastric arteries and the microvessels within tumors, were evaluated and analyzed. The tumor lesions in all 20 patients were supplied with nutrients and connected via the proper hepatic arteries and their branches, but not the left gastric, splenic or gastroduodenal arteries. Therefore, in the present study, the proper hepatic arteries and their branches were considered tumor-associated arteries, while the left gastric, splenic and gastroduodenal arteries were considered non-tumor-associated arteries. The diameters of the portal vein and its branches were evaluated using a contrast-enhanced liver CT scan. Then, the differences in the tumor-associated and non-tumor-associated arteries between pre- and post-sorafenib treatment analyses in the TS group were compared, as were the differences in the arteries between the measurements taken following the first and second TACE sessions in the TACE group. The DSA images were analyzed by 2 radiologists (Zhongshan Hospital Affiliated to Xiamen University) who were blind to the type of treatment administered, according to the following method: The angiography images were saved in the Artis workplace of the DSA system (Artis zee biplane, Siemens AG, Munich, Germany). With this workplace, the radiologists used the Postproc software included in the DSA system to measure the diameter of vessels; prior to measurement, radiologists calibrated the distance using the width of the 4 Fr angiographic catheter as a reference. Microvascular vessels were defined as ≤grade 3 branches of hepatic arteries and these microvascular vessels were able to be detected by DSA.
Statistical analysis
The data are presented as the mean ± standard deviation, and differences were compared using paired Student's t-tests. P<0.05 was considered to indicate a statistically significant difference. Analysis was performed using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism software (version 5.01; GraphPad Software, Inc., La Jolla, CA, USA).
Results
TACE combined with sorafenib decreases the diameters of tumor-associated arteries
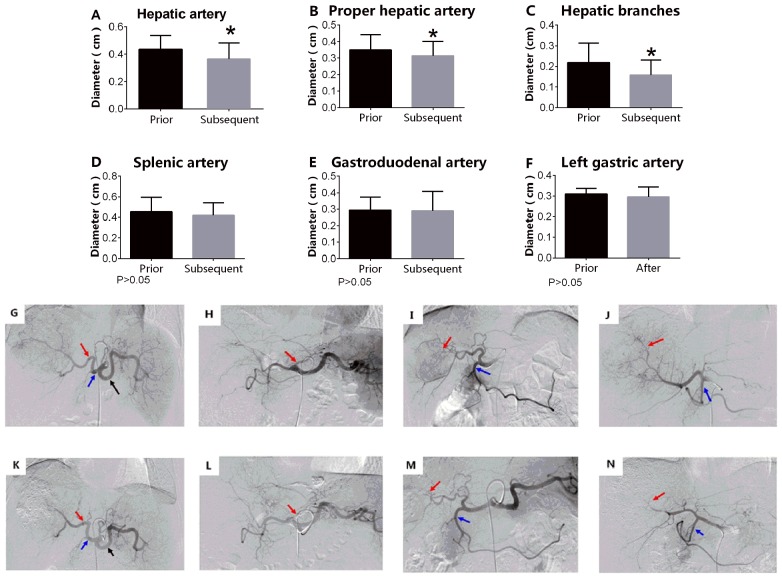
In the TS group, the diameters of tumor-associated arteries prior to sorafenib administration were significantly increased compared with those measured following sorafenib treatment, including the diameters of the hepatic artery (0.218±0.0423 vs. 0.158±0.0321 cm; P<0.05), the proper hepatic artery (0.350±0.042 vs. 0.314±0.040 cm; P<0.05) and the hepatic artery branches (0.218±0.042 vs. 0.158±0.032 cm, P<0.05; Fig. 1).
Figure 1.
TACE combined with sorafenib. TS significantly reduced the diameter of tumor-associated arteries, including the (A) hepatic artery, (B) proper hepatic artery and (C) hepatic branches. *P<0.05. TS did not change the diameter of the non-tumor associated arteries, including the (D) splenic artery, (E) gastroduodenal artery and (F) left gastric artery. P>0.05. G, K, H, L, I, M, J and N represent the same patient prior and subsequent to adminstration of sorafenib respectively. G, H, I and J are images collected in the first TACE procedure; K, L, M and N are images collected in the second TACE procedure. (G) Arrow from left to right indicates the proper hepatic, hepatic and splenic arteries. (H) Arrow indicates left gastric artery. (I) Arrow from left to right indicates the hepatic artery branches and gastroduodenal artery. (J) Arrow from left to right indicates the hepatic artery branches and gastroduodenal artery. (K) Arrow from left to right indicates the proper hepatic, hepatic and splenic arteries. (L) Arrow indicates left gastric artery. (M) Arrow from left to right indicates the hepatic artery branches and gastroduodenal artery. (N) Arrow from left to right indicates hepatic artery branches and gastroduodenal artery. TACE, transarterial chemoembolization; TS group, TACE combined with sorafenib. The magnification for all images in Figure 1 is 1:4.
TACE combined with sorafenib does not decrease the diameters of non-tumor-associated arteries
In the TS group, the diameters of the non-tumor-associated arteries prior to the administration of sorafenib were not significantly different compared with those measured following sorafenib treatment, including the diameters of the splenic artery (0.454±0.063 vs. 0.418±0.055 cm; P>0.05), the gastroduodenal artery (0.294±0.036 vs. 0.29±0.053 cm; P>0.05) and the left gastric artery (0.310±0.012 vs. 0.296±0.022 cm; P>0.05; Fig. 1).
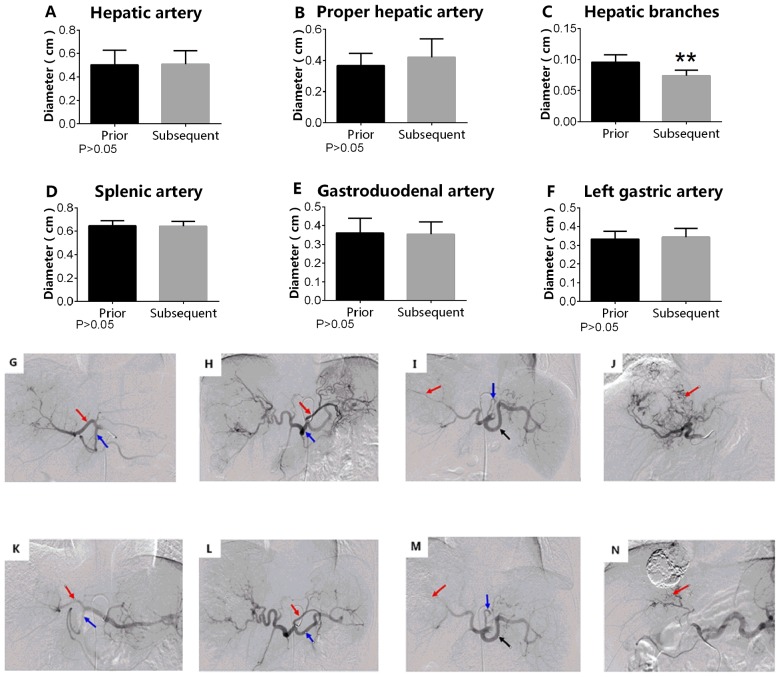
TACE alone does not cause a decrease in diameter of the tumor-associated arteries, with the exception of the hepatic artery branches
In the TACE group, the diameters of the tumor-associated arteries following the first session were not significantly different compared with those following the second session of TACE, including the diameters of the hepatic artery (0.521±0.0272 vs. 0.502±0.0329 cm; P>0.05) and the proper hepatic artery (0.450±0.037 vs. 0.407±0.0419 cm; P>0.05). However, the diameters of the hepatic artery branches were significantly decreased subsequent to the second session of TACE compared with the measurements taken following the first session of TACE (0.110±0.010 vs. 0.087±0.003 cm; P<0.05; Fig. 2).
Figure 2.
TACE alone treatment. TACE did not reduce the diameter of either the tumor-associated and non-tumor-associated arteries (P>0.05) in the (A) hepatic artery and (B) the proper hepatic artery (C) with the exception of the hepatic artery branches (**P<0.05), (D) splenic artery, (E) gastroduodenal artery and (F) left gastric artery. P>0.05. G, H, I and J are images collected in the first TACE procedure; K, L, M and N are images collected in the second TACE procedure. (G) Arrow from left to right indicates the proper hepatic and gastroduodenal arteries. (H) Arrow from left to right indicates the left gastric and splenic arteries. (I) Arrow from left to right indicates the hepatic artery branches, and the left gastric and splenic arteries. (J) Red arrow indicates hepatic artery branches. (K) Arrow from left to right indicates the proper hepatic and gastroduodenal arteries. (L) Arrow from left to right indicates the left gastric and splenic arteries. (M) Arrow from left to right indicates the hepatic artery branches, and the left gastric and splenic arteries. (N) Red arrow indicates hepatic artery branches. TACE, transarterial chemoembolization. The magnification for all images in Figure 2 is 1:4.
TACE alone does not decrease the diameters of non-tumor- associated arteries. In the TACE group, the diameters of non-tumor-associated arteries following the first session of TACE were not significantly different compared with those measured following the second session of TACE, including the diameters of the left gastric artery (0.300±0.018 vs. 0.301±0.021 cm; P>0.05), the gastroduodenal artery (0.352±0.020 vs. 0.338±0.021 cm; P>0.05) and the splenic artery (0.602±0.026 vs. 0.61±0.028 cm; P>0.05; Fig. 2).
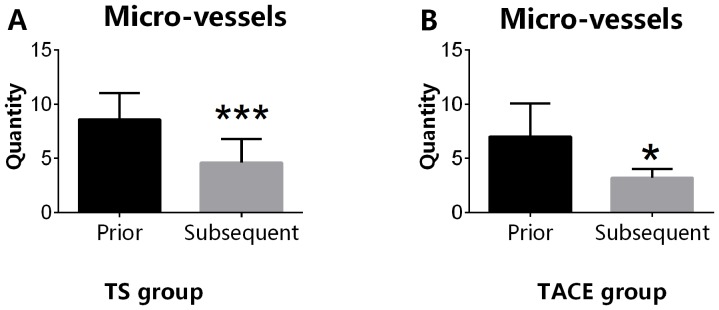
TACE combined with sorafenib and TACE alone significantly decrease the number of tumor microvascular vessels
In the TS group, the number of tumor microvessels prior to the administration of sorafenib was significantly increased compared with the number following the sorafenib treatment (8.60±1.07 vs. 4.6±0.980 cm; P<0.05). In the TACE-alone group, the number of microvessels within the tumor following the first session of TACE was also significantly increased compared with that measured following the second session of TACE (6.00±0.745 vs. 3.80±1.14 cm; P<0.05; Fig. 3).
Figure 3.
Microvascular vessels. (A) TS group. ***P<0.05. (B) TACE-alone group. *P<0.05. TACE, transarterial chemoembolization; TS group, TACE combined with sorafenib.
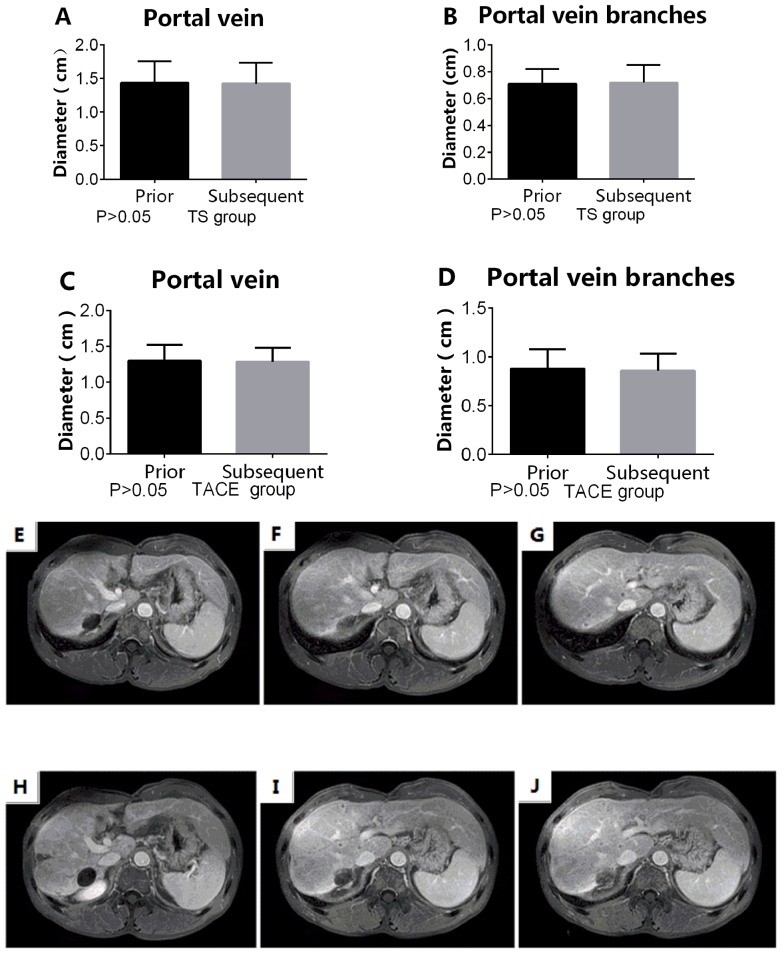
TACE combined with sorafenib and TACE-alone treatments do not affect the diameters of the portal vein and its branches
In the TS group, the diameters of the portal vein and its branches prior to sorafenib treatment were not significantly different compared with those measured following sorafenib treatment (portal vein, 1.44±0.146 vs. 1.426±0.138 cm; portal vein branches, 0.710±0.0491 vs. 0.718±0.0591 cm; both P>0.05). In the TACE group, the diameters of the portal vein and its branches following the first session of TACE were not significantly different compared with those measured following the second session of TACE (portal vein, 1.304±0.069 vs. 1.289±0.060 cm; portal vein branches, 0.879±0.063 vs. 0.859±0.055 cm; both P>0.05; Fig. 4).
Figure 4.
TS and TACE-only treatments do not decrease the diameter of portal vein and its branches. (A) Portal vein and (B) portal vein branches in the TS group. (C) Portal vein and (D) portal vein branches in the TACE group. (E-G) MRI prior to administration of sorafenib. (H-J) MRI subsequent to administration of sorafenib. E, H, F, I, G and J represent the same patient prior and after adminstration of sorafenib respectively. TACE, transarterial chemoembolization; TS group, TACE combined with sorafenib. The magnification for all images in Figure 4 is 1:4.
TACE combined with sorafenib significantly reduces the diameters of tumor-associated arteries and the number of tumor microvascular vessels compared with those in the TACE-only group
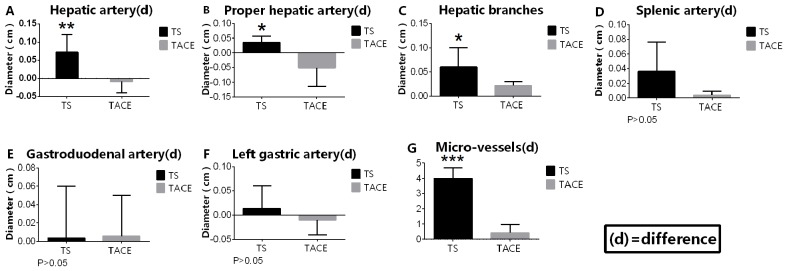
The difference in the diameters of tumor-associated arteries, including the hepatic and the proper hepatic arteries and the hepatic artery branches, between pre-sorafenib and post-sorafenib measurements in the TS group was significantly increased compared with the difference in the diameters measured following the first and the second TACE sessions in the TACE-only group. Concurrently, the number of microvascular vessels following the second session in the TACE-only group was significantly increased compared with that in the TS group (0.400±0.927 vs. 4.00±0.927 vessels respectively; P<0.05). However, the difference in the diameters of non-tumor-associated arteries, including the left gastric, splenic and gastroduodenal arteries, between pre-sorafenib and post-sorafenib measurements in the TS group was not significantly different compared with the difference between the measurements taken following the first and second TACE sessions in the TACE-only group (Fig. 5).
Figure 5.
TS significantly decreases the diameter of tumor-associated arteries and tumor microvascular vessels when compared with those in the TACE group (P<0.05). (A) Hepatic artery (d). (B) Proper hepatic artery (d). (C) Hepatic artery branches (d). (D) Splenic artery (d). (E) Gastroduodenal artery (d). (F) Left gastric artery (d). (G) Microvascular vessels (d). TACE, transarterial chemoembolization; TS group, TACE combined with sorafenib. *P<0.05; **P<0.01; ***P<0.001. (d) difference. TACE, transarterial chemoembolization.
Discussion
The results of the present study demonstrated that TACE combined with sorafenib significantly decreased the diameter of tumor-associated hepatic arteries and their branches, notably following the oral administration of sorafenib, while TACE alone did not alter the diameter of the tumor-associated arteries (20). Furthermore, the number of microvascular vessels in the tumor, as detected by hepatic arteriography, was markedly decreased following combination treatment with sorafenib and TACE. Compared with the first angiography, TACE alone also significantly decreased the number of microvascular vessels within the HCC tumor tissues (21). TACE combined with sorafenib significantly reduced the diameter of tumor-associated arteries and tumor microvascular vessels when compared with TACE alone.
Previous studies have indicated that VEGF expression in patients with HCC is associated with radiological features (21,22). Previous data have also indicated that the degree of enhancement in hepatic arteriography in HCC was closely associated with the pro-angiogenic cytokine level, and that VEGF contributed to the development of dense vascular networks in cancer lesions (23,24). In the present study, following combinatorial therapy with TACE and sorafenib, the number of tumor microvascular vessels was significantly decreased. Previous studies have also demonstrated that tumor-associated hepatic arterial perfusion parameters were additionally decreased following sorafenib treatment (25). Therefore, we hypothesized that sorafenib combined with TACE, compared with TACE alone, may reduce the serum levels of VEGF and bFGF in patients with liver cancer, and inhibit angiogenesis by reducing the production of pro-angiogenic factors, by preventing them from binding to their receptors or by blocking their actions.
Previous data have demonstrated that the level of VEGF facilitated the formation of arteries with larger diameters, which provides increased blood supply to cancer lesions (26). In addition, the present study demonstrated that the diameter of tumor-associated arteries in patients with HCC treated with TACE combined with sorafenib was significantly reduced, and that the microvascular density of HCC tumor tissues was markedly decreased, compared with those in patients treated with TACE only (27). We hypothesize that sorafenib inhibited the upregulation of TACE-induced pro-angiogenic cytokines, including VEGF or PDGF, which are critical during the development of cancer; furthermore, pro-angiogenic cytokines including VEGF serve a key role in stimulating angiogenesis and endothelial cell proliferation. However, the absence of quantified data on these growth factors is a limitation of the present study. In addition, this was a retrospective observational study and HCC biopsies were not routinely performed; diagnoses were usually made according to the results of enhanced CT or MRI-T1 and -T2 diffusion-weighted imaging scans. The absence of HCC biopsy data is an additional limitation of the present study.
Parenchymal arterialization, sinusoidal capillarization and the development of unpaired arteries are among the multiple steps involved in HCC carcinogenesis (28). Ultimately, the blood supply of HCC shifts from the portal vein to the hepatic artery. A total of >95% of the blood supply in HCC originates from the hepatic artery, while the non-tumor liver region obtains 80% of its blood supply from the portal vein (29). Previous studies have demonstrated that when contrast agent was infused via the hepatic artery, the intratumoral contrast concentrations were increased 10-fold compared with those following infusion through the portal vein, and also indicated that the tissue concentration of contrast agents within the tumor was >40 times increased compared with of the surrounding normal liver tissue (30,31). In the present study, it was revealed that TACE combined with sorafenib and TACE alone did not decrease the diameters of the portal vein or its branches. A previous study also revealed that during hepatocarcinogenesis the majority of hepatocellular nodules exhibit a deterioration of arterial blood flow rate and pressure prior to the loss of portal blood flow (32). Subsequently, as a result of a marked increase in neo-vascularized arteries, with the assistance of increased pro-angiogenic cytokine secretion, arterial blood flow becomes dominant (33).
Recent advances in imaging have enabled clinicians to evaluate the hemodynamics of hepatocellular lesions, in particular dysplastic nodules or HCC, using CT or MRI hepatic arteriography and CT or MRI during arterial portography (34). As a tumor with a typically good blood supply, HCC exhibits hyper-enhancement on DSA in the majority of cases; more specifically, it demonstrates marked hyper-enhancement during the arterial phase, with such enhancement gradually decreasing during the venous phase and disappearing during the delayed phase (35). To the best of our knowledge, few studies have been performed to investigate the hemodynamic characteristics of HCC treated with sorafenib. In addition, the hemodynamic changes following combination treatment with sorafenib and TACE have not been well-evaluated radiologically. The present study compared the diameter of different hepatic arteries in patients treated sorafenib combined with TACE or TACE alone (36). Previously, the response of HCC to treatment has been primarily evaluated using the mRECIST criteria. However, the staining of DSA images and the structure of the vascular network prior to and following interventional therapy are priority concerns for interventional radiologists. In the present retrospective study, DSA provided a useful evaluation tool for assessing the response of HCC to combination treatment with TACE and sorafenib (37,38).
In conclusion, TACE and sorafenib are used individually in the treatment of HCC at different stages, and the two treatments have an anti-angiogenic effect, with sorafenib exhibiting broader effects due to its capacity for tyrosine kinase inhibition. The results of the present study indicated improved hepatic hemodynamic outcomes when TACE and sorafenib were used in combination for the treatment of advanced-stage HCC, compared with TACE alone.
Acknowledgements
The authors would like to thank Dr Xinhua Zhou for aiding with data analysis.
Funding
This work was supported by the Project of National Natural Science Foundation of China (no. 81371698).
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YFZ, HZ, HP, QL, XZ, WW, YL, MZ and JW collected and analyzed the data. YLZ, LC and JZ designed the research and wrote the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Zhongshan Hospital Xiamen University. Patients provided written informed consent prior to treatment.
Consent for publication
Patients provided written informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Elserag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Wang JS, Huang T, Su J, Liang F, Wei Z, Liang Y, Luo H, Kuang SY, Qian GS, Sun G, et al. Hepatocellular carcinoma and aflatoxin exposure in Zhuqing Village, Fusui County, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2001;10:143–146. [PubMed] [Google Scholar]
- 3.Alloatti A, Testero SA, Uttaro AD. Chemical evaluation of fatty acid desaturases as drug targets in Trypanosoma cruzi. Int J Parasitol. 2009;39:985–993. doi: 10.1016/j.ijpara.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Boas FE, Kamaya A, Do B, Desser TS, Beaulieu CF, Vasanawala SS, Hwang GL, Sze DY. Classification of hypervascular liver lesions based on hepatic artery and portal vein blood supply coefficients calculated from triphasic CT scans. J Digit Imaging. 2015;28:213–223. doi: 10.1007/s10278-014-9725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brade AM, Ng S, Brierley J, Kim J, Dinniwell R, Ringash J, Wong RR, Cho C, Knox J, Dawson LA. Phase 1 trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2016;94:580–587. doi: 10.1016/j.ijrobp.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Brock TJ, Browse J, Watts JL. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2006;2:e108. doi: 10.1371/journal.pgen.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia ZZ, Jiang GM, Feng YL. Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J. 2011;26:158–162. doi: 10.1016/S1001-9294(11)60041-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zhang WJ, Guo Z, Wang HB, Wang DD, Zhou JJ, Chen QW. pH-responsive iron manganese silicate nanoparticles as T1-T2* dual-modal imaging probes for tumor diagnosis. ACS Appl Mater Interfaces. 2015;7:5373–5383. doi: 10.1021/acsami.5b00727. [DOI] [PubMed] [Google Scholar]
- 10.Das S, Ladell DS, Podgrabinska S, Ponomarev V, Nagi C, Fallon JT, Skobe M. Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases. Cancer Res. 2010;70:1814–1824. doi: 10.1158/0008-5472.CAN-09-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Costanzo GG, Tortora R, Morisco F, Addario L, Guarino M, Cordone G, Falco L, Caporaso N. Impact of diabetes on outcomes of sorafenib therapy for hepatocellular carcinoma. Target Oncol. 2017;12:61–67. doi: 10.1007/s11523-016-0454-5. [DOI] [PubMed] [Google Scholar]
- 12.Enoch HG, Catalá A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Che. 1976;251:5095–5103. [PubMed] [Google Scholar]
- 13.Funami Y, Okuyama K, Koide Y, Kouzu T, Kinoshita H, Shimada Y, Isono K. Anatomical analysis of small bronchial arteries in front of the trachea by 3D-CT and digital subtraction angiography: Implications for esophageal cancer surgery. Nihon Geka Gakkai Zasshi. 1995;96:207–212. (In Japanese) [PubMed] [Google Scholar]
- 14.Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, Park MS, Kim EH, Seong J, Lee DY, Han KH. Applicability of BCLC stage for prognostic stratification in comparison with other staging systems: single centre experience from long-term clinical outcomes of 1717 treatment-naïve patients with hepatocellular carcinoma. Liver Int. 2012;32:1120–1127. doi: 10.1111/j.1478-3231.2012.02811.x. [DOI] [PubMed] [Google Scholar]
- 15.Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327–10335. doi: 10.3748/wjg.v21.i36.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. SOFIA (SOraFenib Italian Assessment) study group: Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–1342. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Igal RA. Roles of stearoylCoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers (Basel) 2011;3:2462–2477. doi: 10.3390/cancers3022462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Brã C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZL. Research on the security and clinical curative effect of TACE combined with sorafenib in the treatment of hepatocellular carcinoma in the middle and late period. China Foreign Med Treat. 2016;1:126–127. [Google Scholar]
- 21.Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY, Chao Y. Interim analysis of START: Study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer. 2013;132:2448–2458. doi: 10.1002/ijc.27925. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77. doi: 10.1002/hep.510280111. [DOI] [PubMed] [Google Scholar]
- 24.Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339–5345. [PubMed] [Google Scholar]
- 25.Maio MD, Daniele B, Perrone F. Targeted therapies: Role of sorafenib in HCC patients with compromised liver function. Nat Rev Clin Oncol. 2009;6:505–506. doi: 10.1038/nrclinonc.2009.114. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: A meta-analysis. PLoS One. 2014;9:e100305. doi: 10.1371/journal.pone.0100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooka Y, Kanai F, Okabe S, Ueda T, Shimofusa R, Ogasawara S, Chiba T, Sato Y, Yoshikawa M, Yokosuka O. Gadoxetic acid-enhanced MRI compared with CT during angiography in the diagnosis of hepatocellular carcinoma. Magn Reson Imaging. 2013;31:748–754. doi: 10.1016/j.mri.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 30.Numata K, Tanaka K, Kiba T, Saito S, Isozaki T, Hara K, Morimoto M, Sekihara H, Yonezawa H, Kubota T. Using contrast-enhanced sonography to assess the effectiveness of transcatheter arterial embolization for hepatocellular carcinoma. AJR Am J Roentgenol. 2001;176:1199–1205. doi: 10.2214/ajr.176.5.1761199. [DOI] [PubMed] [Google Scholar]
- 31.Koito K, Namieno T, Ichimura T, Hirokawa N, Syonai T, Hareyama M, Katsuramaki T, Hirata K, Nishi M. Power Doppler sonography: Evaluation of hepatocellular carcinoma after treatment with transarterial embolization or percutaneous ethanol injection therapy. AJR Am J Roentgenol. 2000;174:337–341. doi: 10.2214/ajr.174.2.1740337. [DOI] [PubMed] [Google Scholar]
- 32.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 33.Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353–2360. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- 34.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: An explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1002/hep.1840380430. [DOI] [PubMed] [Google Scholar]
- 35.Kudo M. Imaging blood flow characteristics of hepatocellular carcinoma. Oncology. 2002;62(Suppl 1):S48–S56. doi: 10.1159/000048276. [DOI] [PubMed] [Google Scholar]
- 36.Woo HY, Heo J, Yoon KT, Kim GH, Kang DH, Song GA, Cho M. Clinical course of sorafenib treatment in patients with hepatocellular carcinoma. Scand J Gastroenterol. 2012;47:809–819. doi: 10.3109/00365521.2012.683040. [DOI] [PubMed] [Google Scholar]
- 37.Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer. 2015;4:165–175. doi: 10.1159/000367739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan X, Zhai X, Yan Z, Yang P, Li J, Wu D, Wang K, Xia Y, Shen F. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget. 2016;7:83806–83816. doi: 10.18632/oncotarget.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, Ferrari D, Cortesi E, Porta C, Mucciarini C, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: A prospective feasibility analysis. Ann Oncol. 2013;24:406–411. doi: 10.1093/annonc/mds343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.