Abstract
Non-small cell lung cancer (NSCLC) is the most common subtype of lung cancer worldwide. The high mortality rate of NSCLC is due to a limited number of diagnosis being made at an early stage of disease. Therefore, the development of a novel biological marker for the diagnosis and prognosis prediction of NSCLC remains urgent. Current literature shows that microRNA-21 (miRNA-21/miR-21), as an oncogenic miRNA, is involved in the growth, metastasis and apoptosis of NSCLC cells through its control of various target molecules and signaling pathways. Notably, a growing body of evidence further shows that miR-21 is closely associated with the prognosis prediction, recurrence and diagnosis of cancer patients, indicating that miR-21 may be a novel promising biomarker for the diagnosis and prognosis prediction of NSCLC. The present review aimed to provide a summary of recent findings on the associated progression toward finding a novel biomarker for NSCLC.
Keywords: microRNA-21, non-small cell lung cancer, prognosis, metastasis, diagnosis
1. Introduction
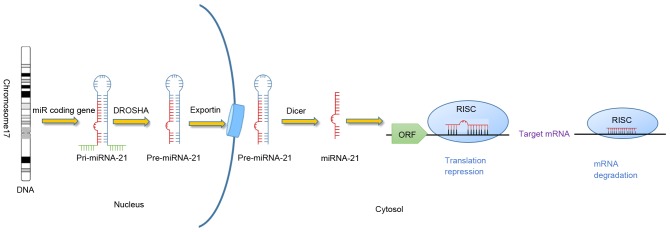
MicroRNA-21 (miRNA-21/miR-21), a member of the miRNA family, is encoded by the MIR21 gene located on chromosome 17q23.2 in humans (1). The mature miR-21, which is formed from endogenous non-coding RNA molecules of ~22 nucleotides, is incorporated into an RNA-induced silencing complex, which binds to the 3′-untranslated region of various target mRNAs through imperfect base pairing with the miRNA (Fig. 1). The expression of miR-21 is significantly increased in a number of solid tumors, including lung, breast, colon, gastric and pancreatic cancer (2–9). Moreover, in previous studies, miR-21 was also upregulated in immune cells, promoted immune-related inflammatory diseases and played important roles in the pathogenesis of autoimmune diseases, including systemic lupus erythematosus, multiple sclerosis and type 1 diabetes (10–13). Emerging studies have shown that miR-21, as an oncogenic miRNA (oncomiR), is upregulated in non-small cell lung cancer (NSCLC) and can regulate the growth, metastasis and apoptosis, as well as the genetic instability, of cancer cells through altering the expression of various target molecules, such as phosphatase and tensin homolog, programmed cell death 4, Purinergic Receptor P2× 7 and phosphoinositide 3-kinase (14–18). Importantly, even though the underlying mechanism of the expression of miR-21 remains largely unknown, a large number of studies have investigated the potential value of miR-21 expression in the prognosis prediction and diagnosis of NSCLC, indicating its potential use as a novel biomarker for cancer diagnosis, recurrence and prognosis prediction, which may harbor relevant clinical implications.
Figure 1.
miR-21 biogenesis. miR-21 is transcribed from chromosome 17 into pri-miR-21 transcripts, which are processed into hairpin precursor molecules (pre-miR-21) inside the nucleus. These are then further processed into mature miR-21 sequences in the cytoplasm of eukaryotic cells, which are incorporated into the RISC and guided to miR-21 target mRNAs to repress their expression. miR, microRNA; pre-miR, precursor miRNA; pri-miR, primary miRNA; RISC, RNA-induced silencing complex.
2. miR-21 and the prognosis of NSCLC
Tissue miR-21 expression and the prognosis prediction of NSCLC
In 2006, Yanaihara et al (19) was the first study to examine miRNA expression in frozen lung adenocarcinoma tissues and normal lung tissues using the microarray technique. The results showed that the expression level of 5 microRNAs, miR-155, miR-17, miR-21, miR-145 and let-7a, differed significantly among the various tissues from NSCLC patients, which may be associated with patient mortality. In particular, the expression of the 3 oncomiRs, miR-21, miR-17 and miR-155, was significantly elevated in the adenocarcinoma tissues. Further analysis (20) suggested that high miR-21 expression was closely associated with the prognosis and progression of early NSCLC [Tumor-Node-Metastasis (TMN) stage I] (21), supporting the hypothesis that abnormal miR-21 expression may be critical for the prognosis prediction of NSCLC (22,23). Furthermore, a recent case-control study performed by Cinegaglia et al (24) measured the expression of miR-21 in 24 fresh frozen tissues (17 lung adenocarcinoma and 7 normal tissues) by TaqMan quantitative polymerase chain reaction (qPCR). The data were subsequently compared with the published database of Mirbase (http://www.mirbase.org) and it was found that high miR-21 expression was closely correlated with a worse prognosis in NSCLC patients, further confirming the potential value of miR-21 expression as a prognostic biomarker in NSCLC.
A study on miRNA-21 expression in formalin-fixed paraffin-embedded tissues (FFPETs) also yielded notable findings. Tian et al (25) determined the level of miRNA-21 expression in 204 pairs of FFPET samples (including cancer tissue samples and their corresponding adjacent normal tissues), using TaqMan qPCR, and found that miR-21 expression was significantly increased in the NSCLC tissues compared with the adjacent normal tissues. The miR-21 expression in late NSCLC (stage IIb-IIIa) tissues [mean ± standard deviation (SD), 7.9±2.1] was much higher than that in early NSCLC (stage Ia-IIa) tissues (mean ± SD, 5.1±1.6). Patients with high miR-21 expression also had higher rates of lymph node metastasis, while no correlation was found between miR-21 expression and tumor size. A Kaplan-Meier survival analysis further confirmed that the overall survival time (OS) and the progression-free survival time (PFS) were significantly reduced in the NSCLC patients with high miR-21 expression compared with those in patients with low miR-21 expression. Finally, multiple logistic regression analysis indicated that high miR-21 expression was a novel independent prognostic factor for the reduced OS of NSCLC patients. Given the reliability of FFPETs in clinical pathology, it may be concluded that these results indicate that the regular measurement of miRNA-21 expression may represent an appropriate approach to the prognosis prediction of NSCLC.
Serum miR-21 expression and the prognosis prediction of NSCLC
Recent studies have suggested that the level of serum-distinct miRNA molecules may be a useful biomarker for the prognosis prediction of various cancer types (26–29). In particular, the association between the level of serum miR-21 and the prognosis prediction of NSCLC has become the focus of a number of studies. The study by Wang et al (30) found that the serum miR-21 level in NSCLC patients were markedly higher than those in healthy individuals. The 3-year survival rate of the NSCLC patients with high serum miR-21 expression was significantly decreased compared with that of the patients with low serum miR-21 expression. Moreover, the study also noted a positive association between high levels of serum miR-21 and TNM staging and lymph node metastases in NSCLC patients. Based on a multiple logistic regression analysis of the OS in NSCLC patients, the serum miR-21 level is an independent prognostic factor for the disease (relative risk, 2.01). Additionally, Liu et al (31) reported that serum level of miR-21 was consistent with the cancer tissue level of miR-21 in the prognosis of NSCLC patients.
Notably, in a recent study, using a receiver operating characteristic curve to evaluate the prognostic value of serum miR-21 expression in NSCLC patients, Zhao et al (32) demonstrated that the sensitivity and specificity of using the relative expression of miR-21 in the prognosis of NSCLC patients were up to 73.8 and 71.1%, respectively, when the cut-off value was set at 1.22. When the NSCLC patients were separated based on this cut-off value, a Kaplan-Meier analysis indicated that the survival time of 23.1 months in the high miR-21 expression group (≥1.22) was significantly reduced compared with the 34.3 months recorded in the low miR-21 expression group (<1.22). In addition, Pearson's correlation analysis demonstrated that serum miR-21 levels were negatively correlated with the survival of NSCLC patients (r=−0.508). Since blood collection is an easy procedure to perform, these findings suggested that combining serum miR-21 detection with the evaluation of associated clinical information may become an important direction in predicting the prognosis of NSCLC.
3. miR-21 expression and cancer recurrence and metastasis in NSCLC patients
In 2013, Yang et al (33) detected 141 miRNAs in the cancer tissue from NSCLC patients and found significantly abnormal expression of 4 of these, namely miR-155, miR-21, miR-34 and let-7. Subsequent hazard ratio (HR) analyses further indicated that miR-21 expression was not only negatively correlated with the OS (HR, 2.32), but that it was also negatively correlated with recurrence-free survival (HR, 2.43), indicating that miR-21 also has a clinical value in predicting cancer recurrence in NSCLC patients (34).
Accumulating evidence has suggested a potential value of cancer cell-derived exosomes in reflecting the development of various types of cancer. Consistent with this research, a recent study by Munagala et al (35) found that miR-21 levels in cancer exosomes could also be a biomarker of the recurrence of lung cancer. The study cultured a normal lung cell line (Beas-2b) and a lung cancer cell line (H1299) in vitro, and analyzed the miRNA expression profiles between cells and their exosomes. Data showed that there were significant changes in the expression of 77 miRNAs (including miR-21) in the H1299 cells compared with that in the Beas-2b cells. Notably, the miR-21 level in the exosomes from the H1299 cells was also significantly increased. Primary and recurrent xenograft lung cancer nude mouse models were then established and the significantly upregulated expression of miR-21 in the recurrent tumor tissues compared with the primary tumor tissues was observed. Moreover, miRNA expression, including miR-21, in the serum exosomes was also detected and a consistent result was obtained. This study suggested that miR-21 expression in serum exosomes could also be employed as a potential biomarker for recurrent lung cancer.
Previous studies have found that miR-21 expression may also be a potential prognostic marker in NSCLC with lymph node metastases. Stenvold et al (36) collected 335 tumor tissues from patients with NSCLC stage I to IIIa and measured the level of miR-21 in the tumor cells and basal cells using in situ hybridization. The study demonstrated that high miR-21 expression in the tumor cells was a positive prognostic indicator in the patients with lymph node metastases. By contrast, high miR-21 expression in the basal cells was an adverse prognostic indicator in the patients without lymph node metastases. A multiple logistic regression analysis further demonstrated that a low miR-21 level in the cancer cells was an independent adverse prognostic indicator in the patients with lymph node metastases (HR, 2.03). However, it is important to note that the findings from this study were inconsistent with those from other studies, in which it had been reported that miR-21 plays a role as an oncogene in regulating lung cancer metastases. Therefore, further studies are required to elucidate the reasons for such disparities. We hypothesize that the mechanism of differential miR-21 expression in the cancer and basal cells, which was associated with the abnormal activity of multiple cancer-related regulators, such as the signal transduction and activation of signal transducer and activator of transcription 3, activator protein 1, transforming growth factor β, and epidermal growth factor receptor (25), may have contributed to this unexpected difference.
4. miR-21 expression and an early diagnosis of NSCLC
Plasma miR-21 level and an early diagnosis of NSCLC
Although the detection of the plasma miR-21 level alone is not useful for an early diagnosis in NSCLC patients (37,38), recent studies have suggested that the combined detection of miR-21 and other associated molecules could improve the accuracy and specificity of an early NSCLC diagnosis. Tang et al (39) examined the abnormal plasma expression of 3 miRNAs (miR-21, miR-145 and miR-155) in 62 lung cancer patients and 60 smokers by qPCR. The area under the curve (AUC) analysis found that the combination of these 3 miRNAs had a high diagnostic rate for lung cancer (AUC, 0.874), with a sensitivity and specificity of up to 69.4 and 78.3%, respectively. The study then validated the diagnostic effect of these 3 miRNAs in 34 lung cancer patients, 30 patients with benign pulmonary nodules and 32 healthy smokers, and found that the combination of these miRNAs not only distinguished the lung cancer patients from the healthy smokers (AUC, 0.872; sensitivity, 76.5%; specificity, 81.3%), but also distinguished the patients with lung cancer from those with benign pulmonary nodules (AUC, 0.841; sensitivity, 76.5%; specificity, 80.0%). This study suggested that the detection of a combination of plasma miR-21, miR-145 and miR-155 could be a novel strategy for the early non-invasive screening of lung cancer.
Furthermore, researchers have also examined the potential diagnostic values of different combinations of miR-21 and other molecules in NSCLC. Geng et al (40) tested the expression of 5 miRNAs (miR-20a, miR-223, miR-21, miR-221 and miR-145) in plasma samples from 126 patients with early lung cancer, 42 patients with non-tumor lung diseases and 60 healthy volunteers, and analyzed the value of this combination in the early diagnosis of NSCLC. The results indicated that the AUC when using these 5 miRNAs independently in the diagnosis of early NSCLC were 0.89, 0.94, 0.77, 0.92 and 0.77, respectively. Furthermore, the plasma levels of miR-20a, miR-223, miR-21 and miR-145 could improve the prediction of NSCLC in smokers. Further analysis also revealed that the combination of these 5 miRNAs could more accurately distinguish the clinical stages and pathological types of NSCLC, in particular the subtypes of squamous cell carcinoma (40). In summary, these studies indicate that the measurement of the plasma miR-21 level could be an important complement to the early diagnosis of NSCLC patients.
Bronchoalveolar lavage fluid (BAL) miR-21 level in sputum and the early diagnosis of NSCLC
A recent study revealed that the combined detection of miR-21 and other associated molecules in the sputum and BAL can be used in the diagnosis of NSCLC. Kim et al (41) examined sputum samples from 27 NSCLC patients and 11 healthy individuals, and a clustering analysis indicated that the sensitivity and specificity of using a combination of 5 miRNAs (miR-21, miR-143, miR-155, miR-210 and miR-372) in the early diagnosis of NSCLC were up to 67.8 and 90%, respectively. Meanwhile, the sensitivity and specificity of this miRNA combination in the BAL group were up to 86.7 and 100%, respectively, indicating that the miRNA expression profiles in sputum and BAL are potential biomarkers for the early diagnosis of NSCLC.
Based on the aforementioned study, a recent study by Razzak et al (42) examined the potential value of using 3 of the aforementioned miRNAs (miR-21, miR-210 and miR-372) in the early diagnosis of NSCLC. Sputum samples were collected from 21 early NSCLC patients (stages I and II), 22 late NSCLC patients (stage III and above) and 10 healthy controls. Clustering analysis further revealed that the sensitivity and specificity of using a combination of these 3 miRNAs in the diagnosis of early NSCLC were up to 67 and 90%, respectively, and that for late NSCLC, the sensitivity and specificity were up to 64 and 100%, respectively. Therefore, this study raised a notable point that, although the sensitivity of using the detection of 3 miRNAs in the diagnosis of late NSCLC was less than that of 5 miRNAs, their sensitivity and specificity in the diagnosis of early NSCLC remained relatively high. In clinical testing, detecting fewer miRNAs reduces the difficulty of performing the test, and thus, this study suggested that optimizing the combination of specific miRNAs in sputum samples for the early diagnosis of NSCLC will be an important focus in future studies.
Serum miR-21 level and an early diagnosis of NSCLC
It is worth mentioning that a recent studies have revealed that serum miR-21 level also have a potential value in the early diagnosis of NSCLC. Yang et al (43) examined the abnormal expression of 4 miRNAs (miR-21, miR-148a, miR-148b and miR-152) in 152 NSCLC patients and 300 healthy individuals using qPCR, and AUC analysis showed that the combination of these miRNAs (AUC, 0.98) had a higher accuracy in the early diagnosis of NSCLC than a single miRNA (AUC, 0.81, 0.86, 0.90 and 0.82, respectively). In particular, the sensitivity and specificity of this combination in the diagnosis of early NSCLC were up to 96 and 91%, respectively, which was significantly higher than the miR-21 combinations from the sputum and the BALs used in the aforementioned studies. These data indicate that, in the future, the detection of an miR-21 combination in serum will be a useful method for the early diagnosis of NSCLC.
5. Conclusion
Recent studies have demonstrated that miR-21 is closely associated with the prognosis, recurrence, metastases and diagnosis of NSCLC, and may be applied as a potential biomarker in the disease (Table I). However, there remain a number of problems that are yet to be elucidated; for example, whether or not miR-21 is associated with drug resistance in NSCLC, the regulatory mechanism of miR-21 in NSCLC, and how to develop miR-21-based clinical and biological therapeutic strategies for treating NSCLC. Further investigation of these questions will not only aid in elucidating the mechanism of NSCLC occurrence, but will also have significant implications for the development of associated clinical diagnostic methods, and novel therapeutic targets and strategies.
Table I.
miR-21 expression in early diagnosis, prognosis and recurrence, as well as metastases, in non-small cell lung cancer patients.
| Type of samples | Early diagnosis | Prognosis | Recurrence | Metastases |
|---|---|---|---|---|
| Plasma | miR-21/145/155 (38) miR-21/221/223/145/20a (39) | – | miR-21 (32,33) | – |
| BAL | miR-21/143/155/210/372 (40) | – | – | – |
| Sputum | miR-21/210/372 (40,41) | – | – | – |
| Serum | miR-21/148a/148b/152 (42) | miR-21 (29,31) | miR-21 (32–34) | miR-21 (29) |
| Frozen tumor tissues | – | miR-21 (19,20) | miR-21 (32,33) | – |
| Quick-frozen tumor tissues | – | miR-21 (23) | miR-21 (32,33) | – |
| FFPETs | – | miR-21 (24) | miR-21 (32,33) | miR-21 (24,35) |
Number in parenthesis () indicates the corresponding reference. BAL, bronchoalveolar lavage fluid; FFPET, formalin-fixed paraffin-embedded tissues; miR, microRNA.
Acknowledgements
The authors would like to thank Professor Wei Xu (Soochow University, Suzhou, Jiangsu, China) for providing valuable suggestions on the writing of the original manuscript.
Funding
This study was supported by Program for Program for High level innovative talents in Guizhou Province (QKH-RC-2016-4031), New Century Excellent Talents in University, Ministry of Education of China (NCET-12-0661), National Natural Science foundation of China (31760258), Program for Excellent Young Talents of Zunyi Medical University (15ZY-001) and Project of Guizhou Provincial Department of Science and Technology (2009C491).
Availability of data and materials
Not applicable.
Authors' contributions
WZ wrote the initial draft and designed the outline of the manuscript. JJZ, YT and MG designed the outline and revised the manuscript. YZ, CC, NQ, JZ and JL revised and expanded the manuscript. LX designed the outline of manuscript and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Liu H, Jin W, Ding Z, Zheng S, Yu Y. Tissue microRNA-21 expression predicted recurrence and poor survival in patients with colorectal cancer-a meta-analysis. Onco Targets Ther. 2016;9:2615–2624. doi: 10.2147/OTT.S103893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y, Cai Q, Huang Y, Li S, Yang H, Sun L, Chen K, Wang Y. MicroRNA-21 as a potential diagnostic biomarker for breast cancer patients: A pooled analysis of individual studies. Oncotarget. 2016;7:34498–34506. doi: 10.18632/oncotarget.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu ZH, Tao ZH, Zhang J, Li T, Ni C, Xie J, Zhang JF, Hu XC. MiRNA-21 induces epithelial to mesenchymal transition and gemcitabine resistance via the PTEN/AKT pathway in breast cancer. Tumour Biol. 2016;37:7245–7254. doi: 10.1007/s13277-015-4604-7. [DOI] [PubMed] [Google Scholar]
- 5.Hu GY, Tao F, Wang W, Ji KW. Prognostic value of microRNA-21 in pancreatic ductal adenocarcinoma: A meta-analysis. World J Surg Oncol. 2016;14:82. doi: 10.1186/s12957-016-0842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang S, Wang R, Yan H, Jin L, Dou X, Chen D. MicroRNA-21 modulates radiation resistance through upregulation of hypoxia-inducible factor-1α-promoted glycolysis in non-small cell lung cancer cells. Mol Med Rep. 2016;13:4101–4107. doi: 10.3892/mmr.2016.5010. [DOI] [PubMed] [Google Scholar]
- 7.Yan J, Liu T, Zhou X, Dang Y, Yin C, Zhang G. FZD6, targeted by miR-21, represses gastric cancer cell proliferation and migration via activating non-canonical wnt pathway. Am J Transl Res. 2016;8:2354–2364. [PMC free article] [PubMed] [Google Scholar]
- 8.Tao YJ, Li YJ, Zheng W, Zhao JJ, Guo MM, Zhou Y, Qin NL, Zheng J, Xu L. Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells. Cancer Cell Int. 2015;15:77. doi: 10.1186/s12935-015-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sha M, Ye J, Luan ZY, Guo T, Wang B, Huang JX. Celastrol induces cell cycle arrest by MicroRNA-21-mTOR-mediated inhibition p27 protein degradation in gastric cancer. Cancer Cell Int. 2015;15:101. doi: 10.1186/s12935-015-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husakova M. MicroRNAs in the key events of systemic lupus erythematosus pathogenesis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:327–342. doi: 10.5507/bp.2016.004. [DOI] [PubMed] [Google Scholar]
- 11.Sanders KA, Benton MC, Lea RA, Maltby VE, Agland S, Griffin N, Scott RJ, Tajouri L, Lechner-Scott J. Next-generation sequencing reveals broad down-regulation of microRNAs in secondary progressive multiple sclerosis CD4+ T cells. Clin Epigenetics. 2016;8:87. doi: 10.1186/s13148-016-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao XY, Shao K. Roles of MicroRNA-21 in the pathogenesis of insulin resistance and diabetic mellitus-induced non-alcoholic fatty liver disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:144–149. doi: 10.3881/j.issn.1000-503X.2016.02.004. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 13.Sekar D, Venugopal B, Sekar P, Ramalingam K. Role of microRNA 21 in diabetes and associated/related diseases. Gene. 2016;582:14–18. doi: 10.1016/j.gene.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Guo Q, Zhang H, Zhang L, He Y, Weng S, Dong Z, Wang J, Zhang P, Nao R. MicroRNA-21 regulates non-small cell lung cancer cell proliferation by affecting cell apoptosis via COX-19. Int J Clin Exp Med. 2015;8:8835–8841. [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Zang A, Jia Y, Zhang J, Fan W, Feng J, Duan M, Zhang L, Huo R, Jiao J, Zhu X. Triptolide reduces proliferation and enhances apoptosis of human non-small cell lung cancer cells through PTEN by targeting miR-21. Mol Med Rep. 2016;13:2763–2768. doi: 10.3892/mmr.2016.4844. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Meng H, Peng Q, Yang X, Gan R, Zhao L, Chen Z, Lu J, Meng QH. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22:23–29. doi: 10.1038/cgt.2014.66. [DOI] [PubMed] [Google Scholar]
- 17.Boldrini L, Giordano M, Ali G, Melfi F, Romano G, Lucchi M, Fontanini G. P2X7 mRNA expression in non-small cell lung cancer: MicroRNA regulation and prognostic value. Oncol Lett. 2015;9:449–453. doi: 10.3892/ol.2014.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB, Luo XL, Zhu XL, Liu C, Xu FP, Luo DL, et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol. 2016;48:471–484. doi: 10.3892/ijo.2015.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Saito M, Schetter AJ, Mollerup S, Kohno T, Skaug V, Bowman ED, Mathé EA, Takenoshita S, Yokota J, Haugen A, Harris CC. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: A retrospective analysis of three cohorts. Clin Cancer Res. 2011;17:1875–1882. doi: 10.1158/1078-0432.CCR-10-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 22.Zhu W, Xu B. MicroRNA-21 identified as predictor of cancer outcome: A meta-analysis. PLoS One. 2014;9:e103373. doi: 10.1371/journal.pone.0103373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma XL, Liu L, Liu XX, Li Y, Deng L, Xiao ZL, Liu YT, Shi HS, Wei YQ. Prognostic role of microRNA-21 in non-small cell lung cancer: A meta-analysis. Asian Pac J Cancer Prev. 2012;13:2329–2334. doi: 10.7314/APJCP.2012.13.5.2329. [DOI] [PubMed] [Google Scholar]
- 24.Cinegaglia NC, Andrade SC, Tokar T, Pinheiro M, Severino FE, Oliveira RA, Hasimoto EN, Cataneo DC, Cataneo AJ, Defaveri J, et al. Integrative transcriptome analysis identifies deregulated microRNA-transcription factor networks in lung adenocarcinoma. Oncotarget. 2016;7:28920–28934. doi: 10.18632/oncotarget.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L, Shan W, Zhang Y, Lv X, Li X, Wei C. Up-regulation of miR-21 expression predicate advanced clinicopathological features and poor prognosis in patients with non-small cell lung cancer. Pathol Oncol Res. 2016;22:161–167. doi: 10.1007/s12253-015-0004-y. [DOI] [PubMed] [Google Scholar]
- 26.Vychytilova-Faltejskova P, Radova L, Sachlova M, Kosarova Z, Slaba K, Fabian P, Grolich T, Prochazka V, Kala Z, Svoboda M, et al. Serum-based microRNA signatures in early diagnosis and prognosis prediction of colon cancer. Carcinogenesis. 2016;37:941–950. doi: 10.1093/carcin/bgw078. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Jiang R, Li J, Wang B, Ma C, Lv Y, Mu N. MicoRNA-425-5p is a potential prognostic biomarker for cervical cancer. Ann Clin Biochem. 2017;54:127–133. doi: 10.1177/0004563216649377. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Du L, Duan W, Wang R, Yan K, Wang L, Li J, Zheng G, Zhang X, Yang Y, Wang C. Serum microRNA expression signatures as novel noninvasive biomarkers for prediction and prognosis of muscle-invasive bladder cancer. Oncotarget. 2016;7:36733–36742. doi: 10.18632/oncotarget.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu HT, Hasan AM, Liu RB, Zhang ZC, Zhang X, Wang J, Wang HY, Wang F, Shao JY. Serum microRNA profiles as prognostic biomarkers for HBV-positive hepatocellular carcinoma. Oncotarget. 2016;7:45637–45648. doi: 10.18632/oncotarget.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J Surg Oncol. 2011;104:847–851. doi: 10.1002/jso.22008. [DOI] [PubMed] [Google Scholar]
- 31.Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ, Wang YK, Zeng F, Zhou JH, Zhang YK. High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29:618–626. doi: 10.1007/s12032-011-9923-y. [DOI] [PubMed] [Google Scholar]
- 32.Zhao W, Zhao JJ, Zhang L, Xu QF, Zhao YM, Shi XY, Xu AG. Serum miR-21 level: A potential diagnostic and prognostic biomarker for non-small cell lung cancer. Int J Clin Exp Med. 2015;8:14759–14763. [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Shen H, Qiu C, Ni Y, Wang L, Dong W, Liao Y, Du J. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur J Cancer. 2013;49:604–615. doi: 10.1016/j.ejca.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Li J, Tong L, Zhang J, Zhai A, Xu K, Wei L, Chu M. The prognostic value of miR-21 and miR-155 in non-small-cell lung cancer: A meta-analysis. Jpn J Clin Oncol. 2013;43:813–820. doi: 10.1093/jjco/hyt084. [DOI] [PubMed] [Google Scholar]
- 35.Munagala R, Aqil F, Gupta RC. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumour Biol. 2016;37:10703–10714. doi: 10.1007/s13277-016-4939-8. [DOI] [PubMed] [Google Scholar]
- 36.Stenvold H, Donnem T, Andersen S, Al-Saad S, Valkov A, Pedersen MI, Busund LT, Bremnes RM. High tumor cell expression of microRNA-21 in node positive non-small cell lung cancer predicts a favorable clinical outcome. BMC Clin Pathol. 2014;14:9. doi: 10.1186/1472-6890-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu R, Jiang Y, Wu Q, Li Q, Cheng D, Xu L, Zhang C, Zhang M, Ye L. Diagnostic value of microRNA-21 in the diagnosis of lung cancer: Evidence from a meta-analysis involving 11 studies. Tumour Biol. 2014;35:8829–8836. doi: 10.1007/s13277-014-2106-7. [DOI] [PubMed] [Google Scholar]
- 38.Meng X, Xiao C, Zhao Y, Jia L, Tang Y, Li D. Meta-analysis of microarrays: Diagnostic value of microRNA-21 as a biomarker for lung cancer. Int J Biol Markers. 2015;30:e282–e285. doi: 10.5301/jbm.5000153. [DOI] [PubMed] [Google Scholar]
- 39.Tang D, Shen Y, Wang M, Yang R, Wang Z, Sui A, Jiao W, Wang Y. Identification of plasma microRNAs as novel noninvasive biomarkers for early detection of lung cancer. Eur J Cancer Prev. 2013;22:540–548. doi: 10.1097/CEJ.0b013e32835f3be9. [DOI] [PubMed] [Google Scholar]
- 40.Geng Q, Fan T, Zhang B, Wang W, Xu Y, Hu H. Five microRNAs in plasma as novel biomarkers for screening of early-stage non-small cell lung cancer. Respir Res. 2014;15:149. doi: 10.1186/s12931-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JO, Gazala S, Razzak R, Guo L, Ghosh S, Roa WH, Bedard ELR. Non-small cell lung cancer detection using microRNA expression profiling of bronchoalveolar lavage fluid and sputum. Anticancer Res. 2015;35:1873–1880. [PubMed] [Google Scholar]
- 42.Razzak R, Bédard EL, Kim JO, Gazala S, Guo L, Ghosh S, Joy A, Nijjar T, Wong E, Roa WH. MicroRNA expression profiling of sputum for the detection of early and locally advanced non-small-cell lung cancer: A prospective case-control study. Curr Oncol. 2016;23:e86–e94. doi: 10.3747/co.23.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu RX, Liu SH, Yi QT, Li J, Song CH. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol. 2015;36:3035–3042. doi: 10.1007/s13277-014-2938-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.